Abstract
Background:
This post hoc analysis evaluated the efficacy and safety of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus (T2DM) from India.
Methods:
Changes from baseline in HbA1c, fasting plasma glucose (FPG), body weight, and blood pressure (BP) with canagliflozin 100 and 300 mg were evaluated in a subgroup of patients from India (n = 124) from 4 randomized, double-blind, placebo- and active-controlled, Phase 3 studies (N = 2313; Population 1). Safety was assessed based on adverse event (AE) reports in these patients and in a broader subgroup of patients from India (n = 1038) from 8 randomized, double-blind, placebo- and active-controlled, Phase 3 studies (N = 9439; Population 2).
Results:
Reductions in HbA1c with canagliflozin 100 and 300 mg were −0.74% and −0.88%, respectively, in patients from India, and −0.81% and −1.00%, respectively, in the 4 pooled Phase 3 studies. In the Indian subgroup, both canagliflozin doses provided reductions in FPG, body weight, and BP that were consistent with findings in the overall population. The incidence of overall AEs in patients from India was generally similar with canagliflozin 100 and 300 mg and noncanagliflozin. The AE profile in patients from India was generally similar to the overall population, with higher rates of genital mycotic infections and osmotic diuresis–related and volume depletion–related AEs with canagliflozin versus noncanagliflozin.
Conclusion:
Canagliflozin provided glycemic control, body weight reduction, and was generally well tolerated in patients with T2DM from India.
Keywords: Fasting blood glucose, HbA1c, hyperglycemia, oral medications, type 2 diabetes
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is increasing globally, particularly in developing countries.[1,2] In India, it is estimated that over 65 million people have T2DM, making it the country with the second highest number of cases, behind only China.[2,3] It is projected that nearly 110 million people in India will have T2DM by 2035.[2] Thus, T2DM poses a significant economic and health care burden in India.
Many Indian patients have been shown to exhibit clinical and biochemical characteristics that predispose them to T2DM, including increased insulin resistance and abdominal obesity despite having lower body weight and body mass index (BMI).[4,5,6,7] These characteristics are collectively referred to as the “Asian Indian Phenotype” and, along with lifestyle changes resulting from increased urbanization, contribute to the rising rates of T2DM in India.[4,5,7] Patients with T2DM in India generally develop the disease at a younger age than those in other parts of the world (i.e., 45–64 years vs. ≥65 years in developed countries).[6,7] The younger age of onset of T2DM increases the chances that patients will develop microvascular and macrovascular complications and comorbidities.[4,5,6]
Canagliflozin is a sodium glucose co-transporter 2 (SGLT2) inhibitor developed for the treatment of adults with T2DM.[8,9,10,11,12,13,14,15,16,17,18,19,20] Canagliflozin lowers plasma glucose via an insulin-independent mechanism by lowering the renal threshold for glucose and promoting urinary glucose excretion (~80–120 g/day), which leads to a mild osmotic diuresis and net caloric loss.[8,21,22,23,24] Across Phase 3 studies, canagliflozin improved glycemic control and reduced body weight and blood pressure (BP) and was generally well tolerated in a broad range of patients with T2DM inadequately controlled by their current treatment regimens.[9,10,11,12,13,14,15,16,17,18,19,20] In a preliminary post hoc analysis, canagliflozin was shown to reduce HbA1c, body weight, and BP in patients with T2DM from India using pooled data from 6 placebo-controlled studies, including the CANagliflozin cardioVascular Assessment Study (CANVAS) add-on to insulin and add-on to sulfonylurea substudies.[25] An additional post hoc analysis was performed excluding the CANVAS substudies, which had a different design and patient population compared with the other studies, such as a shorter duration (18 weeks vs. 26 weeks) and a population of patients with T2DM who had a history or high risk of cardiovascular (CV) disease. This manuscript describes the efficacy findings from this analysis in subgroups of patients with T2DM from India based on pooled data from 4 placebo-controlled studies, as well as an assessment of the safety of canagliflozin based on pooled data from a broader population of patients with T2DM.
