Abstract
The New South Wales Brain Tissue Resource Centre (NSWBTRC) at the University of Sydney (Australia) is an established human brain bank providing tissue to the neuroscience research community for investigations on alcohol-related brain damage and major psychiatric illnesses such as schizophrenia. The NSWBTRC relies on wide community engagement to encourage those with and without neuropsychiatric illness to consent to donation through its allied research programs. The subsequent provision of high-quality samples relies on standardized operational protocols, associated clinical data, quality control measures, integrated information systems, robust infrastructure, and governance. These processes are continually augmented to complement the changes in internal and external governance as well as the complexity and diversity of advanced investigation techniques. This report provides an overview of the dynamic process of brain banking and discusses the challenges of meeting the future needs of researchers, including synchronicity with other disease-focus collections.
Keywords: alcohol, brain bank, brain damage, donor program, neuropathology, schizophrenia
Introduction
Over the past three decades our understanding of the factors that contribute to and the consequences of schizophrenia and alcohol-related brain damage (ARBD) has been advanced through studies of neuroimaging, neuropsychology, genetics, and neuropathology of the human brain. In particular, the field of neuropathology has been transformed by the molecular techniques of the so-called genomic age (Sutherland, Sheedy, & Kril, 2014). For the past decade many of these studies have been facilitated by the New South Wales Brain Tissue Resource Centre (NSWBTRC), a specialized research resource, supported by the National Institutes of Alcohol Abuse and Alcoholism and Schizophrenia Research Institute (Australia), and established to provide clinically and pathologically characterized post-mortem brain tissue to researchers.
The NSWBTRC at The University of Sydney, Sydney, Australia has engaged with the neuroscience community for over 20 years. The NSWBTRC collection is primarily focused on cases with histories of alcohol-use disorder, major neuropsychiatric disorders, and healthy controls. The associated brain donor program, Using our Brains, has engaged the wider community to participate in brain donation and research since being launched in 2002.
Over these years the NSWBTRC has encountered challenges in terms of funding sustainability, researcher expectations, incorporation of technical advances, maintaining quality, and data-sharing management. New management and operational strategies introduced to strengthen the growth of the NSWBTRC are described herein along with contributions made to this research community.
Recently the NSWBTRC has undergone changes to its organizational structure in an effort to safeguard the continuation of the resource. These changes are primarily related to institutional governance, new infrastructural opportunities, and tissue access.
Governance
The NSWBTRC is housed at The University of Sydney and is under the Sydney Local Health Network (Protocol No X15-0199) and University of Sydney Biobank Committee jurisdiction.
In an effort to ensure longevity of brain banking in this research area, a decision to share resources and combine some processes with an affiliated brain bank was accorded. An inter-institutional agreement with the University of New South Wales, which houses the Sydney Brain Bank, was executed to operate collaboratively as the NSW Brain Banks (NSWBB) to review requests from researchers and to facilitate access to this valuable resource. The NSW Brain Banks Inter-Institutional Board is the overarching body that provides governance to the NSW Brain Banks committees and panels.
Funding
The NSWBTRC has funding from the National Institutes of Alcohol Abuse and Alcoholism and the Schizophrenia Research Institute, with infrastructure support by the University of Sydney. Each funding body shapes the focus of the brain collection and research strategy of the NSWBTRC in line with their overall objectives.
Brain banks are expensive to maintain, and understanding the actual costs is imperative to gaining long-term funding. Our recent audit of cost suggests this is AU$ 20,000 per brain collected, a figure comparable with that previously published (Hulette, 2003). Cost recovery for access is implemented by the NSWBB using the Australian Brain Bank Network Access Policy (http://www.austbrainbank.org.au/researchers.html). A recent study by Albert et al. has shown that full cost recovery is not feasible and the expected revenue is between 5–25%, albeit toward the lower estimate if only academic research is supported (Albert, Bartlett, Johnston, Schacter, & Watson, 2014).
Infrastructure
The NSWBTRC has recently relocated to the purpose-built research institute, the Charles Perkins Centre (CPC), at the University of Sydney (http://sydney.edu.au/perkins/). Although the centre's primary research foci relate to chronic systemic disorders (obesity, diabetes, and cardiovascular disease), the brain bank acts as a conduit to understanding the bi-directional relationships between the brain and these disease states. Indeed, various CPC research groups utilize brain bank material while Using our Brains (UoB) donors participate in research projects where both systemic and central phenotypic data are being collected.
Researchers
A major aim of this facility is the promotion of post-mortem human brain tissue for neuroscience research. Traditionally, tissue banks and collections have been used to study morphological aspects of disease. The value of these studies is clear; however, further advances in our knowledge can be gained by coupling histological studies with techniques that examine functional aspects of tissue such as “omics” studies – proteomics, genomics, transcriptomics, and metabolomics (Sutherland, Sheedy, & Kril, 2014). Gaining feedback from researchers via annual evaluations allows the review of methods undertaken and those proposed for future use.
