Abstract
Background:
T-cells are important in the pathogenesis of Type 1 diabetes (T1D). However, the exact role of T-cell subpopulations in this pathway remains unknown. The purpose of this study was to assess the expression pattern of T helper 1 (Th1) interferon-gamma (IFN-γ) and Th2 interleukin-4 (IL-4) cytokines and their relationship with sex and disease duration in T1D patients.
Materials and Methods:
This study was conducted on 21 T1D patients and 22 healthy subjects. Gene expression analysis of peripheral blood mononuclear cells (PBMCs) was performed using real-time reverse transcriptase polymerase chain reaction.
Results:
IFN-γ gene expression was significantly lower in T1D patients compared with controls (P < 0.05). Conversely, IL-4 mRNAs were significantly increased in the PBMCs from patients as compared to controls (P < 0.05). There was no significant difference in the expression of IL-4 and IFN-γ between men and women with T1D (P > 0.05) while IL-4 mRNA expression in male patients was about 1.9 folds higher than female patients. Moreover, IFN-γ mRNA expression in female patients was about 1.8 folds lower than male patients. Patients were divided into two groups regarding their disease duration: <10 years and >10 years. A significant increase in the IL-4 expression was observed between two groups of patients compared to controls (P < 0.0001). Conversely, there was a significant difference in the expression of IFN-γ only between patients with more than 10 years of disease duration (P = 0.02).
Conclusion:
These data propose supplementary implications for the role of Th1/Th2 imbalance in T1D immunopathogenesis. Moreover, factors such as sex and disease duration may have some influence on cytokine mRNA expression.
Keywords: Autoimmunity, cytokine, Type 1 diabetes
INTRODUCTION
Diabetes is a common chronic disease and is now on the rise in many countries. Globally, in 2030, it is estimated that nearly to 552 million people are diabetic.[1] About 5–10% of people suffering from diabetes have the Type 1 form (Type 1 diabetes [T1D]). The disease can occur at any age, but is often observed in adolescence and early adulthood. The etiology of T1D is unknown; nonetheless, genetic, immunologic, and environmental factors (such as viruses) contribute to the pathogenesis of disease.[2,3]
In T1D, insulin deficiency is associated with the destruction of the β-cells of the pancreas that may be caused by the activation of auto-aggressive T helper (Th) lymphocytes and macrophages.[4] Th1 and Th2 cells comprise two functionally separate subpopulations of Th cells and are distinguished by their cytokine profiles and activities. For example, Th1 cells are an important source of interferon-gamma (IFN-γ) and also form a major component of cellular immune response. However, Th2 lymphocytes produce interleukin-4 (IL-4) and are more effective in stimulation of humoral immune system.[5]
There is growing evidence to suggest that altered Th1/Th2 balance and related cytokines play an important role in the pathogenesis of autoimmune diseases such as T1D. However, there is disagreement in the literature about T1D being a Th1 or Th2 - mediated autoimmune disease, or both.[6,7,8] Therefore, elucidating a peculiar role for various Th1 and Th2 subpopulations in T1D may help in identifying specific immune parameters in this important metabolic disease. Moreover, the effect of gender and duration of disease on T-cell immunity and development of insulin-dependent diabetes mellitus (IDDM) remains also controversial.[9,10]
The aim of this study was to analyze IFN-γ and IL-4 mRNA expression patterns in peripheral blood mononuclear cells (PBMCs) of patients with T1D. Moreover, correlations between mRNA levels of these cytokines and clinical parameters of patients (gender and duration of disease) were also evaluated.
MATERIALS AND METHODS
Patients
During 6 months, venous blood samples (5 ml) were drawn from patients (n = 21) with T1D and control subjects (n = 22). The mean age of the patients was 26.67 ± 4.78 years (men 25 ± 4.06 years; women 27.92 ± 5.05 years). All of the healthy controls had no history of T1D or other chronic and autoimmune diseases and their mean ages were 29.18 ± 7.66 years (men, 25.20 ± 5.63 years, women, 30.35 ± 7.91 years).
