Abstract
Background:
Congenital hypothyroidism (CH) is most common preventable cause of mental retardation in children. Cord blood Thyroid Stimulating Hormone (CBTSH) level is an accepted screening tool for CH.
Objectives:
To study CBTSH profile in neonates born at tertiary care referral center and to analyze the influence of maternal and neonatal factors on their levels.
Design:
Cross retrospective sectional study.
Methods:
Study population included 979 neonates (males = 506 to females = 473). The CBTSH levels were estimated using electrochemiluminescence immunoassay on Cobas analyzer. Kit based cut-offs of TSH level were used for analysis. All neonates with abnormal CBSTH levels, were started on levothyroxine supplementation 10 μg/Kg/day and TSH levels were reassessed as per departmental protocol.
Results:
The mean CBTSH was 7.82 μIU/mL (Range 0.112 to 81.4, SD = 5.48). The mean CBTSH level was significantly higher in first order neonates, neonates delivered by assisted vaginal delivery and normal delivery, delivered at term or preterm, neonates with APGAR score <5 and those needing advanced resuscitation after birth. The CBTSH level >16.10 and <1.0 μIU/mL was found in 4.39 % and 1.02 % neonates respectively. The prevalence rate of CBTSH level >16.1 μIU/mL was significantly higher in neonates delivered by assisted vaginal delivery and normal delivery, term and preterm neonates, APAGR score of <5, presence of fetal distress, need for resuscitation beyond initial steps and in those with birth weight of <1.5 Kg. Three neonates were confirmed to have CH after retesting of TSH level.
Conclusions:
The CBTSH estimation is an easy, non-invasive method for screening for CH. The cutoff level of CB TSH (μIU/mL) >16.10 and <1.0 led to a recall of 5.41% of neonates which is practicable given the scenario in our Country. The mode of delivery and perinatal stress factors have a significant impact on CBTSH levels and any rise to be seen in the light of these factors. The prevalence rate of CH after recall was ~3 in 1000 live births.
Keywords: Cord blood thyroid stimulating hormone, congenital hypothyroidism, newborn screening
INTRODUCTION
Congenital hypothyroidism (CH) is the most common preventable cause of mental retardation with a global incidence of 1:2500–1:4000 live births.[1,2,3,4] Clinical diagnosis is difficult at birth, and the time of initiation of therapy is a critical determinant of outcome. Screening for early diagnosis of CH is mandatory in most of the developed countries for early diagnosis and treatment.[4]
Neonatal screening methods measure thyroid stimulating hormone (TSH) level in either cord blood (CB) sample or that obtained from heel prick (HP) sample at 3–4 days of life. CBTSH estimation has the advantages of being easy to collect, noninvasive, and low rates of follow-up loss as the results would be available before the mother leaves the hospital, enabling repeat sampling if needed at the earliest, which is critical for early institution of treatment if necessary. Various maternal and perinatal factors are known to affect the CBTSH levels.[5,6,7,8,9,10,11,12,13] Neonatal TSH level can also be used as one of the indicators for monitoring iodine sufficiency of the population.[14] A study by Seth et al. did not find statistically significant difference in mean TSH values in serum obtained from CB and HP at 4th–7th day-of-life.[15]
There are very few studies in India on screening for CH and the effects of various factors on CBTSH levels.[5,6,7,8,9,10,11] This study presents an analysis of various maternal and neonatal factors on CBTSH level, studied in neonates born at a Tertiary Care Medical College Hospital in Kerala, India.
Objectives
The objectives of the study were to analyze the profile of CBTSH levels and the effect of maternal and neonatal factors on their levels, in neonates born at MES Medical College Hospital, Perinthalmanna, a tertiary care, referral hospital in North Kerala. The factors studied were parity of mother (birth order), maternal medical and obstetric conditions, mode of delivery, indication for cesarean section, birth weight, gestational age, gender, weight, requirement of resuscitation beyond initial steps, and the Apgar score.
MATERIALS AND METHODS
Study design and subjects
This cross-sectional, retrospective study was a study conducted in Neonatology Unit of MES Medical College Hospital, Perinthalmanna, Malappuram, Kerala. Our hospital is tertiary care multi-specialty referral hospital with all specialties and super-specialty departments. All consecutive neonates delivered from January 2013 to January 2014 were included in the study. The neonates with major life-threatening malformations, those with antenatally detected central nervous system malformations and neonates whose mothers were on any known anti-thyroid drugs were excluded from the study.
