Abstract
In the past decade, the number of epidemiological publications addressing environmental chemical exposures and autism has grown tremendously. These studies are important because it is now understood that environmental factors play a larger role in causing autism than previously thought and because they address modifiable risk factors that may open up avenues for the primary prevention of the disability associated with autism. In this review, we covered studies of autism and estimates of exposure to tobacco, air pollutants, volatile organic compounds and solvents, metals (from air, occupation, diet, dental amalgams, and thimerosal-containing vaccines), pesticides, and organic endocrine-disrupting compounds such as flame retardants, non-stick chemicals, phthalates, and bisphenol A. We included studies that had individual-level data on autism, exposure measures pertaining to pregnancy or the 1st year of life, valid comparison groups, control for confounders, and adequate sample sizes. Despite the inherent error in the measurement of many of these environmental exposures, which is likely to attenuate observed associations, some environmental exposures showed associations with autism, especially traffic-related air pollutants, some metals, and several pesticides, with suggestive trends for some volatile organic compounds (e.g., methylene chloride, trichloroethylene, and styrene) and phthalates. Whether any of these play a causal role requires further study. Given the limited scope of these publications, other environmental chemicals cannot be ruled out, but have not yet been adequately studied. Future research that addresses these and additional environmental chemicals, including their most common routes of exposures, with accurate exposure measurement pertaining to several developmental windows, is essential to guide efforts for the prevention of the neurodevelopmental damage that manifests in autism symptoms.
Introduction and Scope of Review
Autism Spectrum Disorder, commonly called autism, is now known to occur in about 1 in 68 children in the U.S.,1 increasing the likelihood that clinicians will care for children, adolescents, and adults with autism. All people with autism have difficulties in social communication and restricted interests and behaviors. The severity of the symptoms and the level of functional impairment vary widely. A review in this journal has covered the important topics of screening/early warning signs, the role of the pediatrician within a multidisciplinary team, and the evidence base for treatments,2 with another review covering pharmacological interventions, genetic testing, and treatment across the life course.3
Herein, we focus on processes occurring earlier in the life history of autism—exploring xenobiotic risk factors that tip the balance to cause the emergence of autism symptoms in a child. We have focused on environmental chemicals; agents that arise outside of the human body; and enter via the routes of ingestion, inhalation, dermal absorption, injection, and placental transport from mother to fetus. Other risk factors fit into the broader definition of environment and are likely important, such as nutrients, medications, obstetric complications, maternal medical conditions, and social/demographic influences, but were not included in this review.
The traditional environmental chemical exposures that we included are important in part because exposure to these factors can be reduced, opening up viable avenues for the primary prevention of autism. Increasingly, clinicians are called upon to play a role in identifying, researching, educating about, and advocating for change regarding these modifiable chemical exposures. For example, parents may desire guidance from clinicians regarding the potential risk to their fetus or infant from living with someone who smokes cigarettes or from the use of plastics or residential pesticides. Exercising behavioral or consumer choices, however, cannot entirely protect a patient from these widespread exposures, especially for chemicals that are ubiquitous, such as air pollution, or for contaminants that are unknown to the patient.
Environmental chemical exposures are increasingly understood to be important in causing autism, with current theories positing that autism is caused by the interplay of multiple genetic and environmental contributions that differ from individual to individual.4,5 While initial studies suggested a strong genetic heritability of autism, recent studies with larger sample sizes have demonstrated a lesser influence, including a study of over 14,000 children with autism in Sweden that demonstrated a heritability of 50%, supporting an equally strong role for environmental risk factors.6 Genetic and environmental factors may combine to disrupt the normal processes of nervous system development, interfering with neuron formation and migration, synapse formation, or neurological connectivity, ultimately causing autism. Environmental chemical exposures may act through pathophysiologies, including the direct disruption of cells and structures of the nervous system, endocrine hormone- or immune system-mediated impacts, epigenetic changes, and more (Table 1). The important role for environmental chemical exposures in these processes has received data support and increased attention.7–9 These calls for research are bolstered by the dearth of understanding of the role of our complex, human-created chemical environment on development, with estimates that, out of a chemical universe topping 80,000 agents, over 1000 have laboratory evidence of neurotoxicity, but only a small fraction have been studied in humans during critical windows of development.10,11 Human exposures to these chemicals are common: 250 xenobiotic chemicals were detected in biological samples from a 2013 representative sample of the U.S. in the National Health and Nutrition Examination Survey.12 Furthermore, chemical mixtures predominate; in earlier data from this same study, 100% of pregnant women had detectable levels of five chemical classes that were examined.13
TABLE 1.
Pathophysiological mechanisms hypothesized to mediate relationships between xenobiotic exposures and autism
| Mechanism | Rationale |
|---|---|
| Immune | Close connection between immune cells and neurons during development, and evidence of immune alterations in children with autism, such as elevated cytokine levels.168–171 |
| Endocrine | The male preponderance of autism, combined with requirements for thyroid and other endocrine hormones for optimal neurodevelopment.146,172 |
| Epigenetic | Epigenetic alterations such as altered methylation act at the intersection between genetic and environmental factors and are implicated in the etiology of autism, including markers of reduced methylation capacity observed in children with autism and their mothers.173–178 |
| Microbiome | Microbiome may regulate host immunity to pathogenic microbial or xenobiotic exposures and microbiota may differ in children with autism.179,180 |
| Mitochondrial | Mitochondrial dysregulation has been shown in children with autism.181,182 |
Although a mere decade ago publications addressing the environment and autism could almost not be found, now epidemiological publications have examined the association of autism with estimates of exposure to tobacco, air pollutants, volatile organic compounds, metals, pesticides, and organic pollutants, such as plasticizers and non-stick chemicals. The state of evidence regarding autism and exposures to some of these pollutants has been reviewed.14,15 In this review, we provide contextual information about these chemicals, along with an assessment of the current state of epidemiological knowledge regarding exposures to these chemicals and autism, with special attention to assessing validity, integrating results, and evaluating evidence for causality. Our goal is to evaluate and summarize the current state of knowledge, while informing clinicians about the complexities surrounding study in this area together with current approaches. We seek to engage clinicians in the continued identification of, and ultimately, the reduction of, environmental factors that harm neurodevelopment, specifically autism (Box 1).
Box 1. Abbreviations.
CDC: United States Centers for Disease Control and Prevention
EPA: United States Environmental Protection Agency
FDA: United States Food and Drug Administration
DSM: Diagnostic and Statistical Manual of the American Psychiatric Association, various versions
ICD: International Classification of Diseases, morbidity and disease codes, various versions
ASD: autism spectrum disorder
AD: autistic disorder
Asp: Asperger’s disorder
ASD w/ID: ASD with co-occurring intellectual disability
ASD w/o ID: ASD without co-occurring intellectual disability
ASD w/Reg.: ASD with developmental regression
ASD-NOS: ASD not otherwise specified
PDD: pervasive developmental disorder
PDD-NOS: PDD not otherwise specified
OR: odds ratio
95% CI: 95% confidence interval
NATA: National-scale Air Toxics Assessment model of the US EPA
CALINE4: California line source air emissions dispersion model
Review Methodology: Evaluating the Epidemiological Evidence
In this review, we present study design details (in tables) and results (in figures) from valid research reports. Our inclusion criteria are listed in Box 2 and described in greater detail following.
Box 2. Review inclusion criteria.
Primary report of population-based human (epidemiological) research.
English-language peer-reviewed publication prior to March 1, 2014 or manuscript under review if one of us was included as an author.
Odds ratio, slope, statistical test, or similar estimate describing the association between an environmental chemical exposure in regard to autism.
Individual-level data on autism diagnoses or a psychometric measurement of the continuous autism phenotype.
Environmental chemical measurements pertaining to exposures during conception, pregnancy, or the 1st postnatal year.
Valid comparison including appropriate sample selection and accounting for confounders.
Adequate sample sizes to generate precise measures of association, defined as a confidence interval ratio or confidence interval difference <10.
Population-Based Human Studies
This review focuses on evidence regarding environmental chemical exposures and autism from human, population-based literature, that is, epidemiological studies, published as primary research reports in English-language, peer-reviewed journals with publication dates prior to March 1, 2014. We also included articles under review when one of us was an author. Findings from controlled laboratory studies on other animals are important in elucidating mechanisms, screening many exposures, and creating highly controlled conditions that strengthen causal inference, but cannot directly address the full spectrum of autism or the real-world human living condition.
We separately included estimates for multiple chemicals within a publication that met our inclusion criteria, for example, reporting on inhaled diesel particles, metals, and volatile organic compounds from one publication.16 We did not, however, include multiple measures for one chemical-autism question from a given publication, but instead included only the measure most illustrative of the findings, as follows: when results were presented for both the continuous autism phenotype and a dichotomous autism classification, we chose the association that had the most precision, or was of the same type of comparison as the other studies of the same chemical. When exposures were categorized into different levels or geographic distances, we chose either the results emphasized by the authors, or the comparisons that were the most extreme, such as presenting the 4th quartile comparison of an air pollutant rather than the 2nd quartile comparison, or the closest geographic distance to a pesticide application rather than a moderate distance.
Individual-Level Data on Autism Diagnoses or Behaviors
We restricted our scope of review to studies that were able to include individual-level data on autism or the dimensional nature of autism-like symptoms in analyses, excluding those studies that only had access to aggregated data on autism occurrence (e.g., community-level or ecological outcome data). Although ecological studies are useful in generating hypotheses, we excluded such studies because of some inherent limitations in this design. In aggregate outcome data, it cannot be known that the individual experiencing the exposure is the same individual within the group that had an autism diagnosis. Furthermore, it is not possible to account for individual-level confounding influences in this type of design, biases that are critically important to remove before concluding that an observed statistical association represents a causal relationship.17,18 In contrast, we did include studies that measured exposure at the community level (ecological exposures). Community-level exposures (e.g., an air toxic pollutant estimated for a census tract) often serve as reasonable proxies of individual-level exposure, although they include some degree of exposure measurement error. Importantly, when these studies include an individual measure of autism, proper confounding control is possible and they are not subject to the ecological fallacy.
The studies included in this review included data from multi-faceted research studies and from pre-existing records (Table 2). Different means of diagnosing autism or measuring autism symptoms and characteristics (the continuous autism phenotype) were included (Table 3). Measures of the continuous autism phenotype in a general population differ from clinical diagnoses because fewer study children will exhibit symptoms at the upper extreme that would lead to an autism diagnosis, and because the behavioral traits measured may differ from the constructs that led a clinician to diagnose autism. We have included these studies because the psychometric autism screening tests used have been validated, the continuous measures allow for robust statistical properties, all children are tested and so an outside comparison group is not needed, and these studies allow greater feasibility for prospective, and therefore more accurate, measurement of prenatal environmental exposures.
TABLE 2.
Research studies and data sources used in two or more studies of environment and autism
| Abbreviation | Full name | Description | Citation |
|---|---|---|---|
| CHARGE | Childhood Autism Risks from Genetics and the Environment | Case–control study of environmental and genetic causes and risk factors for autism and developmental delay in central California. | 183 |
| ADDM | The Autism and Developmental Disabilities Monitoring Network | A group of programs funded by the CDC to estimate the number of children with autism spectrum disorders and other developmental disabilities living in different areas of the United States. | 1,184 |
| California DDS | Department of Developmental Services | The agency through which the state of California provides free diagnostic and therapeutic services to individuals with developmental disabilities. | 185 |
| VSD | Vaccine Safety Datalink | Established by the CDC to monitor immunization safety, includes linked electronic medical data from three Health Maintenance Organizations in Oregon and California. | 186 |
| FiPS-A | Finnish Prenatal Study of Autism and Autism Spectrum Disorders | A study examining prenatal serologic factors, mediating and moderating developmental antecedents, and autism, using government health data linkages and stored serum samples. | 187 |
TABLE 3.
Psychometric tests to measure autism diagnoses and symptoms used in studies of environment and autism
| Abbreviation | Full Name | Method of Administration | Description |
|---|---|---|---|
| ADI-R | Autism Diagnostic Interview-Revised188 | Parent questionnaire | A 93-item scale assessing language and communications; reciprocal social interactions; and restricted, repetitive, and stereotyped behaviors and interests. |
| ADOS | Autism Diagnostic Observation Schedule189 | Interactive observation by trained person | A semi-structured assessment using various activities allowing observation of social and communication behaviors related to the diagnosis of pervasive developmental disorders. |
| CAST | Childhood Asperger Syndrome Test190 | Parent or teacher questionnaire | A 37-item questionnaire of yes/no question regarding a child’s social behaviors and communication tendencies. |
| ASSQ | Autism Spectrum Screening Questionnaire191 | Parent or teacher questionnaire | A 27-item checklist for completion by lay informants when assessing symptoms characteristic of Asperger’s disorder and other high-functioning ASDs in children and adolescents with normal intelligence or mild mental retardation. |
| SRS | Social Responsiveness Scale192 | Parent or teacher questionnaire | A 65-item rating scale that measures the severity of autism spectrum symptoms by assessing social impairments, social awareness, social information processing, capacity for reciprocal social communication, social anxiety/avoidance, and autistic preoccupations and traits. |
| SCQ | Social Communication Questionnaire193 | Parent questionnaire | A total of 40 yes-no questions to evaluate communication skills and social functioning in children who may have autism spectrum disorders. |
| CBCL | Child Behavior Checklist194 | Parent questionnaire | Evaluates maladaptive behavioral and emotional problems across several domains, including internalizing and externalizing behaviors, including a DSM-oriented scale pertaining to PDD. |
Prenatal and Early Postnatal Exposures
We restricted this review to studies that addressed chemical exposures during, or pertaining to, conception, pregnancy, and the 1st postnatal year, to assure that the exposure could have been part of the multifactorial landscape that preceded and caused the emergence of autism symptoms. Although the precise critical periods of susceptibility to environmental factors for autism have not been fully elucidated, evidence from neuroanatomical, animal, and epidemiologic studies support the prenatal and early postnatal origins of autism.19–24 A critical period in early pregnancy was suggested from a study of autism cases suspected to be caused by thalidomide exposure in early pregnancy.22 Later pregnancy and early postnatal life are additionally implicated by accumulating molecular and pathological evidence pointing to altered neuronal connectivity and a resultant perturbation in signaling pathways, systems under intense development in these periods.25,26 These developmental windows are a time of known neurodevelopmental susceptibility, and it is believed to be exposures during these times that can exert causal influences on developmental disorders.14,27,28 By limiting to exposures pertaining to these periods, it is assured that the environmental chemical exposures occurred prior to the development of autism symptoms and could not result from the sequelae of autism. For example, metals in hair during childhood may indicate altered metal metabolism and clearance, or altered diet or medication use, any of which could result from autism, instead of contributing to the development of autism. As illustration, we provided a partial list of publications excluded because of exposure timing criteria (Table 4), but this is not a comprehensive list, especially in the case of metal exposures, for which there is a large literature measuring metal exposure in childhood.
TABLE 4.
Excluded publicationsa
| Environmental exposure | Reason | Citations |
|---|---|---|
| Tobacco | No accounting for socioeconomic status, important in studies of tobacco and autism because of the strong associations between factors like income and education and tobacco use. | 21,165,195–203 |
| Regulated and traffic-related air pollutants VOCs/solvents | Measures of criteria pollutants pertained to postnatal periods after 1 year of age. |
204 None |
| Metals—air, occupation, and diet | Biomarkers in childhood reflected, in part, exposures after the 1st year of life. | 205–210 |
| Metals in acid-digested whole baby teeth included metal exposures up until the tooth was shed.211 | 212 | |
| Limited information about maternal age or other sociodemographic characteristics of cases vs. controls and no adjustment for these factors. | 213 | |
| For placement of dental amalgams containing mercury in pregnancy: did not meet precision criteria—two children whose mothers had dental amalgams containing mercury. For seafood consumption as a source of metals, no description of measurement and no adjustment for confounders. | 212 | |
| Metals—vaccines | Studies using the Vaccine Adverse Event Reporting System (VAERS) that did not account for temporal trends in vaccine formulations or autism prevalence or the voluntary nature of autism reporting, along with other limitations previously discussed.105 | 214–220 |
| A study reported as a letter to the editor without sufficient reporting on methods such as multivariable adjustment. | 221,222 | |
| No information on sociodemographic and maternal age characteristics of the cases and controls without adjustment for these factors. | 223 | |
| A study based on a convenience sample in a genetic clinic that did not account for temporal trends or maternal age. | 224 | |
| Did not meet precision criteria (1 child with autism) | 225 | |
| Pesticides | Did not meet precision criteria. | 226 |
| PCBs, flame retardants, non-stick chemicals, phthalates, and bisphenol A | Measures of phthalates, PBDEs, or PCBs pertained to later childhood. Not meeting precision criteria (5 children with autism), external contamination of PCBs, and no adjustment for confounding. |
227–230 231 |
Valid Comparisons
We included studies without major validity concerns of selection biases and confounding, because when these influences can be ruled out, epidemiological studies reporting associations can suggest a population-level causal association between the risk factor and autism. Across all studies, we evaluated whether the comparison group, typically children without autism, was a valid representation of the underlying population that generated the group of children with autism diagnoses. Selection bias is common in studies of autism because children receiving an autism diagnoses may differ from the set of all children who could, under ideal conditions, receive a diagnosis. For example, disadvantaged racial/ethnic or sociodemographic groups have been shown to have lower rates of diagnosis1,29 and up to 20% of children are not diagnosed by 8 years of age,1 perhaps because of limited health care access or parent knowledge of developmental milestones. We excluded a study when we assessed that the comparison population may be meaningfully different from the underlying population that generated the group of children with autism, when key confounding factors (which differed by chemical group) were not accounted for, or when publications did not provide the details to assess these considerations. We reported on the specific reasons for every publication excluded due to major validity problems that otherwise met inclusion criteria (Table 4).
Adequate Sample Sizes and Statistical Precision
An alternate explanation of an elevated (or reduced) measure of association between an environmental chemical and autism is sampling error, often ruled out using standard statistical testing and p values. In this review, we emphasized the magnitude of the association together with the precision with which it was estimated, where the precision is reflected by the width of the confidence interval. We, and others, prefer this approach over dichotomizing results into those that do or do not pass a statistical significance threshold, because more information on the strength of the evidence is considered.30,31 Study estimates that have greater precision (more narrow confidence intervals) contribute more strongly to the accumulated evidence, for example, they carry more weight when included in a meta-analysis.
We have excluded some estimates with poor precision, which we defined as estimates with a confidence interval ratio or difference (upper 95% CI divided by, for ORs, or with a subtraction of, for slopes, the lower 95% CI) >10. These estimates arose when fewer than five children with autism had the xenobiotic exposure, a commonly used criterion of insufficient sample size. Every publication excluded using precision criteria is listed in Table 4.
Identifying and Reviewing Publications
We used a multi-method, iterative approach to locate all publications that met these inclusion criteria, as follows. We searched our personal libraries and performed online searches in PubMed and Google Scholar, including the PubMed feature “Related Citations.” These searches used the outcome keywords: autism, Pervasive Developmental Disorder, and social impairment, combined with keywords pertaining to the exposure (e.g., for air pollutants: air pollution, air toxic, and traffic). We then searched for additional publications by the authors and parent studies that were generated from the initial search (e.g., CHARGE). We reviewed all citations from each located primary publication, from two recent reviews of environmental factors and autism,14,32 and from reviews of individual exposure groups, such as thimerosal-containing vaccines and autism,33,34 and endocrine-disrupting compounds and autism.35 When publications were located outside of the online literature searches, we then performed repeat online literature searches after adjusting our keywords and approach. We recognized that associations of tobacco and autism were often reported in descriptive tables of publications without being mentioned in the title or abstract, and so we widened our scope of consideration for tobacco publications. For each exposure group (e.g., pesticides) at least two of us performed independent, iterative searches.
Among primary, human population-based publications pertaining to our included chemical groups, we reviewed the abstract or complete publication for ecological outcome data and to determine the developmental timing of the exposure information. This resulted in a set of 58 publications that met inclusion criteria 1–5 (see Box 2). We thoroughly reviewed these 58 publications, excluding 26 due to validity and precision criteria 6–7 (Table 4), resulting in the final set of 32 publications included, several of which reported on multiple environmental chemicals.