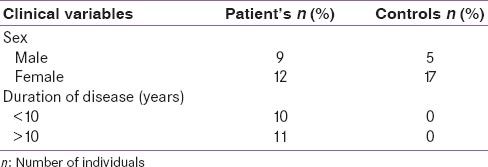
As shown in Table 1, study participants were categorized into two groups on the basis of disease duration - Group 1 (below 10 years, mean = 5.33 ± 3.31) and Group 2 (above 10 years, mean = 16.0 ± 4.98). Samples were also subgrouped according to gender into males and females. Cytokine alterations were analyzed by subgrouping patients and controls according to gender and disease duration.
Table 1.
Classification of individuals according to gender and duration of disease
Ethical clearance
The protocol was in accordance with the guidance of the Research Ethics Board at Tehran University of Medical Sciences. All patients and control subjects gave informed written consent before the beginning of any study-related procedures.
Isolation of peripheral blood mononuclear cells using Ficoll
PBMCs were isolated using Ficoll density-gradient centrifugation (Pharmacia, Uppsala, Sweden) from the whole blood. In brief, the heparinized blood was diluted 1:1 in phosphate-buffered saline (PBS) and layered over 5 ml Ficoll-Paque in a conical tube. The tubes were centrifuged at 400 × g for 20 min at 20°C. The PBMCs layer was transferred to a new canonical tube. The cells were washed twice in PBS (at 20°C for 10 min at 300 × g followed by 10 min for 200 × g) and resuspended in an appropriate amount of buffer. The mixture was then transferred to a 1.5 ml microcentrifuge tube.
RNA extraction and first-strand cDNA synthesis
RNA isolation was performed immediately after PBMC preparation. Purification of total RNA from PBMCs was performed using Ribospin™ (GeneALL, Seoul, Korea). Electrophoresis and spectrophotometry used for the evaluation of quality and quantity of extracted RNA. cDNA was synthesized according to manufacturer's protocol (Fermentas, Germany) and kept frozen at −20°C until use.
Reverse transcription-quantitative polymerase chain reaction analysis
Prepared cDNA samples were subjected to reverse transcriptase polymerase chain reaction (RT-PCR) with specific primer pairs to IL-4, IFN-γ, and β-actin. The exact primers sequences and RT-PCR techniques employed in this study was explained previously.[11] Primers specific for IFN-γ, IL-4 and housekeeping gene β-actin were purchased from TAG Copenhagen (Denmark). It must be mentioned that β-actin gene amplification was used as a reference standard for the normalization of gene expression data. Amplification specificity of each primer set was also controlled by a melting curve and the amount of mRNA target was assessed via the comparative cycle threshold (ΔCt) method.
Statistical analysis
Independent sample t-test was used to compare two or more groups for statistical differences. Data were expressed as mean ± standard deviation. Analysis of variance was also used for identifying genes that were differentially expressed on one or more of the groups. P < 0.05 was considered statistically significant. All statistical calculations were analyzed using SPSS software (version 16, Chicago, IL, USA).
RESULTS
Quantitation of interleukin-4 gene expression in patients with Type 1 diabetes and healthy subjects
The levels of IL-4 mRNA expression was significantly augmented in patients (6.44 ± 1.27) compared to controls (9.09 ± 1.30) (P = 0.001). No significant difference was observed between men and women with T1D in the expression of IL-4 (P > 0.05), while, the mean level of IL-4 expression was higher in PBMCs of both men (P = 0.0001) and women (P = 0.0003) with T1D in comparison to normal healthy men and women.
Comparison of IL-4 ratio between male and female patients also showed that the expression levels of IL-4 mRNA in male patients were about 1.9 folds higher than female patients.
Patients with short (<10 years) and long (more than 10 years) disease duration had higher levels of IL-4 mRNA expression as compared with normal controls (P < 0.0001).
