Abstract
Introduction:
Pregnant women represent a typical group susceptible to dietary and mineral deficiencies. This study was sought to assess the efficacy and safety of various doses of 25-hydroxyvitamin D (25[OH]D) supplementation during pregnancy and ratify the inadequacy of the recommended daily allowance for Vitamin D in vulnerable groups.
Materials and Methods:
A total of 100 pregnant women were included in this open-label, parallel group, prospective, randomized, and controlled trial. Study subjects were assigned to four treatment groups: Group 1 (n = 26), 1000 IU of Vitamin D daily; Group 2 (n = 21), 30,000 IU of Vitamin D monthly; Group 3 (n = 27), 2000 IU of Vitamin D daily; and Group 4 (n = 26), 60,000 IU Vitamin D monthly. Group 1 and 2 were further analyzed together as Group 1K (1000 IU daily and 30,000 IU monthly), and Group 3 and 4 as Group 2K (2000 IU daily and 60,000 IU monthly). The analysis was done on an intention to treat basis.
Results:
A total of 87 patients completed the study; 21 in Group 1, 25 in Group 2, 18 in Group 3, and 23 in Group 4. The levels of 25(OH)D at baseline ranged from 1.3 to 58.0 with a mean of 24.2 ± 15.1 ng/ml. Postsupplementation, 25(OH)D levels ranged from 11.5 to 70.3 with a mean of 40.2 ± 12.2 ng/ml. The postsupplementation levels of 25(OH)D were higher in Group 2K (42.86 ± 12.83) than in Group 1K (36.96 ± 10.56) with P value of 0.023.
Conclusion:
We concluded that Vitamin D supplementation with 2000 IU/day or 60,000 IU/month is very effective and safe in achieving Vitamin D sufficiency in pregnant women.
Keywords: Dose, efficacy, pregnancy, Vitamin D
INTRODUCTION
Global high prevalence of Vitamin D insufficiency and growing scientific evidence linking low circulating 25-hydroxyvitamin D (25[OH]D) to increased risk of osteoporosis, diabetes, cancer, and autoimmune disorders have simulated major research work in this field.[1] Studies throughout the world have confirmed that optimal Vitamin D supply, not only influences the course of pregnancy but is also required for fetal and neonatal calcium homeostasis, bone maturation and mineralization. Breastfed infants born to Vitamin D deficient mothers are at risk of developing Vitamin D deficiency and its metabolic sequels.[2] Despite abundant sunlight, majority of apparently healthy individuals in Kashmir valley in the Northern Indian State of Jammu and Kashmir are Vitamin D deficient. Pregnant Kashmiri women being a vulnerable group due to limited exposure to sunlight because of peculiar cultural issues are even more Vitamin D deficient.[3] Because pregnancy and birth provoke important modifications of mineral homeostasis, the newborn must adapt rapidly to ensure the positive calcium balance necessary for normal skeletal growth and development. Maternal 25(OH)D is believed to cross the placenta, and the fetus is entirely dependent on the mother for its supply. The fetus is affected by the Vitamin D deficiency during pregnancy resulting in decreased birth weight of newborn, impaired bone mineralization and frequent neonatal hypocalcemia.[4] The need, safety and effectiveness of Vitamin D supplementation during pregnancy is contentious as there is limited data available on the subject, especially from the Indian subcontinent. Whereas, there is a clear need of Vitamin D supplementation in Kashmiri pregnant women, there is no data on safety and effectiveness of Vitamin D supplementation during pregnancy in our population. Therefore, the present study was undertaken to study the safety and effectiveness of Vitamin D supplementation during pregnancy in our population.
MATERIALS AND METHODS
This study was an open-label, parallel group, prospective, and randomized controlled trial. The trial was registered with National trial registry India (No. 005967). The study was conducted according to the guidelines in the World Medical Association (2000) declaration of Helsinki: Ethical principles for medical research involving human subjects, with notes of clarification of 2002 and 2004 and all procedures involving human subjects/patients were approved by the Ethical Committee of the Institute. A written informed consent was obtained from all subjects participating in the study. Subjects were mainly recruited from the out-patient department (OPD) of Obstetrics and Gynaecology, SKIMS, during summer of 2013, and some subjects from maternity hospital (Lal Ded, Srinagar) were also included.