Evidence Regarding Environmental Chemical Pollutants
Tobacco
Tobacco use, despite its known harmful health impacts, is still common: more than 13% of women smoked cigarettes during pregnancy in the U.S. in 1999–2006 and even more infants live with a parent who smokes.36 Both direct smoke and secondhand smoke are complex mixtures including thousands of chemicals,37 including nicotine, volatile organic compounds, and metals such as cadmium and lead,38,39 and so tobacco smoke overlaps with chemical groups covered later in this review. Increasingly, it is understood that there are important differences in the chemical make-up of smoke inhaled by a smoker (direct) vs. smoke inhaled by a non-smoker (secondhand smoke), with each representing a different toxic load with suspected different impacts on neurodevelopment. These exposures are strongly suspected to disrupt the developing nervous system, with evidence supporting links with attention-deficit disorders and intellectual disabilities.40–42 Mechanisms responsible for the harmful effects of tobacco include direct interaction of nicotine with the receptor of the neurotransmitter acetylcholine, impacts on the immune system, interference with folate homeostasis, general oxidative stress, epigenetic alterations, and placental blood flow impacts.43–49
Tobacco exposure is currently one of the more-studied chemical exposures in relation to autism, in part because it is reported on birth certificates and in medical registries, and so is often studied in concert with other obstetric factors (e.g., C-section and preterm birth). Because of the strong associations between tobacco use and lower income or education, which are related to many other health-detracting factors such as stress and poor nutrition, socioeconomic status is an especially important potential confounder of this question, and so we excluded studies that did not account for this (Table 4). We included seven studies (Table 5), taking place in the U.S., Canada, Sweden, Finland, Norway, and England/Wales. These studies included cohort and nested case–control samples and varying measures of autism, ranging from parent report of diagnosis to direct evaluation of children. Tobacco use in pregnancy was measured by parent recall, which is subject to some degree of under-reporting because of stigma, and by medical data (e.g., birth certificates) that was collected prospectively and considered reasonably valid for measuring in utero tobacco exposure,50 but was still subject to under-reporting.36
TABLE 5.
Included epidemiological publications of autism and maternal smoking in pregnancya
| Citation | Geographic location |
Birth years | Sample sizeb |
Study design |
Autism data sourcec |
Autism measurementd | Autism classification |
Adjustment variablese | Exposure measurement | Exposure timing |
|---|---|---|---|---|---|---|---|---|---|---|
| Indredavik et al.53 | Norway | 1986–1988 | 77 | Cohort | Part of a multicenter pregnancy cohort study | Autism Spectrum Screening Questionnaire (ASSQ) | Continuous autism phenotype | Gender, birth weight, socioeconomic status, single parent, mothers’ use of alcohol, mothers’ age, and mothers’ mental health. | Daily smoking at the time of conception, reported by mothers during pregnancy. | Conception |
| Larsson et al.58 | Sweden | 1994–1999 | 72/4779 | Cohort | Dampness in Buildings and Health Study | Parent report | ASD and Tourette’s syndrome | Flooring material in parents’ bedroom (PVC vs. wood), condensation on windows, child’s sex and age, child’s asthma status, and financial problems. | Retrospective maternal report of smoking in pregnancy, the child’s 1st year, or at the time of reporting (when child was 1–6 years old). | Pregnancy + early childhood combined |
| Ronald et al.54 | England and Wales | 1994–1996 | 13,690 | Cohort | Twins Early Development Study | Childhood Asperger Syndrome Test (CAST) Parent rating using questionnaire based on DSM-IV Teacher rating using questionnaire based on DSM-IV |
Continuous autism phenotype | Child cognitive ability and social class composite based on maternal education, paternal education, maternal occupation level, paternal occupation level, and maternal age at 1st child. | Retrospective parent questionnaire of number of cigarettes smoked by mom in pregnancy. | Pregnancy |
| Burstyn et al.57 | Alberta, Canada | 1998–2004 | 1122/215,217 | Cohort | Record linkages in Alberta | ICD-9 codes 299.0 and 299.8 from records of Alberta Health and Wellness | ASD | Maternal age, maternal weight, pre-pregnancy diabetes, gestational diabetes, bleeding in pregnancy, poor weight gain, parity, socioeconomic status, breech presentation, induction of labor, C-section, Apgar score, child’s gender, and birth year (low birth weight, prematurity, and pre-eclampsia). | Collection by hospital upon admission for delivery, recorded in Alberta Perinatal Health Program. | Pregnancy |
| Lee et al.51 | Sweden | 1984–2003 | 3958/38,983 | Nested case–control | Stockholm Youth Cohort (Swedish registry based) | Screening followed by specialist diagnosis according to regional protocol | ASD ASD with comorbid ID ASD without comorbid ID |
Birth year, gender, maternal age, paternal age, parity, education, occupational class, family income, and maternal origin of birth. | Medical Birth Register, self-reported to midwife at 1st prenatal visit. | First trimester |
| Kalkbrenner et al.52 | Several sites in USA | n 1992, 1994, 1996, and 1998 | 3315/633,989 | Nested case–control | ADDM surveillance | DSM-IV-R criteria applied to developmental evaluations | ASD Lower-functioning ASD Higher-functioning ASD |
Maternal education, race/ethnicity, marital status, and maternal age. | Birth certificate report. | Pregnancy |
| Tran et al.59 | Finland | 1990–2005 | 4020/16,185 | Nested case–control | FiPS-A | ICD codes in the Finish Hospital Discharge Register | ASD Autistic disorder Asperger’s disorder PDD-NOS |
Maternal age, maternal social class based on occupation or education, infant’s weight for gestational age, and presence of maternal psychiatric diagnosesl. | Collection by maternity clinic nurses and reported to the Finish Medical Birth Register. | First trimester Entire pregnancy |
Results in Figure 1.
Sample size is presented by a single number when statistical models included a continuous measure of autism phenotype, or, otherwise, as the number of cases/controls.
Autism data sources are described in Table 2.
Autism diagnostic and psychometric tools are described in Table 3.
Variables in parentheses were evaluated but not included in final models because they did not impact results.
Results of these studies were scattered above and below the null, with most confidence intervals crossing the null, consistent with no association between direct maternal smoking in pregnancy and Autism Spectrum Disorder diagnosis, overall (Fig. 1). Some studies also examined autism subtypes, including lower-functioning groups (e.g., autistic disorder and Autism Spectrum Disorder with co-occurring intellectual disability) and higher-functioning groups (e.g., Asperger’s Disorder and Autism Spectrum Disorder without co-occurring intellectual disabilities). Associations between maternal smoking during pregnancy and higher-functioning autism were elevated compared to associations with lower-functioning autism for two studies, including a study for which one of us (A.K.) was an author (Fig. 1, green and purple).51,52 Two studies examining the continuous autism phenotype also suggested that tobacco may impact higher-functioning autism (Fig. 1).53,54 These associations between tobacco and higher-functioning autism and continuously measured autism behaviors could (1) indicate that direct maternal smoking causes elevation of some domains of autism, such as social functioning; (2) reflect random error; (3) be caused by confounding bias that acts differently for different autism subtypes; or (4) actually reflect associations with other neurodevelopmental disorders, for example, attention-deficit problems, which are suspected to be influenced by tobacco, and are highly comorbid with autism.55,56
Fig 1.
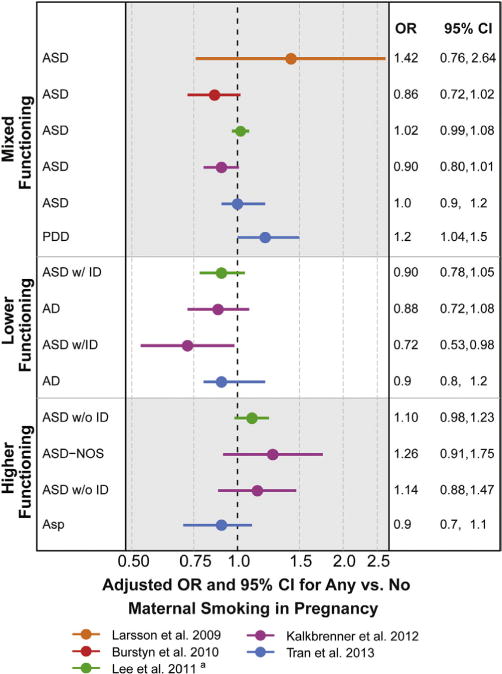
Associations between autism and maternal smoking in pregnancy. Other results not reported as measures of association with confidence intervals included the following: Ronald et al.54—small positive adjusted correlations between smoking in pregnancy and autism symptoms reported by parents and teachers—and Indredavik et al.53—adjusted elevations of 1.4 points when mothers smoked during pregnancy compared to not smoking on the ASSQ, a scale with a maximum of 54 points. aAuthor provided information not included in the publication.
The importance of social class confounding for studies of tobacco and autism was affirmed in the results: associations were altered after adjustment for factors such as maternal education or income. The direction of this confounding differed by country. In the U.S. and Canada, associations were higher after adjusting for sociodemographic variables,52,57 whereas in European countries (Sweden, Finland, and Norway), associations were attenuated after adjustment.51,53,58,59 These patterns may reflect different practices of autism screening and health services access between the U.S. vs. Scandinavian countries, and this hypothesis has direct data support.60
In summary, evidence does not support a strong causal link between direct maternal smoking during pregnancy and autism. A possible link with higher-functioning autism was suggested, but complicated by sociodemographic confounding and the phenotypic overlap with other neurodevelopmental disabilities. The published literature on the role of tobacco is currently limited by the absence of any measures of secondhand smoke exposure to the mother during pregnancy. This is an important gap, given that (1) secondhand smoke is a different chemical mixture than primary smoke, (2) there is evidence that secondhand smoke exhibits neurodevelopmental toxicity,61,62 and (3) it is a very common exposure. Given the complexity of tobacco mixtures and the biologic plausibility of impact, future studies should evaluate the role of secondhand exposure and consider biomarker measures of tobacco; these measures are objective, quantitative, and integrate all sources of exposure and, although not yet used in this literature due to the preponderance of retrospective designs, could be used when biologic materials have been stored or can be prospectively collected. Research on the neurotoxicity of tobacco should additionally resolve different components of the combustion mixture, which may open up more customized direction for preventive actions. Lastly, future studies should consider the possibility of confounding by genetics.59 Women who continue to smoke when pregnant may be genetically predisposed toward addictive behavior, which in turn may pass along increased genetic loading for neuropathologies that are related to autism risk (e.g., through dopamine or serotonin pathways), complicating the study of tobacco and neurodevelopment.
Regulated and Traffic-Related Air Pollutants
Outside air pollutants include hundreds of chemicals, present as particulates, droplets, or gases. These pollutants arise mostly from human activities, conventionally grouped into (1) point pollutants arising from spatially separated large plumes (factories, power plants, and waste incineration); (2) area pollutants associated with population density (gas stations, dry cleaners, and residential and commercial heating and cooling); and (3) mobile pollutants associated with vehicles, primarily from roadway traffic. After entering the atmosphere, pollutants move and undergo chemical reactions, creating new pollutants. In this section, we discuss air pollutants that arise from traffic or that are regulated in the U.S., such as particulate matter (PM), nitrogen dioxide (NO2), and ozone. In the next section, we cover volatile organic compounds (VOCs) in the outside air together with indoor sources of VOCs. Lastly, we cover airborne metals such as mercury, lead, and manganese in the section on metals. Although levels of some air pollutants have declined in the U.S. because of the Clean Air Act and vehicle emissions standards, these declines have not put an end to concerns about the health impacts of air pollutants, in part because the developmental impacts of current levels of ambient air pollutants have not been adequately studied. Furthermore, recent reductions may be offset by future population growth, climate change63 and international transport of air pollutants.64
Air pollutants enter the human body via inhalation and by direct translocation from the nose to the brain via the olfactory bulb.65 Particulate pollutants have mixed chemical properties and are defined by size, such as particulate matter <2.5 μm (PM2.5) and particulate matter <10 μm in diameter (PM10). Smaller particulate pollutants penetrate more deeply into the lungs than larger particulates and have greater surface area, increasing their biological activity. Chemical constituents of air pollution can enter blood circulation; the level of absorption and half-life in the body vary from chemical to chemical. Some air pollutants are known to induce oxidative stress and cause a systemic inflammatory reaction, a proposed mechanism whereby air pollutant exposures may perturb neurodevelopment.66–68 Fetuses can be exposed to the chemical constituents of the air pollutant itself (e.g., metals) and also to the elevated levels of inflammatory cytokines (or other resultant biologic factors) in the maternal circulation. Given the chemical and physical complexity of air pollutants, some likely cause additional pathophysiologies that have not yet been fully explored, such as epigenetic alterations.69
Air pollution exposures are widespread. While urban areas are hotspots for air pollutant mixtures—given that air pollutants arise from traffic and population density—rural areas can also have high levels of air pollutants when they are close to factories, waste incineration facilities, and the like. The spatial patterns of air pollutant exposures coincide with other drivers of autism occurrence and detection, necessitating the strong control of socioeconomic confounding for questions about air pollutants and autism. As an example, individuals with high pollutant exposures may be living near highways in neighborhoods that are impoverished, full of social stressors, and reduced access to healthy food or health care.
Seven publications of traffic-related and criteria air pollutants met our inclusion criteria for further consideration (Table 6). These studies all took place in the U.S. and included case–control studies with controls sampled from a population base and one prospective cohort study.70 The studies had adequate sample sizes (ranging from 284 to 7594 children with autism) and controlled for appropriate confounders such as maternal age and parents’ education level. Although confounding by such family social characteristics was expected, the degree of this confounding was not strong and acted in opposite directions across studies, attenuating ORs in some studies71,72 and producing elevated ORs in other studies.73,74 Season of birth is also an important potential confounder because the occurrence of autism varies by season of conception for unknown reasons, and because criteria air pollutant concentrations exhibit strong seasonal gradients, so that an observed association between an air pollutant and autism may actually reflect another seasonal risk factor (e.g., influenza infections and vitamin D levels). Season of birth was accounted for in one of the included studies.74
TABLE 6.
Included epidemiological publications of autism and traffic-related and regulated (criteria) air pollutantsa
| Citation | Geographic location |
Birth years | Sample size | Study design | Autism data sourceb |
Autism measurementc |
Autism classifica- tion |
Adjustment variables | Exposure measurement |
Exposure timing |
|---|---|---|---|---|---|---|---|---|---|---|
| Windham et al.90 | San Francisco Bay Area, California, USA | 1994 | 284/687 | Nested case–control | ADDM surveillance | DSM-IV-R criteria applied to developmental evaluations | ASD | Maternal age, education, and child race | National-Scale Air Toxics Assessment (NATA) (1996) | Annual average for year close to birth year |
| Kalkbrenner et al.16 | West Virginia and Central North Carolina, USA | 1992, 1994, and 1996 | 383/2829 | Nested case–control | ADDM surveillance | DSM-IV-R criteria applied to developmental evaluations | ASD | State, year, race, maternal education, maternal age, smoking in pregnancy, census median income, census urbanity, and 33 other air toxics | National-Scale Air Toxics Assessment (NATA) (1996) | Annual average for year close to birth year |
| Kalkbrenner et al.74 | San Francisco Bay Area, California and Central North Carolina, USA | 1994, 1996, 1998, and 2000 | 979/14,666 | Nested case–control | ADDM surveillance | DSM-IV-R criteria applied to developmental evaluations | ASD | State, birth year, race/ethnicity, maternal education, maternal age, census median income, census urbanity, and season of birth | Space-time interpolation between regulatory monitors | Pre-conception through 1st birthday |
| Volk et al.232 | Central California, USA | 1997–2006 | 305/259 | Case–control | CHARGE | ADOS + ADI-R | Autistic disorder | Child’s gender, child’s ethnicity, education of parents, maternal age, and maternal smoking in pregnancy | Distance to roadway | Pregnancy trimesters and at birth |
| Volk et al.233 | Central California, USA | 1997–2006 | 279/245 | Case–control | CHARGE | ADOS + ADI-R | Autistic disorder | Child’s gender, child’s ethnicity, education of parents, maternal age, and maternal smoking in pregnancy | CALINE4 traffic-based emissions model Interpolation between up to four regulatory monitors |
Pregnancy trimesters and 1st year of life |
| Becerra et al.73 | Los Angeles, California, USA | 1995–2006 | 7594/,635 | Nested case–control | California DDS | DSM-IV-R determined by DDS staff | Autistic disorder | Birth year, sex, maternal age, race/ethnicity, maternal place of birth, type of birth, parity, insurance type, and gestational weeks at birth | Concentration from nearest regulatory monitor Land use regression model with seasonal adjustment |
Pregnancy trimesters and entire pregnancy |
| Roberts et al.70 | All 50 states, USA | 1987–2002 | 325/22,101 | Cohort | Children of Nurses’ Health Study | Parent report of a diagnosis via questionnaire | ASD | Maternal age, year of birth, maternal parents’ education, census tract median income, census tract percent college educated, and NATA model year | National-Scale Air Toxics Assessment (NATA) (1990, 1996, 1999, and 2002) | Annual average for a year within 2 years of birth year |
One potential confounding influence was not explored in any study: that of noise due to traffic. Noise can cause a stress response or interfere with sleep and has been associated with cognitive performance.75 It is possible that associations observed between autism and air pollutants from traffic could be due wholly or in part to traffic noise, although one attempt to adjust for noise in studies of traffic pollution has not borne this out.76
Results for the included studies showed elevated associations between autism and measures of mixed air pollutant exposures and diesel particulate matter (Fig. 2) and for the individual air pollutants PM2.5, PM10, and NO2, with less consistently elevated associations for NO, ozone, and CO (Fig. 3). The size of the ORs was not consistent across studies, despite the standardized comparison of air pollutant concentrations that we calculated, for example, ORs were around 1.5 per 10 ppb increase in NO2 for the California CHARGE study (Fig. 3, red),72 but only 1.04 for the California Department of Developmental Services study (Fig. 3, green).73 Reasons for this different size in impact could reflect air pollutant differences between California regions, a greater degree of exposure measurement error in the DDS study, or that this study adjusted for a possible mediating factor, gestational age, which did result in the attenuation of ORs for some air pollutants.73 For almost every pollutant and every study, associations were stronger for exposures in the 3rd trimester of pregnancy and the 1st year of life compared to in earlier pregnancy (Fig. 3).
Fig 2.
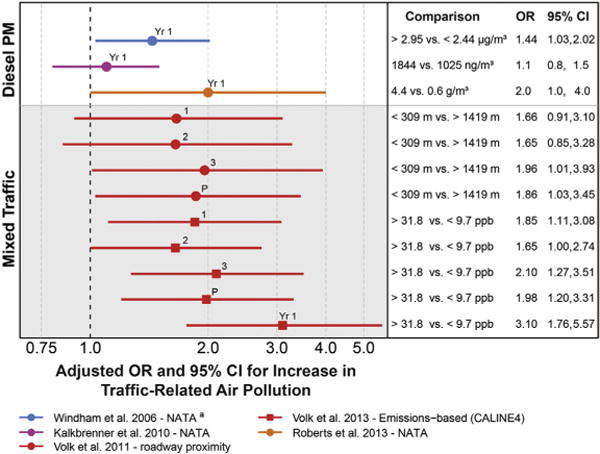
Associations between autism and estimates of exposure to mixed traffic and diesel air pollutants. Exposure measured during developmental windows: 1, trimester 1; 2, trimester 2; 3, trimester 3; P, pregnancy; Yr 1, 1st postnatal year. aAuthor provided information not included in the publication.
Fig 3.