METHODS
Study design, patient populations, and treatments
This post hoc analysis for efficacy was based on pooled data from patients with T2DM (N = 2313; Population 1) enrolled in four 52-week, double-blind, placebo- and active-controlled, Phase 3 studies, including canagliflozin as monotherapy,[13] add-on to metformin,[15] add-on to metformin plus sulfonylurea,[17] and add-on to metformin plus pioglitazone.[18] In each study, patients were randomized to receive canagliflozin 100 or 300 mg or placebo once daily. The add-on to metformin study included a sitagliptin treatment arm that was not included in this analysis. In the monotherapy, add-on to metformin, and add-on to metformin plus pioglitazone studies, patients in the placebo group were switched to sitagliptin 100 mg after 26 weeks. The safety and tolerability of canagliflozin 100 and 300 mg were assessed in Population 1 and in a broader population of patients with T2DM enrolled in 8 double-blind, placebo- and active-controlled, Phase 3 studies (N = 9439; Population 2). Population 2 included 26-week data from the studies described above, as well as the 52-week study of canagliflozin as add-on to metformin versus glimepiride,[14] the 26-week study in older patients aged ≥55–≤80 years,[20] the 26-week study in patients with moderate renal impairment (baseline estimated glomerular filtration rate ≥30–<50 mL/min/1.73 m2),[12] and CANVAS.[26] Safety analyses for CANVAS, an ongoing event-driven study, were performed using data up to a cut-off date of September 15, 2011.
At screening, eligible patients must have had inadequately controlled T2DM with diet and exercise (monotherapy study) or while on the protocol-designated background antihyperglycemic agent (AHA) therapy. Key inclusion criteria for most studies included HbA1c ≥7.0% and ≤10.5% at screening and repeated fasting plasma glucose (FPG) <15.0 mmol/L during the pretreatment phase. The age range for most studies was ≥18–≤80 years; exceptions include the study in older patients aged ≥55–≤80 years; CANVAS, which enrolled patients aged ≥30 years (with CV history) or ≥50 years (with presence of CV risk factors) and had no upper age limit; and the study in patients with moderate renal impairment, which enrolled patients aged ≥25 years with no specified upper age limit. Common exclusion criteria included a history of diabetic ketoacidosis or type 1 diabetes; severe renal impairment; history of myocardial infarction, unstable angina, revascularization procedure, or a cerebrovascular accident within 3 months of screening; uncontrolled hypertension; and alanine aminotransferase level >2 times the upper limit of normal (ULN) or total bilirubin >1.5 times the ULN at screening. Details of the study design, including randomization, blinding, and glycemic rescue therapy, have previously been reported for the individual studies included in these pooled datasets.[12,13,14,15,17,18,20,26]
All studies included in this analysis were conducted in accordance with ethical principles that comply with the Declaration of Helsinki and were consistent with Good Clinical Practices and applicable regulatory requirements. Study protocols and amendments were approved by the Institutional Review Boards and Independent Ethics Committees. All patients provided written informed consent prior to participation in the studies.
Study endpoints and assessments
This post hoc analysis evaluated changes from baseline in HbA1c, FPG, body weight, and systolic and diastolic BP at week 52 in Population 1 (N = 2313) and in a subgroup of patients from India (n = 124); safety and tolerability were assessed in these patients based on adverse event (AE) reports through week 52. Since therapy with canagliflozin and sitagliptin were not concurrently initiated in Population 1 (i.e., patients in the placebo groups of the monotherapy, add-on to metformin, and add-on to metformin plus pioglitazone studies switched to sitagliptin 100 mg after 26 weeks), direct comparisons for efficacy parameters at week 52 cannot be made as the placebo/sitagliptin group served as a control group for safety purposes only. Therefore, efficacy findings are reported for canagliflozin 100 and 300 mg, while safety findings are reported for canagliflozin 100 and 300 mg and placebo/sitagliptin. Safety and tolerability were also assessed in Population 2 (N = 9439) and in a subgroup of patients from India (n = 1038) through the primary time point of studies (i.e., week 26 or 52, or the cut-off date of September 15, 2011 for CANVAS). Documented hypoglycemia episodes, including biochemically documented episodes (≤3.9 mmol/L) and severe episodes (i.e., requiring the assistance of another individual or resulting in seizure or loss of consciousness), were also evaluated separately in patients from India in Population 2 in the pooled placebo-controlled studies by baseline use of AHAs associated with hypoglycemia, and in the individual add-on to metformin versus glimepiride study.