The NSWBTRC provides not only access to tissue but also experience in the application of research techniques which can assist researchers with their studies and help troubleshoot projects. This is an important interaction as many researchers have developed their skills using animal models. The availability of tissue samples to refine methods using human tissue is invaluable and allows for better use of the collection. Details of the NSW Brain Banks (NSWBB) may be accessed at https://nswbrainbank.org.au/. Guidelines for the researcher are available on this site. Principal and co-investigators who utilize tissue are invited to serve on the Tissue Review Panel that assists the NSWBB Scientific Review Committee to assess applications.
Donor programs
Brains donated to the NSWBTRC come from two sources. The first is the New South Wales Department of Forensic Medicine (DOFM) (equivalent to the Medical Examiners office), that receives all sudden, unexpected, accidental, institutional, or violent deaths from the greater Sydney region (~5 million people). Approximately 60% of the next of kin contacted consent to brain donation (Glaw et al., 2009). This source provides the majority of alcoholic cases as well as controls.
The second source is prospectively consented and characterized brain donors. Here, members of the community are encouraged to donate their brains by joining our Using our Brains (UoB) donor program. This source provides cases with variable alcohol consumption and variable co-existing illnesses. Staff share the 24-hour ‘on call pager’ for brain collection of donors who have consented through this program.
The ‘Using our Brains’ brain donor program has encountered positive community support. Maintaining contact is important from the initial consent process, allowing a relationship to be built with the donor and their family, which aids in longitudinal data collection. Donors are invited to participate in a variety of allied research projects, and data sharing from these studies enhances the overall program.
Methods
NSWBTRC staff attend the DOFM daily and assess the reports of death to the coroner for potential donations using exclusion and inclusion criteria. They then call the next of kin of the deceased person to discuss the donation and consenting process.
Potentially suitable cases are donors who are over the age of 18 with no developmental or neurological disorders, infections or disease such as HIV or hepatitis C, and no history of substance dependence/abuse (except for alcohol and/or nicotine). Those that have died as a result of significant head injury or have undergone assisted ventilation for more than 24 hours or that have had a poor agonal status due to other causes are excluded from the collection.
The consenting process requires initially obtaining a recorded verbal consent and later written consent from the senior next of kin of the deceased. Written consent is required from the case pathologist, the coroner, and designated officer (an authorized person appointed by the Australian Department of Health responsible for tissue and organ donation from a deceased person). Using our Brains donors have pre-consented to the donation process, including consent from their next of kin and therefore only require written consent from a designated officer for the donation to proceed.
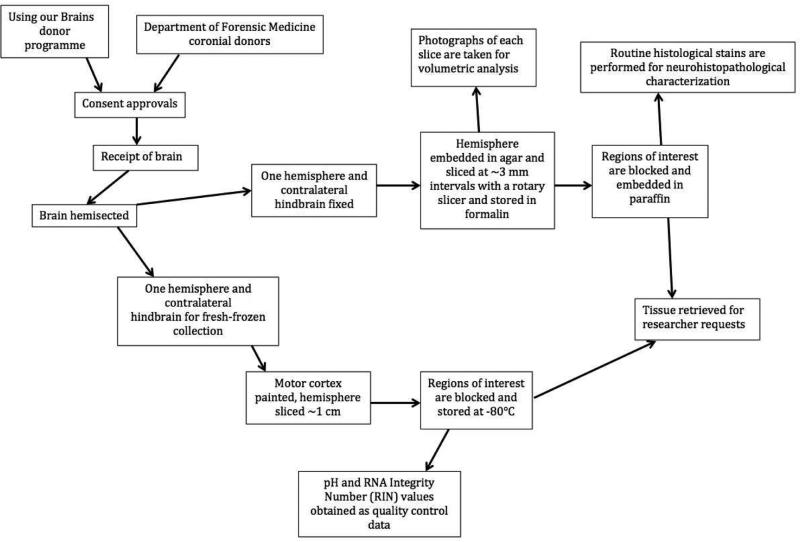
Brains are prepared at the time of autopsy by technicians trained in anatomical dissection. The NSWBTRC brain collection protocol (Fig. 1) ensures that the maximum range and number of specimens become available to researchers. Both fresh-frozen and formalin-fixed tissue is collected and stored with the fixed hemisphere being cut at 3 mm by a rotary slicer to allow whole and regional brain volumetric analysis. The regions dissected from the fixed and frozen hemispheres (Table 1) are evidence-based and updated to reflect the current research zeitgeist. In particular, fixed blocks of commonly requested regions are paraffin-embedded at approximately 3 weeks, providing a uniform source of tissue for immunohistochemical studies. Additional regions can be requested, subject to Scientific Review Committee approval, but tissue storage times will vary as per time of donation.
Fig. 1.
Brain collection procedures schematic
Table 1.
