Abstract
Substantial ischemia-reperfusion injury (IRI) to the transplanted kidney occurs in 30% to 50% of transplantation patients who receive the organ from a deceased donor. IRI usually manifests as delayed graft function (DGF) and, in severe cases, results in primary nonfunction. Previous studies, primarily experimental, have demonstrated sex-specific susceptibility to IRI in kidney and other organs. In this issue of the JCI, Aufhauser Jr., Wang, and colleagues further demonstrate the importance of donor and recipient sex in IRI and elucidate the role of estrogen receptors in a murine model. Furthermore, analysis of data from 46,691 renal transplant patients in the United Network for Organ Sharing (UNOS) database revealed that sex affects DGF outcomes in humans. Manipulation of sex-driven molecular pathways offers a fertile opportunity to increase the number of organs available for transplantation and to reduce IRI in kidney and, likely, other organs.
Sexual dimorphism and delayed graft function
Ischemia reperfusion injury (IRI) occurs in all deceased donor organ transplants and, when severe, leads to substantial graft dysfunction. For patients undergoing a kidney transplantation, IRI manifests as delayed graft function (DGF), which is defined as the need for dialysis in the first week after transplantation (dialysis within the first 24 hours is not included in some DGF definitions). DGF occurs in approximately 30% to 50% of deceased donor kidney recipients and leads to increased patient morbidity, hospital costs, and risk of rejection, as well as decreased long-term transplant survival (1–4). Many established molecular pathways, including the generation of ROS, cellular and soluble inflammation, apoptosis, and metabolic disruption, along with endothelial, epithelial, and mitochondrial dysregulation, are involved in kidney IRI (refs. 2, 5–7; Figure 1). Kidney IRI is an important factor that limits the number of organs that can be transplanted, as kidneys with severe IRI, such as those from uncontrolled cardiac death, are usually discarded. New methods to limit kidney IRI could increase the number of donor organs that are used for the thousands of patients waiting for a kidney transplant and improve both short- and long-term outcomes.
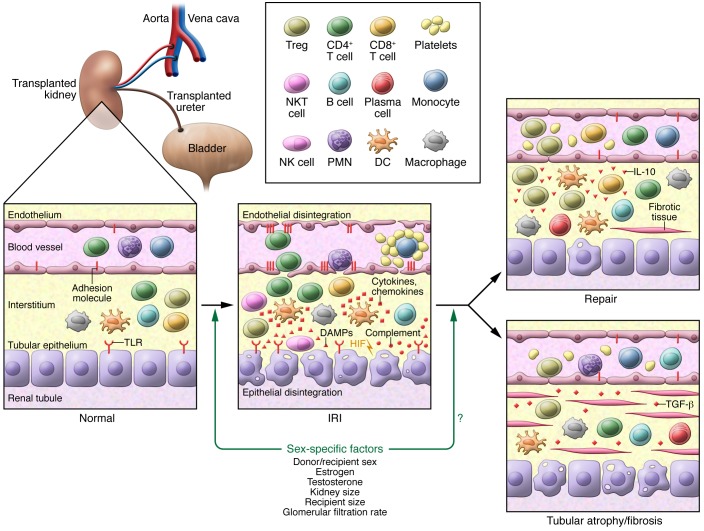
Figure 1. Sex affects the susceptibility and pathogenesis of IRI in a single transplanted kidney.
Exposure of a kidney transplant to warm and/or cold IR results in the activation of multiple pathological processes, including uncontrolled ROS production, activation of immune cells, proinflammatory cytokine production, endothelial and epithelial disintegration, and mitochondrial dysregulation. These effects result in acute loss of kidney function that often resolves following a complete and normal repair. Alternatively, persistent inflammation and further injury, along with defective and inefficient repair processes, can lead to tubular atrophy and fibrosis, which subsequently result in the loss of graft function. In this issue, Aufhauser Jr., Wang, and colleagues demonstrate that sex and estrogen mediate the response from the normal kidney to the IRI kidney. It remains unknown whether these factors affect recovery back toward normal kidney function or the development of atrophic and fibrosed kidney. In addition to estrogen, other factors, such as testosterone, kidney size, recipient size, and sex-specific differences in glomerular filtration rates, could also modulate recovery following IRI. It is not understood how these sex-specific factors interact with other identified pathophysiological mechanisms to determine DGF outcomes. Adapted with permission from Nature Reviews Nephrology (5). DAMPs, damage-associated molecular patterns; TLR, Toll-like receptor.
The effect of sex on immunity is well established (8). Furthermore, sex alters kidney size and function (9), and the importance of sex hormones and sex-specific differences in kidney allograft rejection has led to recommendations by funding agencies such as the NIH to advise that both male and female animals and cells be included in preclinical studies to ensure reproducibility and successful translational outcomes (10, 11). Nonetheless, the mechanisms that underlie sex-dependent effects on kidney IRI are still incompletely understood, and little is known about the sex-specific effects on human DGF.