Following variables were recorded and analyzed:
Neonatal factors: CBTSH level, birth weight, length at birth, birth order, date and time of delivery, gender, mode of delivery, Apgar score, MORO reflex, need for resuscitation, respiratory distress syndrome, neonatal jaundice, and hemoglobin level
Maternal factors: Maternal age, maternal medical history (diabetes mellitus, hypertension, and thyroid diseases).
CB samples of all the babies born were collected and sent for CBTSH estimation. Blood samples (2 mL) were collected in a sterile container drawn from the umbilical vein with the help of a 5cc syringe, from 15 to 20 cm length of the umbilical cord severed at the time of birth of the baby. The samples were analyzed within 4 h using electrochemiluminescence immunoassay on Cobas analyzer. Normal value of TSH in neonates as per the kit was 1.00–16.10 µIU/mL. Values >16.1 and <1.0 μIU/mL were considered abnormal. All neonates who had CBTSH values in abnormal range were advised repeat TSH assessment within 14 days of life.
The neonates with abnormal CBTSH levels (<1 or >16.1 µIU/mL) were started on supplemental levothyroxine of 10 µg/kg/day and TSH levels were recalled at 1st, 3rd, 6th, and 12th months after delivery, to confirm thyroid illness. CH was diagnosed if the values if TSH >10 µIU/mL along with T4 <6.5 µg/dl and FT4 <0.8 ng/dl.
Statistical analysis was performed using SPSS (version 17) by SPSS Inc., Illinois, USA for Windows. The quantitative variables (CBTSH level, birth weight) have been described as mean ± standard deviation (SD) and range. The birth order, mode of delivery, resuscitation (routine or advanced), gestational and maternal age categories, Apgar score categories, timing of CB sampling, and maternal comorbidities were summarized as counts and percentages. One-way ANOVA test was used to assess the effect of maternal and neonatal factors on mean CBTSH level. The Chi-square test was used to assess the trends in the prevalence of abnormal CBTSH level and its relation to neonatal and maternal factors. The multivariate regression analysis of variables was done to find out their effect on CBTSH level. A P < 0.05 was taken as statistically significant. The study was approved by Hospital Ethics Committee.
RESULTS
A total of 1012 neonates were born at MES Medical College Hospital, Perinthalmanna, India, during the study period from January 2013 to January 2014 of which 979 whose records were complete with all the necessary variables as per protocol were included in the study. The clinical profile of subjects according to neonatal and maternal factors is summarized in Table 1.
Table 1.
Profile of subjects according to neonatal factors along with mean CBTSH levels
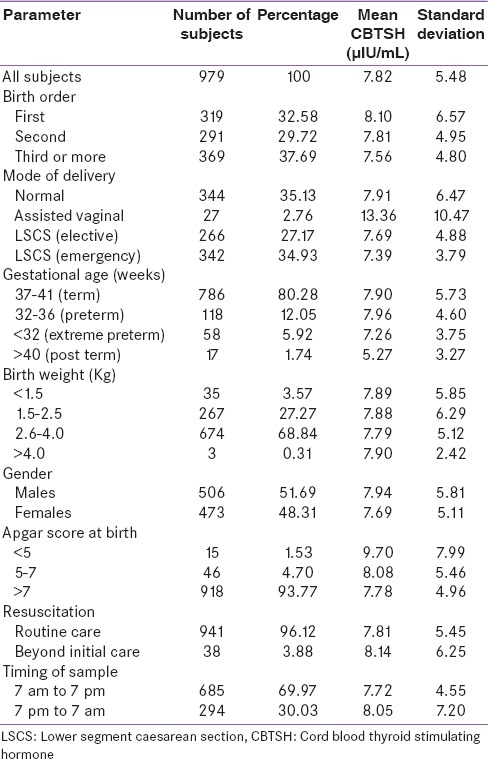
The mean value of CBTSH was 7.82 µIU/mL (range 0.112–81.4, SD = 5.48). Mean birth weight of the neonates was 2.75 kg (range 0.72–4.32 kg, SD = 0.57). The cord TSH levels from 1.00 to 16.10 µIU/mL was taken as normal. The males (506) to females (473) ratio was 1.07:1.