Fixed and Fresh Standard Blocks
| Region | Brodmann area | Fixed | Frozen | Notes |
|---|---|---|---|---|
| Prefrontal cortex | 9 | ✓ | ✓ | |
| Anterior cingulate cortex | 24 | ✓ | ✓ | Includes corpus callosum on block |
| Posterior cingulate cortex | 23, 31 | ✓ | Includes corpus callosum on block | |
| Primary motor cortex | 4 | ✓ | ✓ | |
| Striatum | ✓ | ✓ | Both caudate nucleus and the putamen are removed as a single block. Also includes globus pallidus. | |
| Amygdala | ✓ | ✓ | ||
| Thalamus | ✓ | ✓ | ||
| Hippocampus | ✓ | ✓ | ||
| Superior temporal gyrus | 22 | ✓ | ✓ | |
| Primary visual cortex | 17 | ✓ | ✓ | Also includes Brodmann area 18 on block |
| Pons | ✓ | ✓ | ||
| Medulla | ✓ | ✓ | ||
| Mammillary body & Hypothalamus | ✓ | |||
| Midbrain | ✓ | ✓ | ||
| Cerebellum | ✓ | ✓ | ||
| Vermis | ✓ | ✓ | ||
| Supramarginal Gyrus | 40 | ✓ | ||
| Meninges | ✓ | |||
| Choroid plexus | ✓ | |||
| Pineal gland | ✓ |
Gross anatomical examination is performed by a neuropathologist with neurohistopathological characterization primarily performed using H&E-stained sections with additional stains including cresyl violet, luxol fast blue, and Bodian silver. Immunohistochemical assays for (pathological) tau, ubiquitin, alpha synuclein, and beta amyloid are performed when indicated by relevant clinical information; these stains help to diagnose dementias such as Alzheimer's disease. All cases over the age of 50 years undergo a modified Braak staging screen (Halliday et al., 2002). The remainder of the fixed material is stored indefinitely in 10% formalin until required for research purposes.
The neuropathological screening for Alzheimer-type pathology in controls and psychiatric disease cases, including those with a history of depression or schizophrenia, eliminates confounding factors and allows for better cohort selection (Schmitt, Parlapani, Bauer, Heinsen, & Falkai, 2008). Late-onset depression, late-onset schizophrenia, and elderly schizophrenia cases do exhibit some degree of Alzheimer's disease- (AD) related neuropathology (Bozikas, Kovari, Bouras, & Karavatos, 2002; Rapp et al., 2010). The presence of cortical neuritic plaques and hippocampal neurofibrillary tangles may influence the degree of dementia severity. Therefore, obtaining thorough clinical histories of these donors is essential to research outcomes (Bozikas et al., 2002).
A retrospective or post-mortem clinical diagnosis of each case is determined primarily through extensive review of medical records from hospitals and general practitioners (physicians) of the donor, plus confirmation through donor history questionnaires from the donor's next of kin. Data collected incorporates demographic, social, medical, pathological, cognitive, psychiatric, medications, lifestyle factors (e.g., drug, alcohol, and smoking) information, and family history of the donor.
Clinical personnel then format this information into a structured clinical overview of the donor. Clinical characterization of alcohol use is based on DSM-IV Diagnostic Criteria Alcohol-Related Disorders-Alcohol Dependence (303.90) and Alcohol Abuse (305.00), while psychiatric diagnosis is confirmed by DSM-IV Diagnostic Criteria and then verified by a consultant psychiatrist for final diagnosis authorization.
Questionnaires are sent to next of kin that include a combination of Audit-C screening questions and DSM-IV diagnostic criteria to determine classification of either substance abuse and/or dependence. Seven or more criteria over a 12-month period were required for a ‘dependence’ diagnosis, whereas those that met four or more diagnostic criteria within a 12-month period would receive the ‘abuse’ diagnosis. The alcohol-use phenotype data available are outlined in Table 2.
Table 2.
Alcohol phenotype data available
| Field | Data |
|---|---|
| Category | Current Drinker, Current Abstainer, Lifetime Abstainer |
| Age commenced and ceased drinking | Years |
| Periods of abstinence: total | Years |
| Total drinking period | Years |
| Alcohol Type | Beer, wine, spirits combination |
| Frequency last 12 months | 01-99 |
| Alcohol withdrawal attempted | Yes/No |
| No. of standard drinks/week | Number of drinks |
| Grams of ethanol/day | Grams |
| DSM-IV questions specific to abuse/dependence | DSM-IV criteria |
| DSM-5 Criterion | Criteria 1-11 |
| Thiamine supplementation | Yes/No |
| Family history | Relative/s |
The revision to the diagnostic classification for alcohol consumption, DSM-5 (released May 2013) integrates the two DSM-IV disorders, ‘alcohol abuse’ and ‘alcohol dependence’, into a single disorder called ‘alcohol-use disorder’ (AUD). The distinction is based on sets of diagnostic criteria and classified in terms of severity (mild, moderate, and severe sub-classifications) and based on the number of criteria endorsed: mild AUD – 2+ criteria required, moderate AUD – 4-5 criteria required, and severe AUD – 6+ criteria required. ‘Recurrent legal problems’, a criterion for abuse in DSM-IV, has been removed from DSM-5. A new criterion for AUD has been added: ‘craving or a strong desire or urge to use a substance’. The NSWBTRC continues to use DSM-IV for the primary classification criteria, but cases are also classified according to DSM-5 to assist the analysis of phenotype data.
Access to control tissues is also an essential component of the NSWBTRC collection. Controls are donors with no neurological or psychiatric disorders and are without neuropathological abnormalities (e.g., stroke, Alzheimer's disease) and who consume less than 20 g of absolute alcohol per day.