Sample size calculation and enrollment of subjects
An online calculator provided by the Massachusetts General Hospital Mallinckrodt General Clinical Research Center was used to calculate sample size. For 90% power of study and 5% margin of error (α = 0.05), a minimum of 22 patients per group were required to detect a statistically significant increase in 25(OH)D by 10 ng/ml between two groups. This calculation assumed that standard deviation of 25(OH)D measurements at a single time point was approximately 10 and that there would be a low correlation between the baseline and final measurements. Finally, 100 subjects were enrolled for the study, considering that a substantial proportion of participants may be lost during follow-up because of either withdrawal from participation or termination of care. Enrollment was done from the first trimester to starting of the second trimester, depending upon when the pregnancy was confirmed.
Initial study visits
Completion of questionnaires
At the first visit, a detailed proforma was recorded which included subject's sociodemographic profile, medical history, obstetric history, exposure to sunlight, and dietary intake.
Anthropometric measurements
Prepregnancy height and weight were recorded at first OPD visit. During subsequent visits, only the subject's weight was recorded. Birth weight was recorded for each infant.
Baseline blood sample and clinical parameters
A baseline whole blood sample was taken during and up to 16 weeks of gestation after an overnight fasting in a clot activator vial followed by subsequent separation of serum. Baseline investigations that included complete blood count, kidney function tests, liver function tests, blood glucose, serum calcium, and other relevant biochemical parameters were done on an autoanalyzer. One aliquot of serum sample was used for estimation of baseline 25(OH)D.
Vitamin D supplementation
The schematic flowchart showing recruitment and follow-up of study subjects is shown in Figure 1. After the initial workup, the subjects were randomly assigned to four treatment groups: (i) Group 1 (n = 26), 1000 IU of Vitamin D daily; (ii) Group 2 (n = 21), 30,000 IU of Vitamin D monthly (iii) Group 3 (n = 27), 2000 IU of Vitamin D daily and (iv) Group 4 (n = 26), 60,000 IU Vitamin D monthly. The subjects were also analyzed as Group 1K and Group 2K irrespective of the daily or monthly supplementation regime. Group 1K included both 1000 IU daily, and 30,000 IU monthly subjects whereas Group 2K included 2000 IU daily and 60,000 IU monthly subjects.
Figure 1.
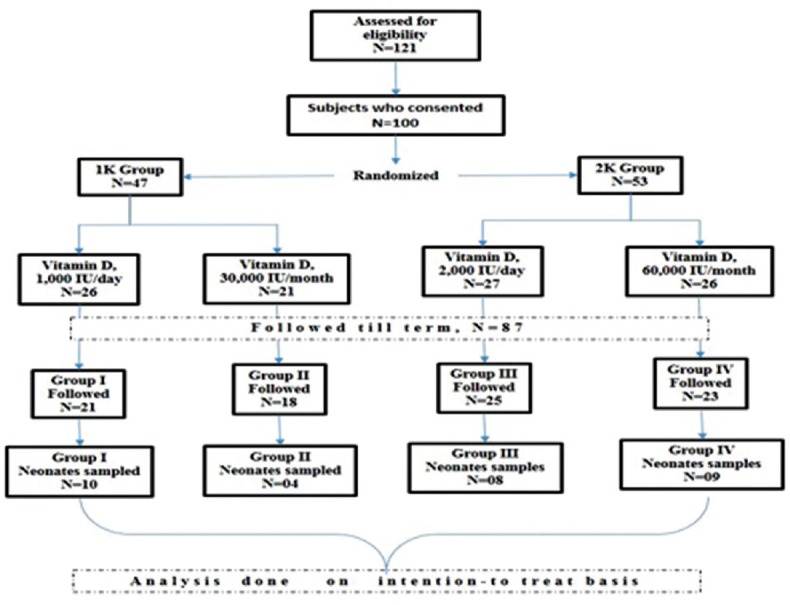
Flowchart showing recruitment and follow-up of study subjects
Vitamin D supplementation was started after 16 weeks; it was ensured that none of the subjects received Vitamin D before the 16th week of pregnancy. All Vitamin D supplementation was given in the form of tablets; the dose depended upon the group to which the subject was consigned.