CBTSH >16.10 µIU/mL was found in 43 (4.39%) neonates with range of 16.4–81.40 µIU/mL (mean = 26.17). CBTSH level <1.0 was found in 1.02% (10) of neonates with range of 0.113–0.917 µIU/mL (mean = 0.57).
The cord blood thyroid stimulating hormone level and neonatal factors
The relation of neonatal factors and mean CBTSH level are shown in Table 1. The mean CBTSH (µIU/mL) was higher in neonates delivered by assisted vaginal (13.36) and normal vaginal mode (7.91) or than lower segment caesarean section (LSCS) (elective [7.69] or emergency [7.39]); the difference was that it was statistically significant (P < 0.01). The mean CBTSH was higher in neonates belonging to term (7.90) and preterm (7.96) gestational age than postterm (5.27) or extreme preterm (7.26); it was statistically significant (P = 0.01).
The mean CBTSH was higher in the first (8.10) and second (7.81) child than the third or more (7.56) birth order; it was statistically significant (P = 0.03). The mean CBTSH was higher in neonates with fetal distress or needing advanced resuscitation after birth (8.14) than neonates receiving routine care (7.81); it was statistically significant (P < 0.01). The mean CBTSH was higher in those with Apgar score of <5 (9.70) than those with score of 5–7 (8.08) and >7 (7.78); it was statistically significant (P < 0.01).
The mean CBTSH was marginally higher in those with birth weight of more than 4 kg (7.90) than those belonging to categories of 2.6–4.0 kg (7.79), <1.5 kg (7.89), and 1.5–2.5 kg (7.88), and the variation was statistically not significant (P = 0.65). The mean CBTSH was slightly higher males (7.94) than females (7.69); it was statistically not significant (P = 0.13). The mean CBTSH was slightly higher when time of birth was between 7 pm to 7 am (8.05) and 7 am to 7 pm (7.72); however, it was statistically not significant (P = 0.54).
The relation of neonatal factors and CBTSH level-based categories are shown in Table 2. CBTSH more than 16.1 and >1.0 µIU/mL was noted in 4.39% (43) and 1.02% (10) of neonates, respectively. The prevalence rate of CBTSH >16.1 µIU/mL was higher in neonates delivered by normal delivery (5.23%) and assisted vaginal delivery (22.22%) as compared to cesarean section (elective [4.51] or emergency [2.04]), and it was statistically significant (P < 0.01).
Table 2.
Effect of neonatal factors on CBTSH levels
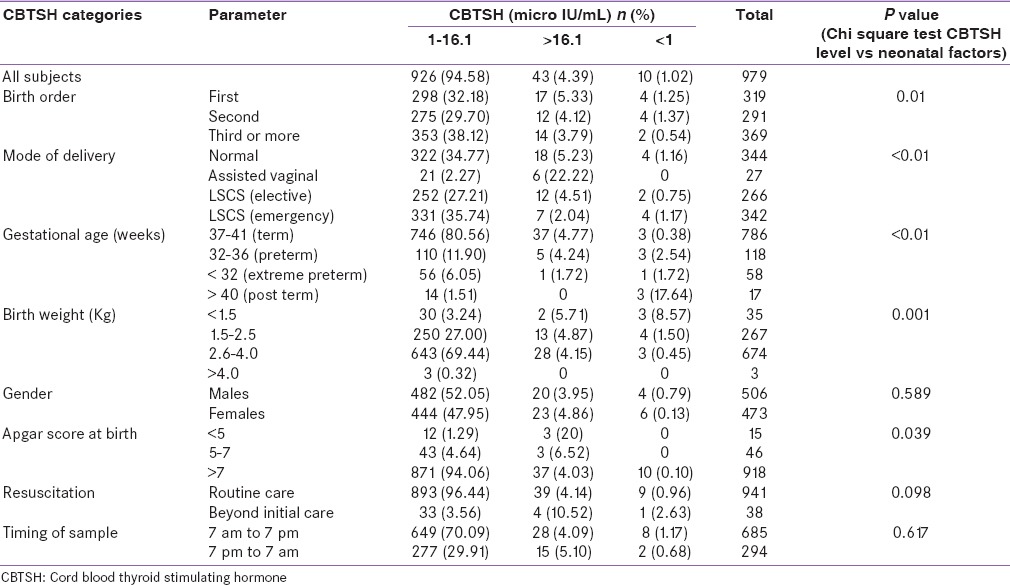
The prevalence rate CBTSH >6.1 µIU/mL was more in neonates belonging to term (4.77%) and preterm (4.24%) gestational age than extreme preterm (1.72%) or postterm (0%); it was statistically significant (P < 0.01).