Statistical analyses
Efficacy analyses were conducted using the modified intent-to-treat population, which included all randomized patients who received ≥1 dose of double-blind study drug. The last observation carried forward approach was used to impute missing data; for patients who received glycemic rescue therapy, the last postbaseline value prior to initiation of rescue was used for analysis. An analysis of covariance model, with treatment and stratification factors as fixed effects and the corresponding baseline value for each endpoint as a covariate, was used to assess primary endpoints. The least squares (LS) mean changes from baseline were estimated. Safety analyses included all reported AEs, regardless of rescue therapy, and included all randomized patients who received ≥1 dose of double-blind study drug.
RESULTS
Patient disposition and baseline characteristics
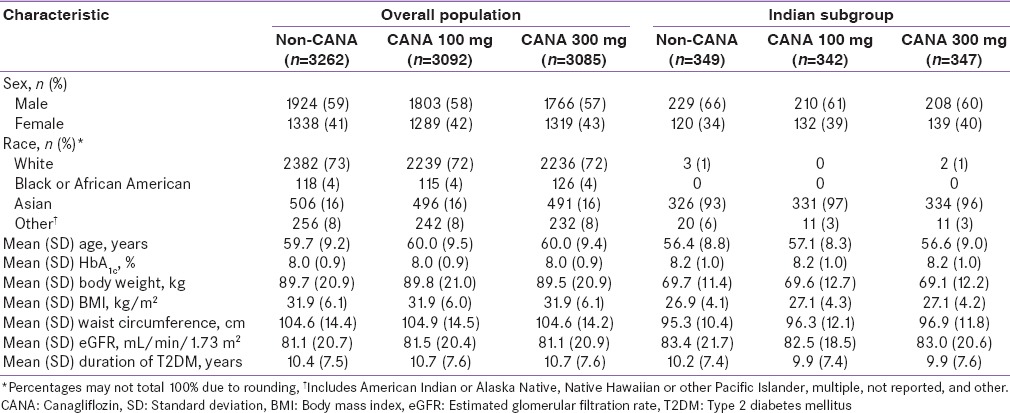
Baseline demographic and disease characteristics were generally similar across treatment groups within each population [Tables 1 and 2]. Patients from India were younger and tended to have a lower baseline body weight and BMI compared with patients in the overall populations. In Population 1, 23.9% of patients in the overall population discontinued the study compared with 16.9% of patients from India; rates of discontinuation with canagliflozin 100 and 300 mg and noncanagliflozin were 21.4%, 20.4%, and 31.7%, respectively, in the overall population and 14.3%, 15.9%, and 22.6%, respectively, in patients from India. In Population 2, rates of discontinuation were similar in the overall population and Indian subgroup (15.3% and 14.2%, respectively).
Table 1.
Baseline demographic and disease characteristics in the overall population and in the Indian subgroup (Population 1)
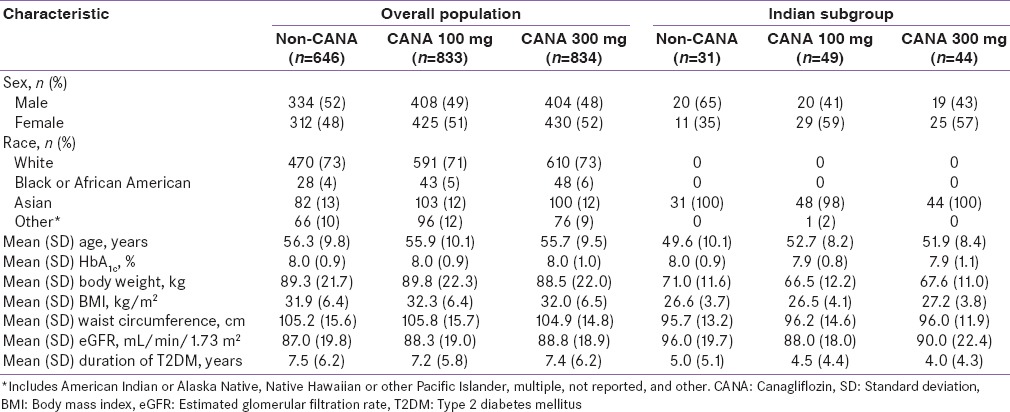
Table 2.