Subsequent study visits
Subjects were followed monthly either personally or through telephonic calls till delivery, coinciding with their routine obstetrical visits with an obstetrician. Subsequent blood samples of the study subjects were collected at the end of the second trimester and at term for assessing Vitamin D. A sample of fetal cord blood was also collected at the time of delivery for assessing fetal 25(OH)D levels.
Adherence to medication regimen
Adherence to the prescribed Vitamin D supplementation regimen was assessed by maternal self-report and pill count that was provided on each visit. The monthly pill was taken in front of doctor during her routine visit.
Clinical measures
Major clinical measures included pregnancy health status, labor and delivery characteristics, and postnatal complications.
Laboratory measures
Chief laboratory measures included maternal serum Vitamin D levels (postsupplementation) and neonatal Vitamin D levels. Levels were measured by a radioimmunoassay (RIA) technique using the commercially available kit as per the manufacturers’ instructions. The DiaSorin 25(OH)D assay consists of a two-step procedure. The first procedure involves a rapid extraction of 25(OH) D and other hydroxylated metabolites from serum or plasma with acetonitrile. Following extraction, the treated sample is then assayed using an equilibrium RIA procedure.
Statistical analysis
Statistical analyses were performed with Statistical Package for Social Sciences (SPSS), version 11.0, for Windows software (SPSS Inc.). Results are reported as means ± standard deviations unless stated otherwise. Categorical variables were compared by group using the Chi-square test whereas differences between groups on continuous variables were assessed by using the independent-samples t-test and where the data were normally distributed, Mann–Whitney U-test was used. Effects of Vitamin D supplement on 25(OH)D levels in the study subjects and the neonates were examined without adjustment for potential confounders by using repeated-measures analysis of variance (ANOVA). Associations between variables were evaluated by using Pearson's correlation analysis. Values at P ≤ 0.05 were considered statistically significant.
RESULTS
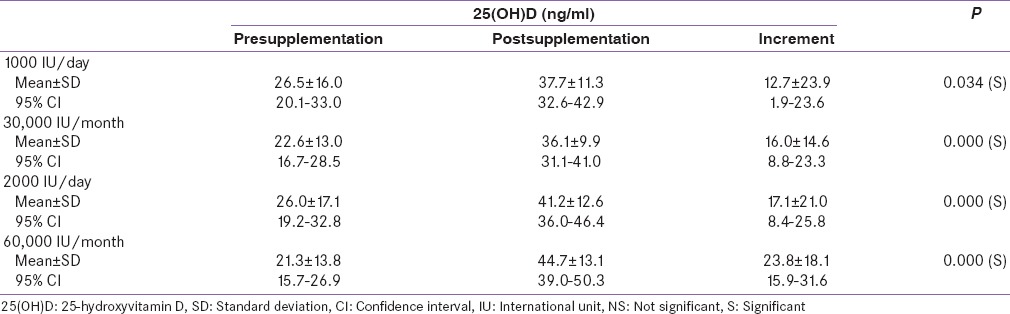
A total of 121 women were interviewed and 100 consenting women who fulfilled the inclusion criteria were recruited for the study. These 100 study subjects were randomly assigned into four treatment groups: (i) Group 1 (n = 26), 1000 IU of Vitamin D daily, (ii) Group 2 (n = 21), 30,000 IU of Vitamin D monthly, (iii) Group 3 (n = 27), 2000 IU of Vitamin D daily, and (iv) Group 4 (n = 26), 60,000 IU Vitamin D monthly. Mean age for cases was 27.7 years ranging from 20 to 30 years. Complete age distribution and demographic characteristics are shown in Table 1. Majority of our subjects were unemployed homemakers with limited exposure to sunlight. Exposure to sunlight was observed in cases belonging to urban dwelling though the comparison was nonsignificant. It is important to mention here that most of the recruitment was done in the summer months of May–July. The mean 25(OH)D level at baseline was 24.2 ± 15.1 ng/ml. Only 33% women were Vitamin D sufficient if a criteria of 25(OH)D of 30 ng/ml or more is followed, but 56% were Vitamin D sufficient with criteria of 25(OH)D of 20 ng/ml or more. Postsupplementation, Vitamin D levels increased to 40.2 ± 12.2 ng/ml. There was a quite variable effect on circulating levels of Vitamin D, postsupplementation as shown in Table 2. Postsupplementation, the overall status of vitamin sufficiency was better in Group 3 (2000 IU/day) irrespective of the regimen used monthly or daily. Vitamin D level significantly increased in all four groups with mean increment of 12.7 ± 23.9 ng/ml with 1000 IU/day, 17.1 ± 21.0 ng/ml with 2000 IU/day, 16.0 ± 14.6 ng/ml with 30,000 IU monthly, and 23.8 ± 18.1 ng/ml with 60,000 IU monthly. Though there was a significant increase in 25(OH)D levels in all the treatment groups, compared to baseline, there was no significant difference in the postsupplementation 25(OH)D levels in between the four groups by ANOVA.