Cases that do not meet clinical diagnostic criteria or do not meet pathological screening criteria are not used in research cohorts but are retained in the collection, as such tissue is useful for researchers wishing to establish new research methodologies or test new experimental protocols.
Over the past 5 years the average rate of collection has been 32 brains per year, with 70–80% becoming part of the target research cohorts. The remainder were either unable to be clinically characterized or showed additional neuropathology (e.g., stroke, degenerative pathology). A strength of the NSWBTRC is the rigorous clinical and pathological screening of cases before they are made available to researchers. Extensive medical and lifestyle histories are obtained with over 120 features recorded for each case (Table 3). Cases from the Department of Forensic Medicine undergo a full autopsy so additional information such as comorbidities, liver pathology, toxicological screening, and blood alcohol levels at death is available. Pre-mortem or agonal information is also collected, including the rapidity of death, hypoxic events, neurotoxic substance ingestion, organ failure, head injury, coma, pyrexia, and sepsis.
Table 3.
Types and range of clinical data available on cases from NSWBTRC
| Group | Examples of variables | No. Items |
|---|---|---|
| Demographic | Date of birth; Country of birth; Date of death; Gender; Ethnicity; Place of residence | 8 |
| Physical | Height; Weight; Body mass index; Handedness | 4 |
| Smoking history | Age commenced smoking; Periods of abstinence; Age ceased smoking; Daily/weekly/monthly consumption; Pack years | 6 |
| Medical history | Systemic disorders; CNS disorders; Medication history: type, duration, and dose; Medications at time of death; Cognitive function; Hospitalizations/operations; Treating doctors and specialists; Pathology and Diagnostic test results | 37 |
| Social history | Education; Occupation; Marital status | 6 |
| Family history | Mental health disorders; Substance-related disorders (alcohol/drugs); Medical disorders (e.g., cardiac, diabetes) | 10 |
| Lifestyle factors | Frequency and type of exercise; Dietary habits; Vitamin supplements | 3 |
| DSM-IV | Schizophrenia and other psychotic disorders; Positive and Negative symptoms; Substance-related disorders; Depressive disorders | 16 |
| Tissue features | Post-mortem interval; Agonal state; Neuropathological findings and diagnosis; pH; RNA integrity number | 25 |
| Total No. of data points for each subject | 115 |
Staff undertake research activities to enhance data on the cases, such as the effect of co-existing liver disease on brain pH and RNA integrity number (RIN) in alcoholics, particularly in those with hepatic encephalopathy (Sheedy, Say, Stevens, Harper, & Kril, 2012). This information is highly important in the allocation of cohorts for projects and allows further in-depth analysis by the researcher.
Routine measurement of brain RNA integrity was introduced to supplement the measurement of brain pH that has been undertaken for many years. RIN is tightly correlated to brain pH and is largely affected by events in the agonal period (Barton, Pearson, Najlerahim, & Harrison, 1993; Durrenberger et al., 2010; Monoranu et al., 2009). Brain pH is negatively correlated with the number and severity of hypoxic events in the pre-mortem period (Hardy et al., 1985). Low pH or RNA measurements from brain tissue are a proxy for a more severe agonal period and in the case of AUD, liver cirrhosis and hepatic encephalopathy (D. Sheedy, Say, Stevens, Harper, & Kril, 2012). Their effects on gene expression can exceed the disease itself and are therefore important confounders or covariates in transcriptomic studies (Sutherland, Sheedy, Sheahan, Kaplan, & Kril, 2014).
Research cohorts
The associated clinical information provided by the NSWBTRC is important for analysis of the phenotypic characteristics and determining whether detected differences reflect susceptibility to, or are consequences of, alcohol use. Having toxicology reports at time of death potentially allows attribution of pathology to alcohol-induced neuroadaptations or exposure effects. In terms of covariates, the NSWBTRC alcohol research cohorts are developed to only include cases that do not have a history of illicit drug abuse, apart from tobacco smoking. It is estimated that 80% of alcoholics smoke tobacco, and neuroimaging (Durazzo et al., 2014) and animal studies (Tong et al., 2015) suggest that tobacco smoke has additive or synergistic effects with alcohol on alcohol-related brain disorders (ARBD). However, in a recent pathological study, all the atrophy seen in ARBD, and largely in white matter, was centrally attributable to alcohol (McCorkindale et al., under review). The consideration of smoking status and volume is a relatively recent trend in alcohol research, with most research to date comparing cases with high levels of alcohol abuse, or specific organ damage (e.g., cirrhosis), to normal controls. Our aim is to develop additional case-control cohorts that, along with the impact of tobacco smoking on ARBD, allow a wider range of research questions to be addressed. Cohorts in development are subjects with different drinking patterns or pathologies, such as heavy drinkers who do not endorse DSM-IV criteria for addiction, older drinkers with comorbidities (i.e., diabetes), alcoholic smokers and non-smokers, and alcoholics with suicidal behavior.