The prevalence rate CBTSH >16.1 µIU/mL was higher in the first (5.33%) child than the second (4.12%) and third or more (3.79%) birth order; however, the variation was not statistically significant (P = 0.66). The prevalence rate CBTSH >16.1 µIU/mL was higher in females (4.86%) than males (3.95%); it was statistically not significant (P = 0.59).
The prevalence rate CBTSH >16.1 µIU/mL was higher in those with Apgar score of <5 (20%) than those with score of 5–7 (6.52%) and >7 (4.03%); it was statistically significant (P = 0.039). The prevalence rate CBTSH >16.1 µIU/mL was higher in neonates with fetal distress or needing advanced resuscitation after birth (10.52%) than neonates receiving routine care (4.14%); it was not statistically significant (P = 0.098).
The prevalence rate CBTSH >16.1 µIU/mL was the highest in those with birth weight of <1.5 kg (5.71%), followed by than those weighing 1.5–2.5 kg (4.87%), 2.6–4.0 kg (4.15%), and >4.0 kg (0%); variation was statistically significant (P = 0.001). The prevalence rate CBTSH >16.1 µIU/mL was slightly higher when time of birth was between 7 pm to 7 am (5.10%) and 7 am to 7 pm (4.09%); however, it was statistically not significant (P = 0.617).
The cord blood thyroid stimulating hormone level and maternal factors
The relation of maternal factors and mean CBTSH level are shown in Table 3. The mean CBTSH (µIU/mL) was slightly higher if the maternal age (years) is more than 35 (7.94) in comparison to age groups of 26–34 (7.79) and 18–25 (7.84) but was not statistically significant (P = 0.42).
Table 3.
Effect of neonatal factors on cord blood thyroid stimulating hormone levels
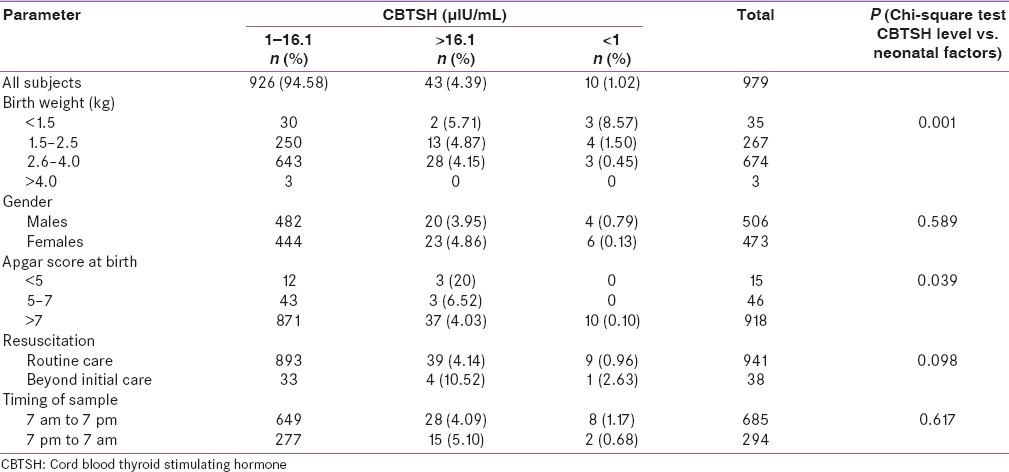
The mean CBTSH level was higher in neonates whose mothers had hypertension (8.95) followed by those with of both hypertension and diabetes mellitus (8.40), no maternal comorbidities (7.66), diabetes mellitus only (7.46) and was least in those with hypothyroidism (7.45); however, it was statistically insignificant (P = 0.56).