Baseline demographic and disease characteristics in the overall population and in the Indian subgroup (Population 2)
Efficacy
Glycemic parameters
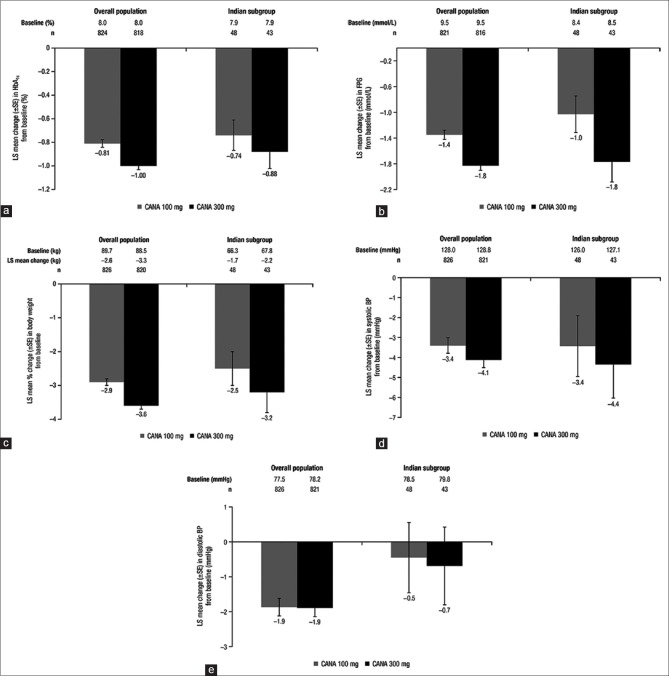
Efficacy parameters were assessed in a pooled population of patients from 4 Phase 3 studies (N = 2313; Population 1) and in a subgroup of patients from India (n = 124). Canagliflozin 100 and 300 mg provided clinically meaningful reductions in HbA1c in the overall population and in the Indian subgroup [Figure 1a]. In the overall population, LS mean reductions in HbA1c with canagliflozin 100 and 300 mg were −0.81% and −1.00%, respectively. In patients from India, reductions in HbA1c with canagliflozin 100 and 300 mg were −0.74% and −0.88%, respectively. Canagliflozin 100 and 300 mg also provided reductions in FPG in the overall population and in the Indian subgroup [Figure 1b]. In the overall population, LS mean reductions in FPG with canagliflozin 100 and 300 mg were −1.4 mmol/L and −1.8 mmol/L, respectively. In patients from India, reductions in FPG with canagliflozin 100 and 300 mg were −1.0 mmol/L and −1.8 mmol/L, respectively.
Figure 1.
Changes from baseline in (a) HbA1c, (b) FPG, (c) body weight, (d) systolic BP, and (e) diastolic BP in the overall population and in the Indian subgroup at week 52 (Population 1). FPG: Fasting plasma glucose, BP: Blood pressure, CANA: Canagliflozin, LS: Least squares, SE: Standard error
Body weight and blood pressure
Both canagliflozin doses were associated with reductions in body weight in the overall population and in the Indian subgroup [Figure 1c]. In the overall population, LS mean percent reductions in body weight with canagliflozin 100 and 300 mg were −2.9% and −3.6%, respectively. In patients from India, mean percent reductions in body weight with canagliflozin 100 and 300 mg were −2.5% and −3.2%, respectively. Both canagliflozin doses also provided reductions in BP in the overall population and in the Indian subgroup [Figure 1d and e]. In the overall population, LS mean reductions in systolic BP with canagliflozin 100 and 300 mg were −3.4 mmHg and −4.1 mmHg, respectively. In patients from India, reductions with canagliflozin 100 and 300 mg were −3.4 mmHg and −4.4 mmHg, respectively. Reductions in diastolic BP with canagliflozin 100 and 300 mg were −1.9 mmHg and −1.9 mmHg, respectively, in the overall population, and −0.5 mmHg and −0.7 mmHg, respectively, in the Indian subgroup.