Interest in understanding molecular pathways and potential biomarkers for neuropsychiatric disorders has led to advances in neuroscience and genetic research (Kalia & Costa, 2015). The AUD cohorts have been studied using advanced techniques including MALDI Imaging (Yalcin & de la Monte, 2015) and RNA Sequencing (Farris, Arasappan, Hunicke-Smith, Harris, & Mayfield, 2015). Furthermore, shared genome-wide association studies (GWAS) data by NSWBTRC researchers who have accessed different brain regions enhances the potential of data analysis and productivity of this facility (Edenberg et al., 2010; McClintick et al., 2013; Okvist et al., 2007; Wang, Gelernter, & Zhang, 2013). Data sharing on platforms such as GeneWeaver and GeneNetwork provides an integration opportunity for research on other species. Other methods applied to NSWBTRC tissue include digitization of the fixed slices for comparative volumetric studies (McCorkindale et al., under review, Zheng et al., under review).
A primary schizophrenia cohort has been developed for researchers affiliated with the Schizophrenia Research Institute and international research groups (Weickert et al., 2010). The additional clinical data obtained for this cohort concerning medications, positive and negative symptoms, substance abuse, and treatment history has assisted in a more discriminating analysis (Fillman et al., 2013). A secondary cohort for comparative studies is in development. These researchers have extensively accessed post-mortem tissue and are applying a variety of techniques (Fillman et al., 2013; Ishiguro et al., 2010; Newell, Zavitsanou, & Huang, 2005; Weickert et al., 2010). A recent review proposed future directions for the use of post-mortem tissue in the study of schizophrenia (McCullumsmith, Hammond, Shan, & Meador-Woodruff, 2014). These include techniques employed at a cellular level, such as kinome array, protein qPCR, capillary electrophoresis, laser capture microdissection, fractionation, and immunohistochemical localization methods. More novel techniques such as Fluorescence Activated Cell Sorting (FACS) and single-cell mass cytometry are feasible although there is a need to develop protocols and validation criteria (MacDonald et al., 2012).
Outcomes
Over the past 15 years the NSWBTRC has distributed more than 90,000 fixed and frozen samples for 500 projects. The type of sample requested has varied over the years and has been dependent upon available techniques. In the late 1990s, 85% of samples supplied were formalin-fixed, whereas from 2000–2004 63% were frozen samples. More recently the distribution has been 46% fixed and 54% frozen. Over the past few years approximately 20% of researchers have requested both fixed and frozen samples for their projects.
Conference presentations and peer-reviewed journals by researchers and staff also allow for promotion of the NSWBTRC. As of the most current report period (mid-2015), there have been 413 peer-reviewed journals and 590 conference presentations. Higher-degree (graduate) student involvement in projects has been encouraged by many of the research groups. On average, 41% of projects over the last decade have engaged one or more students.
A requirement of the NSWBB is the submission of an annual evaluation of research project progress and outcomes. This assists with the management of the brain banks and reporting requirements of the relevant funding bodies. Researchers accessing the NSWBTRC are located worldwide. Collaborative networks of researchers studying AUD have emerged to share data, and to utilize expertise and technologies.
UoB demographics
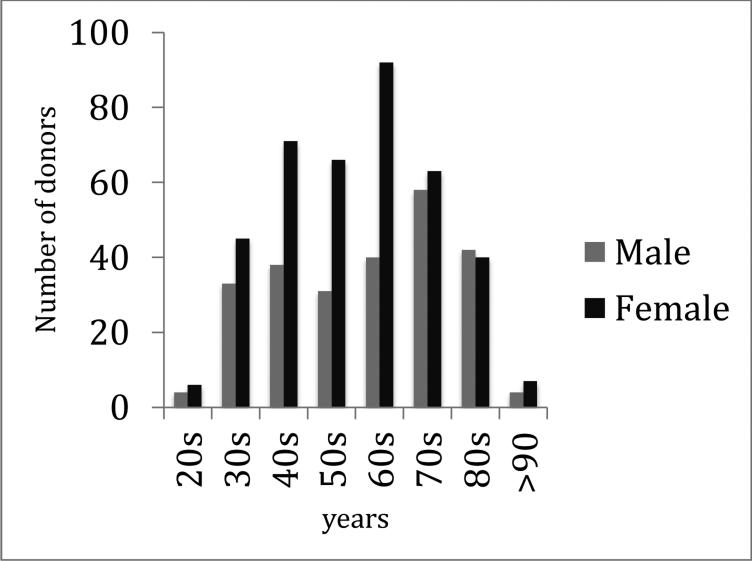
The UoB program currently has 640 community participants, of which 31% have been enrolled since its commencement in 2002. The majority of donors are female (60%) with an age range of 23–95 years; males are aged 27–101 years (Fig. 2). Longitudinal data about medical history, lifestyle, and cognition are collated, which enhances the characterization of the cases. Since 2003, 1100 cognitive assessments and 104 MRI scans have been performed. Of the successful donations (120), 55 donors have had cognitive assessments, of which 11 have also had an MRI. More than half of the current participants have expressed an interest in engaging in research opportunities offered.
Fig. 2.