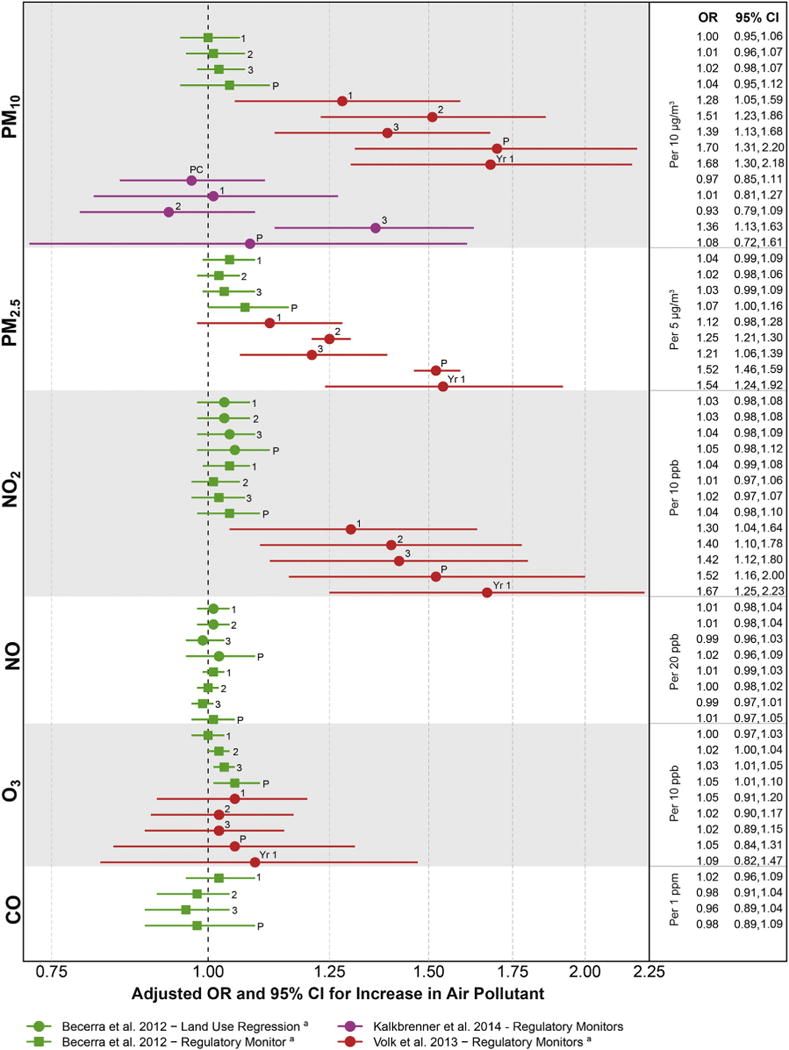
Associations between autism and estimates of exposure to individual traffic-related and criteria air pollutants. PM10, particulate matter <10 m in diameter; PM2.5, particulate matter <2.5 m in diameter; NO2, nitrogen dioxide; NO, nitrogen oxide; O3, ozone; CO, carbon monoxide. Exposure measured during developmental windows: PC, peri-conceptual; 1, trimester 1; 2, trimester 2; 3, trimester 3; P, pregnancy; Yr 1, 1st postnatal year. aWe re-calculated parameters to reflect a change in the exposure comparison to be consistent with other comparisons in the figure, involving calculations assuming that parameters were normally distributed.
All of these studies estimated exposure to outside levels of these air pollutants using historical data that could be related geographically to the home residence of the pregnant woman or infant. Direct person-based air sampling was not possible in these retrospective studies. The residence-based metrics included measurement error due to inaccuracies in geo-locating residences, air modeling assumptions, and the inability to include time spent away from home, measurement error that would be expected to attenuate findings. Yet elevated associations were found for some air pollutants. As pointed out by the authors of these studies, the likelihood is low that exposure measurement error could have resulted in overestimated associations (possible if measurement error was related to autism status). It is therefore possible that these study results are conservative, that is, these air pollutants may have stronger associations with autism than suggested by the observed data.
In summary, the published literature shows associations between traffic-related and criteria air pollutants and the development of autism and possibly increased susceptibility when exposures occur in late pregnancy or early postnatal life. These findings are bolstered by strong study designs and consistent findings across different means of measuring pollutant concentration and identifying children with autism. Reasons for these findings include a true causal relationship, a consistent bias such as residual social class confounding, or the impacts of traffic noise. Questions about the size of the impact and the individual chemical pollutant most responsible for these findings remain, as most studies have not accounted for the correlations between multiple chemical pollutants or the differences in air pollutant mixtures between the West and East coasts of the U.S.
Volatile Organic Compounds/Solvents
Volatile organic compounds (VOCs) include a large group of chemicals that become gaseous close to room temperature and are released/emitted from products or processes (sometimes called “off-gassing” or “out-gassing”), chemicals such as formaldehyde and benzene. VOCs overlap partly with other chemical groups: organic solvents (a group defined by their ability to dissolve other chemicals) and some air toxics (defined as chemicals detectable in outside air that are known to harm human health). VOCs are emitted from many human products and activities, including indoor residential and workplace paints, furnishings, and air fresheners; vehicle fuel combustion (benzene, toluene, ethyl benzene, and xylene, abbreviated BTEX); dry cleaning (tetrachloroethylene, also known as perchloroethylene or PERC); refrigeration; cigarette smoke (benzene, ethyl benzene, formaldehyde, and xylenes); hair and nail salons (toluene); and in other industries involving fuels, lubricants, paints, and more. Organic solvents have been reported as among the most prevalent occupational chemical exposures for women.77 VOC concentrations are generally 2–5 times higher in indoor compared to outdoor air,78,79 and have been hypothesized to cause Sick Building syndrome.80 VOCs are ubiquitous and occur in combination with other VOCs and pollutants covered elsewhere in this review: tobacco, traffic-related criteria air pollutants, and pesticides. Petroleum-based VOCs like benzene, toluene, ethyl benzene, and xylenes have been widely detected in biological samples from a U.S. representative sample in 2013.12 These body burdens are especially notable given that the biological half-lives of VOCs range from minutes to days, indicating frequent exposure to these chemicals.
The primary route of exposure to VOCs is by inhalation, although VOCs can be ingested through contaminated drinking water and can be dermally absorbed.81 Biologic mechanisms of harm include oxidative stress after metabolism by P450 enzymes and direct interference with cell membrane integrity for the many VOCs that are lipophilic.82 VOCs interact with many organ systems, including the nervous system, as demonstrated by evidence in rats of neurodevelopmental toxicity.83 Epidemiological studies of the impacts of early-life VOC exposures are few, but have demonstrated that women occupationally exposed to multiple VOCs have children with cognitive and visual-motor functioning deficits84,85 and symptoms of attention-deficit disorder.86
The seven studies of VOCs included in our review (Table 7) measured exposures with the following methods: (1) self-report or birth certificate report of parental exposure in the workplace and occupation, which was coded into multiple chemical classes based on the work duties and products manufactured87,88; (2) parent report of exposure to “textiles” and “newly built homes”89; and (3) a vehicle and factory emissions-based air pollutant model of the U.S. Environmental Protection Agency that models census tract levels of air toxics for selected years, the National-scale Air Toxics Assessment (NATA), including a study for which one of us (A.K.) was an author.16,70,90 The NATA model predicted concentrations of over 30 air toxics in 1996 and over 180 air toxics in 1999, 2002, and 2005. In summary, these exposure methods had the following strengths: (1) corresponding to the pertinent prenatal and early postnatal time periods; (2) independent expert verification/coding of occupational exposures87,88; and (3) prospective measurement, along with a reduced chance of a recall bias, in the studies of air toxics and the occupation study based on birth certificate-reported occupation.16,70,88,90
TABLE 7.
Included epidemiological publications of autism and volatile organic compounds/solventsa
| Citation | Geographic location |
Birth years | Sample size | Study design |
Autism data sourceb |
Autism measurementc |
Autism classifica- tion |
Adjustment variables | Exposure measurement | Exposure timing |
|---|---|---|---|---|---|---|---|---|---|---|
| Windham et al.90 | Described in Table 6. | |||||||||
| Windham et al.88 | San Francisco Bay Area, California, USA | 1994 | 284/659 | Case–control | ADDM surveillance | DSM-IV-R criteria applied to developmental evaluations | ASD | Child’s race, maternal age, and maternal education | Occupational medicine-certified physician-coded exposures from maternal usual occupation from California birth certificate into broad categories. Solvents = electronics, custodian, lab work, dry cleaning, cosmetology, painter, construction, mechanic. Disinfectants = hands-on medical occupations | Pregnancy |
| Kalkbrenner et al.16 | Described in Table 6. | |||||||||
| Kim et al.89 | Korea | In elementary school -<2010 | 106/324 | Case–control | Newly recruited | Diagnosis by child psychiatrist, confirmed by government agency | ASD | No statistical adjustment, but no differences between cases and controls in maternal age, maternal education, socioeconomic status, breastfeeding, or alcohol consumption in pregnancy | Retrospective parent questionnaire of pregnancy exposure to “newly built houses” and “textiles” | Pregnancy |
| McCanlies et al.87 | Central California, USA | 1998–2003 | 93/81 | Case–control | CHARGE | ADOS + ADI-R | ASD | Duration of breast-feeding, mother’s age, mother’s education, regional center, child’s gender, child’s age, and payment method | Retrospective phone questions of mothers’ and fathers’ occupations including list of specific substance exposures. Blinded industrial hygienist coding of parent report |
3 Months prior to pregnancy until birth or weaning |
| Roberts et al.70 | Described in Table 6. | |||||||||
Results for VOC exposures and autism were scattered above and below the null, a pattern expected to result from sampling error when no causal relationship exists (Fig. 4). Upon closer inspection, a few groups of VOCs (paints and chlorinated solvents) showed elevated associations compared to other groups. Some individual VOCs had consistently elevated associations, although the VOC concentration comparisons differed somewhat across studies: methylene chloride, trichloroethylene, and styrene. Given this consistency, and that some of these associations had the statistical power to rule out no association, these VOCs deserve future study as candidate risk factors for autism.
Fig 4.
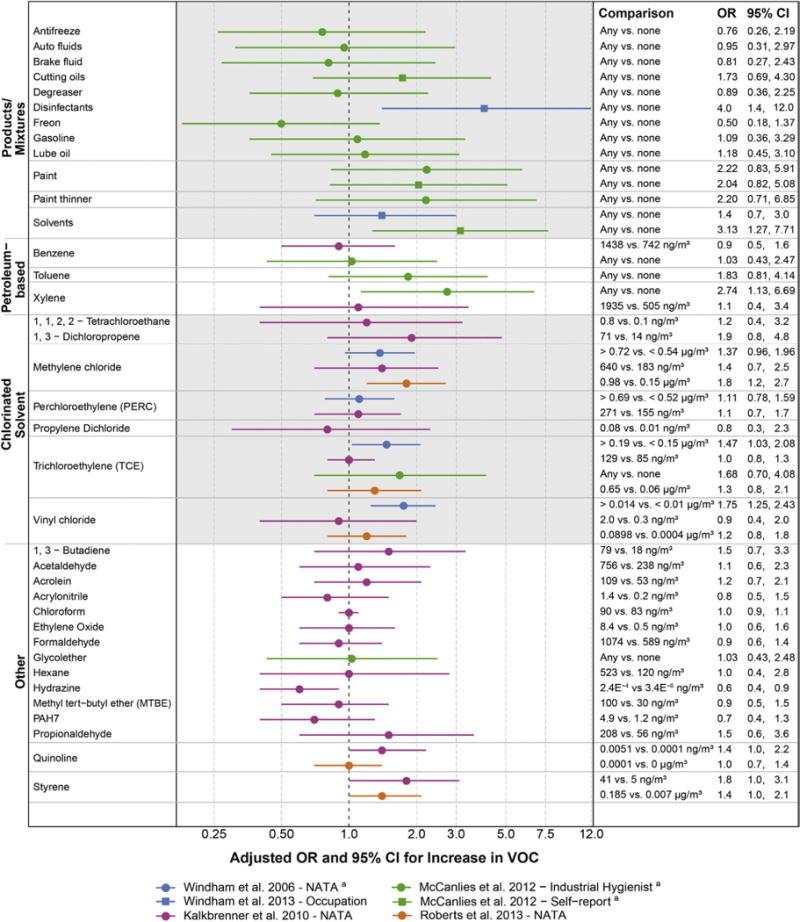
Associations between autism and estimates of exposure to volatile organic compounds/solvents. Other results not reported as measures of association with confidence intervals included Kim et al.89: higher retrospective reports of pregnancy exposures to “newly built homes” and “textiles” for children with autism compared to other children. VOC, volatile organic compounds; PAH7, mixed group of 7 polycyclic aromatic hydrocarbons. aAuthor provided information not included in the publication.
Published results for this research question often had wide confidence intervals reflecting small numbers of exposed children with autism, especially for the occupational studies. This imprecision and uncertainty in estimates also resulted from the inherent measurement error of VOCs in these studies. Despite the strengths of the exposure methods, all of these methods provided only a rough approximation of the internal dose of VOCs to the developing nervous system. The emissions-based NATA model is especially problematic for VOCs because it relies on proximity to sources emitting VOCs outdoors, such as traffic and large factories, ignoring the important unique indoor sources for several VOCs.79
In summary, studies of VOCs and autism, despite being underpowered with some degree of exposure measurement error, have shown patterns suggestive of causal associations that point to the need for more research on certain VOCs (e.g., methylene chloride, trichloroethylene, and styrene). Other VOCs are not cleared from culpability, but instead have simply not been adequately studied. Given that VOCs themselves occur in mixtures and also occur together with other pollutants, large studies with multiple detailed measured exposures would be required to disentangle a potential causal impact of an individual VOC, ubiquitous chemicals with high biologic plausibility of damaging the developing nervous system.
Metals From Air, Occupation, Dental Amalgams, and Diet
Lead and mercury have strong and consistent evidence of harming the developing nervous system, resulting in impairments such as the loss of IQ points and behavioral problems.42 Other metals, including arsenic, fluoride, and manganese, are increasingly recognized as also being human neurodevelopmental toxicants.11,91–93 These health impacts are not restricted to high-exposure scenarios, but have been seen with typical exposure levels, leading to “silent toxicity.”94 Metals cross the placenta and the blood–brain barrier, accumulate in developing brains, and interact directly at the cellular level through a variety of mechanisms, including interfering with key cellular receptors and causing reactive oxygen species.
Metals are widespread in the environment, each metal with different sources of exposure, via ingestion and inhalation, illustrated here using the examples of lead, arsenic, and mercury. Historically, leaded gasoline and lead-containing paint resulted in the contamination of homes and soils, and many families in the U.S. remain exposed to lead hazards, especially those living in poorly maintained older homes. Sociodemographic and neighborhood factors, therefore, are important potential confounders when studying lead (and other metal) exposures. Lead can still be measured in outside air, and air may constitute an important source of lead exposure for some families.95 Arsenic is found in contaminated drinking water resulting from arsenical soil, including in some parts of the U.S., with secondary sources including arsenic-treated wood, arsenic-containing pesticides, and some dietary sources (e.g., rice). Mercury exposure, especially the most toxic form, methylmercury, occurs primarily from diet, because it bioaccumulates in the environment. Persistent environmental chemicals like mercury often have affinity for fatty tissues (they are lipophilic) and so are not readily broken down. After widespread use and distribution, such chemicals deposit in waterways and concentrate toward the top of the food chain in predatory fish and human milk. The same fish that contain methylmercury accumulate other persistent organic pollutants, such as those covered later in this review, and also contain beneficial nutrients (e.g., fatty acids), leading to problems of negative and positive confounding from the mixtures of chemicals present in some fish. Less important sources of mercury exposure include dental amalgams (during placement and from continual release of trace amounts of mercury vapor for the life of the amalgam) and outside air near coal combustion and some industrial facilities.
Five epidemiological studies of metal exposure from air, occupation, dental amalgams, and diet were included (Table 8). The studies addressed multiple airborne metals among a panel of other air pollutant exposures measured using the emissions-based air toxics model, NATA,16,70,90 metals from parental occupation in the California-based CHARGE Study,87 mercury from dental amalgams present in mothers during pregnancy, and mercury in maternal hair studied in the Republic of Seychelles, a high fish-consuming island country in the Indian Ocean near Madagascar.96
TABLE 8.
Included epidemiological publications of autism and metals from air, occupation, dental amalgams, and dieta
| Citation | Geographic location |
Birth years | Sample size | Study design |
Autism data source |
Autism measurementb | Autism classification |
Adjustment variablesc |
Exposure measurement |
Exposure timing |
|---|---|---|---|---|---|---|---|---|---|---|
| Windham et al.90 | Described in Table 6. | |||||||||
| Geier et al.97 | USA | 1990–1999 | Autistic disorder: 40 ASD: 60 | Cohort | Genetic Centers of America | Physician diagnosis of ICD-9R 299.00 (A.D.) or 299.80 (ASD) based on child observation and Autism Treatment Evaluation Checklist completed by parents | Severe (ICD-9R 299.00) or mild (299.80) | Child’s age, gender, race, and region of the U.S. Study restricted to children without exposure to Rho (D) immune globulines or influenza vaccinations during pregnancy. | Medical history of number of dental amalgams present in moms during pregnancy. | Pregnancy |
| Kalkbrenner et al.16 | Described in Table 6. | |||||||||
| McCanlies et al.87 | Described in Table 7. | |||||||||
| Roberts et al.70 | Described in Table 6. | |||||||||
| Van Wijngaarden et al.96 | Republic of Seychelles | 1986–1990 and 2001 | SCQ 149/1784 SRS 182/537 |
Cohort | Seychelles Child Development Study | SCQ 4 14 SRS 4 69 (males) SRS 4 64 (females) |
Continuous autism phenotype | Child’s sex, recruitment cohort (maternal and paternal age) | Laboratory analysis of methylmercury in maternal hair collected around birth | Pregnancy |
Across all metals in these studies, results were predominantly above the null, a pattern consistent with either higher risk due to metal exposure or consistent residual confounding across designs, confounding not highly likely given that all of these studies adjusted for socioeconomic status and maternal age, but possible (Fig. 5). The pattern of elevated associations was especially striking given the measurement error inherent in the emissions-based air pollutant model used in three of these studies, which would be expected to attenuate observed ORs.16,70,90
Fig 5.
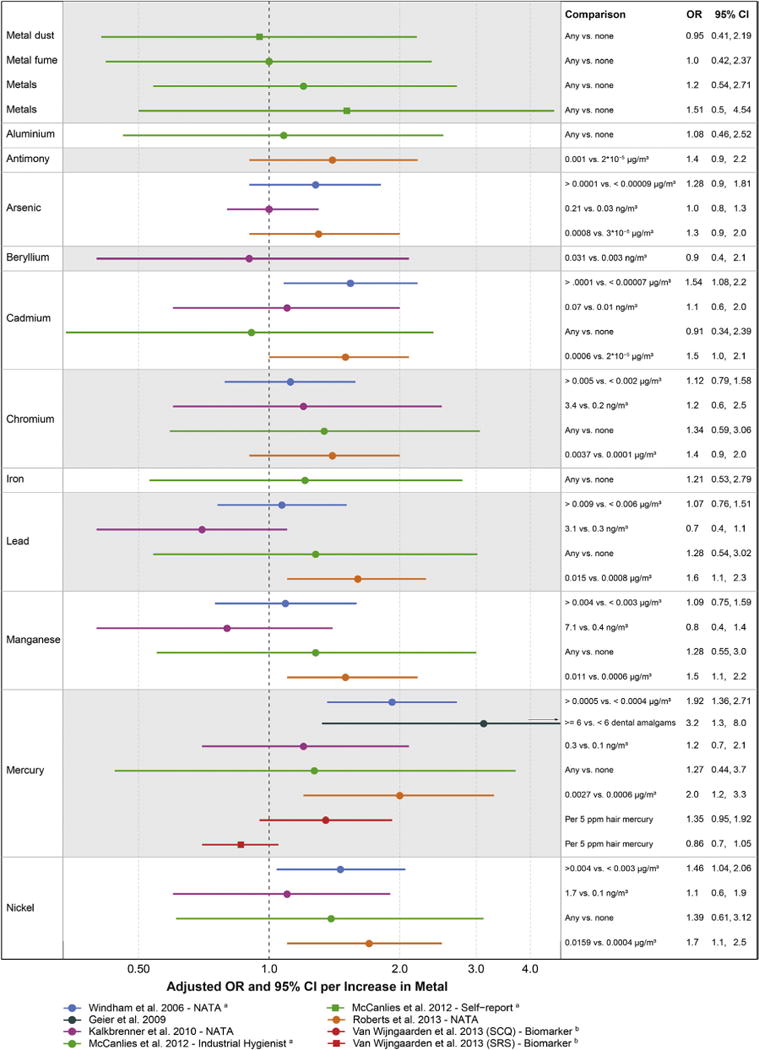
Associations between autism and estimates of exposure to metals from air, occupation, dental amalgams, and diet. aAuthor provided information not included in the publication. bWe re-calculated parameters to reflect a change in the exposure comparison to be consistent with other comparisons in the figure, involving calculations assuming that parameters were normally distributed.