Safety and tolerability
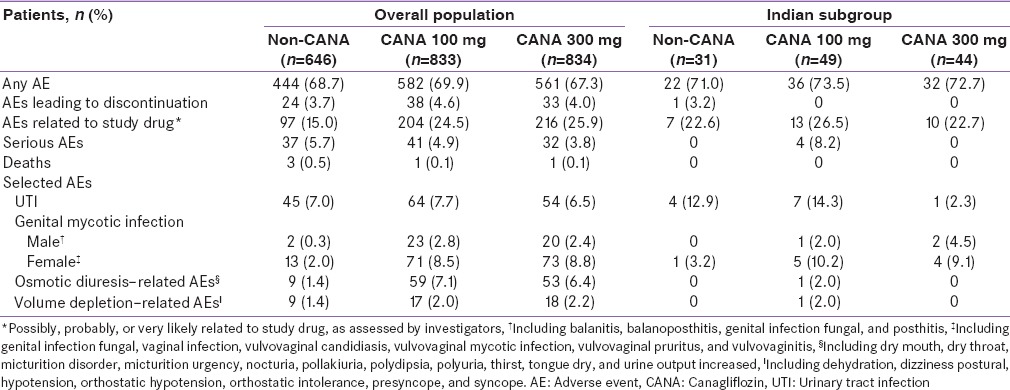
In Population 1, the overall incidence of AEs at week 52 was similar across treatment groups in the overall population and in the Indian subgroup [Table 3]. In the overall population, the incidence of AEs leading to discontinuation with canagliflozin 100 and 300 mg and noncanagliflozin was 4.6%, 4.0%, and 3.7%, respectively; the incidence of serious AEs was 4.9%, 3.8%, and 5.7%, respectively. In the Indian subgroup, 1 patient in the noncanagliflozin group and no patients treated with canagliflozin experienced AEs that led to discontinuation; 4 patients in the canagliflozin 100 mg group and no patients in the canagliflozin 300 mg and noncanagliflozin groups reported serious AEs.
Table 3.
Summary of overall adverse events and selected adverse events in the overall population and in the Indian subgroup at week 52 (Population 1)
In Population 2, the overall incidence of AEs was higher with canagliflozin 300 mg compared with canagliflozin 100 mg and noncanagliflozin in both the overall population and in the Indian subgroup; the incidence of AEs was lower overall in patients from India [Table 4]. In the overall population, the incidence of AEs leading to discontinuation was 4.2%, 5.6%, and 3.7% with canagliflozin 100 and 300 mg and noncanagliflozin, respectively; the incidence of serious AEs was 7.7%, 8.1%, and 8.3%, respectively. In the Indian subgroup, the incidence of AEs leading to discontinuation was 1.8%, 4.0%, and 1.4% with canagliflozin 100 and 300 mg and noncanagliflozin, respectively; the incidence of serious AEs was 7.0%, 4.6%, and 6.9%, respectively.
Table 4.
