Abstract
Current treatment of metastatic bone prostate cancer with Docetaxel chemotherapy per CHAARTED trial is standard of care. Timing of CT and bone scintigraphy for evaluation of successful treatment of lytic lesions is not available in the literature. We present a case of a 70 year old male with PSA of 586 and wide spread metastatic bone lytic lesions, who underwent androgen deprivation therapy and six cycles of Docetaxel chemotherapy. The patient had clinically successful treatment. Contrast enhanced CT scan demonstrated sclerotic bone lesions with PSA 2.5 at this point in treatment; however, 99mTc-MDP bone scintigraphy remained positive for metastatic lesions.
Keywords: Prostate, Lytic, Bone-scan, Docetaxel
Introduction
Current treatment of metastatic bone prostate cancer with Docetaxel chemotherapy per CHAARTED trial is the standard of care. Timing of bone scintigraphy for evaluation of successful treatment of lytic lesions is not available in the literature. We present a case with clinically successful treatment but positive bone scan results 1 month after chemotherapy.
Case presentation
A 70 year old male presented with prostate specific antigen (PSA) of 586 and a baseline PSA of 3.7 a year prior. Biopsy and staging: T4, G4+3 prostate cancer with perineural invasion. Staging contrast CT scan revealed wide spread lytic lesions with large 11th rib and left ileum lytic lesions, bladder soft tissue mass effect, and pelvic lymphadenopathy. 99mTc-MDP bone scintigraphy showed multifocal lytic lesions with increased blood pool/intense hyperemia activity at 5 minutes and 2 hours after hydration, consistent with aggressive appearing lytic metastatic disease. 11th rib biopsy was consistent with metastatic prostate cancer. The patient underwent immediate androgen deprivation therapy (ADT), 6 cycles of systemic Docetaxel, and pelvis/pubic ramus radiation for bone pain palliation. He had clinical improvement post-Docetaxel with PSA of 2.5 (Figure 1, Figure 2).
Figure 1.
Composite photomicrograph showing the morphologic features of prostatic tissue. A) Low power view showing a prostate needle biopsy demonstrating back-to-back glands with an infiltrative growth pattern, glandular fusion, and cribiform architecture (arrow) consistent with Gleason pattern 4 (H&E, original magnification ×10). B) High power view showing the cribiform architecture and cytologic features. The tumor cells have large nuclei (arrow) with prominent large nucleoli (H&E, original magnification ×10). C) Prostatic adenocarcinoma with perineural invasion (arrow) in a core needle biopsy (H&E, original magnification ×40).
Figure 2.

A) Photomicrograph of the left rib core needle biopsy demonstrating metastatic prostate carcinoma involving bone; woven bone deposition (arrow) and novel bone formation upon sites of prior resorption creating a marked reversal front (arrowhead) appear consistent with a lesion exhibiting increased bone breakdown and repair. H&E, original magnification ×20. B (inset) Fine needle aspiration of left rib lesion demonstrating positive immunoreactivity for prostate specific acid phosphatase (PSAP) (immunohistochemistry, original magnification ×40).
One month follow up CT scan demonstrated sclerotic lesions with minimal evidence of active metastatic disease; however bone scan still showed multiple uptake areas consistent with active disease. Current literature for hormone sensitive prostate cancer with lytic bone lesions stems from breast cancer management; CT scan in this patient provides evidence of successful treatment suggested by sclerotic lesions as is seen in breast and prostate cancer patients post-chemotherapy.1, 2, 3 However, there is no data with timing recommendations for bone scan to evaluate successful treatment outcome of prostate cancer with metastatic lytic lesions (Fig. 3).