Age distribution of Using our Brains donors by gender
Discussion
Not only is the human brain anatomically and functionally complex, but also a wide array of factors must be taken into consideration when designing and interpreting the results of studies of the human brain. Humans are genetically diverse with each individual having a unique exposure pattern to disease risk factors, including alcohol, tobacco, malnutrition, stress, and co-morbidities that may directly or indirectly affect the brain. Complex disorders such as alcoholism and schizophrenia that result from both intrinsic and extrinsic influences pose a range of scientific questions that need to be addressed by a combination of clinical, social, pathophysiological, and genetic approaches. While animal and other experimental systems may be able to model some of these factors, they are not able to fully reproduce the human condition. However, the convergence of animal and human study data can provide important insights in the understanding of the behavioral and mechanistic measures and molecular deficits relating to both addiction and mental illness.
In this respect NSWBTRC staff have played key roles on the Scientific Advisory Board of the Monkey Alcohol Tissue Research Resource in terms of their autopsy protocols, researcher access, and data management procedures (Daunais et al., 2014; Davenport, Grant, Szeliga, Friedman, & Daunais, 2014). This intricacy of the human condition also extends to the need for the standardized characterization of medical, family, and social history, risk factors, and co-existing diseases for cases used in research studies.
The worldwide ranking of global burden of mental, neurological, and substance-use disorders has AUD as 2nd and schizophrenia as 3rd (Collins et al., 2011). Alcohol use is associated with over 200 diseases and injury-related health conditions, most notably alcohol dependence, liver cirrhosis, cancers, and injuries (Lim et al., 2012). The 2014 World Health Organization global status report on alcohol and health reported that 3.3 million deaths, or 5.9% of all global deaths (7.6% for men and 4% for women), were attributable to alcohol consumption (WHO, 2014).
Recent reviews have demonstrated the value of modern brain banking for studies of the effects of long-term alcohol disorders, schizophrenia, bipolar disorders, and neurodegenerative diseases (Beach, 2013; Farris, Pietrzykowski, et al., 2015; Lewohl et al., 2004; MacDonald et al., 2012; Schmitt et al., 2008; Sutherland, Sheedy, & Kril, 2014). In terms of NSWBTRC, the support of the NIH's National Institute on Alcohol Abuse and Alcoholism and associated alcohol research society members has strengthened the importance of this resource (Reilly, Noronha, & Warren, 2014).
Engagement with the broad community allows a forum to discuss brain donation and present the opportunity to contribute to health outcomes of future generations (Harmon & McMahon, 2014). Those participating in the UoB program are sent an annual survey to update their longitudinal medical and lifestyle histories. This data collection by the NSWBTRC has evolved to assist with the research analysis. Information attained from the medical record reviews, donor, and next of kin surveys and post-mortem reports gives an insight to social, health, and lifestyle variables of the research cohorts. Retrospective data collection relies on obtaining access to multiple institutional medical records, follow-up interviews with next of kin, and reliability of lifetime alcohol consumption patient reports (Haeny, Littlefield, & Sher, 2014; Jacob, Seilhamer, Bargeil, & Howell, 2006). Dedicated staff at NSWBTRC build a rapport with donors, medical staff, and family members to gain constructive insight into the donor. This insight has been beneficial during the current investigation of methods for quantifying adverse agonal events in order to better inform our collection procedures. Future collaborations with clinical researchers undertaking programs with our donors and feedback data will strengthen this resource and future outcomes. Promotion to various research clinicians, and particularly those affiliated with the CPC for their participants to consider brain donation, is currently in progress.
Conclusion
The ultimate goal of medical research is to reduce the burden of disease that encompasses personal, family, social, and economic costs. This can be achieved in many ways, including through the study of tissues from affected individuals. Evidence obtained directly from these laboratory-based studies can be used to investigate susceptibility to disease, explore pathogenic mechanisms of organ damage, and validate studies performed in other model systems. Indirectly, these data can be used to refine treatment strategies, develop new therapies, and inform public-health policy aimed at preventing and minimizing harm. Biobanks are an important contributor to the research effort and sit alongside animal colonies, data repositories, and other multi-user research facilities as essential resources for the scientific community. However, brain banks are particularly important because the complexity of the human brain in combination with the psychological uniqueness of our species creates a disparity with animal models that exceeds any other organ system.
As such, brain banks have an obligation to promote the use of their resource to those in the neuroscience community for projects based on sound scientific, ethical, and legal standards (Harmon & McMahon, 2014).
Highlights.