Summary of overall adverse events and selected adverse events in the overall population and in the Indian subgroup (Population 2)
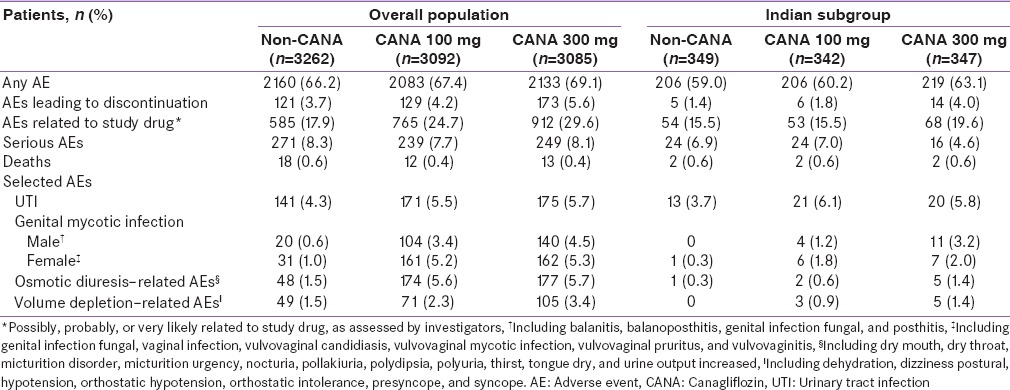
In Populations 1 and 2, incidences of genital mycotic infections in men and women were higher with canagliflozin compared with noncanagliflozin in the overall populations and in the Indian subgroups; these AEs were mild to moderate in intensity, and few led to study discontinuation. In Population 2, the incidence of genital mycotic infections was lower across treatment groups in patients from India than in the overall population. Rates of urinary tract infections (UTIs) were generally similar across treatment groups in the overall population of Population 1; in patients from India in Population 1 (n = 124), UTI rates were lower with canagliflozin 300 mg (2.3%) versus canagliflozin 100 mg (14.3%) and noncanagliflozin (12.9%). In Population 2, UTI rates were higher with canagliflozin versus noncanagliflozin in both the overall population and in the Indian subgroup. The incidence of osmotic diuresis–related and volume depletion–related AEs was higher with canagliflozin versus noncanagliflozin in the overall population of Populations 1 and 2 and in the Indian subgroup of Population 2; in the Indian subgroup of Population 1, osmotic diuresis–related and volume depletion–related AEs were each reported by only 1 patient with canagliflozin 100 mg.
Among patients from India in the placebo-controlled studies of Population 2 not on a background AHA associated with hypoglycemia (n = 200), 4 patients experienced a documented hypoglycemia episode with canagliflozin 100 mg over 26 weeks; no documented hypoglycemia episodes were reported with canagliflozin 300 mg or placebo, and no severe episodes of hypoglycemia were reported in any treatment group [Supplemental Table 1 (452.9KB, tif) ]. Among those on a background AHA associated with hypoglycemia (n = 650), higher rates of documented hypoglycemia were seen with canagliflozin 100 and 300 mg versus placebo over 26 weeks (16.5%, 20.5%, and 11.8%, respectively); the incidence of severe hypoglycemia was 1.4%, 1.9%, and 0%, respectively. In patients from India in the study of canagliflozin as add-on to metformin (n = 109), the incidence of documented hypoglycemia was lower with canagliflozin 100 and 300 mg versus glimepiride over 52 weeks (5.9%, 2.9%, and 24.4%, respectively), despite HbA1c reductions of 0.71%, 0.65%, and 0.50%, respectively; the incidence of severe hypoglycemia was low and similar across treatment groups.