Transplanted kidney protection in females
In this issue, Aufhauser Jr., Wang, and colleagues report that being female is an important factor that favorably influences the outcome of kidney transplantation in both mice and humans (12). This team performed rigorous and innovative experiments to induce ischemic injury in native and transplanted kidneys in hormonally intact and spayed or neutered mice. In addition, they analyzed United Network for Organ Sharing (UNOS) data pertaining to the role of sex as a risk factor for DGF. Aufhauser Jr., Wang, and colleagues sought to answer several key questions relating to sex and kidney IRI. Is the tolerance to IRI in the kidney due to the sex of the organ donor or to alterations in the host’s response as a result of the sex of the recipient? As kidneys in female mice were better protected from IRI, do sex hormones, especially estrogen, play any role in this protection, and, if so, can these effects be modified by altering the hormonal background of the subject? Finally, does sexual dimorphism affect human DGF?
Using a series of murine renal IRI and transplant models, Aufhauser Jr., Wang, and colleagues showed profound protection from IRI in female mice compared with that seen in male mice. Neutering of either sex resulted in an intermediate phenotype that presented as increased IRI in female animals and reduced IRI in males. Mice transplanted with kidneys from the opposite sex demonstrated that the host environment influences recovery from ischemic injury, as kidneys transplanted into females showed less IRI, regardless of the sex of the donor. Supplemental 17-β-estradiol (estrogen) administered over a short time course prior to the induction of warm IRI protected kidney function in female mice and neutered males but was not protective in hormonally intact males. In addition to confirming the previously known relationship between sexual dimorphism and kidney transplant outcomes, this study by Aufhauser Jr., Wang, and colleagues further demonstrates that female sex–specific protection extends to both cold and warm ischemia. This new information could have wide implications and relevance that extend to the transplantation of other organs.
Aufhauser Jr., Wang, and colleagues investigated related effects in humans by analyzing UNOS data and found that male recipients had a greater risk of developing DGF relative to that of female recipients (odds ratio of 1.39). The authors also determined that the hormonal environments of both the donor and the recipient contribute to renal IRI tolerance in humans. While previous studies have focused on the sex-dependent effects on long-term human renal transplant outcomes, Aufhauser Jr., Wang, and colleagues examined short-term IRI-related effects on DGF outcomes and found that in postmenopausal recipients, the impact of sex was reduced and could not be explained by a donor-recipient size mismatch or by nephron dosing. However, the recipient effects were dominant in human transplant outcomes, whereas in mice, the donor effects appeared to be more robust. As pointed out by the Aufhauser Jr., Wang, and colleagues, this information on donor and recipient effects could provide critical guidance for future human hormonal interventions in DGF.
Conclusions and future directions
An improved understanding of donor-specific (brain death, cold and warm ischemic injury, inflammatory signaling) and recipient-specific (reperfusion injury, oxidative stress, innate and adaptive immune responses) pathophysiologic factors is critical for designing strategies that effectively reduce DGF in transplant patients. The current findings by Aufhauser Jr., Wang, and colleagues (12) suggest a potential opportunity for effectively reducing IR-induced injury during transplantation. It should be noted that the authors did not evaluate whether estrogen administered after IRI is protective. It will be an important preclinical finding to address, because organs and patients are frequently taken care of after IRI starts. Additional strategies, such as hypothermic machine perfusion for a prolonged cold ischemia time, reduction of organ extraction time, and inclusion of older (≥60 years) female donors in conjunction with administration of estrogen, can significantly improve graft function and survival (13–17). The effect of estrogen administration needs to be investigated in large animals before human interventions are studied, as the role of the estrogen receptor in the protective mechanism remains undefined. Moreover, the safety of this approach must be demonstrated, especially given that estrogen therapy in older females has been shown to increase the risk of deep vein thrombosis, heart disease, and breast cancer (18). Additionally, multiple laboratory-based animal studies suggest that renal hemodynamics and immune responses differ between male and female animals and therefore may differentially affect transplant outcomes in humans (19, 20). Future preclinical and human studies should use serum creatinine to estimate kidney function, as this measure is closer to actual glomerular filtration rates than is the blood urea nitrogen (BUN) measure in most circumstances. Future studies should also evaluate whether the current algorithm used to predict deceased donor risk in the US, the kidney donor profile index, should be modified to include sex (21).
The opportunity to generalize the findings of Aufhauser Jr., Wang, and colleagues beyond the field of kidney transplantation should not be overlooked. Acute kidney injury occurs in 2% to 5% of hospitalized patients and carries a high incidence of mortality in the intensive care unit setting. Patients undergoing cardiopulmonary bypass surgery have increased rates of acute kidney injury as well. These scenarios and others are excellent opportunities to examine the role of sex-specific differences in IRI and determine whether estrogen therapy can be beneficial toward protecting the kidney. Furthermore, IRI in other organs such as the heart and brain (22, 23) are active areas of investigation in which sex-directed therapeutics could have an important impact.