A description of a human brain bank for Alcohol-related research
The Provision of high quality and well-characterised tissue
Historic and current use of human brain tissue in research
The challenges of meeting the future needs of researchers
Acknowledgments
The New South Wales Brain Tissue Resource Centre at the University of Sydney is supported by The University of Sydney and the Schizophrenia Research Institute. The research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health (R28AA012725). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- NSWBTRC
New South Wales Brain Tissue Resource Centre
- DOFM
Department of Forensic Medicine
- NOK
next of kin
- UoB
Using our Brains Donor Program
- ARBD
alcohol-related brain damage
- AUD
alcohol-use disorder
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert M, Bartlett J, Johnston RN, Schacter B, Watson P. Biobank bootstrapping: is biobank sustainability possible through cost recovery? Biopreservation and Biobanking. 2014;12:374–380. doi: 10.1089/bio.2014.0051. [DOI] [PubMed] [Google Scholar]
- Barton AJ, Pearson RC, Najlerahim A, Harrison PJ. Pre- and postmortem influences on brain RNA. Journal of Neurochemistry. 1993;61:1–11. doi: 10.1111/j.1471-4159.1993.tb03532.x. [DOI] [PubMed] [Google Scholar]
- Beach TG. Alzheimer's disease and the “Valley Of Death”: not enough guidance from human brain tissue? Journal of Alzheimer's Disease. 2013;33(Suppl 1):S219–233. doi: 10.3233/JAD-2012-129020. [DOI] [PubMed] [Google Scholar]
- Bozikas VP, Kövari E, Bouras C, Karavatos A. Neurofibrillary tangles in elderly patients with late onset schizophrenia. Neuroscience Letters. 2002;324:109–112. doi: 10.1016/s0304-3940(02)00189-1. [DOI] [PubMed] [Google Scholar]
- Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30. doi: 10.1038/475027a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunais JB, Davenport AT, Helms CM, Gonzales SW, Hemby SE, Friedman DP, et al. Monkey alcohol tissue research resource: banking tissues for alcohol research. Alcoholism: Clinical and Experimental Research. 2014;38:1973–1981. doi: 10.1111/acer.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport AT, Grant KA, Szeliga KT, Friedman DP, Daunais JB. Standardized method for the harvest of nonhuman primate tissue optimized for multiple modes of analyses. Cell and Tissue Banking. 2014;15:99–110. doi: 10.1007/s10561-013-9380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Pennington D, Abé C, Gazdzinski S, Meyerhoff DJ. Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addiction Biology. 2014;19:132–143. doi: 10.1111/j.1369-1600.2012.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrenberger PF, Fernando S, Kashefi SN, Ferrer I, Hauw JJ, Seilhean D, et al. Effects of antemortem and postmortem variables on human brain mRNA quality: a BrainNet Europe study. Journal of Neuropathology and Experimental Neurology. 2010;69:70–81. doi: 10.1097/NEN.0b013e3181c7e32f. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Koller DL, Xuei X, Wetherill L, McClintick JN, Almasy L, et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcoholism: Clinical and Experimental Research. 2010;34:840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, Arasappan D, Hunicke-Smith S, Harris RA, Mayfield RD. Transcriptome organization for chronic alcohol abuse in human brain. Molecular Psychiatry. 2015;20:1438–1447. doi: 10.1038/mp.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, Pietrzykowski AZ, Miles MF, O'Brien MA, Sanna PP, Zakhari S, et al. Applying the new genomics to alcohol dependence. Alcohol. 2015;49:825–836. doi: 10.1016/j.alcohol.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Molecular Psychiatry. 2013;18:206–214. doi: 10.1038/mp.2012.110. [DOI] [PubMed] [Google Scholar]
- Glaw XM, Garrick TM, Terwee PJ, Patching JR, Blake H, Harper C. Brain donation: who and why? Cell and Tissue Banking. 2009;10:241–246. doi: 10.1007/s10561-009-9121-8. [DOI] [PubMed] [Google Scholar]
- Haeny AM, Littlefield AK, Sher KJ. Repeated diagnoses of lifetime alcohol use disorders in a prospective study: insights into the extent and nature of the reliability and validity problem. Alcoholism: Clinical and Experimental Research. 2014;38:489–500. doi: 10.1111/acer.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G, Ng T, Rodriguez M, Harding A, Blumbergs P, Evans W, et al. Consensus neuropathological diagnosis of common dementia syndromes: testing and standardising the use of multiple diagnostic criteria. Acta Neuropathologica. 2002;104:72–78. doi: 10.1007/s00401-002-0529-5. [DOI] [PubMed] [Google Scholar]
- Hardy JA, Wester P, Winblad B, Gezelius C, Bring G, Eriksson A. The patients dying after long terminal phase have acidotic brains; implications for biochemical measurements on autopsy tissue. Journal of Neural Transmission. 1985;61:253–264. doi: 10.1007/BF01251916. [DOI] [PubMed] [Google Scholar]
- Harmon SH, Mcmahon A. Banking (on) the brain: from consent to authorisation and the transformative potential of solidarity. Medical Law Review. 2014;22:572–605. doi: 10.1093/medlaw/fwu011. [DOI] [PubMed] [Google Scholar]