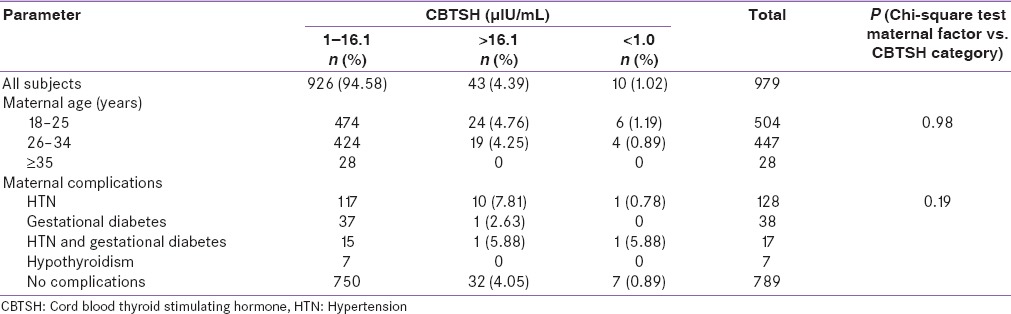
The relation of maternal factors and CBTSH level categories are shown in Table 4. The prevalence rate CBTSH >16.1 µIU/mL was slightly higher if the maternal age (years) is 18–25 (4.76%) followed by 26–34 (4.25%) and >35 (0%) but was statistically insignificant (P = 0.98). The prevalence rate CBTSH >16.1 µIU/mL was higher in neonates whose mothers had hypertension (7.81%) followed by those with of both hypertension and diabetes mellitus (5.88%), no maternal comorbidities (4.05%), diabetes mellitus only (2.63%) and was least in those with hypothyroidism (0%); however, it was statistically insignificant (P = 0.19).
Table 4.
Effect of maternal factors on cord blood thyroid stimulating hormone levels
All neonates with abnormal CBSTH levels were started on levothyroxine supplementation 10 µg/kg/day, and TSH levels were reassessed as per the protocol. Three neonates were confirmed to have CH and are on regular follow-up in the outpatient department; they are euthyroid on levothyroxine supplements. The prevalence rate of CH in the present study is 3 (1 male and 2 females) out of 979 (~3/1000).
DISCUSSION
CH is the most common preventable cause of mental retardation in children. The CBTSH estimation has the advantages of being easy to collect, noninvasive, and low rates of follow-up loss as the results would be available before the mother leaves the hospital, enabling repeat sampling if needed at the earliest, which is critical for early institution of treatment if necessary. This study presents an analysis of effect of maternal and neonatal factors on CBTSH level, studied in neonates born at MES Medical College Hospital, Perinthalmanna, Kerala, India.
In our study, the mean TSH level was 7.82 µIU/mL comparable to other studies with levels of mean TSH ranging from 6.13 to 10.[5,6,8,10] The mean CBTSH was higher than 10 µIU/mL few studies.[7,9,11]
In our study, mean CBTSH was significantly raised in neonates delivered by assisted vaginal delivery and normal delivery than those delivered by LSCS finding correlating with other studies.[10,12] Neonates born at full-term gestational age had significantly higher mean CBTSH than those born at age of pre- and post–term or extreme preterm. Neonates who had fetal distress or low Apgar score (<5) or those requiring resuscitation beyond initial steps had significantly higher CBTSH, correlating with previous studies.[10,12]
Gender had varying effect on CBTSH level significant in a few[10,12] and insignificant in other[9,11] studies. Gender had no significant effect on mean CBTSH level in the present study. The mean CBTSH level was significantly higher in the first and second child than the third or more finding similar to an earlier study.[10] Birth weight also shown to have varying effect on CBTSH level did not have significant effect in the present study similar to one of the studies;[9] CBTSH level was found to be higher in small for gestational age neonates in another study.[12] Low birth weight newborns had significantly lower TSH than normal or high birth weight in one of the studies.[8]
Maternal comorbidities, birth weight, and gestational age did not have significant effect on CBTSH level in the present study, findings correlating with previous studies.[8,10] However; in one study, CBTSH level showed an increasing trend with maternal age.[11]
Researchers have used different CBTSH cut-off levels ranging from 10 to 20 µIU/mL for recall and rescreening, to make it more cost-effective.[5,7,8,10,11] The kit cut-off values were used in the present study as there were no normative data for the local population. The previous studies also had used cut-off values based on the kit for analysis.[5,7,10,11,12]
