Abstract
Tregs are critical for control of self-reactive T cells that escape thymic selection and end up in the periphery. Treg subsets suppress effector T cell populations through the secretion of immunosuppressive molecules and inhibitory cytokines as well as cell contact–dependent mechanisms. In this issue of the JCI, Wen and colleagues describe another mechanism by which Tregs suppress effector T cell populations. Specifically, the authors reveal that CD8+ T cells in close contact with target T cells release NADPH oxidase 2–containing microvesicles that inhibit TCR activation by elevating ROS and thereby reducing phosphorylation of the TCR-associated kinase ZAP70. Together, the results of this study provide important insight into CD8+ Treg function and into the development of autoimmunity in older individuals.
Tregs: mediators of peripheral tolerance
During development, deletion of self-reactive T cells in the thymus is incomplete, and escape of these potentially autoaggressive T cells into the periphery is not the exception, but the rule. Control of these T cells in peripheral tissues (peripheral tolerance) is key to prevent autoimmunity — a task that is fulfilled by so-called Tregs. Individuals with dysfunction of the transcriptional regulator FOXP3 lack Tregs and develop severe autoimmune disease, a testament to the importance of this population in maintaining peripheral tolerance (1).
Regulation of peripheral tolerance has been extensively studied in CD4+ T cells; however, suppressive capacity is not strictly confined to the CD4+ T cell compartment. CD8+ Tregs, which have been characterized by various investigators via a range of cell surface phenotypes, have been described in both humans and mice (2). The capacity to suppress T cell proliferation and/or function is common to all Treg subsets. Similar to their CD4+ counterparts, CD8+ Tregs have been reported to exert inhibition through cell-cell contact or via secretion of immunosuppressive molecules and inhibitory cytokines, such as IL-10. Alterations in the CD8+ Treg compartment have been described in human autoimmune diseases, including multiple sclerosis, type 1 diabetes, rheumatoid arthritis, and systemic lupus erythematodes (2). As systemic inflammation affects many of the phenotypic markers used to track Tregs and as there is an absence of a genetically defined disease associated with a selective lack of CD8+ Tregs, the in vivo relevance of this regulatory subset has been discussed with some skepticism.
In this issue, Wen and colleagues show that human CD39+CD26–CD8+ Tregs, which are representative of the FOXP3+CCR7+ subset, directly inhibit T cell activation ex vivo (3). Expanding on previously reported suppressive mechanisms of CD4+ and CD8+ Tregs, Wen et al. found that CD8+ Tregs deliver NADPH oxidase 2 (NOX2) and, thereby, ROS-containing membrane vesicles (microvesicles) into target T cells. CD8+ Treg-derived NOX2/ROS in turn inhibited T cell receptor (TCR) signal transduction by reducing phosphorylation of the TCR-associated tyrosin kinase ZAP70. Experiments in which CD8+ Tregs and CD4+ T cells were transfered into mice lacking T cells established that CD8+ Tregs modulate CD4+ T cell expansion in vivo. Importantly, knockdown and overexpression of NOX2 modulated the suppressive function CD8+ Tregs accordingly.
CD8+ Treg microvesicles target CD4+ T cells
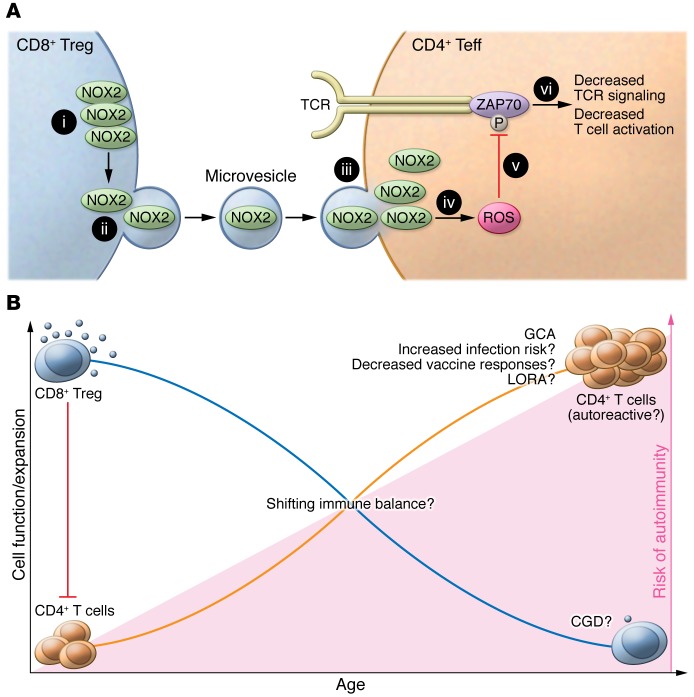
Wen et al. have described a fascinating mechanism by which CD8+ Tregs suppress CD4+ T cells (ref. 3 and Figure 1A). The authors demonstrated that CD8+ Tregs form a synapse, with clustering of NOX2 in the contact area (3). These NOX2-enriched areas of the membrane are released as extracellular vesicles and fuse with the neighboring CD4+ T cells, thus delivering NOX2 and hence ROS to reduce ZAP70 phosphorylation. NOX2/ROS-mediated cell suppression occurred very rapidly (within minutes after NOX2 microvesicle transfer) yet left targeted CD4+ T cells stunned for several days. These observations add another facet to our understanding of the various functions of ROS. Given the broad spectrum of potential ROS targets (4), it is likely that NOX2 microvesicles have additional effects beyond ZAP70 phosphorylation. Furthermore, in addition to the delivery of NOX2 and ROS, microvesicles have been speculated to harbor many more biologically important molecules, such as specific RNAs or signaling molecules (5, 6). The study by Wen et al. thus extends the list of candidate microvesicles that might become interesting therapeutic targets, with the aim of modulating the immune system (7).