For the metals chromium and nickel, the ORs were of consistent magnitude across studies, although the exposure concentration comparisons differed somewhat; if combined in a meta-analysis, the expectation would be that precision would improve, resolving elevated associations (Fig. 5). In one study, lead, and, to a lesser extent, manganese, appeared inversely related to autism risk, which could have been caused by a stronger association with the comparison group in this design: children with speech and language impairment (Fig. 5, purple).16 Autism risk due to mercury exposure was supported by the studies of airborne mercury,70,90 but not from the Seychelles longitudinal biomarker study reflecting all sources of mercury (Fig. 5, red).96 One possible reason for the lack of observed elevated association in the Seychelles study is that mercury exposure was almost entirely from fish consumption in this population, so that the impacts of brain-enhancing nutrients contained in fish may have counteracted any toxic impact of mercury. Mercury from dental amalgams was also associated with increased autism severity (Fig. 5, dark green).97 In this study, some typical confounders were not accounted for, but were unlikely to strongly bias results: maternal age, while a strong risk factor for autism diagnosis, may not be relevant for autism severity,98 and sociodemographics were somewhat homogenous in this study population in which all study children had received an autism diagnosis and were attending a genetic clinic.
In summary, the existing studies of autism due to metals from air, occupation, dental amalgams, and diet are suggestive that metal exposure may cause autism but are, for the most part, limited in scope. The only biomarker study took place in a high fish-consuming population with limited generalizability to other populations. The other studies only pertained to less important routes of metal exposure (e.g., air), and so are unlikely to represent the full impact of these metals on autism risk. For example, although three studies measured arsenic in ambient air, no studies addressed the most common source of arsenic: drinking water, and no studies addressed lead exposure from ingestion of items contaminated with house dust and soil. Given the established neurotoxicity of many metals and the suggested trends shown here, future research to address the common sources of metal exposure and autism is essential.
Metal (Ethylmercury) From Vaccinations
Metal exposure, specifically ethylmercury from the vaccine preservative thimerosal, has been studied in relation to autism and we reviewed these publications. Thimerosal contains ethylmercury, considered a less toxic form of mercury compared to methylmercury.99 Because of the biologic plausibility that thimerosal may pose neurotoxic harm, the U.S. Food and Drug Administration suggested removing, or reducing, thimerosal in childhood vaccines in 2001. Currently, the only vaccines containing more than trace amounts of thimerosal are some influenza vaccines, to which both infants and pregnant women may be exposed.100 Of note, additional community and research attention regarding vaccines and autism has focused on measles, mumps, and rubella (MMR) vaccine, which never contained thimerosal and so is outside the scope of this review.
We included six epidemiological studies of thimerosal-containing vaccines and autism; these were conducted in the U.S., United Kingdom, and Denmark (Table 9). The included studies utilized pre-existing data from government health registries or linked medical records from health maintenance organizations participating in the Vaccine Safety Datalink or the U.S. National Health Interview Survey and were all cohort designs or case–control designs nested within these cohorts (Table 3). We included studies that adjusted for suspected confounders. Additionally, for this exposure (but not some others in this review), we also included studies that did not account for the potential confounding influences of maternal age or sociodemographics. This decision was based on our assessment that these factors did not act as strong confounders in the four studies that were able to adjust for these factors: ORs were identical after adjusting for maternal age (OR of 1.12 and 1.12),101 similar when adjusting for education [OR of 3.0 vs. 2.8 (unadjusted)],102 and similar in two studies that adjusted for both maternal age and education, among other factors [OR of 1.0 vs. 1.1 (unadjusted)]103 and [OR of 1.12 vs. 1.13 (unadjusted)].104
TABLE 9.
Included epidemiological publications of autism and ethylmercury from thimerosal in vaccinesa
| Citation | Geographic location |
Birth years |
Sample size | Study design | Autism data sourceb |
Autism measurementc |
Autism classification |
Adjustment variablesd |
Exposure measurement |
Exposure timing |
|---|---|---|---|---|---|---|---|---|---|---|
| Hviid et al.101 | Denmark | 1990–1996 | 467,450 (2,986,654 person-years) 440 Autistic Disorder 787 Other ASD |
Cohort | Danish Psychiatric Central Register | ICD-8 or ICD-10 F84.0 or F84.1-F84.9 | Autistic disorder Other ASD |
Child’s sex, child’s place of birth, birth weight, 5-min Apgar, gestational age, mother’s age, mother’s country of birth, child’s age, and calendar period. | Sum of mercury from pertussis vaccinations prior to thimerosal removal as reported to Danish National Board of Health, at about 1, 2, and 10 months of life | First year of life |
| Verstraeten et al.234 | West Coast of USA | 1991–1997 | 202 (Autistic Disorder), 108 (PDD-NOS)/110,883 | Cohort | Vaccine Safety Datalink | ICD-9 299.0 or 299.8 | Autistic disorder Asperger’s disorder/PDD-NOS |
Child’s sex, birth month and year, usual clinic, and restriction to those with consistent health care seeking behavior. | Sum of mercury content from infant vaccines: HBV, DPT, and Hib, by month, from computerized databases of included HMOs. |
Postnatal: Birth to 1 month Birth to 3 months Birth to 7 months |
| Andrews235 | United Kingdom | 1988–1997 | 106/107,152 | Cohort | General Practitioner Research Database (GPRD) | ICD-8 or ICD-9 code 299.0 | Autistic disorder | Child’s sex and year of birth (month of birth). | Number of DTP/DT doses by age from the linked patient, medical, and prevention databases of the Office for National Statistics |
Postnatal: Birth to 3 months Birth to 4 months All postnatal |
| Croen et al.103 | Northern California, USA | 1995–1999 | 400/410 | Nested case–control | Membership in health plan: Kaiser Permanente | ICD-9-CM 299.0 or 299.8 | Autistic disorder Asperger’s disorder/PDD-NOS |
Child’s sex, birth year, hospital of birth, birth order, plurality, maternal age, maternal race/ethnicity, and maternal education. | Maternal Rh immunoglobulin or influenza vaccine abstracted from the prenatal medical record. | Pregnancy |
| Price et al.104 | West Coast of USA | 1994–1999 | 256 ASD/752 187 Autistic Disorder/724 49 ASD with regression/652 | Nested case–control | Vaccine Safety Datalink | ICD-9 299.0 or 299.8 confirmed with ADOS and ADI-R | ASD Autistic disorder ASD with regression |
Birth year, child’s gender, managed care organization, birth weight, maternal age, and family income (maternal education, birth order, breastfeeding duration, adequacy of prenatal care, use of cholesterol screening, use of Pap test, maternal alcohol consumption, maternal folic acid use, maternal viral infection, child lead exposure, child anemia, and child pica).e | Sum of mercury from prenatal maternal and postnatal vaccinations, obtained from computerized medical records, medical charts, and maternal interviews, and linked with manufacturer information | Pregnancy Postnatal: Birth to 1 month Birth to 7 months |
| Gallagher and Good-man102 | USA | <1999 And in NHIS | 30/7044 | Cohort | National Health Interview Survey (NHIS) | Parent response to inquiry of whether health professional ever said that child had autism. | ASD | Race, two-parent household, and maternal education. Study restricted to males. | Interviewer transcription of dates and type of vaccination from vaccination record |
Postnatal: Birth to 1 month |
Results in Figure 6.
Autism data sources are described in Table 2.
Autism diagnostic and psychometric tools are described in Table 3.
Variables in parentheses were evaluated but not included in final models because they did not impact results.
In Price et al., variables in parentheses were included in models of one, but not all, autism subgroups.
For all but two of the associations between thimerosal and autism, results were most consistent with a pattern due to chance, demonstrated by ORs of small magnitude (most between 0.9 and 1.2), above and below the null, and with confidence intervals spanning the null, suggesting that thimerosal does not increase autism risk (Fig. 6). This assessment is consistent with previous reviews, including the Institute of Medicine review from 2004, which concluded that “the body of epidemiological evidence favors rejection of a causal relationship between thimerosal-containing vaccines and autism,”105 several other reviews,33,34,106–109 and a meta-analysis published in 2014.110 One exception to the pattern of no association was an estimate of thimerosal from multiple vaccines and Autism Spectrum Disorder with regression; this result was markedly protective/inverse but imprecise and inconsistent with biologic plausibility (Fig. 6, orange).104 The second exception was from a study of thimerosal from hepatitis B vaccination in the 1st month of life, limited to males, that showed an elevated association (Fig. 6, purple).102 This result has not been included in prior reviews. Alternate explanations of this elevated result include (1) the failure to adjust for temporal trends, (2) random error and a consequent bias away from the null given that only 30 cases were included, or (3) that non-mercury constituents of the hepatitis B vaccine could increase autism risk.
Fig 6.
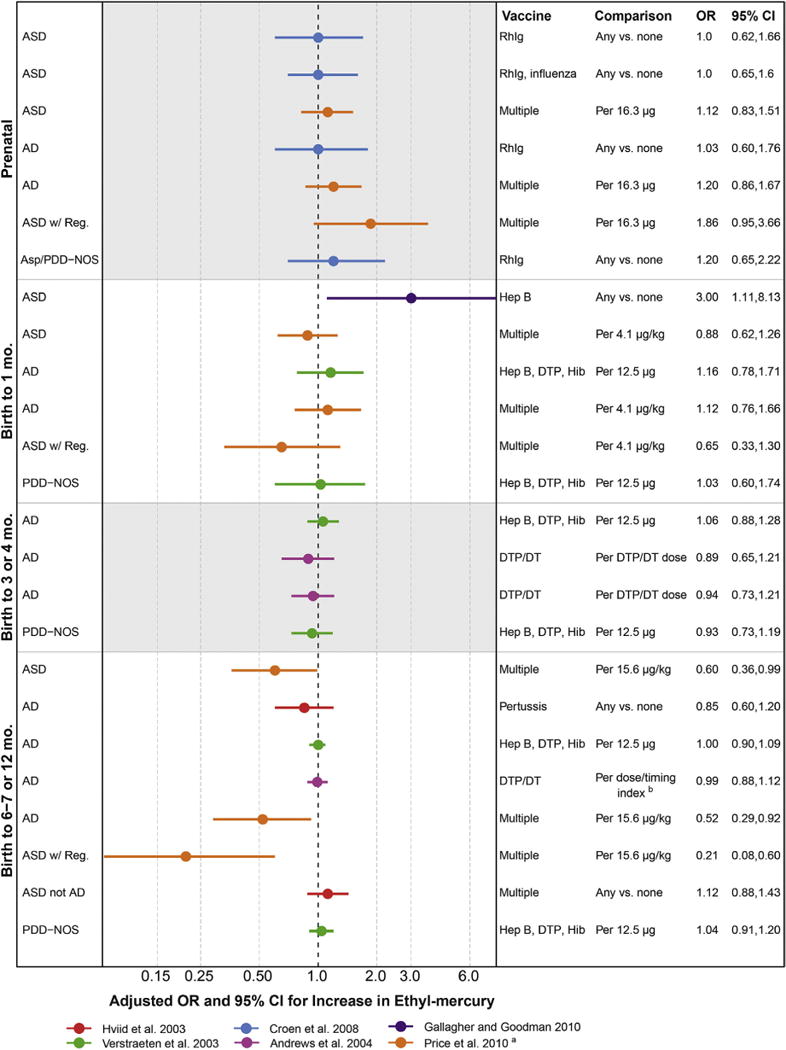
Associations between autism and estimates of exposure to mercury from thimerosal in vaccines. RhIg, anti-D immune globulin injection; Hep B, hepatitis B vaccine; DTP, combined diphtheria, tetanus, and Pertussis vaccine; Hib, Haemophilus influenzae type B vaccine; DT, diphtheria–tetanus vaccine; aVaccine exposure measured as μg of mercury per kg of child’s body weight. bVaccine exposure measured as an index where higher numbers indicated more doses received earlier.
Concluding that a factor like thimerosal is not causative using epidemiological studies is difficult, however, and requires ruling out biases that may artificially attenuate ORs. Although the studies we included met our validity criteria, some minor flaws may have created such an influence. For example, in a California study,103 it was assumed that all children obtaining Rh immunoglobulin received thimerosal exposure even though only about 50% of market formulations contained thimerosal.15 This situation could lead to attenuated observed ORs comparing between children who did and did not receive any RhIg vs. the ORs that would have been observed if thimerosal content was measured directly and was related to autism status. Another example of complications in this literature is that the healthiest babies may be given vaccines earlier, and may be independently less likely to develop autism, possibly leading to an artificial influence where earlier vaccines are associated with less autism.15
When patterns of autism risk were examined by the timing of the vaccine, more associations for prenatal vaccines were above null compared to the later postnatal vaccines, such as in the case–control study conducted using the Vaccine Safety Datalink (Fig. 6, orange).104 Whether these data support that the earlier, prenatal, vaccines deserve more research for causing autism is tenuous, but warrants mention because the only vaccines currently containing more than trace levels of thimerosal (influenza vaccines given to pregnant women) can be given prenatally.
In summary, the published literature on thimerosal, although not consistently adjusted for some potential confounders and with other potential biases, does not suggest an association with autism. The public health importance of this controversial issue has diminished given the prophylactic removal of thimerosal from pediatric vaccines, although pregnant women may still receive thimerosal-containing influenza vaccines. Of note, these studies have not examined the potential influence of vaccine co-factors. Vaccines contain carriers and adjuvants (e.g., aluminum), each of which could possibly cause toxicity, and the active ingredient is an immune trigger that, itself, may be deleterious for the developing nervous system.
Pesticides
Over 600 unique chemical pesticides and 20,000 commercial pesticide products, categorized by function as insecticide, herbicide, fungicide, rodenticide, fumigant, and repellant, are on the market, and over one billion pounds are used in the U.S. each year.111 Though individual pesticides enter and leave the market quickly, pesticide classes remain the same, and of these, organophosphates (OP) and organochlorines (OC) have the most evidence of neurotoxicity. Pesticide exposure is widespread, for example, in a representative sample in the U.S., OC pesticides were detected in over 97% of bio-samples, while OP pesticide metabolites were detected in 70%.12 Pesticides can pass through the placenta and the blood–brain barrier.113
Many pesticides act to control insects through direct impacts on the insect nervous system, with suspected analogous impacts on humans. Demonstrated biological mechanisms also suggest pesticides as candidate risk factors for autism.112 OP pesticides inhibit acetylycholinesterase production in the brain,114 restricting neurotransmission in the peripheral and central nervous systems, ultimately impacting synapse formation, axon transmission, cell maturation, and programmed cell death.115 In contrast, OC pesticides act on the nervous system via gamma aminobutyric acid (GABA) receptor-mediated chloride ion channels116 and may also derail neurodevelopment through their endocrine-disrupting properties.117 Population-based human studies have shown some pesticides to cause neurodevelopmental decrements.118–121
OP and OC pesticides are currently used most often in agricultural applications,122 and so the primary route of exposure to OP and OC pesticides is via diet.123,124 Many different pesticides have been detected in fruits, vegetables, grains, and breast milk.125,126 Secondary routes, especially for areas close to farms, include inhalation from pesticide drift after agricultural applications127 and dermal absorption from direct contact with a pesticide, most common in occupational settings. OC pesticides are persistent pollutants and some are detectable in the environment and human tissues decades after they have been banned, such as the OC pesticide DDT.128
Pesticide exposures occur together with factors that may harm or benefit the developing nervous system, factors that can act as confounders in studies of pesticides and autism. Examples of pollutants that co-occur with pesticides in diet include metals from soil and chemicals in non-stick cookware (perfluorinated compounds or PFCs, discussed below).129 Pesticide drift co-exposures include aerosols from high-pressure cleaning,130 and ammonia from fertilizer and animal waste.131 Beneficial co-exposures include vitamins and minerals from the fruits, vegetables, and grains that contain pesticide residues. Sociodemographics are also important to consider because dietary patterns, including the consumption of an organic diet, determine pesticide exposure and also the ability to obtain an autism diagnosis. Maternal age is another potential confounder because total body burden of pesticides increases with age132 and advanced maternal age is a well-supported risk factor for autism.133,134
Seven epidemiological studies of pesticides and autism met our criteria for inclusion in this review (Table 10). All of these studies exhibited reliable information on autism diagnosis or characteristics, used suitable controls with limited selection bias, and accounted for important confounders such as social class and maternal age. OP and OC pesticides and their metabolites, as well as some other pesticides, were measured using maternal and child biological samples,135–137 residential proximity to agricultural applications,138,139 and retrospective parent questionnaires.87,140 These studies generally supported an association with elevated risk of autism with pesticide exposure, with most point estimates above the null, many with large enough impacts and statistical precision to rule out sampling error (Fig. 7). Elevated associations were not limited to one pesticide class, but were found for OC, OP, and other types of pesticides, and were not limited to one exposure route, but seen across studies addressing agricultural drift, use of flea/tick pet treatments, and dietary sources as reflected by biomarkers (Fig. 7). The largest measured impacts were seen in a study of agricultural drift for two developmental windows: a period in the 1st trimester of pregnancy corresponding to central nervous system development (pregnancy weeks: 1–7), and a period that emerged, a posteriori, from the data (pregnancy weeks: 4–12) (Fig. 7, orange).138
TABLE 10.
Included epidemiological publications of autism and pesticidesa
| Citation | Geographic location |
Birth years |
Sample sizeb |
Study design |
Autism data sourcec |
Autism measurementd |
Autism classification |
Adjustment variablese | Exposure measurement | Exposure timing | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eskenazi et al.135 | Salinas Valley, California, USA | 2000–2001 | 51/304 | Cohort | Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) | >97% On PDD scale of CBCL | ASD | Child’s sex and exact age at assessment, breastfeeding duration, HOME score,3 household income above poverty threshold, parity, maternal PPVT,4 maternal depression, and maternal age5 | Maternal and child urine specimens, maternal prenatal blood sample | Pregnancy and birth to 24 months | ||
| Roberts et al.138 | Central Valley, California, USA | 1996–1998 | 465/6975 | Nested case–control | California DDS | DSM-IV-R determined by DDS staff | Autistic disorder | Maternal education, race, and DDS regional center of diagnosis, and maternal age | Agricultural pesticide application data from the California Department of Pesticide Regulation linked to children using crop information and a geographic information system based on residential address. | Pregnancy: CNS development: gestational days 7–49. A posteriori gestational period: days 2–81. All of gestation: gestational days 14 to DOB | ||
| Roberts and English139 | Central Valley, California, USA | 1996–1998 | 465/6975 | Nested case–control | California DDS | DSM-IV-R determined by DDS staff | Autistic disorder | Maternal race/ethnicity and educational attainment, and DDS regional center of diagnosis, and maternal age | Agricultural pesticide application data from the California Department of Pesticide Regulation linked to children using crop information and a geographic information system based on residential address. | Window in pregnancy: days 38–163 postfertilization, Postnatal window: days 346–529 postfertilization | ||
| McCanlies et al.87 | Described in Table 6. | |||||||||||
| Cheslack-Postava et al.137 | Finland | 1991–2000 | 75/75 | Case–control | FiPS-A | ICD code F84.0 from record linkages of the Finnish Medical Birth Registry and the Finnish Hospital and Outpatient Discharge Registry | Autistic disorder | Child’s sex, birth year, maternal age, urban/Socioeconomic status was not associated with any persistent pollutant in this sample. ORs for DDE and HCB were also adjusted for co-pollutant: total PCBs. | Laboratory analysis of stored serum collected during pregnancy, usually in the 1st trimester. | Early pregnancy | ||
| Keil et al.140 | Central California, USA | 1997–2006 | 407/262 | Case–control | CHARGE | ADOS + ADI-R | Autistic disorder | Child’s sex, regional center of birth, and age, maternal education, race/ethnicity, and parity, and pet ownership during prenatal period | Retrospective Environmental Exposure Questionnaire via maternal phone interview | 3 Months prior to pregnancy through termination of breast-feeding | ||
| Braun étal.136 | Cincinnati, Ohio, USA | 2003–2006 | 175 | Cohort | Health Outcomes and measures of the Environment (HOME) Study | Social Responsiveness Scale at ages 4 and 5 years | Continuous autism phenotype | Maternal age at delivery, race, marital status, education, insurance status, IQ, serum cotinine, and employment, parity, household income, prenatal vitamin use, depressive symptoms in 2nd trimester, and HOME score | Laboratory analysis of serum sample during gestation, usually around 16 weeks, lipid normalized | Pregnancy | ||
Results in Figure 7.