Table 1.
Baseline and demographic characteristics of study subjects
Table 2.
Vitamin D levels of the study population pre- and post-supplementation, and the increment in levels with respect to supplementation regimen
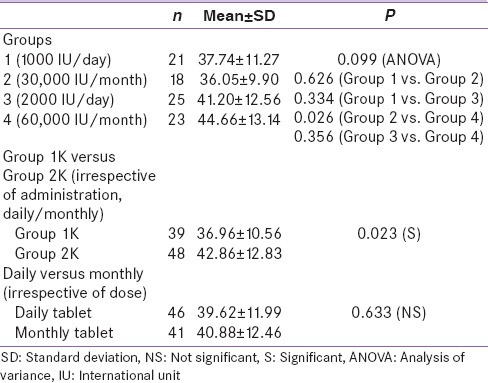
On analysis, no statistical difference was observed in the postsupplementation 25(OH)D levels between the groups [Table 3] except between Group 2 and 4. The comparison showed that Group 4 (60,000 IU/month) subjects had significantly higher levels than those of Group 2 (30,000 IU/month) subjects. One of the primary objectives of the study was to find the difference in the postsupplementation levels of 25(OH)D between Group 1K and 2K regimen. As shown in Table 3, there was a significant difference (P = 0.023) between these two groups with Group 2K showing higher levels. With respect to the safety of Vitamin D supplementation, overall there were few adverse outcomes in the study population; however the difference in pregnancy outcomes between various study groups did not reach statistical significance. Pregnancy-induced hypertension (PIH) and 25(OH)D levels in the mother showed no significant association between baseline Vitamin D or postsupplementation levels at term, yet subjects with PIH had statistically low Vitamin D in newborns. There was no statistically significant difference in maternal and neonatal levels of 25(OH)D between subjects with or without other adverse outcomes such as gestational diabetes mellitus, postpartum hemorrhage, rate of lower segment cesarean section.
Table 3.
Comparison of postsupplementation 25-hydroxyvitamin D levels in relation to study group, dose, and mode of administration
DISCUSSION
The present study was carried out in a Tertiary Care Hospital in the Northern Indian State of Jammu and Kashmir to assess the effectiveness and safety of various supplementation doses of Vitamin D in correcting Vitamin D deficiency and its effect on pregnancy outcome. Considering 25(OH)D level of 30 ng/ml or more as criteria of Vitamin D sufficiency, we found about two-third of pregnant Kashmiri women Vitamin D insufficient or deficient. This is not surprising as Vitamin D deficiency is very common here. Previously we have reported the prevalence of vitamin deficiency in as many as 80% of healthy Kashmiri individuals,[3] and 82% of pregnant Kashmiri women (unpublished data). Many studies among pregnant women from south and north India have reported high Vitamin D deficiency levels ranging from 67% to 96%. Our entire cohort was recruited from summer 2013, and this could be responsible for the slightly lower percentage of Vitamin D deficiency in our study. The study by Sahu et al. showed a significant effect of season on Vitamin D deficiency, recording 54% in the period from May to October and in 93% from November to April.[5] Marwaha et al. also recorded significantly lower values of 25(OH)D in winter as compared to summer.[6]
There was a quite variable effect on circulating levels of Vitamin D, postsupplementation. Vitamin D level significantly increased in all four groups with mean increment of 12.7 ± 23.9 ng/ml with 1000 IU/day, 17.1 ± 21.0 ng/ml with 2000 IU/day, 16.0 ± 14.6 ng/ml with 30,000 IU monthly, and 23.8 ± 18.1 ng/ml with 60,000 IU monthly. Whereas there was a significant increase in 25(OH)D levels in all the treatment groups, compared to baseline, there was no significant difference in the postsupplementation 25(OH)D levels in between the four groups by ANOVA. On comparing the dose of 1K versus 2K irrespective of regimen used (daily or monthly), a significant difference (P = 0.023) was observed although there was no statistical association in the postsupplementation 25(OH)D levels between Group 1 (1000 IU/day) and Group 3 (2000 IU/day). The significant association between 1K and 2K regimen can be attributed to supplementation availability.