Recall rates were varied widely based on cut-off levels of CBTSH levels and geographic location of study, 1.83–11.45%[10] if the CBTSH cut-off >20 µIU/mL.[5,7,10] The recall rate was 29.53% in one of the studies using CBTSH cut-off >13.2 µIU/mL.[11] When the CBTSH cut-off level was raised to 40.0 μIU/mL, none of the variables examined were significant.[12]
In the present study, CBTSH >16.1 and <1.0 μIU/mL were considered abnormal as per the kit. The abnormal CBTSH levels of >16.1 μIU/mL in 43 and <1.0 μIU/mL in 10 neonates were leading to a recall of 5.41% (53 out of 979). The recall rate reduces to 0.6% (~6 neonates) and 0.31% (~3 neonates) if the CBTSH cut-off is >30 and >40 μIU/mL, respectively; however, higher cut-off has risk of underestimate the risk of CH. A 5-year prospective study from Thailand used cut-off value of 30 to begin with and had a recall rate of 1.1% in a large sample size of 35,390 neonates. Modification in cut-off value to 40 lead to a fall in recall rate to 0.43%.[16]
The neonates with abnormal CBTSH were started on levothyroxine supplements, and TSH was reassessed as per the protocol at 1st, 3rd, 6th, and 12th months after delivery. CH was confirmed in 3 neonates, with prevalence rate of ~3 in 1000, during follow-up as per the protocol mentioned above. Higher cut-off values of CBTSH for recall lowers the recall rate and improves the economic and practical logistics. Our figures have shown a comparable trend as with the normative data for CBTSH values as reported by various workers.[12,15]
The prevalence rate of higher CBTSH level (>16.1 µIU/mL) was significantly more in neonates delivered by assisted vaginal delivery followed by normal delivery and LSCS. The prevalence rate of higher CBTSH was more in full-term neonates than and pre- or post-term. The prevalence rate of higher CBTSH level was significantly more in those with birth weight of <1.5 kg or Apgar score of <5 or with fetal distress or needing advanced resuscitation after birth. The birth order/parity, gender, time of birth, maternal age, and maternal comorbidities did not have any significant effect on prevalence rate of CBTSH >16.1 µIU/mL.
On multivariate regression analysis, mode of delivery, gestational age, and Apgar score had significant effect on prevalence rate of higher CBTSH (>16.1 µIU/mL). Gender, time of CB sampling/birth time, birth weight, parity, maternal age, and maternal comorbidities did not have significant effect on prevalence of higher CBTSH level.
CH was confirmed in 3 neonates (females: 2, males: 1) during the follow-up. The prevalence rate of CH in the present study was ~ 3 in 1000 neonates, with female to male ratio of 2:1. The previous studies from India have reported varying rates prevalence of CH 3:430, 1:476, 1:1700, 1:2481, and 1:2804.[11,17,18,19,20]
Limitations
Our study is based on single-center experience; hence, we need large population-based or multicenter studies in future to estimate the prevalence of CH in India. The present study used kit based cut-off for CBTSH, we also need multicenter national data using uniform analysis kits find out the normative range for the population. We need national protocol based on consensus for screening and treatment of CH, which is nonexistent at present.
CONCLUSIONS
The CBTSH estimation has the advantages of being easy to collect, noninvasive method for screening for CH. The cut-off level of CBTSH (µIU/mL) >16.10 and <1.0 led to a recall of 5.41% which is practicable give the scenario in our country. Higher cut-off values can further reduce the recall rates and needs to be studied.
The assisted vaginal or normal mode of delivery, presence of fetal distress or low Apgar score (<5) or requirement resuscitation beyond initial steps had significant effect on CBTSH level. Gender, parity and birth weight, maternal age, and maternal comorbidities did not have significant effect on CBTSH level.