Acknowledgments
The authors’ work is supported by R01 grant DK104662 from the NIH.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Reference information:J Clin Invest. 2016;126(5):1643–1645. doi:10.1172/JCI87428.
See the related article beginning on page 1968.
Contributor Information
Sanjeev Noel, Email: snoel6@jhmi.edu.
Hamid Rabb, Email: hrabb1@jhmi.edu.
References
- 1.Chaumont M, et al. Delayed graft function in kidney transplants: time evolution, role of acute rejection, risk factors, and impact on patient and graft outcome. J Transplant. 2015;2015: doi: 10.1155/2015/163757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menke J, Sollinger D, Schamberger B, Heemann U, Lutz J. The effect of ischemia/reperfusion on the kidney graft. Curr Opin Organ Transplant. 2014;19(4):395–400. doi: 10.1097/MOT.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 3.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu WK, Famure O, Li Y, Kim SJ. Delayed graft function and the risk of acute rejection in the modern era of kidney transplantation. Kidney Int. 2015;88(4):851–858. doi: 10.1038/ki.2015.190. [DOI] [PubMed] [Google Scholar]
- 5.Jang HR, Rabb H. Immune cells in experimental acute kidney injury. Nat Rev Nephrol. 2015;11(2):88–101. doi: 10.1038/nrneph.2014.180. [DOI] [PubMed] [Google Scholar]
- 6.Noel S, et al. T lymphocyte-specific activation of Nrf2 protects from AKI. J Am Soc Nephrol. 2015;26(12):2989–3000. doi: 10.1681/ASN.2014100978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martina MN, et al. Double-negative αβ T cells are early responders to AKI and are found in human kidney. J Am Soc Nephrol. 2016;27(4):1113–1123. doi: 10.1681/ASN.2014121214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oertelt-Prigione S. The influence of sex and gender on the immune response. Autoimmun Rev. 2012;11(6–7):A479–A485. doi: 10.1016/j.autrev.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 9.Sabolic I, Asif AR, Budach WE, Wanke C, Bahn A, Burckhardt G. Gender differences in kidney function. Pflugers Archiv. 2007;455(3):397–429. doi: 10.1007/s00424-007-0308-1. [DOI] [PubMed] [Google Scholar]
- 10.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandberg K, Umans JG, Georgetown Consensus Conference Work Group Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research. FASEB J. 2015;29(5):1646–1652. doi: 10.1096/fj.14-269548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aufhauser DD, Jr, et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J Clin Invest. 2016;126(5):1968–1977. doi: 10.1172/JCI84712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemann CU, et al. Therapeutic hypothermia in deceased organ donors and kidney-graft function. N Engl J Med. 2015;373(5):405–414. doi: 10.1056/NEJMoa1501969. [DOI] [PubMed] [Google Scholar]
- 14.Xia Y, Friedmann P, Cortes CM, Lubetzky ML, Kayler LK. Influence of cold ischemia time in combination with donor acute kidney injury on kidney transplantation outcomes. J Am Coll Surg. 2015;221(2):532–538. doi: 10.1016/j.jamcollsurg.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Lam VW, Laurence JM, Richardson AJ, Pleass HC, Allen RD. Hypothermic machine perfusion in deceased donor kidney transplantation: a systematic review. J Surg Res. 2013;180(1):176–182. doi: 10.1016/j.jss.2012.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Osband AJ, James NT, Segev DL. Extraction time of kidneys from deceased donors and impact on outcomes. Am J Transplant. 2016;16(2):700–703. doi: 10.1111/ajt.13457. [DOI] [PubMed] [Google Scholar]
- 17.Matos AC, et al. Expanding the pool of kidney donors: use of kidneys with acute renal dysfunction. Einstein (Sao Paulo) 2015;13(2):319–325. doi: 10.1590/S1679-45082015RW3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett-Connor E, Stuenkel CA. Hormone replacement therapy (HRT) — risks and benefits. Int J Epidemiol. 2001;30(3):423–426. doi: 10.1093/ije/30.3.423. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka R, Tsutsui H, Ohkita M, Takaoka M, Yukimura T, Matsumura Y. Sex differences in ischemia/reperfusion-induced acute kidney injury are dependent on the renal sympathetic nervous system. Eur J Pharmacol. 2013;714(1–3):397–404. doi: 10.1016/j.ejphar.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Kang KP, et al. Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol Med Rep. 2014;9(6):2061–2068. doi: 10.3892/mmr.2014.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israni AK, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842–1848. doi: 10.1681/ASN.2013070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostadal B, Ostadal P. Sex-based differences in cardiac ischaemic injury and protection: therapeutic implications. Br J Pharmacol. 2014;171(3):541–554. doi: 10.1111/bph.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broughton BR, et al. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke. 2014;45(3):835–841. doi: 10.1161/STROKEAHA.113.001499. [DOI] [PubMed] [Google Scholar]