Our study demonstrates that daily or monthly doses are equally effective in correcting Vitamin D level at term. This improvement in Vitamin D status was achieved without any evidence of hypervitaminosis D or an increase in adverse events during pregnancy and with optimization of 25(OH) D. Similar to our analysis Hollis et al. found a dose of 2000 IU Vitamin D3 corrected around two-third of patients, and Vitamin D raised from 34 to 46 ng/ml.[7] We found minor differences between pregnant participants receiving the daily or monthly bolus dose. Although monthly bolus dose proved to be better than daily dose, the difference was insignificant. Based on the present analysis, we could conclude slightly better 25(OH)D response to a monthly dose of Vitamin D than daily dose which may be related to better compliance. Delvin et al. reported that a daily dose of 1000 IU Vitamin D3 administered to 15 French women during the third trimester modestly raised mean maternal serum (25[OH]D) from 55 nmol/l to 65 nmol/l.[8] A unique aspect of our study was the comparing daily dose with monthly equivalent dose, and our data showed a statistically insignificant association between daily dose or their monthly equivalent nevertheless monthly dose proved to be better than the daily dose at the end of the term. Although the study may have been too small to detect minor inter-dose fluctuations in (25[OH]D), the data supported the appropriateness of administering monthly doses of 30,000 IU or 60,000 IU instead of daily administration 1000 IU or 2000 IU.
One recent study on Vitamin D supplementation in pregnancy from India reported a high prevalence of Vitamin D deficiency, used intermittently very large doses of 120,000 IU Vitamin D in the fifth and seventh months.[9] Other recent study by Dawodu et al. supplemented pregnant women with 2000 IU and 4000 IU of Vitamin D in the second and third trimester of pregnancy.[10] Mean serum 25(OH)D concentrations at delivery and in cord blood were significantly higher in 2000 and 4000 IU than in 400 IU/day group (P < 0.001) and was highest in the 4000 IU/day group. Kalra et al. in the study of Vitamin D (D3) supplementation during pregnancy randomized subjects in the second trimester were divided into three groups.[11] Group 1 received one oral dose of 1500 μg Vitamin D3; Group 2 received doses of 3000 μg Vitamin D3 each in the second and third trimesters. Median maternal 25(OH) D at term was higher in Group 2 - 23.48 ng/ml than in Group 1-10.48 ng/ml.[11] Only 34% of the subjects in the study Sahu et al., compared with 80.5% of subjects on 2000 IU/day in our study achieved serum 25(OH)D of >30 ng/ml at delivery.[12]
Recent observational studies indicated that serum 25(OH)D concentrations >30 ng/ml (75 nmol/l) is associated with a reduced risk of nonskeletal health disorders, such as preeclampsia[13] and gestational diabetes.[14] Furthermore, serum 25(OH)D >30 ng/mL in the cord blood has been associated with an improved newborn innate immune response.[15] We found no differences in birth weight and gestational diabetes, PIH between 1000 IU and 2000 IU groups that may be related to small sample size of our cohort.
CONCLUSION
Starting at 16 weeks of gestation, Vitamin D supplementation of 2000 IU daily or the monthly equivalent dose is more effective in achieving higher Vitamin D levels throughout pregnancy without increased risk of toxicity in the mother or the neonate. Our findings suggest that a daily 2000 IU or monthly 60,000 IU supplementation of Vitamin D started from the second trimester onward is effective and safe in achieving ideal Vitamin D levels throughout pregnancy.