Expression profile of interferon-gamma in Type 1 diabetes patients and controls
Quantitative cytokine mRNA expression revealed significantly reduced IFN-γ mRNA levels in the PBMCs of patients (11.25 ± 1.91) in comparison with those of control (10.17 ± 1.29) (P = 0.001). There was no significant difference between men and women with T1D in the expression of IFN-γ (P > 0.05). However, comparison of IFN-γ ratio between male and female patients showed that the expression levels of IFN-γ mRNA in female patients was about 1.8 folds lower than male patients.
The mean level of IFN-γ expression was lower in PBMCs of both men and women with T1D in comparison to normal healthy men and women. Nonetheless, significant between-group differences were only observed in male participants (P = 0.05).
Patients with short (<10 years) and long (more than 10 years) disease duration had lower levels of IFN-γ mRNA expression as compared with normal controls. However, significant between-group differences were only observed in patients with long disease duration (P = 0.02).
CONCLUSION
T1D is an important endocrine disorder that causes the elimination of insulin- producing beta cells, in the pancreatic islets.[12] The exact cause of the autoimmune attack in this debilitating illness remains unknown but T-cells appear to play a pivotal role in the destruction process of the insulin-producing beta cells.
While the function of different subpopulations of CD4+ Th lymphocytes is elucidated in T1D, the potential contributions of Th1/Th2 subsets in the disease process seem to be substantial. These cells can participate in disease process through different ways. However, aberrations of their cytokines appear to play a pivotal role in the pathogenesis of T1D.[13]
Hence, in this study, cytokine gene expression for IFN-γ and IL-4, Th1 and Th2 signature cytokines, respectively were compared. Our results revealed a significant decrease in IFN-γ mRNA level of patients compared to normal subjects. In contrast, the levels of IL-4 specific mRNA showed a substantial increase in diabetic patients compared to healthy controls. These data point to a shift from a Th1 to a Th2 type of immune response and are consistent with some previous studies that find evidence for a protective role of IL-4 in T1D. The precise mechanism of IL-4-mediated protection is unknown at this time, and different pathways have been proposed to be involved. It may act through the elimination of specific memory cells against islet cell antigens and down-regulation of autoreactive responses.[14]
The results of this study, however, is not consistent with previous reports indicating that Th1 cells are involved in the pathogenesis of T1D.[15,16] These disparities may be dependent upon patient related factors such as their gender, duration of disease, and different phases of the autoimmune response that may affect some aspects of the immune system by altering the balance of Th1/Th2 cells and the cytokines they release. Therefore, in the next part of this study, the profile of Th1/Th2 - related cytokine was analyzed according to the gender and disease duration of participants [Table 2]. The present study showed no significant difference in the expression of IL-4 and IFN-γ between men and women with T1D (P > 0.05). Nonetheless, women with T1D demonstrate a significantly higher degree of IL-4 expression in peripheral blood compared to healthy females. The same results were obtained when male patients and controls were compared. Moreover, male patients had higher level of IL-4 expression than female patients (1.9 fold).
Table 2.
Comparative cycle threshold of interferon gamma and interleukin-4 in different groups of patients and controls
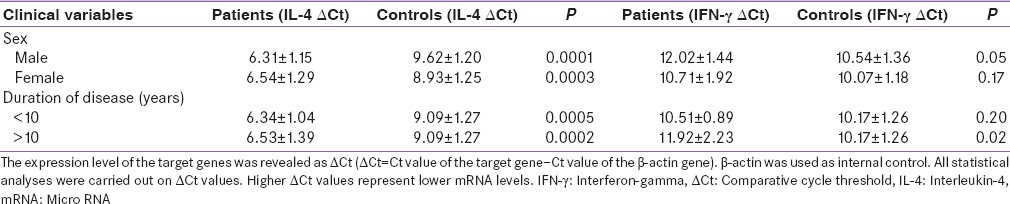
Analysis of IFN-γ gene expression also indicates that there is only significant statistical difference between male patients and male controls. In addition, the expression levels of IFN-γ mRNA in female patients were about 1.8 folds lower than male patients.