- Hulette CM. Brain banking in the United States. Journal of Neuropathology and Experimental Neurology. 2003;62:715–722. doi: 10.1093/jnen/62.7.715. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Koga M, Horiuchi Y, Noguchi E, Morikawa M, Suzuki Y, et al. Supportive evidence for reduced expression of GNB1L in schizophrenia. Schizophrenia Bulletin. 2010;36:756–765. doi: 10.1093/schbul/sbn160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob T, Seilhamer RA, Bargeil K, Howell DN. Reliability of Lifetime Drinking History among alcohol dependent men. Psychology of Addictive Behaviors. 2006;20:333–337. doi: 10.1037/0893-164X.20.3.333. [DOI] [PubMed] [Google Scholar]
- Kalia M, Costa E, Silva J. Biomarkers of psychiatric diseases: current status and future prospects. Metabolism. 2015;64(3 Suppl 1):S11–15. doi: 10.1016/j.metabol.2014.10.026. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Van Dyk DD, Craft GE, Innes DJ, Mayfield RD, Cobon G, et al. The application of proteomics to the human alcoholic brain. Annals of the New York Academy of Sciences. 2004;1025:14–26. doi: 10.1196/annals.1316.002. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald ML, Ciccimaro E, Prakash A, Banerjee A, Seeholzer SH, Blair IA, et al. Biochemical fractionation and stable isotope dilution liquid chromatography-mass spectrometry for targeted and microdomain-specific protein quantification in human postmortem brain tissue. Molecular & Cellular Proteomics. 2012;11:1670–1681. doi: 10.1074/mcp.M112.021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintick JN, Xuei X, Tischfield JA, Goate A, Foroud T, Wetherill L, et al. Stress-response pathways are altered in the hippocampus of chronic alcoholics. Alcohol. 2013;47:505–515. doi: 10.1016/j.alcohol.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith RE, Hammond JH, Shan D, Meador-Woodruff JH. Postmortem brain: an underutilized substrate for studying severe mental illness. Neuropsychopharmacology. 2014;39:65–87. doi: 10.1038/npp.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monoranu CM, Apfelbacher M, Grünblatt E, Puppe B, Alafuzoff I, Ferrer I, et al. pH measurement as quality control on human post mortem brain tissue: a study of the BrainNet Europe consortium. Neuropathology and Applied Neurobiology. 2009;35:329–337. doi: 10.1111/j.1365-2990.2008.01003a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Huang XF. Ionotropic glutamate receptor binding in the posterior cingulate cortex in schizophrenia patients. Neuroreport. 2005;16:1363–1367. doi: 10.1097/01.wnr.0000174056.11403.71. [DOI] [PubMed] [Google Scholar]
- Okvist A, Johansson S, Kuzmin A, Bazov I, Merino-Martinez R, Ponomarev I, et al. Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system. PLoS One. 2007;2:e930. doi: 10.1371/journal.pone.0000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Purohit DP, Reichenberg A, McGurk SR, Haroutunian V, et al. Cortical neuritic plaques and hippocampal neurofibrillary tangles are related to dementia severity in elderly schizophrenia patients. Schizophrenia Research. 2010;116:90–96. doi: 10.1016/j.schres.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MT, Noronha A, Warren K. Perspectives on the neuroscience of alcohol from the National Institute on Alcohol Abuse and Alcoholism. Handbook of Clinical Neurology. 2014;125:15–29. doi: 10.1016/B978-0-444-62619-6.00002-1. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Parlapani E, Bauer M, Heinsen H, Falkai P. Is brain banking of psychiatric cases valuable for neurobiological research? Clinics (São Paulo) 2008;63:255–266. doi: 10.1590/s1807-59322008000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy D, Say M, Stevens J, Harper CG, Kril JJ. Influence of liver pathology on markers of postmortem brain tissue quality. Alcoholism: Clinical and Experimental Research. 2012;36:55–60. doi: 10.1111/j.1530-0277.2011.01580.x. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Sheedy D, Kril JJ. Using autopsy brain tissue to study alcohol-related brain damage in the genomic age. Alcoholism: Clinical and Experimental Research. 2014;38:1–8. doi: 10.1111/acer.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GT, Sheedy D, Sheahan PJ, Kaplan W, Kril JJ. Comorbidities, confounders, and the white matter transcriptome in chronic alcoholism. Alcoholism: Clinical and Experimental Research. 2014;38:994–1001. doi: 10.1111/acer.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Yu R, Silbermann E, Zabala V, Deochand C, de la Monte SM. Differential Contributions of Alcohol and Nicotine-Derived Nitrosamine Ketone (NNK) to White Matter Pathology in the Adolescent Rat Brain. Alcohol and Alcoholism (Oxford, Oxfordshire) 2015;50:680–689. doi: 10.1093/alcalc/agv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gelernter J, Zhang H. Differential Expression of miR-130a in Postmortem Prefrontal Cortex of Subjects with Alcohol Use Disorders. Journal of Addiction Research & Therapy. 2013;4(155) doi: 10.4172/2155-6105.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert CS, Sheedy D, Rothmond DA, Dedova I, Fung S, Garrick T, et al. Selection of reference gene expression in a schizophrenia brain cohort. The Australian and New Zealand Journal of Psychiatry. 2010;44:59–70. doi: 10.3109/00048670903393662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Global status report on alcohol and health 2014. 2014 Retrieved from http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
- Yalcin EB, de la Monte SM. Review of matrix-assisted laser desorption ionization-imaging mass spectrometry for lipid biochemical histopathology. The Journal of Histochemistry and Cytochemistry. 2015;63:762–771. doi: 10.1369/0022155415596202. [DOI] [PMC free article] [PubMed] [Google Scholar]