Sample size is presented by a single number when statistical models included a continuous measure of autism phenotype, or, otherwise, as the number of cases/controls.
Autism data sources are described in Table 2.
Autism diagnostic and psychometric tools are described in Table 3.
Variables in parentheses were evaluated but not included in final models because they did not impact results.
Fig 7.
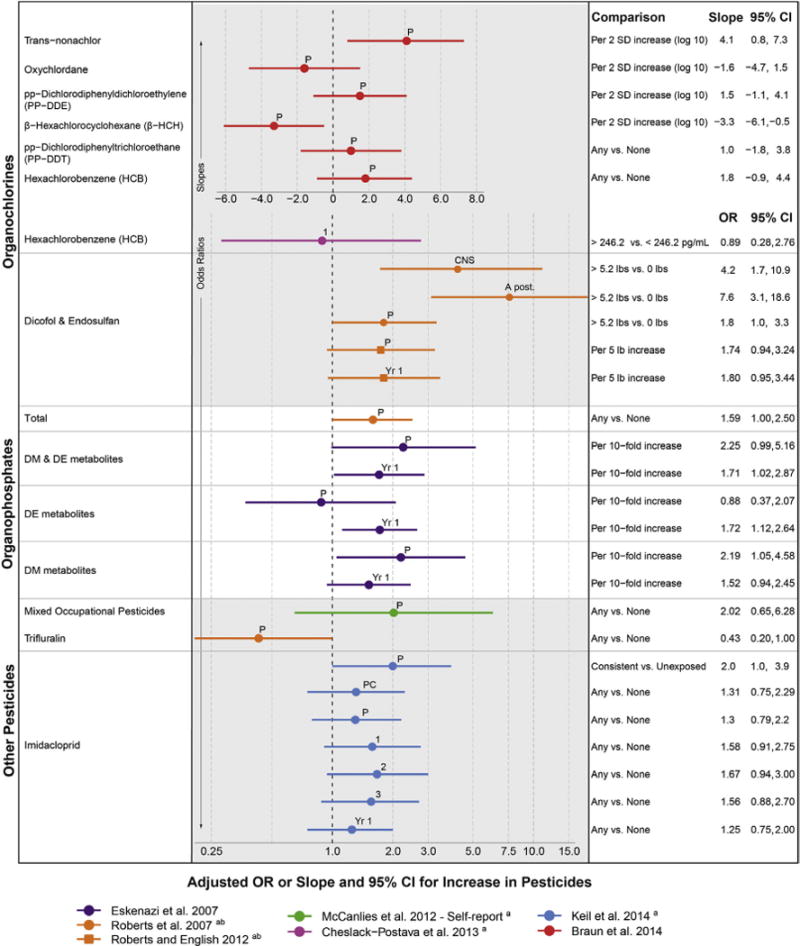
Associations between autism and estimates of exposure to pesticides. DE metabolites, diethyl phosphate metabolites of organophosphate pesticides; DM metabolites, dimethyl phosphate metabolites of organophosphate pesticides. Exposure measured during developmental windows: CNS, a priori period of central nervous system development (7 days pre-fertilization to 49 days postfertilization); A post., a posteriori period of development (26–81 days postfertilization); 1, trimester 1; 2, trimester 2; 3, trimester 3; P, pregnancy; Yr 1, 1st postnatal year. Other results not reported as measures of association with confidence intervals included pesticides that were determined not to be associated with increased autism risk and were not included in subsequent analyses from Roberts et al.138: pesticide classes (cholinesterase inhibitors, copper-containing compounds, fumigants, avermectins, halogenated organics, N-methyl carbamates, pyrethroids, and thiocarbamates) and individual pesticide compounds (1,3-dichloropropene, chloropicrin, cypermethrin, fenarimol, methyl bromide, norflurazon, bromacil acid, chlorpyrifos, dazomet, glyphosate, molinate, oxadiazon, bifenthrin, diuron, metam-sodium, myclobutanil, and paraquat). aAuthor provided information not included in the publication. bWe re-calculated parameters to reflect a change in the exposure comparison to be consistent with other comparisons in the figure, involving calculations assuming that parameters were normally distributed.
Limitations in these studies include that none adjusted for other agricultural exposures, and it is therefore possible that observed associations between pesticides and autism may, in actuality, be reflecting a causal influence of another chemical along for the ride. While possible, this co-pollutant confounding influence is not likely to be large: pesticides are chemicals with demonstrated neurotoxicity and these studies measured pesticides using different methods and across different settings. In one study among a largely Hispanic, agricultural community, the association between pesticides and autism may have been overestimated because of unusual performance of the psychometric test in this population and because the study used an OR, which is best suited for rare diseases, for a common outcome.15
The elevated associations observed across this literature may also be attenuated compared to the true relationship, given that pesticide exposure measurement methods included error. For example, residential proximity to agricultural applications may incur error from wind patterns, time spent away from home, or limitations in the pesticide application data sources.138,139 Another example is that blood and urine pesticide/metabolite biomarkers used in some studies only reflected the past 48 h of exposure,135 so that even repeated measures in pregnancy may not have accurately reflected the true dose to developing neurological structures. Another attenuating influence may be negative confounding by nutrients in the fruit and vegetables that contribute pesticides, which could counter-balance the toxic impact of the pesticide given some evidence that nutrients like folate are associated with reduced risk for autism.141,142
In summary, the results from these studies suggest that several types of pesticide exposures may, for some children, be part of what causes the emergence of autism. A period of susceptibility in early pregnancy was suggested by one study, but this trend did not hold in other studies and requires confirmation. Importantly, many pesticides have not been studied in relation to autism, though most give rise to neurotoxicity.143 Continued study of varying pesticides, combinations, and developmental windows is necessary to substantiate the current evidence of the deleterious effects of pesticide exposure.
Polychlorinated Biphenyls, Flame Retardants, Non-Stick Chemicals, Phthalates, and Bisphenol A
Persistent organic pollutants are synthetic, carbon-based chemicals that accumulate in the environment and in human tissues, including legacy chemicals that have since been banned along with chemicals currently used. Persistent organic pollutants that were banned by the Stockholm Convention in 2001 include PCBs (heat-stable lubricants used throughout industry) and some dioxins (such as 2,3,7,8-tetrachlorodibenzodioxin, TCDD, the dioxin present as a contaminant in the Vietnam-era defoliation compound, Agent Orange).144 Currently used chemicals include polybrominated diphenyl ethers (PBDEs, used as flame-retardant additives to textiles and plastic casings of electronics) and perfluorinated compounds (PFCs, used as non-stick coatings in cookware and outdoor gear). Some of the pesticides reviewed in the previous section are persistent organic pollutants (e.g., DDT).
Also covered in this section are two groups of non-persistent organic pollutants: bisphenol A (BPA) and phthalates. Although these chemicals are not persistent, that is, they are readily cleared from the human body in part because they are water soluble, exposure is ubiquitous and continuous, so is important to examine for chronic effects. BPA is used in plastics and resins, including linings of canned foods, receipts, toys, and medical equipment. High-molecular-weight phthalates are plasticizers, used in medical tubing, food packaging, and polyvinyl chloride (PVC) plastics and flooring. Low-molecular-weight phthalates are used in cosmetics, fragrances, and medication coatings.
These persistent and non-persistent chemical groups are all suspected to interfere with hormone systems and have thus been termed endocrine-disrupting chemicals. Some chemicals covered in other sections of this review, such as pesticides, also act to disrupt the endocrine system. Such chemicals have been shown to mimic, block, or alter hormone signaling of steroid hormones, oxytocin, or thyroid hormones.145 For example, PBDEs are structurally similar to thyroid hormone, which is required during discrete periods of neurodevelopment.146 Other pathophysiological mechanisms of these organic pollutants include neurotoxicity or immunotoxicity.147–149 Epigenetic mechanisms are suggested by a mouse study that demonstrated transgenerational impacts on social recognition following BPA exposure,150 and the involvement of mitochondrial impairment is supported by the demonstration that a flame retardant interfered with brain mitochondrial function in mice.151 Human epidemiological studies have widely examined a role for these chemicals in various neurodevelopmental end points, with reviews concluding a plausible role in attention-deficit disorders121 and autism.152
Diet is a predominant source of exposure for many persistent pollutants (PCBs, PBDEs, and PFCs), because they bioaccumulate in predatory fish and breast milk. In addition, these chemicals can be widely present in interior spaces because they are not chemically bound in the products in which they are used, and so are readily liberated into the environment, leading to further ingestion or inhalation. Examples here include PBDEs in indoor dust after release from computers or carpets and phthalates released from fragrances and scented products. All of these chemicals, whether banned or not, or persistent or not, are near-universally detected in representative human biological samples from the U.S.12 Some chemicals are even increasing in body burden, for example, PBDE flame retardants153 and some, but not all, types of phthalates.154 Body burdens of PBDE flame retardants may correspond to regulatory standards: dust biomarker levels are especially high in California, where flammability standards had until recently been more strict than in the U.S. as a whole.155 Additional drivers of exposure include lifestyle patterns, such as greater consumption of canned food or having older home upholstery or electronic equipment, and neighborhood, because of proximity to polluting sources, and so sociodemographic and neighborhood factors are plausible confounders for this question.156 Calendar time and child-rearing practices are similarly important: maternal age, because older moms were living in times prior to chemical bans and incurred more years to accumulate body burdens, and birth order and breast-feeding practices, in part because breast-feeding is one of the most effective methods of excreting lipophilic chemicals.157
Five studies, from different countries and using different valid measures of autism diagnosis or the continuous autism phenotype, met our inclusion criteria (Table 11). Two studies used parent questionnaire to measure exposures,58,89 and three studies used prospective biomarkers pertaining to pregnancy, including a study for which one of us (A.K.) was an author.136,137,158 Pregnancy biomarkers are especially valid for the persistent pollutants (PCBs, PBDEs, and PFCs) because they have long half-lives and reflect exposures over the entire relevant period. In contrast, biomarkers for phthalates and BPA may incur more measurement error because they only reflect exposure for a few days instead of the entire relevant period of development. Still, biomarkers of phthalates and BPA have strengths of being objective, quantitative measures, and in one study were collected more than one time in pregnancy.136 All of these studies adjusted for important sociodemographic confounders, and all except for one adjusted for maternal age.58
TABLE 11.
Included epidemiological publications of autism and polychlorinated biphenyls, flame retardants, non-stick chemicals, phthalates, and bisphenol Aa
| Citation | Geographic location |
Birth years | Sample sizeb |
Study design |
Autism data source |
Autism measurementc |
Autism classification |
Adjustment Variablesd | Exposure Measurement | Exposure Timing |
|---|---|---|---|---|---|---|---|---|---|---|
| Larsson et al.58 | Described in Table 5. | Condensation on windows, maternal smoking, child’s sex and age, child’s asthma status, and financial problems | Parent questionnaire at study baseline about type of flooring in the parent’s and child’s bedrooms. | Pregnancy + early childhood combined | ||||||
| Kim et al.89 | Described in Table 7. | Retrospective parent questionnaire of exposure to “canned food,” “plastics,” “waste incinerators,” “seafoods,” “polyurethane foams,” “fast food,” “hot canned beverages.” “old electronics,” and “microwaveable food” | Pregnancy | |||||||
| Miodovnik et al.158 | New York City, New York, USA | 1998–2002 | 137 | Cohort | Mt. Sinai Children’s Environmental Health Study | SRS when child was 7–9 years | Continuous autism phenotype | Child race and sex, caregiver marital status, and urinary creatinine, a measure of urine dilution. (Maternal age, IQ, education, child IQ, and age at SRS) | Laboratory analyses of maternal spot urine samples from 25 to 40 weeks of pregnancy | Mid–late pregnancy |
| Cheslack-Postava et al.137 | Described in Table 10. | |||||||||
| Braun et al.136 | Described in Table 10. | PCBs, BFRs, and PFCs measured from laboratory analysis of serum (usually at 16 weeks of gestation) and lipid normalized. Phthalates and BPA measured in urine samples from pregnancy (around 16 and 26 weeks) and creatinine standardized. | Pregnancy | |||||||
Results in Figure 8.
Sample size is presented by a single number when statistical models included a continuous measure of autism phenotype, or, otherwise, as the number of cases/controls.
Autism diagnostic and psychometric tools are described in Table 3.
Variables in parentheses were evaluated but not included in final models because they did not impact results.
Associations between PCBs (individual congeners and mixtures) and autism were scattered above and below the null and so were not consistent with a causal relationship. Associations between PCBs and autism from a pilot study in Finland were mostly elevated; however, the modest sample (75 children with autism) did not allow for precise, conclusive results, and the lack of adjustment for birth order may have slightly biased these findings (Fig. 8, purple).137 Results for flame-retardant (PBDE) and non-stick (PFCs) chemicals, all from one publication, showed mixed findings, with suggestions that some congeners may be associated with more autistic behaviors (e.g., PBDE-28), whereas others may be associated with fewer autistic behaviors (e.g., PBDE-85 and PFOA) (Fig. 8, red).136 Associations between autism and BPA from two studies were not consistent with a causal association.136,158 Several phthalate congeners, in contrast, appeared associated with increased autism across three different studies, although results were not consistent across these studies and most lacked the statistical precision for conclusive results.58,136,158
Fig 8.
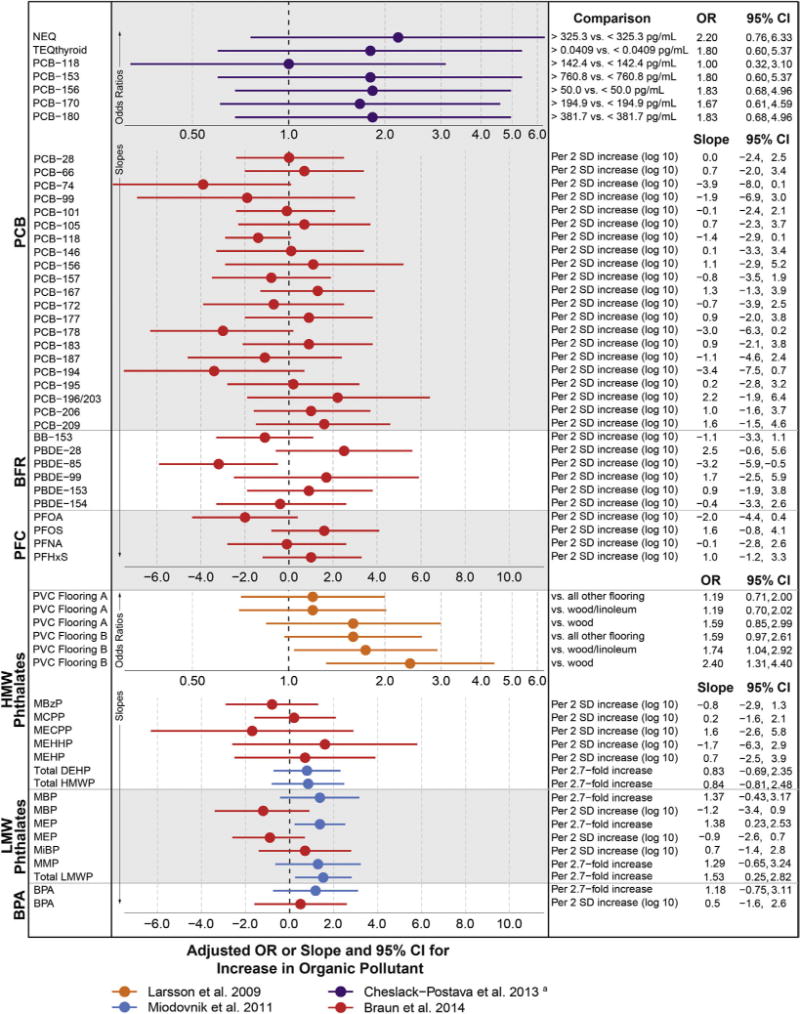
Associations between autism and estimates of exposure to polychlorinated biphenyls, flame retardants, non-stick chemicals, phthalates, and bisphenol A. PCB, polychlorinated biphenyls; NEQ, neurotoxic equivalents, a weighted index of PCB congeners; TEQthyroid, thyroid hormone-based toxic-equivalent index of PCB congeners; BFR, brominated flame retardants, including: BB, brominated biphenyl; PBDE, polybrominated diphenyl ethers; PFC, polyfluorinated compounds (non-stick chemicals), including: PFOA, perfluorooctanoate; PFOS, perfluorooctane sulfate; PFNA, perfluorononanoate; PFHxS, perfluorohexane sulfonate; HMW phthalates, high-molecular-weight phthalates, including: from PVC, polyvinyl chloride; MBzP, mono-benzyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono(2-ethylhexyl) phthalate; Total DEHP, di(2-ethylhexyl) phthalates = [MECPP, MEHHP, MEOHP, mono(2-ethyl-5-oxohexyl) phthalate and MEHP]; Total HMWP = MBzP and MCPP; LMW phthalates, low-molecular-weight phthalates, including: MBP, monobutyl phthalate; MEP, monoethyl phthalate; MiBP, mono-iso-butyl phthalate; MMP, monomethyl phthalate; Total LMW = MMP, MEP, MBP, MiBP. PVC Flooring A was in the child’s bedroom, PVC Flooring B was in the parents’ bedroom. Other results not reported as measures of association with confidence intervals included, for Kim et al.89: higher parent report of various non-specific exposures likely to include persistent and endocrine-disrupting compounds for children with autism. aAuthor provided information not included in the publication.
In summary, the emerging literature on autism and persistent and non-persistent endocrine-disrupting compounds does not allow definitive conclusions regarding a role for PCBs, flame retardants, non-stick chemicals, or BPA, while results for phthalates are more suggestive that exposures to some phthalates may be risk factors for autism. Given the difficulty in measuring exposure, especially for phthalates and BPA, which have short half-lives, it is not possible to rule out any of these chemicals from causing neurodevelopmental harm. Their known endocrine-disrupting properties are also a reason not to dismiss concern regarding these chemicals. Two caveats deserve mention. Studies to date have focused on the important prenatal period, yet if transgenerational impacts are implicated via epigenetic mechanisms, these studies may completely miss the relevant exposures that occurred for parents or grandparents. Lastly, the studies to date have not addressed numerous other chemicals that disrupt hormone signaling and are ubiquitous in the environment, such as dioxin and the anti-bacterial compound, triclosan.
Susceptibilities and Heterogeneity of Response to Xenobiotic Exposures
Greater Susceptibility in Males or Females
Being a male is one of the strongest predictors of being diagnosed with autism, especially higher-functioning autism, and while reasons for this have been explored,159–162 there is currently no definitive explanation. Given the link between autism and child’s sex together with the endocrine activity of many environmental chemicals, there is good reason to explore sex-specific susceptibility to the impacts of xenobiotic exposures (i.e., sex modification or interaction). While greater susceptibility may be a priori expected in males as a driver of their higher autism prevalence, either sex may be more susceptible. Additionally, whether males or females are more susceptible may vary from one chemical to another.
Some studies reviewed here have addressed susceptibilities in males vs. females, without finding clear trends.70,136 The very strength of the child sex-autism association presents a research challenge because the number of females with autism in any given study will be much smaller than the number of males, limiting the statistical power to assess sex × environment interactions. This is an important research area, and future studies may need to over-sample females with autism or pool data across studies to better answer questions about sex-specific susceptibilities.