These findings concur with previous literature suggesting a relationship between gender differences and the risk of T1D. For instance Fitzpatrick et al. indicated the increased incidence of T1D in castrated nonobese diabetic (NOD) (animal model of T1D) male mice.[17] Moreover, treatment of female NOD mice with androgen delay the onset of disease.[18] Several studies have shown a marked predominance of Th1 cytokines in females patients than males.[19]
Since duration of disease may modify cytokine pattern and levels in patients with T1D, patients were categorized in two subgroups according to the times from the onset of symptoms. Participants in both patient groups had higher level of IL-4 mRNA in their PBMCs than the group of healthy subjects (P < 0.05). PBMCs of both patients group also showed a lower level of IFN-γ in comparison to controls. However, there was a statistically significant difference in the expression of IFN-γ only between patients with more than 10 years of disease duration (P = 0.02). These findings suggest a possible relationship between immune alterations and the long duration of disease. Although the exact mechanism behind this effect is not clear, it may be relevant to alteration of cytokine profiles during disease. For instance, Chatzigeorgiou et al. studied plasma levels of twenty cytokines in T1D patients and a group of normal individuals. They showed a close correlation between disease duration and the serum levels of Th1 cytokines.[20] The variation found in the expression of these cytokines may help to explain differences of beta cell destruction between young and adults patients.[21] We must also not ignore the role of other T-cell subpopulations in the outcome of the diabetogenic response. For instance, Yarde et al. showed a negative correlation between the proportion of CD28− CD8+ T-cells and disease progression.[10] The role of Th17, iNKT, regulatory T-cells, and cytokines secreted by non – T-cells must undoubtedly be considered.[22,23,24,25,26]
Overall, our results indicate a Th2-skewed immune response in adult patients with IDDM. Moreover, factors such as gender and duration of disease seem to favor T1D by contributing to alterations in cytokine levels.
Future studies with a larger sample size are required to confirm this finding.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are declared their thanks and appreciation to all patients with T1D and Gabric Diabetes information Association that helped us in conducting this research project.
REFERENCES
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Morran MP, Vonberg A, Khadra A, Pietropaolo M. Immunogenetics of type 1 diabetes mellitus. Mol Aspects Med. 2015;42:42–60. doi: 10.1016/j.mam.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galleri L, Sebastiani G, Vendrame F, Grieco FA, Spagnuolo I, Dotta F. Viral infections and diabetes. Adv Exp Med Biol. 2012;771:252–71. doi: 10.1007/978-1-4614-5441-0_20. [DOI] [PubMed] [Google Scholar]
- 4.Szablewski L. Role of immune system in type 1 diabetes mellitus pathogenesis. Int Immunopharmacol. 2014;22:182–91. doi: 10.1016/j.intimp.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Zhang Y, Gu W, He L, Sun B. Th1/Th2 cell's function in immune system. Adv Exp Med Biol. 2014;841:45–65. doi: 10.1007/978-94-017-9487-9_3. [DOI] [PubMed] [Google Scholar]
- 6.Azar ST, Tamim H, Beyhum HN, Habbal MZ, Almawi WY. Type I (insulin-dependent) diabetes is a Th1- and Th2-mediated autoimmune disease. Clin Diagn Lab Immunol. 1999;6:306–10. doi: 10.1128/cdli.6.3.306-310.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson JT, Cornelius JG, Jarpe AJ, Winter WE, Peck AB. Insulin-dependent diabetes in the NOD mouse model. II. Beta cell destruction in autoimmune diabetes is a TH2 and not a TH1 mediated event. Autoimmunity. 1993;15:113–22. doi: 10.3109/08916939309043886. [DOI] [PubMed] [Google Scholar]