Financial support and sponsorship
The study was funded by the research grant provided by Sher-i-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India. Vitamin D supplementation was provided free of cost to subjects by M/S Eris Life sciences and Myer pharmaceuticals.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledged the voluntary participation and support of all the study subjects, their babies, and their families.
REFERENCES
- 1.Wagner CL, Taylor SN, Dawodu A, Johnson DD, Hollis BW. Vitamin D and its role during pregnancy in attaining optimal health of mother and fetus. Nutrients. 2012;4:208–30. doi: 10.3390/nu4030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaludjerovic J, Vieth R. Relationship between Vitamin D during perinatal development and health. J Midwifery Womens Health. 2010;55:550–60. doi: 10.1016/j.jmwh.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Zargar AH, Ahmad S, Masoodi SR, Wani AI, Bashir MI, Laway BA, et al. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad Med J. 2007;83:713–6. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salle BL, Delvin E, Glorieux F. Vitamin D and pregnancy. Bull Acad Natl Med. 2002;186:369–76. [PubMed] [Google Scholar]
- 5.Sahu M, Bhatia V, Aggarwal A, Rawat V, Saxena P, Pandey A, et al. Vitamin D deficiency in rural girls and pregnant women despite abundant sunshine in Northern India. Clin Endocrinol (Oxf) 2009;70:680–4. doi: 10.1111/j.1365-2265.2008.03360.x. [DOI] [PubMed] [Google Scholar]
- 6.Marwaha RK, Tandon N, Chopra S, Agarwal N, Garg MK, Sharma B, et al. Vitamin D status in pregnant Indian women across trimesters and different seasons and its correlation with neonatal serum 25-hydroxyvitamin D levels. Br J Nutr. 2011;106:1383–9. doi: 10.1017/S000711451100170X. [DOI] [PubMed] [Google Scholar]
- 7.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26:2341–57. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delvin EE, Salle BL, Glorieux FH, Adeleine P, David LS. Vitamin D supplementation during pregnancy: Effect on neonatal calcium homeostasis. J Pediatr. 1986;109:328–34. doi: 10.1016/s0022-3476(86)80396-1. [DOI] [PubMed] [Google Scholar]
- 9.Madelenat P, Bastian H, Menn S. Winter supplementation in the 3rd trimester of pregnancy by a dose of 80,000 IU of Vitamin D. Obstet Gynecol. 2011;118:197–8. [PubMed] [Google Scholar]
- 10.Dawodu A, Saadi HF, Bekdache G, Javed Y, Altaye M, Hollis BW. Randomized controlled trial (RCT) of Vitamin D supplementation in pregnancy in a population with endemic Vitamin D deficiency. J Clin Endocrinol Metab. 2013;98:2337–46. doi: 10.1210/jc.2013-1154. [DOI] [PubMed] [Google Scholar]
- 11.Kalra P, Das V, Agarwal A, Kumar M, Ramesh V, Bhatia E, et al. Effect of Vitamin D supplementation during pregnancy on neonatal mineral homeostasis and anthropometry of the newborn and infant. Br J Nutr. 2012;108:1052–8. doi: 10.1017/S0007114511006246. [DOI] [PubMed] [Google Scholar]
- 12.Sahu M, Das V, Aggarwal A, Rawat V, Saxena P, Bhatia V. Vitamin D replacement in pregnant women in rural north India: A pilot study. Eur J Clin Nutr. 2009;63:1157–9. doi: 10.1038/ejcn.2009.27. [DOI] [PubMed] [Google Scholar]
- 13.Baker AM, Haeri S, Camargo CA, Jr, Espinola JA, Stuebe AM. A nested case-control study of midgestation Vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metab. 2010;95:5105–9. doi: 10.1210/jc.2010-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Qiu C, Hu FB, David RM, van Dam RM, Bralley A, et al. Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus. PLoS One. 2008;3:e3753. doi: 10.1371/journal.pone.0003753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker VP, Zhang X, Rastegar I, Liu PT, Hollis BW, Adams JS, et al. Cord blood Vitamin D status impacts innate immune responses. J Clin Endocrinol Metab. 2011;96:1835–43. doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]