Genetic Susceptibilities
It is likely that the autism risk associated with a given xenobiotic exposure will differ based on the child’s genes or the mother’s genes. Genetic polymorphisms of possible relevance include those encoding proteins involved in the metabolism or biological activation of the xenobiotic, genes allowing improved resiliency to neurodevelopmental damages, or genes that confer some degree of autism risk that, when combined with xenobiotic damage, result in autism. Some examples of research in this area include a study demonstrating interaction between traffic-related air pollutants and a functional variant of the Met receptor tyrosine kinase (MET) gene with regard to autism risk,163 and a study finding no interaction between OP pesticide exposure and polymorphisms in the gene encoding the detoxifying enzyme, paraoxonase 1 (PON1) with autism risk.164
In this report, we did not provide a comprehensive review of studies of gene–environment interaction in autism, but instead point to ways in which this area influences our knowledge about the main effects of a xenobiotic exposure. Failing to account for genetic susceptibilities may result in the missed identification of an environmental risk factor: If a genetically susceptible subgroup is combined with a non-susceptible group, the overall impact of the xenobiotic exposure on autism risk may be diluted or obscured. Future research is needed in this area, and will require expanded knowledge of biological pathways to select candidate polymorphisms and sufficiently large sample sizes required to detect gene × environment interactions.
Different Responses Across the Spectrum of Neurodevelopmental Phenotypes
Epidemiological studies of environmental chemicals and autism are also complicated by the fact that children diagnosed under the autism umbrella exhibit a very wide range of symptoms, level of disability, and co-occurring conditions. Furthermore, autism, like other psychiatric disorders, is diagnosed based on observable symptom clustering, not causal pathways, and so diagnostic definitions may not be ideal for causal research, a phenomena termed “etiological heterogeneity.”165–167 The environmental chemical risk factors for a given phenotype, such as high-functioning autism, may be quite different from risk factors for children with severe autism and intellectual disability. When multiple autism subtypes are combined in a given study, true causal impacts may be diluted or masked. However, defining a discrete disorder that reflects a common causal pathway remains elusive. The defining variables are not known.
A corollary to etiologic heterogeneity is that autism is one manifestation of developmental neurological damage, part of the broader set of neurodevelopmental problems including cognitive impairments, attention-deficit disorders, sensory processing disorders, and more. Instead of studying autism in isolation, a more holistic approach would comprehensively define neurodevelopmental impairments along multiple cross-cutting dimensions of behavior and abilities, and this nuanced “phenome” approach may aid in the further discovery of modifiable, environmental chemical risk factors that may impact neurodevelopment.
Conclusions
The environment is not one entity, and the question about whether environmental exposures/compounds, as a whole, cause autism, is not sufficient to guide the primary prevention of autism. Instead, important questions address individual environmental chemicals, during discrete critical periods of development for the fetus or infant, in relation to specific phenotypic domains of autism symptoms. Definitive causal conclusions cannot yet be drawn from this emerging body of literature. So far, evidence suggests that some criteria air pollutants, some metals, and several pesticides, with suggestions that some volatile organic compounds (e.g., methylene chloride, trichloroethylene, and styrene) and phthalates, may be linked to autism. These associations have emerged despite difficulties of study in this area, most notably the error in accurately measuring exposure concentrations and obtaining sufficient sample sizes.
Increasingly, studies that examine exposures by developmental time period suggest that all windows are not equal. Although evidence for a discrete developmental window of susceptibility for autism is in its early stages, results shown here suggest that early pregnancy may be a period of susceptibility to pesticides, whereas later pregnancy may be a period of susceptibility to criteria air pollutants. Different critical windows for different exposures is not unexpected given the various biological activities of environmental chemicals and the many, interwoven events of neurodevelopment, taking place during pregnancy and postnatal life, which, if disrupted, could manifest in autism symptoms.
The volume of questions that remain are staggering, constituting an important gap in pediatric health. The number of chemicals not yet studied is large—chemicals that are widely detectable in humans and have animal or mechanistic evidence of the endocrine, immune, epigenetic, and other pathophysiologies that may be involved in causing autism. Even more daunting is the large number of permutations of relevant research questions when it is considered that each chemical exposure is best studied in combination with many critical windows of exposure, several autism phenotypic subdomains, each child’s gender, and a myriad of genetic susceptibilities, many of which remain undiscovered. Lastly, the issue of potentiation due to chemical mixtures and autism, that is, whether the neurodevelopmental response to chemical X differs when combined with chemical Y (and Z, and so on), has only been addressed in a handful of reports.
In the meantime, clinicians have an important role to play in protecting the vulnerable brains of fetuses, infants, and children. Clinicians are on the front lines of autism diagnosis and can advocate for the equitable early diagnoses that are so important in assisting children toward appropriate therapy, diagnoses that ultimately support the validity of research studies in this area. The demonstrated racial/ethnic and social disparities in the timely receipt of an autism diagnosis remain pressing public health problems. Clinicians serve as the experts on autism symptomatology, raising observations that may provide the clues for future study, refining the understanding of autism phenotype, and participating as autism experts in epidemiological research. Clinicians advise pregnant women and parents toward avoiding harmful substances in their environment. Yet more powerful than these is the leadership role of the clinician in advocating for a healthy environment and the societal prevention activities that have the most impact. Clinicians increasingly must have a strong voice in advocating for policies that protect the public’s health, including calls for research into environmental chemicals, potentially modifiable factors that may contribute to autism or other neurodevelopmental problems in childhood.
Acknowledgments
We gratefully acknowledge the assistance of Brian Thayer, who created all the figures; Kim Yolton, Paul Auer, and Hongbo Ma, who provided consultation; Nancy Mathiowetz, Joseph Braun, and Sharon Sagiv, who provided feedback on the draft manuscript; and the authors of included publications who provided additional information used in the figures: Gayle Windham, Eric Roberts, Erin McCanlies, Alex Keil, Keely Cheslack-Postava, Angelica Ronald, Andre Sourander, and Brian Lee.
References
- 1.Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(suppl 2):1–21. [PubMed] [Google Scholar]
- 2.Manning-Courtney P, Murray D, Currans K, et al. Autism spectrum disorders. Curr Probl Pediatr Adolesc Health Care. 2013;43(1):2–11. doi: 10.1016/j.cppeds.2012.08.001. http://dx.doi.org/10.1016/j.cppeds.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Coury DL, Swedo SE, Thurm AE, et al. Treating the whole person with autism: the proceedings of the Autism Speaks National Autism Conference. Curr Probl Pediatr Adolesc Health Care. 2014;44(2):26–47. doi: 10.1016/j.cppeds.2013.12.002. http://dx.doi.org/10.1016/j.cppeds.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Engel SM, Daniels JL. On the complex relationship between genes and environment in the etiology of autism. Epidemiology. 2011;22(4):486–8. doi: 10.1097/EDE.0b013e31821daf1c. http://dx.doi.org/10.1097/EDE.0b013e31821daf1c. [DOI] [PubMed] [Google Scholar]
- 5.Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22(2):219–25. doi: 10.1097/MOP.0b013e328336eb9a. http://dx.doi.org/10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 6.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. J Am Med Assoc. 2014;311(17):1770–7. doi: 10.1001/jama.2014.4144. http://dx.doi.org/.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallmayer J. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095. doi: 10.1001/archgenpsychiatry.2011.76. http://dx.doi.org/10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawler CP. The “environment” for autism research: signs of improvement? Environ Health Perspect. 2008;116(10):A416–A417. doi: 10.1289/ehp.12107. http://dx.doi.org/10.1289/ehp.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniels JL. Guest editorial: autism and the environment. Environ Health Perspect. 2006;114(7):A396. doi: 10.1289/ehp.114-a396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368(9553):2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 11.Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8. doi: 10.1016/S1474-4422(13)70278-3. http://dx.doi.org/10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables. 2013 Available at: http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Sep2013.pdf. Accessed 16.12.13.
- 13.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ Health Perspect. 2011;119(6):878–85. doi: 10.1289/ehp.1002727. http://dx.doi.org/10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyall K, Schmidt RJ, Hertz-Picciotto I. Maternal lifestyle and environmental risk factors for autism spectrum disorders. Int J Epidemiol. 2014 doi: 10.1093/ije/dyt282. http://dx.doi.org/10.1093/ije/dyt282. [DOI] [PMC free article] [PubMed]
- 15.Hertz-Picciotto I. Environmental risk factors in autism: results from large-scale epidemiologic studies. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism Spectrum Disorders. Oxford: Oxford University Press; 2011. pp. 827–62. [Google Scholar]
- 16.Kalkbrenner AE, Daniels JL, Chen J-C, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology. 2010;21(5):631–41. doi: 10.1097/EDE.0b013e3181e65d76. http://dx.doi.org/10.1097/EDE.0b013e3181e65d76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S, Robins J. Invited commentary: ecologic studies —biases, misconceptions, and counterexamples. Am J Epidemiol. 1994;139(8):747–60. doi: 10.1093/oxfordjournals.aje.a117069. [DOI] [PubMed] [Google Scholar]
- 18.Braun JM, Kalkbrenner A. Autism prevalence and precipitation: the potential for cross-level bias. Arch Pediatr Adolesc Med. 2009;163(5):492. doi: 10.1001/archpediatrics.2009.83. [author reply 492–493] [DOI] [PubMed] [Google Scholar]
- 19.Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–54. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- 20.Herbert MR, Ziegler DA, Deutsch CK, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain J Neurol. 2005;128(Pt 1):213–26. doi: 10.1093/brain/awh330. http://dx.doi.org/10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- 21.Hultman CM, Sparén P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology. 2002;13(4):417–23. doi: 10.1097/00001648-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370:247–61. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23(1):297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoner R, Chow ML, Boyle MP, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370(13):1209–19. doi: 10.1056/NEJMoa1307491. http://dx.doi.org/10.1056/NEJMoa1307491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamou M, Streifel KM, Goines PE, Lein PJ. Neuronal connectivity as a convergent target of gene × environment interactions that confer risk for Autism Spectrum Disorders. Neurotoxicol Teratol. 2013;36:3–16. doi: 10.1016/j.ntt.2012.12.001. http://dx.doi.org/10.1016/j.ntt.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119(4):747–54. doi: 10.1172/JCI37934. http://dx.doi.org/10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodier PM. The early origins of autism. Sci Am. 2000;282:56–63. doi: 10.1038/scientificamerican0200-56. [DOI] [PubMed] [Google Scholar]
- 28.London E, Etzel RA. The environment as an etiologic factor in autism: a new direction for research. Environ Health Perspect. 2000;108(suppl 3):401–4. doi: 10.1289/ehp.00108s3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandell DS, Wiggins LD, Carpenter LA, et al. Racial/ethnic disparities in the identification of children with autism spectrum disorders. Am J Public Health. 2009;99:493–8. doi: 10.2105/AJPH.2007.131243. http://dx.doi.org/10.2105/AJPH.2007.131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savitz DA. Is statistical significance testing useful in interpreting data? Reprod Toxicol. 1993;7(2):95–100. doi: 10.1016/0890-6238(93)90242-y. [DOI] [PubMed] [Google Scholar]
- 31.Nuzzo R. Scientific method: statistical errors. Nature. 2014;506(7487):150–2. doi: 10.1038/506150a. http://dx.doi.org/10.1038/506150a. [DOI] [PubMed] [Google Scholar]
- 32.Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry. 2014;4(2):e360. doi: 10.1038/tp.2014.4. http://dx.doi.org/10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurley AM, Tadrous M, Miller ES. Thimerosal-containing vaccines and autism: a review of recent epidemiologic studies. J Pediatr Pharmacol Ther. 2010;15(3):173–81. [PMC free article] [PubMed] [Google Scholar]
- 34.Dórea JG. Making sense of epidemiological studies of young children exposed to thimerosal in vaccines. Clin Chim Acta. 2010;411(21–22):1580–6. doi: 10.1016/j.cca.2010.07.008. http://dx.doi.org/10.1016/j.cca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 35.De Cock M, Maas YGH, van de Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review: exposure to EDCs and neurodevelopmental disorders. Acta Paediatr. 2012;101(8):811–8. doi: 10.1111/j.1651-2227.2012.02693.x. http://dx.doi.org/10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- 36.Dietz PM, Homa D, England LJ, et al. Estimates of nondisclosure of cigarette smoking among pregnant and non-pregnant women of reproductive age in the United States. Am J Epidemiol. 2011;173(3):355–9. doi: 10.1093/aje/kwq381. http://dx.doi.org/10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- 37.Löfroth G. Environmental tobacco smoke: overview of chemical composition and genotoxic components. Mutat Res Toxicol. 1989;222(2):73–80. doi: 10.1016/0165-1218(89)90021-9. http://dx.doi.org/10.1016/0165-1218(89)90021-9. [DOI] [PubMed] [Google Scholar]
- 38.McElroy JA, Shafer MM, Trentham-Dietz A, Hampton JM, Newcomb PA. Urinary cadmium levels and tobacco smoke exposure in women age 20–69 years in the United States. J Toxicol Environ Health A. 2007;70(20):1779–82. doi: 10.1080/15287390600754953. http://dx.doi.org/10.1080/15287390600754953. [DOI] [PubMed] [Google Scholar]
- 39.Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in U.S. children. Epidemiology. 2003;14(6):719–27. doi: 10.1097/01.EDE.0000081998.02432.53. http://dx.doi.org/10.1097/.EDE.0000081998.02432.53. [DOI] [PubMed] [Google Scholar]
- 40.Braun JM, Daniels JL, Kalkbrenner A, Zimmerman J, Nicholas JS. The effect of maternal smoking during pregnancy on intellectual disabilities among 8-year-old children. Paediatr Perinat Epidemiol. 2009;23(5):482–91. doi: 10.1111/j.1365-3016.2009.01056.x. http://dx.doi.org/10.1111/j.1365-3016.2009.01056.x. [DOI] [PubMed] [Google Scholar]
- 41.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–15. [PubMed] [Google Scholar]
- 42.Mendola P, Selevan SG, Gutter S, Rice D. Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment Retard Dev Disabil Res Rev. 2002;8:188–97. doi: 10.1002/mrdd.10033. [DOI] [PubMed] [Google Scholar]
- 43.Breton CV, Byun H-M, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med. 2009;180(5):462–7. doi: 10.1164/rccm.200901-0135OC. http://dx.doi.org/10.1164/rccm.200901-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ozerol E, Ozerol I, Gökdeniz R, Temel I, Akyol O. Effect of smoking on serum concentrations of total homocysteine, folate, vitamin B12, and nitric oxide in pregnancy: a preliminary study. Fetal Diagn Ther. 2004;19(2):145–8. doi: 10.1159/000075139. http://dx.doi.org/10.1159/000075139. [DOI] [PubMed] [Google Scholar]
- 45.Burstyn I, Wang X, Yasui Y, Sithole F, Zwaigenbaum L. Autism spectrum disorders and fetal hypoxia in a population-based cohort: accounting for missing exposures via Estimation-Maximization algorithm. BMC Med Res Methodol. 2011;11(1):2. doi: 10.1186/1471-2288-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. http://dx.doi.org/10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Soothill PW, Morafa W, Ayida GA, Rodeck CH. Maternal smoking and fetal carboxyhaemoglobin and blood gas levels. Br J Obstet Gynaecol. 1996;103:78–82. doi: 10.1111/j.1471-0528.1996.tb09519.x. [DOI] [PubMed] [Google Scholar]
- 48.Abbott LC, Winzer-Serhan UH. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol. 2012;42(4):279–303. doi: 10.3109/10408444.2012.658506. http://dx.doi.org/10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- 49.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5):372–7. doi: 10.1038/nri803. http://dx.doi.org/10.1038/nri803. [DOI] [PubMed] [Google Scholar]
- 50.Vinikoor LC, Messer LC, Laraia BA, Kaufman JS. Reliability of variables on the North Carolina birth certificate: a comparison with directly queried values from a cohort study. Paediatr Perinat Epidemiol. 2010;24(1):102–12. doi: 10.1111/j.1365-3016.2009.01087.x. http://dx.doi.org/10.1111/j.1365-3016.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee BK, Gardner RM, Dal H, et al. Brief report: maternal smoking during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2011;42(9):2000–5. doi: 10.1007/s10803-011-1425-4. http://dx.doi.org/10.1007/s10803-011-1425-4. [DOI] [PubMed] [Google Scholar]