- 8.Shimada A, Charlton B, Rohane P, Taylor-Edwards C, Fathman CG. Immune regulation in type 1 diabetes. J Autoimmun. 1996;9:263–9. doi: 10.1006/jaut.1996.0033. [DOI] [PubMed] [Google Scholar]
- 9.Young EF, Hess PR, Arnold LW, Tisch R, Frelinger JA. Islet lymphocyte subsets in male and female NOD mice are qualitatively similar but quantitatively distinct. Autoimmunity. 2009;42:678–91. doi: 10.3109/08916930903213993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarde DN, Lorenzo-Arteaga K, Corley KP, Cabrera M, Sarvetnick NE. CD28− CD8+ T cells are significantly reduced and correlate with disease duration in juveniles with type 1 diabetes. Hum Immunol. 2014;75:1069–74. doi: 10.1016/j.humimm.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanaki E, Ataei M, Sanati MH, Mansouri P, Mahmoudi M, Zarei F, et al. Expression patterns of Th1/Th2 transcription factors in patients with guttate psoriasis. Acta Microbiol Immunol Hung. 2013;60:163–74. doi: 10.1556/AMicr.60.2013.2.7. [DOI] [PubMed] [Google Scholar]
- 12.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778–86. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rabinovitch A, Suarez-Pinzon WL. Roles of cytokines in the pathogenesis and therapy of type 1 diabetes. Cell Biochem Biophys. 2007;48:159–63. doi: 10.1007/s12013-007-0029-2. [DOI] [PubMed] [Google Scholar]
- 14.Mueller R, Bradley LM, Krahl T, Sarvetnick N. Mechanism underlying counterregulation of autoimmune diabetes by IL-4. Immunity. 1997;7:411–8. doi: 10.1016/s1074-7613(00)80362-3. [DOI] [PubMed] [Google Scholar]
- 15.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–7. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 16.Walldén J, Honkanen J, Ilonen J, Ludvigsson J, Vaarala O. No evidence for activation of T(H) 1 or T(H) 17 pathways in unstimulated peripheral blood mononuclear cells from children with ß-cell autoimmunity or T1D. J Inflamm Res. 2008;1:11–7. doi: 10.2147/jir.s3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick F, Lepault F, Homo-Delarche F, Bach JF, Dardenne M. Influence of castration, alone or combined with thymectomy, on the development of diabetes in the nonobese diabetic mouse. Endocrinology. 1991;129:1382–90. doi: 10.1210/endo-129-3-1382. [DOI] [PubMed] [Google Scholar]
- 18.Fox HS. Androgen treatment prevents diabetes in nonobese diabetic mice. J Exp Med. 1992;175:1409–12. doi: 10.1084/jem.175.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao M, Yang Y, Jun HS, Yoon JW. Molecular mechanisms for gender differences in susceptibility to T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Immunol. 2002;168:5369–75. doi: 10.4049/jimmunol.168.10.5369. [DOI] [PubMed] [Google Scholar]
- 20.Chatzigeorgiou A, Harokopos V, Mylona-Karagianni C, Tsouvalas E, Aidinis V, Kamper EF. The pattern of inflammatory/anti-inflammatory cytokines and chemokines in type 1 diabetic patients over time. Ann Med. 2010;42:426–38. doi: 10.3109/07853890.2010.495951. [DOI] [PubMed] [Google Scholar]
- 21.Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 22.Alnek K, Kisand K, Heilman K, Peet A, Varik K, Uibo R. Increased blood levels of growth factors, proinflammatory cytokines, and Th17 cytokines in patients with newly diagnosed type 1 diabetes. PLoS One. 2015;10:e0142976. doi: 10.1371/journal.pone.0142976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker LS, von Herrath M. CD4 T cell differentiation in type 1 diabetes. Clin Exp Immunol. 2016;183:16–29. doi: 10.1111/cei.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ElEssawy B, Li XC. Type 1 diabetes and T regulatory cells. Pharmacol Res. 2015;98:22–30. doi: 10.1016/j.phrs.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Kis J, Engelmann P, Farkas K, Richman G, Eck S, Lolley J, et al. Reduced CD4+subset and Th1 bias of the human iNKT cells in Type 1 diabetes mellitus. J Leukoc Biol. 2007;81:654–62. doi: 10.1189/jlb.1106654. [DOI] [PubMed] [Google Scholar]
- 26.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]