Figure 1. Schematic of the mechanism by which CD8+ Tregs suppress CD4+ T cells and implications for autoimmune disease.
(A) CD8+ Tregs inhibit expansion of CD4+ T cells. (i) CD8+ Tregs form NOX2-enriched synapses at the plasma membrane. (ii) NOX2-containing microvesicles form and are (iii) delivered to target CD4+ T cells. (iv) This leads to increased ROS in the target cells, (v) which mediates reduction of ZAP70 phosphorylation and (vi) inhibition of TCR signaling. Teff, effector T cell. (B) The capacity of CD8+ Tregs to produce NOX2 declines with age. This loss of CD8+ Treg functionality may facilitate the expansion of potentially autoreactive CD4+ T cells. As proof of this concept, Wen et al. observed a prominent loss of CD8+ Treg function in patients suffering from GCA, a CD4+ T cell–mediated disease. These observations raise a series of interesting questions. What is the role of CD8+ Tregs in other age-associated autoimmune conditions, such as late-onset rheumatoid arthritis (LORA), in chronic infections, and in vaccine responses? How is CD8+ Treg function affected in individuals with genetic NOX2 deficiency, such as those with CGD? Finally, what impact does the loss of CD8+ Treg function have on CD4+ Treg and/or Th subset compartments?
Having unraveled the cellular mechanisms, Wen and colleagues went on to demonstrate that the function of CD8+ Tregs inversely correlates with age (3). In aged, healthy individuals, T cells have an augmented activation threshold (8); therefore, the reduction of CD8+ Treg function may reflect a physiologic balancing response to maintain protective immunity. However, if this balancing act fails, dysfunctional CD8+ Tregs may allow self-reactive T cell clones to expand. Wen et al. explored this possibility in a clinical context by studying giant cell arteritis (GCA). GCA is a paradigm of autoimmune disease in the elderly that is characterized by inflammation of large arteries. In support of the notion that loss of CD8+ Treg function may be linked to development of autoimmune disease, NOX2 coexpressing CD8+ Tregs were almost completely lacking in patients with GCA. T cells are thought to be important for establishing and/or maintaining vascular inflammation in GCA, and immunophenotypic, histopathologic, and genetic studies all point to Th1 and Th17 CD4+ T cell–mediated damage of the vessel wall (9). A model in which age-related loss of peripheral tolerance due to a decline of CD8+ Treg function that allows expansion of autoreactive T cells is therefore an intriguing possibility. Additionally, recent translational studies found normal or only slightly reduced CD4+ Treg numbers (10–12) but normal CD4+ Treg function (11) in patients with GCA.