- 52.Kalkbrenner AE, Braun JM, Durkin MS, et al. Maternal smoking during pregnancy and the prevalence of autism spectrum disorders, using data from the autism and developmental disabilities monitoring network. Environ Health Perspect. 2012;120(7):1042–8. doi: 10.1289/ehp.1104556. http://dx.doi.org/10.1289/ehp.1104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Indredavik MS, Brubakk A-M, Romundstad P, Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr. 2007;96(3):377–82. doi: 10.1111/j.1651-2227.2006.00148.x. http://dx.doi.org/10.1111/j.1651-2227.2006.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ronald A, Happé F, Dworzynski K, Bolton P, Plomin R. Exploring the relation between prenatal and neonatal complications and later autistic-like features in a representative community sample of twins. Child Dev. 2010;81(1):166–82. doi: 10.1111/j.1467-8624.2009.01387.x. [DOI] [PubMed] [Google Scholar]
- 55.Reiersen AM, Constantino JN, Volk HE, Todd RD. Autistic traits in a population-based ADHD twin sample. J Child Psychol Psychiatry. 2007;48(5):464–72. doi: 10.1111/j.1469-7610.2006.01720.x. http://dx.doi.org/10.1111/j.1469-7610.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 56.St Pourcain B, Mandy WP, Heron J, Golding J, Davey Smith G, Skuse DH. Links between co-occurring social-communication and hyperactive-inattentive trait trajectories. J Am Acad Child Adolesc Psychiatry. 2011;50(9):892–902. doi: 10.1016/j.jaac.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can. 2010;30:125–34. [PubMed] [Google Scholar]
- 58.Larsson M, Weiss B, Janson S, Sundell J, Bornehag C-G. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30(5):822–31. doi: 10.1016/j.neuro.2009.01.011. http://dx.doi.org/10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tran PL, Lehti V, Lampi KM, et al. Smoking during pregnancy and risk of autism spectrum disorder in a Finnish national birth cohort: smoking during pregnancy and risk of autism. Paediatr Perinat Epidemiol. 2013;27(3):266–74. doi: 10.1111/ppe.12043. http://dx.doi.org/10.1111/ppe.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rai D, Lewis G, Lundberg M, et al. Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. J Am Acad Child Adolesc Psychiatry. 2012;51(5):467–76. doi: 10.1016/j.jaac.2012.02.012. http://dx.doi.org/10.1016/j.jaac.2012.02.012:(e6) [DOI] [PubMed] [Google Scholar]
- 61.Liu J, Leung PWL, McCauley L, Ai Y, Pinto-Martin J. Mother’s environmental tobacco smoke exposure during pregnancy and externalizing behavior problems in children. Neurotoxicology. 2013;34:167–74. doi: 10.1016/j.neuro.2012.11.005. http://dx.doi.org/10.1016/j.neuro.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen R, Clifford A, Lang L, Anstey KJ. Is exposure to secondhand smoke associated with cognitive parameters of children and adolescents?—a systematic literature review. Ann Epidemiol. 2013;23(10):652–61. doi: 10.1016/j.annepidem.2013.07.001. http://dx.doi.org/10.1016/j.annepidem.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 63.Jacob DJ, Winner DA. Effect of climate change on air quality. Atmospheric Environ. 2009;43(1):51–63. http://dx.doi.org/10.1016/j.atmosenv.2008.09.051. [Google Scholar]
- 64.Lin J, Pan D, Davis SJ, et al. China’s international trade and air pollution in the United States. Proc Natl Acad Sci U S A. 2014;111(5):1736–41. doi: 10.1073/pnas.1312860111. http://dx.doi.org/10.1073/pnas.1312860111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6–7):437–45. doi: 10.1080/08958370490439597. http://dx.doi.org/10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 66.Levesque S, Taetzsch T, Lull ME, et al. Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect. 2011;119(8):1149–55. doi: 10.1289/ehp.1002986. http://dx.doi.org/10.1289/ehp.1002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calderón-Garcidueñas L, Engle R, Mora-Tiscareño A, et al. Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn. 2011;77(3):345–55. doi: 10.1016/j.bandc.2011.09.006. http://dx.doi.org/10.1016/j.bandc.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Sunyer J. The neurological effects of air pollution in children. Eur Respir J. 2008;32(3):535–7. doi: 10.1183/09031936.00073708. http://dx.doi.org/10.1183/09031936.00073708. [DOI] [PubMed] [Google Scholar]
- 69.Baccarelli A, Wright RO, Bollati V, et al. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–8. doi: 10.1164/rccm.200807-1097OC. http://dx.doi.org/10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Roberts AL, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect. 2013 doi: 10.1289/ehp.1206187. http://dx.doi.org/10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed]
- 71.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–7. doi: 10.1289/ehp.1002835. http://dx.doi.org/10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. J Am Med Assoc Psychiatry. 2013;70(1):71–7. doi: 10.1001/jamapsychiatry.2013.266. http://dx.doi.org/10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles County, California. Environ Health Perspect. 2012;121(3):380–6. doi: 10.1289/ehp.1205827. http://dx.doi.org/10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalkbrenner AE, Windham GC, Serre ML, et al. Exposure to ambient coarse particulate matter by gestational period and autism spectrum disorders in North Carolina and California. Am J Epidemiol. 2013;177(S11):156. [Google Scholar]
- 75.Clark C, Stansfeld SA. The effect of transportation noise on health and cognitive development: a review of recent evidence. Int J Comp Psychol. 2007;20 Available at: http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl&08893667&AN=37168563&h=MJFYFG34hmjdyVl3JLXNLkjTSa5FJYeitBtjt6ilYVsMDX2Ph%2FHi1madrjbo9b7a1qO6t%2F4qEq0P1yrorQpksw%3D%3D&crl=c. Accessed 21.2.14. [Google Scholar]
- 76.Guxens M, Aguilera I, Ballester F, et al. Prenatal exposure to residential air pollution and infant mental development: modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012;120(1):144–9. doi: 10.1289/ehp.1103469. http://dx.doi.org/10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bentur Y, Koren G. The three most common occupational exposures reported by pregnant women: an update. Am J Obstet Gynecol. 1991;165(2):429–37. doi: 10.1016/0002-9378(91)90112-5. [DOI] [PubMed] [Google Scholar]
- 78.Wang S, Ang HM, Tade MO. Volatile organic compounds in indoor environment and photocatalytic oxidation: state of the art. Environ Int. 2007;33(5):694–705. doi: 10.1016/j.envint.2007.02.011. http://dx.doi.org/10.1016/j.envint.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Payne-Sturges DC, Burke TA, Breysse P, Diener-West M, Buckley TJ. Personal exposure meets risk assessment: a comparison of measured and modeled exposures and risks in an urban community. Environ Health Perspect. 2004;112:589–98. doi: 10.1289/ehp.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rios JL, Boechat JL, Gioda A, dos Santos CY, de Aquino Neto FR, Lapa e Silva JR. Symptoms prevalence among office workers of a sealed versus a non-sealed building: associations to indoor air quality. Environ Int. 2009;35(8):1136–41. doi: 10.1016/j.envint.2009.07.005. http://dx.doi.org/10.1016/j.envint.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 81.Brown HS, Bishop DR, Rowan CA. The role of skin absorption as a route of exposure for volatile organic compounds (VOCs) in drinking water. Am J Public Health. 1984;74(5):479–84. doi: 10.2105/ajph.74.5.479. http://dx.doi.org/10.2105/AJPH.74.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barr DB, Ryan PB, Buckley B. Volatile organic compounds. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford: Oxford University Press; 2014. pp. 314–24. [Google Scholar]
- 83.Gospe SM, Jr, Zhou SS. Toluene abuse embryopathy: longitudinal neurodevelopmental effects of prenatal exposure to toluene in rats. Reprod Toxicol. 1998;12(2):119–26. doi: 10.1016/s0890-6238(97)00128-7. [DOI] [PubMed] [Google Scholar]
- 84.Laslo-Baker D, Barrera M, Knittel-Keren D, et al. Child neurodevelopmental outcome and maternal occupational exposure to solvents. Arch Pediatr Adolesc Med. 2004;158(10):956. doi: 10.1001/archpedi.158.10.956. [DOI] [PubMed] [Google Scholar]
- 85.Till C, Koren G, Rovet JF. Prenatal exposure to organic solvents and child neurobehavioral performance. Neurotoxicol Teratol. 2001;23(3):235–45. doi: 10.1016/s0892-0362(01)00141-6. [DOI] [PubMed] [Google Scholar]
- 86.Pelé F, Muckle G, Costet N, et al. Occupational solvent exposure during pregnancy and child behaviour at age 2. Occup Environ Med. 2013;70(2):114–9. doi: 10.1136/oemed-2012-100892. http://dx.doi.org/10.1136/oemed-2012-100892. [DOI] [PubMed] [Google Scholar]
- 87.McCanlies EC, Fekedulegn D, Mnatsakanova A, et al. Parental occupational exposures and autism spectrum disorder. J Autism Dev Disord. 2012;42(11):2323–34. doi: 10.1007/s10803-012-1468-1. http://dx.doi.org/10.1007/s10803-012-1468-1. [DOI] [PubMed] [Google Scholar]
- 88.Windham GC, Sumner A, Li SX, et al. Use of birth certificates to examine maternal occupational exposures and autism spectrum disorders in offspring. Autism Res. 2013;6(1):57–63. doi: 10.1002/aur.1275. http://dx.doi.org/10.1002/aur.1275. [DOI] [PubMed] [Google Scholar]
- 89.Kim SM, Han DH, Lyoo HS, Min KJ, Kim KH, Renshaw P. Exposure to environmental toxins in mothers of children with autism spectrum disorder. Psychiatry Investig. 2010;7(2):122. doi: 10.4306/pi.2010.7.2.122. http://dx.doi.org/10.4306/pi.2010.7.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco Bay Area. Environ Health Perspect. 2006;114(9):1438–44. doi: 10.1289/ehp.9120. http://dx.doi.org/10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu F, Parvez F, Graziano JH, Chen Y. Arsenic. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford: Oxford University Press; 2014. pp. 281–9. [Google Scholar]
- 92.Lucchini R, Benedetti C. Other metals. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford: Oxford University Press; 2014. pp. 290–5. [Google Scholar]
- 93.Wasserman GA, Liu X, Loiacono NJ, et al. A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environ Health. 2014;13(1):23. doi: 10.1186/1476-069X-13-23. http://dx.doi.org/10.1186/1476-069X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lanphear BP. Lead. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford: Oxford University Press; 2014. pp. 262–72. [Google Scholar]
- 95.Richmond-Bryant J, Meng Q, Davis A, et al. The influence of declining air lead levels on blood lead-air lead slope factors in children. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307072. http://dx.doi.org/10.1289/ehp.1307072. [DOI] [PMC free article] [PubMed]
- 96.Van Wijngaarden E, Davidson PW, Smith TH, et al. Autism spectrum disorder phenotypes and prenatal exposure to methylmercury. Epidemiology. 2013;24(5):651–9. doi: 10.1097/EDE.0b013e31829d2651. http://dx.doi.org/10.1097/EDE.0b013e31829d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Geier DA, Kern JK, Geier MR. A prospective study of prenatal mercury exposure from maternal dental amalgams and autism severity. Acta Neurobiol Exp (Wars) 2009;69(2):189–97. doi: 10.55782/ane-2009-1744. [DOI] [PubMed] [Google Scholar]
- 98.Baxter AC, Lotspeich LJ, Spiker D, Martin JL, Grether JK, Hallmayer JF. Brief report: effect of maternal age on severity of autism. J Autism Dev Disord. 2007;37(5):976–82. doi: 10.1007/s10803-006-0217-8. http://dx.doi.org/10.1007/s10803-006-0217-8. [DOI] [PubMed] [Google Scholar]
- 99.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med. 2003;349(18):1731–7. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 100.U.S. Food and Drug Administration. Thimerosal in Vaccines. Table 3. http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/VaccineSafety/UCM096228#t1; updated June 20, 2012.Accessed28.3.14.
- 101.Hviid A, Stellfeld M, Wohlfahrt J, Melbye M. Association between thimerosal-containing vaccine and autism. J Am Med Assoc. 2003;290(13):1763–6. doi: 10.1001/jama.290.13.1763. http://dx.doi.org/10.1001/jama.290.13.1763. [DOI] [PubMed] [Google Scholar]
- 102.Gallagher CM, Goodman MS. Hepatitis B vaccination of male neonates and autism diagnosis, NHIS 1997–2002. J Toxicol Environ Health A. 2010;73(24):1665–77. doi: 10.1080/15287394.2010.519317. http://dx.doi.org/10.1080/15287394.2010.519317. [DOI] [PubMed] [Google Scholar]
- 103.Croen LA, Matevia M, Yoshida CK, Grether JK. Maternal Rh D status, anti-D immune globulin exposure during pregnancy, and risk of autism spectrum disorders. Am J Obstet Gynecol. 2008;199(3):e1–6. doi: 10.1016/j.ajog.2008.04.044. http://dx.doi.org/10.1016/j.ajog.2008.04.044:(234) [DOI] [PubMed] [Google Scholar]
- 104.Price CS, Thompson WW, Goodson B, et al. Prenatal and infant exposure to thimerosal from vaccines and immunoglobulins and risk of autism. Pediatrics. 2010;126(4):656–64. doi: 10.1542/peds.2010-0309. http://dx.doi.org/10.1542/peds.2010-0309. [DOI] [PubMed] [Google Scholar]
- 105.Institute of Medicine (US) Immunization Safety Review Committee. Immunization Safety Review: Vaccines and Autism. Washington, DC: National Academies Press; 2004. http://www.ncbi.nlm.nih.gov/books/NBK25344/. [Accessed 18.3.14] [PubMed] [Google Scholar]
- 106.Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics. 2004;114:793–804. doi: 10.1542/peds.2004-0434. [DOI] [PubMed] [Google Scholar]
- 107.Doja A, Roberts W. Immunizations and autism: a review of the literature. Can J Neurol Sci. 2006;33(4):341–6. doi: 10.1017/s031716710000528x. [DOI] [PubMed] [Google Scholar]
- 108.DeStefano F. Vaccines and autism: evidence does not support a causal association. Clin Pharmacol Ther. 2007;82(6):756–9. doi: 10.1038/sj.clpt.6100407. http://dx.doi.org/10.1038/sj.clpt.6100407. [DOI] [PubMed] [Google Scholar]
- 109.Schultz ST. Does thimerosal or other mercury exposure increase the risk for autism? A review of current literature. Acta Neurobiol Exp (Wars) 2010;70(2):187–95. doi: 10.55782/ane-2010-1790. [DOI] [PubMed] [Google Scholar]
- 110.Taylor LE, Swerdfeger AL, Eslick GD. Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.04.085. http://dx.doi.org/10.1016/j.vaccine.2014.04.085. [DOI] [PubMed]
- 111.Karr CJ, Rauh VA. Pesticides. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford: Oxford University Press; 2014. pp. 296–302. [Google Scholar]
- 112.Shelton JF, Hertz-Picciotto I, Pessah IN. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environ Health Perspect. 2012;120(7):944–51. doi: 10.1289/ehp.1104553. http://dx.doi.org/10.1289/ehp.1104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111(14):1779–82. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nigg HN, Knaak JB. Blood cholinesterases as human biomarkers of organophosphorus pesticide exposure. Rev Environ Contam Toxicol. 2000;163:29–111. doi: 10.1007/978-1-4757-6429-1_2. [DOI] [PubMed] [Google Scholar]
- 115.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–2. doi: 10.1016/j.cca.2005.10.008. http://dx.doi.org/10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 116.Ma DQ, Whitehead PL, Menold MM, et al. Identification of significant association and gene-gene interaction of GABA receptor subunit genes in autism. Am J Hum Genet. 2005;77(3):377–88. doi: 10.1086/433195. http://dx.doi.org/10.1086/433195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Soto AM, Chung KL, Sonnenschein C. The pesticides endosulfan, toxaphene, and dieldrin have estrogenic effects on human estrogen-sensitive cells. Environ Health Perspect. 1994;102(4):380–3. doi: 10.1289/ehp.94102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Engel SM, Berkowitz GS, Barr DB, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165:1397–404. doi: 10.1093/aje/kwm029. http://dx.doi.org/10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- 119.Eskenazi B, Rosas LG, Marks AR, et al. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102(2):228–36. doi: 10.1111/j.1742-7843.2007.00171.x. http://dx.doi.org/10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 120.Rauh V, Arunajadai S, Horton M, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119(8):1196–201. doi: 10.1289/ehp.1003160. http://dx.doi.org/10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Polańska K, Jurewicz J, Hanke W. Review of current evidence on the impact of pesticides, polychlorinated biphenyls and selected metals on attention deficit/hyperactivity disorder in children Int. J Occup Med Environ Health. 2013;26(1):16–38. doi: 10.2478/s13382-013-0073-7. http://dx.doi.org/10.2478/s13382-013-0073-7. [DOI] [PubMed] [Google Scholar]
- 122.Roberts JR, Reigart JR. Recognition and Management of Pesticide Posionings. 6th. DIANE Publishing; 2013. http://www2.epa.gov/pesticide-worker-safety/recognition-and-management-pesticide-poisonings. [Google Scholar]
- 123.Bouchard MF, Bellinger DC, Wright RO, Weisskopf MG. Attention-deficit/hyperactivity disorder and urinary metabolites of organophosphate pesticides. Pediatrcs. 2010;125(6):e1270–7. doi: 10.1542/peds.2009-3058. http://dx.doi.org/10.1542/peds.2009-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yolton K, Xu Y, Sucharew H, et al. Impact of low-level gestational exposure to organophosphate pesticides on neurobehavior in early infancy: a prospective study. Environ Health. 2013;12(1):79. doi: 10.1186/1476-069X-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.USDA Agricultural Marketing Service. Pesticide Data Program. USDA; 2014. [Google Scholar]
- 126.Weldon RH, Barr DB, Trujillo C, Bradman A, Holland N, Eskenazi B. A pilot study of pesticides and PCBs in the breast milk of women residing in urban and agricultural communities of California. J Environ Monit. 2011;13(11):3136–44. doi: 10.1039/c1em10469a. http://dx.doi.org/10.1039/c1em10469a. [DOI] [PubMed] [Google Scholar]
- 127.Lee S, McLaughlin R, Harnly M, Gunier R, Kreutzer R. Community exposures to airborne agricultural pesticides in California: ranking of inhalation risks. Environ Health Perspect. 2002;110(12):1175–84. doi: 10.1289/ehp.021101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xu X, Dailey AB, Talbott EO, Ilacqua VA, Kearney G, Asal NR. Associations of serum concentrations of organochlorine pesticides with breast cancer and prostate cancer in U.S. adults Environ Health Perspect. 2010;118(1):60–6. doi: 10.1289/ehp.0900919. http://dx.doi.org/10.1289/ehp.0900919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.EPA. Pesticide News Story: EPA Releases Risk Assessments for Organic Arsenic Herbicides. Seeks Risk Management Ideas. 2006 Available at: http://www.epa.gov/oppfead1/cb/csb_page/updates/organic-herbicid.htm.