We therefore conclude that perinatal stress factors and mode of delivery have a significant impact on CBTSH levels, and any rise in CBTSH should be seen in the light of these factors and those with higher CBTSH needs to be recalled for retesting at later date. CH was confirmed in 3 neonates with prevalence rate of ~3 in 1000 live births.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Desai MP. Congenital hypothyroidism: Screening dilemma. Indian J Endocrinol Metab. 2012;16(Suppl 2):S153–5. doi: 10.4103/2230-8210.104027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sareen N, Pradhan R. Need for neonatal screening program in India: A national priority. Indian J Endocrinol Metab. 2015;19:204–20. doi: 10.4103/2230-8210.149315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal P, Philip R, Saran S, Gutch M, Razi MS, Agroiya P, et al. Congenital hypothyroidism. Indian J Endocrinol Metab. 2015;19:221–7. doi: 10.4103/2230-8210.131748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics. Rose SR, et al. Section on Endocrinology and Committee on Genetics, American Thyroid Association, Brown RS; Public Health Committee, Lawson Wilkins Pediatric Endocrine Society, Foley T, et al. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–303. doi: 10.1542/peds.2006-0915. [DOI] [PubMed] [Google Scholar]
- 5.Manglik AK, Chatterjee N, Ghosh G. Umbilical cord blood TSH levels in term neonates: A screening tool for congenital hypothyroidism. Indian Pediatr. 2005;42:1029–32. [PubMed] [Google Scholar]
- 6.Meghana KP, Kamble BD, Suryawanshi PB, Melinkeri RR. Umbilical cord TSH levels in term small for gestational age neonates. Indian J Res. 2013;2:294–5. [Google Scholar]
- 7.Sangeeta N, Kamala L, Paras K, Gomi B, Ajitkumar Y, Ranbir SL, et al. Assessment of umbilical cord TSH in term neonates in Manipur. IOSR J Dent Med Sci. 2013;9:14–7. [Google Scholar]
- 8.Chandrika DN, Madhava K, Dinesh MN, Nagesha KM. Status of pituitary-thyroid axis of newborns and its relationship with anthropometry and maternal factors at birth. Int J Pharma Bio Sci. 2012;3:B51–7. [Google Scholar]
- 9.Rashmi, Seth A, Sekhri T, Agarwal A. Effect of perinatal factors on cord blood thyroid stimulating hormone levels. J Pediatr Endocrinol Metab. 2007;20:59–64. doi: 10.1515/jpem.2007.20.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Srivastava S, Bhatnagar A. Cord blood thyroid stimulating hormone level – Interpretation in light of perinatal factors. Indian Pediatr. 2014;51:32–6. doi: 10.1007/s13312-014-0330-2. [DOI] [PubMed] [Google Scholar]
- 11.Raj S, Baburaj S, George J, Abraham B, Singh S. Cord blood TSH level variations in newborn – Experience from a rural centre in Southern India. J Clin Diagn Res. 2014;8:PC18–20. doi: 10.7860/JCDR/2014/9058.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan LY, Leung TN, Lau TK. Influences of perinatal factors on cord blood thyroid-stimulating hormone level. Acta Obstet Gynecol Scand. 2001;80:1014–8. doi: 10.1034/j.1600-0412.2001.801108.x. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto N, Tsuji M, Imataki T, Nagamachi N, Hirose S, Hamada Y. Influence of mode of delivery on fetal pituitary-thyroid axis. Acta Paediatr Jpn. 1991;33:363–8. doi: 10.1111/j.1442-200x.1991.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Eastman CJ. Neonatal TSH screening: Is it a sensitive and reliable tool for monitoring iodine status in populations? Best Pract Res Clin Endocrinol Metab. 2010;24:63–75. doi: 10.1016/j.beem.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Seth A, Rashmi M, Bhakhri BK, Sekri T. Neonatal thyroid screening: Relationship between cord blood thyroid stimulating hormone levels and thyroid stimulating hormone in heel prick sample on 4th to 7th day-of-life. Indian J Endocrinol Metab. 2014;18:125–6. doi: 10.4103/2230-8210.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahachoklertwattana P, Phuapradit W, Siripoonya P, Charoenpol O, Thuvasethakul P, Rajatanavin R. Five-year thyrotropin screening for congenital hypothyroidism in Ramathibodi Hospital. J Med Assoc Thai. 1999;82(Suppl 1):S27–32. [PubMed] [Google Scholar]
- 17.Rama Devi AR, Naushad SM. Newborn screening in India. Indian J Pediatr. 2004;71:157–60. doi: 10.1007/BF02723099. [DOI] [PubMed] [Google Scholar]
- 18.Desai MP, Colaco MP, Ajgaonkar AR, Mahadik CV, Vas FE, Rege C, et al. Neonatal screening for congenital hypothyroidism in a developing country: Problems and strategies. Indian J Pediatr. 1987;54:571–81. doi: 10.1007/BF02749056. [DOI] [PubMed] [Google Scholar]
- 19.Desai MP, Upadhye P, Colaco MP, Mehre M, Naik SP, Vaz FE, et al. Neonatal screening for congenital hypothyroidism using the filter paper thyroxine technique. Indian J Med Res. 1994;100:36–42. [PubMed] [Google Scholar]
- 20.Mathew J. Burden of thyroid diseases in India. Need for aggressive diagnosis. Med Update. 2008;18:334–41. [Google Scholar]