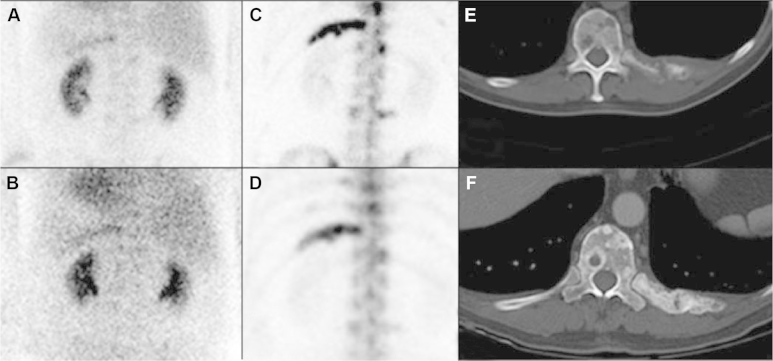
Figure 3.
Pre-chemotherapy blood pool (A) and delay (C) shows increased uptake in the left 11th rib corresponding to lytic metastasis on axial CT (E). One month post-chemotherapy blood pool (B) and delay (D) shows persistent, but decreased uptake on left 11th rib with positive post-treatment sclerosis on axial CT (F).
Discussion
There is no current determination for when bone scintigraphy would become negative after Docetaxel therapy for lytic lesions. Although current literature suggests repeat bone scans coupled with PSA levels at 3 and 6 months following chemotherapy to detect flare phenomenon and repeat CT scans every 2–4 months following Docetaxel therapy, there is no current consensus on the timing of bone scintigraphy for evaluation of treatment response, yet experts recommend that it be performed regularly.4, 5 It is increasingly recognized that techniques combining morphological and functional data are the most sensitive and specific, with PET/CT and PET/MRI emerging as important future tools.6 In our case, CT scan provides evidence of clinical successful treatment by demonstration of sclerotic lesions; however, bone scintigraphy still depicts active disease, a discrepancy that is consistent with treatment response and the associated flare phenomenon.1, 3, 4 Novel imaging-based biomarkers such as function diffusion mapping are being investigated for early evaluation of treatment response with encouraging results that may even indicate greater utility for monitoring response to treatment in the setting of metastatic lytic bone disease.2, 7
Conclusion
There is a need for specific radiographic studies to differentiate between active metastatic bone lesion and active sclerotic non-malignant bone lesion. While the literature describes many new imaging methods, claiming increased sensitivity and specificity, these have yet to be appropriately tested in clinical trials.5 Additionally, precise criteria are lacking for the identification of flare phenomenon in response to treatment.
Conflicts of interest
None.
Footnotes
Funding: None.
Informed consent: Obtained from patient.
References
- 1.Colemann R.E., Mashiter G., Whitaker K.B. Bone scare flare predicts successful systemic therapy for bone metastases. J Nucl Med. 1988;29(8):1354–1359. [PubMed] [Google Scholar]
- 2.Lee K.C., Bradley D.A., Hussain M. A feasibility study evaluating the functional diffusion map as a predictive imaging biomarker for detection of treatment response in a patient with metastatic prostate cancer to the bone. Neoplasia. 2007;9(12):1003–1011. doi: 10.1593/neo.07954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messiou C., Cook G., Reid A.H. The CT flare response of metastatic bone disease in prostate cancer. Acta Radiol. 2011;52(5):557–561. doi: 10.1258/ar.2011.100342. [DOI] [PubMed] [Google Scholar]
- 4.Pollen J.J., Witztum K.F., Ashburn W.L. The flare phenomenon on radionuclide bone scan in metastatic prostate Cancer. Am J Roentgenol. 1984;142:773–776. doi: 10.2214/ajr.142.4.773. [DOI] [PubMed] [Google Scholar]
- 5.Gillessen S., Omlin A., Attard G. Management of patients with advanced prostate cancer: Recommendations of the St Gallen Advanced Prostate Cancer Consensus Conference (APCCC) 2015. Ann Oncol. 2015;26(8):1589–1604. doi: 10.1093/annonc/mdv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Sullivan G.J., Carty F.L., Cronin C.G. Imaging of bone metastasis: An update. World J Radiol. 2015;7(8):202–211. doi: 10.4329/wjr.v7.i8.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelloff G.J., Choyke P., Coffey D.S. Challenges in Clinical Prostate Cancer: Role of Imaging. AJR Am J Roentgenol. 2009;192(6):1455–1470. doi: 10.2214/AJR.09.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]