- 130.Madsen AM, Matthiesen CB. Exposure to aerosols during high-pressure cleaning and relationship with health effects. Ann Agric Environ Med. 2013;20(3):420–5. [PubMed] [Google Scholar]
- 131.Stokstad E. Air pollution. Ammonia pollution from farming may exact hefty health costs. Science. 2014;343(6168) doi: 10.1126/science.343.6168.238. http://dx.doi.org/10.1126/science.343.6168.238. [DOI] [PubMed] [Google Scholar]
- 132.Polder A, Skaare JU, Skjerve E, Loken KB, Eggesbo M. Levels of chlorinated pesticides and polychlorinated biphenyls in Norwegian breast milk (2002–2006), and factors that may predict the level of contamination. Sci Total Environ. 2009;407(16):4584–90. doi: 10.1016/j.scitotenv.2009.04.032. http://dx.doi.org/10.1016/j.scitotenv.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 133.Durkin MS, Maenner MJ, Newschaffer CJ, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168(11):1268–76. doi: 10.1093/aje/kwn250. http://dx.doi.org/10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res. 2010;3:30–9. doi: 10.1002/aur.116. http://dx.doi.org/10.1002/aur.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eskenazi B, Marks AR, Bradman A, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115(5):792–8. doi: 10.1289/ehp.9828. http://dx.doi.org/10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Braun JM, Kalkbrenner AE, Just AC, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME Study. Environ Health Perspect. 2014 doi: 10.1289/ehp.1307261. http://dx.doi.org/10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed]
- 137.Cheslack-Postava K, Rantakokko PV, Hinkka-Yli-Salomäki S, et al. Maternal serum persistent organic pollutants in the Finnish prenatal study of autism: a pilot study. Neurotoxicol Teratol. 2013;38:1–5. doi: 10.1016/j.ntt.2013.04.001. http://dx.doi.org/10.1016/j.ntt.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California central valley. Environ Health Perspect. 2007 doi: 10.1289/ehp.10168. http://dx.doi.org/10.1289/ehp.10168. [DOI] [PMC free article] [PubMed]
- 139.Roberts EM, English PB. Bayesian modeling of time-dependent vulnerability to environmental hazards: an example using autism and pesticide data. Stat Med. 2013;32(13):2308–19. doi: 10.1002/sim.5600. http://dx.doi.org/10.1002/sim.5600. [DOI] [PubMed] [Google Scholar]
- 140.Keil AP, Daniels JL, Hertz-Picciotto I. Autism spectrum disorder, flea and tick medication, and adjustments for exposure misclassification: the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Environ Health. 2014;13(1):3. doi: 10.1186/1476-069X-13-3. http://dx.doi.org/10.1186/1476-069X-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schmidt RJ, Tancredi DJ, Ozonoff S, et al. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012;96(1):80–9. doi: 10.3945/ajcn.110.004416. http://dx.doi.org/10.3945/ajcn.110.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suren P, Roth C, Bresnahan M, et al. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. J Am Med Assoc. 2013;309:570–7. doi: 10.1001/jama.2012.155925. http://dx.doi.org/10.1001/jama.2012.155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Roberts J, Karr C. Council on Environmental Health. Pesticide exposure in children. Pediatrics. 2012;130:e1765–88. doi: 10.1542/peds.2012-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Patterson DG, Jr, Wong L-Y, Turner WE, et al. Levels in the U.S population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long-range transboundary air pollution agreements. Environ Sci Technol. 2009;43(4):1211–8. doi: 10.1021/es801966w. http://dx.doi.org/10.1021/es801966w. [DOI] [PubMed] [Google Scholar]
- 145.Stroustrup A, Swan S. Endocrine disruptors. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford: Oxford University Press; 2014. pp. 325–32. [Google Scholar]
- 146.Messer A. Mini-review: polybrominated diphenyl ether (PBDE) flame retardants as potential autism risk factors. Physiol Behav. 2010;100(3):245–9. doi: 10.1016/j.physbeh.2010.01.011. http://dx.doi.org/10.1016/j.physbeh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 147.Hertz-Picciotto I, Park H-Y, Dostal M, Kocan A, Trnovec T, Sram R. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development: prenatal exposures and immune development. Basic Clin Pharmacol Toxicol. 2008;102(2):146–54. doi: 10.1111/j.1742-7843.2007.00190.x. http://dx.doi.org/10.1111/j.1742-7843.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 148.Kajta M, Wójtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–9. doi: 10.1016/s1734-1140(13)71524-x. [DOI] [PubMed] [Google Scholar]
- 149.Landrigan PJ. Polychlorinated biphenyls, dioxins, furans, DDT, polybrominated compounds, polyfluorinated compounds, and other halogenated hydrocarbons. In: Landrigan PJ, Etzel RA, editors. Textbook of Children’s Environmental Health. Oxford: Oxford University Press; 2014. pp. 303–13. [Google Scholar]
- 150.Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64(5):833–9. doi: 10.1016/j.yhbeh.2013.09.007. http://dx.doi.org/10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Napoli E, Hung C, Wong S, Giulivi C. Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuronal progenitor striatal cells enhanced by a PTEN-deficient background. Toxicol Sci. 2013;132(1):196–210. doi: 10.1093/toxsci/kfs339. http://dx.doi.org/10.1093/toxsci/kfs339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Cock M, Maas YGH, van de Bor M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatr. 2012;101(8):811–8. doi: 10.1111/j.1651-2227.2012.02693.x. http://dx.doi.org/10.1111/j.1651-2227.2012.02693.x. [DOI] [PubMed] [Google Scholar]
- 153.Betts KS. Rapidly rising PBDE levels in North America. Environ Sci Technol. 2002;36(3):50A–2A. doi: 10.1021/es022197w. [DOI] [PubMed] [Google Scholar]
- 154.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the national health and nutrition examination survey, 2001–2010. Environ Health Perspect. 2014;122(3):235–41. doi: 10.1289/ehp.1306681. http://dx.doi.org/10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ Sci Technol. 2008;42(21):8158–64. doi: 10.1021/es801792z. http://dx.doi.org/10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 156.Braun JM, Kalkbrenner AE, Calafat AM, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119:131–7. doi: 10.1289/ehp.1002366. http://dx.doi.org/10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hardell E, Carlberg M, Nordström M, van Bavel B. Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breastfeeding and parity. Sci Total Environ. 2010;408(20):4412–9. doi: 10.1016/j.scitotenv.2010.06.029. http://dx.doi.org/10.1016/j.scitotenv.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 158.Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32(2):261–7. doi: 10.1016/j.neuro.2010.12.009. http://dx.doi.org/10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Baron-Cohen S. Empathizing, systemizing, and the extreme male brain theory of autism. Prog Brain Res. 2010;186:167–75. doi: 10.1016/B978-0-444-53630-3.00011-7. http://dx.doi.org/10.1016/B978-0-444-53630-3.00011-7. [DOI] [PubMed] [Google Scholar]
- 160.Cheslack-Postava K, Jordan-Young RM. Autism spectrum disorders: toward a gendered embodiment model. Soc Sci Med. 2012;74(11):1667–74. doi: 10.1016/j.socscimed.2011.06.013. http://dx.doi.org/10.1016/j.socscimed.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 161.Lai M-C, Lombardo MV, Suckling J, et al. Biological sex affects the neurobiology of autism. Brain J Neurol. 2013;136(Pt 9):2799–815. doi: 10.1093/brain/awt216. http://dx.doi.org/10.1093/brain/awt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schaafsma SM, Pfaff DW. Etiologies underlying sex differences in autism spectrum disorders. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.03.006. http://dx.doi.org/10.1016/j.yfrne.2014.03.006. [DOI] [PubMed]
- 163.Volk HE, Kerin T, Lurmann F, Hertz-Picciotto I, McConnell R, Campbell DB. Autism spectrum disorder: interaction of air pollution with the met receptor tyrosine kinase gene. Epidemiology. 2014;25(1):44–7. doi: 10.1097/EDE.0000000000000030. http://dx.doi.org/10.1097/EDE.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eskenazi B, Huen K, Marks A, et al. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ Health Perspect. 2010;118(12):1775–81. doi: 10.1289/ehp.1002234. http://dx.doi.org/10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Visser JC, Rommelse N, Vink L, et al. Narrowly versus broadly defined autism spectrum disorders: differences in pre-and perinatal risk factors. J Autism Dev Disord. 2012;43(7):1505–16. doi: 10.1007/s10803-012-1678-6. http://dx.doi.org/10.1007/s10803-012-1678-6. [DOI] [PubMed] [Google Scholar]
- 166.Bill BR, Geschwind DH. Genetic advances in autism: heterogeneity and convergence on shared pathways. Curr Opin Genet Dev. 2009;19(3):271–8. doi: 10.1016/j.gde.2009.04.004. http://dx.doi.org/10.1016/j.gde.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hu VW. From genes to environment: using integrative genomics to build a “systems-level” understanding of autism spectrum disorders: integrative genomics of ASD. Child Dev. 2013;84(1):89–103. doi: 10.1111/j.1467-8624.2012.01759.x. http://dx.doi.org/10.1111/j.1467-8624.2012.01759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Croonenberghs J, Wauters A, Devreese K, et al. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32(8):1457–64. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- 169.Molloy CA, Morrow AL, Meinzen-Derr J, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 170.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 171.Noriega DB, Savelkoul HFJ. Immune dysregulation in autism spectrum disorder. Eur J Pediatr. 2013 doi: 10.1007/s00431-013-2183-4. http://dx.doi.org/10.1007/s00431-013-2183-4. [DOI] [PubMed]
- 172.Colborn T. Neurodevelopment and endocrine disruption. Environ Health Perspect. 2004;112(9):944–9. doi: 10.1289/ehp.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jiang Y-H, Sahoo T, Michaelis RC, et al. A mixed epigenetic/genetic model for oligogenic inheritance of autism with a limited role for UBE3A. Am J Med Genet A. 2004;131(1):1–10. doi: 10.1002/ajmg.a.30297. http://dx.doi.org/10.1002/ajmg.a.30297. [DOI] [PubMed] [Google Scholar]
- 174.Hogart A, Nagarajan RP, Patzel KA, Yasui DH, Lasalle JM. 15q11–13 GABAA receptor genes are normally biallelically expressed in brain yet are subject to epigenetic dysregulation in autism-spectrum disorders. Hum Mol Genet. 2007;16(6):691–703. doi: 10.1093/hmg/ddm014. http://dx.doi.org/10.1093/hmg/ddm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schanen NC. Epigenetics of autism spectrum disorders. Hum Mol Genet. 2006;15(Spec2):R138–50. doi: 10.1093/hmg/ddl213. http://dx.doi.org/1093/hmg/ddl213. [DOI] [PubMed] [Google Scholar]
- 176.James SJ, Cutler P, Melnyk S, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80(6):1611–7. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 177.James SJ, Jill James S, Melnyk S, et al. Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. J Autism Dev Disord. 2008;38(10):1966–75. doi: 10.1007/s10803-008-0591-5. http://dx.doi.org/10.1007/s10803-008-0591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berko ER, Suzuki M, Beren F, et al. Mosaic epigenetic dysregulation of ectodermal cells in autism spectrum disorder. PLoS Genet. 2014;10(5):e1004402. doi: 10.1371/journal.pgen.1004402. http://dx.doi.org/10.1371/journal.pgen.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hornig M. The role of microbes and autoimmunity in the pathogenesis of neuropsychiatric illness. Curr Opin Rheumatol. 2013;25(4):488–795. doi: 10.1097/BOR.0b013e32836208de. http://dx.doi.org/10.1097/BOR.0b013e32836208de. [DOI] [PubMed] [Google Scholar]
- 180.Mulle JG, Sharp WG, Cubells JF. The gut microbiome: a new frontier in autism research. Curr Psychiatry Rep. 2013;15(2):1–9. doi: 10.1007/s11920-012-0337-0. http://dx.doi.org/10.1007/s11920-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang B, Angelidou A, Alysandratos KD, et al. Mitochondrial DNA and antimitochondrial antibodies in serum of autistic children. J Neuroinflammation. 2010;7:80. doi: 10.1186/1742-2094-7-80. http://dx.doi.org/10.1186/1742-2094-7-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Giulivi C, Zhang YF, Omanska-Klusek A, et al. Mitochondrial dysfunction in autism. J Am Med Assoc. 2010;304:2389–96. doi: 10.1001/jama.2010.1706. http://dx.doi.org/10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–25. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Van Naarden Braun K, Pettygrove S, Daniels J, et al. Evaluation of a methodology for a collaborative multiple source surveillance network for autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56(1):29–40. [PubMed] [Google Scholar]
- 185.Croen LA, Grether JK, Hoogstrate J, Selvin S. The changing prevalence of autism in California. J Autism Dev Disord. 2002;32(3):207–15. doi: 10.1023/a:1015453830880. http://dx.doi.org/10.1023/A:1015453830880. [DOI] [PubMed] [Google Scholar]
- 186.Chen RT, Glasser JW, Rhodes PH, et al. Vaccine safety datalink project: a new tool for improving vaccine safety monitoring in the United States. Pediatrics. 1997;99(6):765–73. doi: 10.1542/peds.99.6.765. http://dx.doi.org/10.1542/peds.99.6.765. [DOI] [PubMed] [Google Scholar]
- 187.Lampi KM, Banerjee PN, Gissler M, et al. Finnish prenatal study of autism and autism spectrum disorders (FIPS-A): overview and design. J Autism Dev Disord. 2011;41(8):1090–6. doi: 10.1007/s10803-010-1132-6. http://dx.doi.org/10.1007/s10803-010-1132-6. [DOI] [PubMed] [Google Scholar]
- 188.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 189.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule—generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–23. [PubMed] [Google Scholar]
- 190.Scott FJ, Baron-Cohen S, Bolton P, Brayne C. The CAST (Childhood Asperger Syndrome Test): preliminary development of a UK screen for mainstream primary-school-age children. Autism Int J Res Pract. 2002;6(1):9–31. doi: 10.1177/1362361302006001003. [DOI] [PubMed] [Google Scholar]
- 191.Ehlers S, Gillberg C, Wing L. A screening questionnaire for Asperger syndrome and other high-functioning autism spectrum disorders in school age children. J Autism Dev Disord. 1999;29:129–41. doi: 10.1023/a:1023040610384. [DOI] [PubMed] [Google Scholar]
- 192.Constantino JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–33. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 193.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. Br J Psychiatry. 1999;175:444–51. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- 194.Achenbach TM, Rescorla L. ASEBA Preschool Forms & Profiles: An Integrated System of Multi-informant Assessment. ASEBA. 2000 [Google Scholar]
- 195.Torrey EF, Hersh SP, McCabe KD. Early childhood psychosis and bleeding during pregnancy. J Autism Child Schizophr. 1975;5(4):287–97. doi: 10.1007/BF01540676. [DOI] [PubMed] [Google Scholar]
- 196.Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics. 2001;107:E63. doi: 10.1542/peds.107.4.e63. [DOI] [PubMed] [Google Scholar]
- 197.Williams G, Oliver JM, Allard A, Sears L. Autism and associated medical and familial factors: a case control study. J Dev Phys Disabil. 2003;15(4):335–49. [Google Scholar]
- 198.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161:916–25. doi: 10.1093/aje/kwi123. [discussion 926-8] [DOI] [PubMed] [Google Scholar]
- 199.Maimburg RD, Vaeth M. Perinatal risk factors and infantile autism. Acta Psychiatr Scand. 2006;114(4):257–64. doi: 10.1111/j.1600-0447.2006.00805.x. http://dx.doi.org/10.1111/j.1600-0447.2006.00805.x. [DOI] [PubMed] [Google Scholar]
- 200.Zhang X, Lv C-C, Tian J, et al. Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord. 2010;40(11):1311–21. doi: 10.1007/s10803-010-0992-0. http://dx.doi.org/10.1007/s10803-010-0992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hvidtjorn D, Grove J, Schendel D, et al. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health. 2010;65(6):497–502. doi: 10.1136/jech.2009.093823. http://dx.doi.org/10.1136/jech.2009.093823. [DOI] [PubMed] [Google Scholar]
- 202.Bilder D, Pinborough-Zimmerman J, Miller J, McMahon W. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics. 2009;123(5):1293–300. doi: 10.1542/peds.2008-0927. http://dx.doi.org/10.1542/peds.2008-0927. [DOI] [PubMed] [Google Scholar]
- 203.Nijmeijer JS, Hartman CA, Rommelse NNJ, et al. Perinatal risk factors interacting with catechol O-methyltransferase and the serotonin transporter gene predict ASD symptoms in children with ADHD: G×E for ASD symptoms in ADHD. J Child Psychol Psychiatry. 2010;51(11):1242–50. doi: 10.1111/j.1469-7610.2010.02277.x. http://dx.doi.org/10.1111/j.1469-7610.2010.02277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jung C-R, Lin Y-T, Hwang B-F. Air pollution and newly diagnostic autism spectrum disorders: a population-based cohort study in Taiwan. PLoS One. 2013;8(9):e75510. doi: 10.1371/journal.pone.0075510. http://dx.doi.org/10.1371/journal.pone.0075510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Desoto MC, Hitlan RT. Blood levels of mercury are related to diagnosis of autism: a reanalysis of an important data set. J Child Neurol. 2007;22(11):1308–11. doi: 10.1177/0883073807307111. http://dx.doi.org/10.1177/0883073807307111. [DOI] [PubMed] [Google Scholar]
- 206.Majewska MD, Urbanowicz E, Rok-Bujko P, Namyslowska I, Mierzejewski P. Age-dependent lower or higher levels of hair mercury in autistic children than in healthy controls. Acta Neurobiol Exp (Wars) 2010;70(2):196–208. doi: 10.55782/ane-2010-1791. [DOI] [PubMed] [Google Scholar]
- 207.Blaurock-Busch E, Amin OR, Rabah T. Heavy metals and trace elements in hair and urine of a sample of Arab children with autistic spectrum disorder. Maedica. 2011;6(4):247–57. [PMC free article] [PubMed] [Google Scholar]
- 208.Wright B, Pearce H, Allgar V, et al. A comparison of urinary mercury between children with autism spectrum disorders and control children. PloS One. 2012;7(2):e29547. doi: 10.1371/journal.pone.0029547. http://dx.doi.org/10.1371/journal.pone.0029547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Albizzati A, Morè L, Di Candia D, Saccani M, Lenti C. Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatr. 2012;64(1):27–31. [PubMed] [Google Scholar]
- 210.Adams JB, Audhya T, McDonough-Means S, et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biol Trace Elem Res. 2013;151(2):171–80. doi: 10.1007/s12011-012-9551-1. http://dx.doi.org/10.1007/s12011-012-9551-1. [DOI] [PubMed] [Google Scholar]
- 211.Arora M, Austin C. Teeth as a biomarker of past chemical exposure. Curr Opin Pediatr. 2013;25(2):261–7. doi: 10.1097/MOP.0b013e32835e9084. http://dx.doi.org/10.1097/MOP.0b013e32835e9084. [DOI] [PubMed] [Google Scholar]
- 212.Adams JB, Romdalvik J, Ramanujam VMS, Legator MS. Mercury, lead, and zinc in baby teeth of children with autism versus controls. J Toxicol Environ Health A. 2007;70(12):1046–51. doi: 10.1080/15287390601172080. http://dx.doi.org/10.1080/15287390601172080. [DOI] [PubMed] [Google Scholar]
- 213.Abdullah MM, Ly AR, Goldberg WA, et al. Heavy metal in children’s tooth enamel: related to autism and disruptive behaviors? J Autism Dev Disord. 2011;42(6):929–36. doi: 10.1007/s10803-011-1318-6. http://dx.doi.org/10.1007/s10803-011-1318-6. [DOI] [PubMed] [Google Scholar]
- 214.Geier DA, Geier MR. An assessment of the impact of thimerosal on childhood neurodevelopmental disorders. Pediatr Rehabil. 2003;6:97–102. doi: 10.1080/1363849031000139315. [DOI] [PubMed] [Google Scholar]
- 215.Geier MR, Geier DA. Neurodevelopmental disorders after thimerosal-containing vaccines: a brief communication. Exp Biol Med. 2003;228(6):660–4. doi: 10.1177/153537020322800603. [DOI] [PubMed] [Google Scholar]
- 216.Geier DA, Geier MR. A comparative evaluation of the effects of MMR immunization and mercury doses from thimerosal-containing childhood vaccines on the population prevalence of autism. Med Sci Monit. 2004;10:PI33–9. [PubMed] [Google Scholar]
- 217.Geier DA, Geier MR. A two-phased population epidemiological study of the safety of thimerosal-containing vaccines: a follow-up analysis. Med Sci Monit. 2005;11(4):CR160–70. [PubMed] [Google Scholar]
- 218.Geier DA, Geier MR. A meta-analysis epidemiological assessment of neurodevelopmental disorders following vaccines administered from 1994 through 2000 in the United States. Neuro Endocrinol Lett. 2006;27(4):401–13. [PubMed] [Google Scholar]
- 219.Geier DA, Geier MR. An evaluation of the effects of thimerosal on neurodevelopmental disorders reported following DTP and Hib vaccines in comparison to DTPH vaccine in the United States. J Toxicol Environ Health A. 2006;69(15):1481–95. doi: 10.1080/15287390500364556. http://dx.doi.org/10.1080/15287390500364556. [DOI] [PubMed] [Google Scholar]
- 220.Geier DA, Hooker BS, Kern JK, King PG, Sykes LK, Geier MR. A two-phase study evaluating the relationship between thimerosal-containing vaccine administration and the risk for an autism spectrum disorder diagnosis in the United States. Transl Neurodegener. 2013;2(1):25. doi: 10.1186/2047-9158-2-25. http://dx.doi.org/10.1186/2047-9158-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jick H, Kaye JA. Epidemiology and possible causes of autism. Pharmacotherapy. 2003;23(12):1524–30. doi: 10.1592/phco.23.15.1524.31955. [DOI] [PubMed] [Google Scholar]
- 222.Jick H, Kaye JA. Autism and DPT vaccination in the United Kingdom. N Engl J Med. 2004;350(26):2722–3. doi: 10.1056/NEJM200406243502623. http://dx.doi.org/10.1056/NEJM200406243502623. [DOI] [PubMed] [Google Scholar]
- 223.Miles JH, Takahashi TN. Lack of association between Rh status, Rh immune globulin in pregnancy and autism. Am J Med Genet A. 2007;143A(13):1397–407. doi: 10.1002/ajmg.a.31846. http://dx.doi.org/10.1002/ajmg.a.31846. [DOI] [PubMed] [Google Scholar]
- 224.Geier DA, Geier MR. A prospective study of thimerosal-containing Rho(D)-immune globulin administration as a risk factor for autistic disorders. J Matern Fetal Neonatal Med. 2007;20(5):385–90. doi: 10.1080/14767050701228057. http://dx.doi.org/10.1080/14767050701228057. [DOI] [PubMed] [Google Scholar]
- 225.Tozzi AE, Bisiacchi P, Tarantino V, et al. Neuropsychological performance 10 years after immunization in infancy with thimerosal-containing vaccines. Pediatrics. 2009;123(2):475–82. doi: 10.1542/peds.2008-0795. http://dx.doi.org/10.1542/peds.2008-0795. [DOI] [PubMed] [Google Scholar]
- 226.Rauh VA, Garfinkel R, Perera FP, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–59. doi: 10.1542/peds.2006-0338. http://dx.doi.org/10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Testa C, Nuti F, Hayek J, et al. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. ASN Neuro. 2012;4(4):223–9. doi: 10.1042/AN20120015. http://dx.doi.org/10.1042/AN20120015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Stein TP, Schluter MD, Steer RA, Ming X. Autism and phthalate metabolite glucuronidation. J Autism Dev Disord. 2013;43(11):2677–85. doi: 10.1007/s10803-013-1822-y. http://dx.doi.org/10.1007/s10803-013-1822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hertz-Picciotto I, Bergman A, Fängström B, et al. Polybrominated diphenyl ethers in relation to autism and developmental delay: a case-control study. Environ Health. 2011;10(1):1. doi: 10.1186/1476-069X-10-1. http://dx.doi.org/10.1186/1476-069X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mitchell MM, Woods R, Chi L-H, et al. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagen. 2012;53(8):589–98. doi: 10.1002/em.21722. http://dx.doi.org/10.1002/em.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Otake T, Yoshinaga J, Seki Y, et al. Retrospective in utero exposure assessment of PCBs using preserved umbilical cords and its application to case-control comparison. Environ Health Prev Med. 2006;11(2):65–8. doi: 10.1007/BF02898144. http://dx.doi.org/10.1007/BF02898144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect. 2011;119:873–7. doi: 10.1289/ehp.1002835. http://dx.doi.org/10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. J Am Med Assoc Psychiatry. 2013;70:71–7. doi: 10.1001/jamapsychiatry.2013.266. http://dx.doi.org/10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Verstraeten T, Davis RL, DeStefano F, et al. Safety of thimerosal-containing vaccines: a two-phased study of computerized health maintenance organization databases. Pediatrics. 2003;112:1039–48. [PubMed] [Google Scholar]
- 235.Andrews N. Thimerosal exposure in infants and developmental disorders: a retrospective cohort study in the United Kingdom does not support a causal association. Pediatrics. 2004;114(3):584–91. doi: 10.1542/peds.2003-1177-L. http://dx.doi.org/10.1542/peds.2003-1177-L. [DOI] [PubMed] [Google Scholar]