Clinical implications and future directions
With the spotlight on NOX2, studying CD8+ Tregs in the pathology of chronic granulomatous disease (CGD) may be highly informative. Patients with CGD harbor mutations in genes encoding for NOX (most commonly gp91-PHOX on the X chromosome) (13) and suffer from recurrent infections and bowel inflammation reminiscent of Crohn’s disease. Other autoimmune manifestations — typically lupus-like autoinflammatory syndromes — occur in roughly 1 of 10 patients with CGD (14). Further, case reports of children with CGD have described the presence of Kawasaki disease, a vasculitis of the coronary arteries (14). Immunologically, while patients with CGD have lower numbers of CD4+ and CD8+ T cells compared with age-matched controls (15, 16), these individuals have an expanded population of Th17 cells (17) — an observation that would be compatible with insufficient CD8+ Treg function due to absence of NOX2. Large-vessel vasculitis, such as Takayasu’s arteritis or GCA, however, has not been described in patients with CGD to date. It will be important to define the role of CD8+ Tregs in additional age-related inflammatory conditions, such as late-onset rheumatoid arthritis or atherosclerosis. In particular, late-onset rheumatoid arthritis might be a good starting point, as NOX-deficient mice develop spontaneous, age-related arthritis that involves CD4+ T cell expansion (18). In addition to these inflammatory conditions, many more interesting and clinically important questions need to be revisited in light of this report (Figure 1B). For example, do viruses that establish chronic persistence — such as HIV or hepatitis C virus — induce CD8+ Tregs and thus exploit these cells to their benefit? How does suppression of naive CD4+ T cells by CD8+ Tregs affect CD4+ Treg numbers or other CD4+ T cell subsets? Why do elderly individuals have reduced vaccine responses, despite the proposed reduction of CD8+ Treg function proposed by Wen et al.? Addressing these questions will be challenging in humans, especially as it is becoming clearer that T cell subsets can have substantial plasticity and may change phenotype and function (19). Eventually, sophisticated comprehensive network analyses that incorporate dynamic changes of cellular functions over time may be required to firmly dissect the appropriate targets, possibly including CD8+ Tregs, for high-precision immunoregulatory therapies.
Irrespective of how these intriguing issues are approached, it should be remembered that is has taken three decades to appreciate the importance of CD4+ Tregs — we should be more open-minded this time around.
Acknowledgments
C.T. Berger and C. Hess are both supported by the Swiss National Science Foundation (PZ00P3-148000 and SNSF 310030_153059/CRSII3_160766, respectively).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2016;126(5):1646–1648. doi:10.1172/JCI87429.
See the related article beginning on page 1953.
References
- 1.Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol. 2007;120(4):744–750. doi: 10.1016/j.jaci.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 2.Filaci G, Fenoglio D, Indiveri F. CD8(+) T regulatory/suppressor cells and their relationships with autoreactivity and autoimmunity. Autoimmunity. 2011;44(1):51–57. doi: 10.3109/08916931003782171. [DOI] [PubMed] [Google Scholar]
- 3.Wen Z, et al. NADPH oxidase deficiency underlies dysfunction of aged CD8+ Tregs. J Clin Invest. 2016;126(5):1953–1967. doi: 10.1172/JCI84181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13(5):349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridgeman A, et al. Viruses transfer the antiviral second messenger cGAMP between cells. Science. 2015;349(6253):1228–1232. doi: 10.1126/science.aab3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 7.Gyorgy B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14(5):428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol. 2013;9(12):731–740. doi: 10.1038/nrrheum.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM. Th17 and Th1 T-cell responses in giant cell arteritis. Circulation. 2010;121(7):906–915. doi: 10.1161/CIRCULATIONAHA.109.872903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samson M, et al. Th1 and Th17 lymphocytes expressing CD161 are implicated in giant cell arteritis and polymyalgia rheumatica pathogenesis. Arthritis Rheum. 2012;64(11):3788–3798. doi: 10.1002/art.34647. [DOI] [PubMed] [Google Scholar]
- 12.Terrier B, et al. Interleukin-21 modulates Th1 and Th17 responses in giant cell arteritis. Arthritis Rheum. 2012;64(6):2001–2011. doi: 10.1002/art.34327. [DOI] [PubMed] [Google Scholar]
- 13.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 2000;79(3):170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Magnani A, et al. Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J Allergy Clin Immunol. 2014;134(3):655–662. doi: 10.1016/j.jaci.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Heltzer M, Jawad AF, Rae J, Curnutte JT, Sullivan KE. Diminished T cell numbers in patients with chronic granulomatous disease. Clin Immunol. 2002;105(3):273–278. doi: 10.1006/clim.2002.5291. [DOI] [PubMed] [Google Scholar]
- 16.Hasui M, et al. Decreased CD4+CD29+ (memory T) cells in patients with chronic granulomatous disease. J Infect Dis. 1993;167(4):983–985. doi: 10.1093/infdis/167.4.983. [DOI] [PubMed] [Google Scholar]
- 17.Horvath R, et al. Expansion of T helper type 17 lymphocytes in patients with chronic granulomatous disease. Clin Exp Immunol. 2011;166(1):26–33. doi: 10.1111/j.1365-2249.2011.04449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K, Won HY, Bae MA, Hong JH, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc Natl Acad Sci U S A. 2011;108(23):9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16(3):149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]