Summary of documented hypoglycemia episodes in the Indian subgroup (Population 2)*, †
DISCUSSION
Findings from this post hoc analysis of pooled Phase 3 studies demonstrated that canagliflozin provided glycemic improvements and reductions in body weight and BP in patients with T2DM from India. In contrast to what was seen in the initial analysis that included the CANVAS substudies,[25] canagliflozin provided dose-dependent reductions in HbA1c in patients from India, which is consistent with findings across Phase 3 studies of canagliflozin.[9,10,11,12,13,14,15,16,17,18,19,20] Similar to what has been reported in studies of canagliflozin in other Asian populations,[27,28,29,30] canagliflozin was associated with reductions in body weight in patients from India, despite the relatively lower baseline body weight and BMI of these patients. The reductions in body weight were consistent with those observed with canagliflozin in the overall population and in other Phase 3 studies of canagliflozin in broader populations.[9,10,11,12,13,14,15,16,17,18,19,20]
Because canagliflozin lowers blood glucose through an insulin-independent mechanism, the glycemic improvements and reductions in body weight and BP provided by canagliflozin may be particularly beneficial in treating Asian patient populations that generally have a higher prevalence of insulin resistance and beta-cell dysfunction.[31] As canagliflozin does not directly affect insulin secretion or insulin sensitivity, it is expected that canagliflozin would be similarly efficacious in Asian patients compared with a broader population of patients with T2DM, as demonstrated in this analysis. Incretin-based therapies, such as dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, may lose effectiveness over time as insulin resistance worsens and beta cells deteriorate.[32] Canagliflozin provides reductions in HbA1c comparable to what has been reported with DPP-4 and GLP-1 agonists in Asian patients with T2DM[33,34,35,36,37] and has been shown to improve model-based indices of insulin sensitivity and beta-cell function with sustained treatment.[38]
Canagliflozin was generally well tolerated in patients with T2DM from India with a safety profile similar to that seen in previous Phase 3 studies and in the overall populations reported in the current manuscript.[9,10,11,12,13,14,15,16,17,18,19,20] In the broader safety population (Population 2), the incidence of male and female genital mycotic infections was lower in patients from India compared with the overall population and other Phase 3 studies of canagliflozin.[9,10,11,12,13,14,15,16,17,18,19,20] A similar pattern of genital mycotic infections has been reported in studies of canagliflozin in other Asian populations,[27,28,29,30] as well as in studies of other SGLT2 inhibitors in Asian patients.[39,40,41] The incidence of osmotic diuresis–related and volume depletion–related AEs was also lower across treatment groups in patients from India than in the overall Population 2.
In Population 2, the incidence of documented hypoglycemia among patients from India not on background AHAs associated with hypoglycemia was low across treatment groups. Among patients from India on a background AHA associated with hypoglycemia (i.e., insulin and/or sulfonylurea), the incidence of documented hypoglycemia was higher with both canagliflozin doses compared with placebo; the incidence of severe hypoglycemia episodes was low across groups. These findings are consistent with the overall population and other Phase 3 studies of canagliflozin, in which the incidence of hypoglycemia was low when canagliflozin was used in conjunction with background therapies that are not associated with hypoglycemia, and higher with background therapies associated with hypoglycemia.[9,10,13,14,15,16,17,18,19,20]
Limitations of this study include the relatively small sample size of patients from India, the lack of a control group for week 52 efficacy data, and the post hoc analysis of data. Longer-term prospective studies would provide a better assessment of the durability of canagliflozin in patients with T2DM from India and would confirm that the efficacy and safety findings from studies of canagliflozin in broader patient populations also apply to these patients.
CONCLUSIONS
In summary, canagliflozin provided glycemic improvements and reductions in body weight and BP and was generally well-tolerated in patients with T2DM from India on a range of background therapies.
Financial support and sponsorship
This study was sponsored by Janssen Research and Development, LLC. Editorial support was provided by Kimberly Fuller, Ph.D., of MedErgy, and was funded by Janssen Global Services, LLC.
Conflicts of interest
K.M.P.K., V.M., B.S., P.G., and G.B. have no competing financial interests. J.X., G.M., and R.Q. are full-time employees of Janssen Research and Development, LLC.
Acknowledgments
Canagliflozin has been developed by Janssen Research and Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
This study was previously presented, in part, in abstract form at the 10th International Diabetes Federation-Western Pacific Region Congress; 21–24 November 2014; Suntec, Singapore.
REFERENCES
- 1.Rawal LB, Tapp RJ, Williams ED, Chan C, Yasin S, Oldenburg B. Prevention of type 2 diabetes and its complications in developing countries: A review. Int J Behav Med. 2012;19:121–33. doi: 10.1007/s12529-011-9162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 4.Ramachandran A, Snehalatha C, Viswanathan V. Burden of type 2 diabetes and its complications – The Indian scenario. Curr Sci. 2002;83:1471–6. [Google Scholar]
- 5.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 6.Sosale A, Prasanna Kumar KM, Sadikot SM, Nigam A, Bajaj S, Zargar AH, et al. Chronic complications in newly diagnosed patients with type 2 diabetes mellitus in India. Indian J Endocrinol Metab. 2014;18:355–60. doi: 10.4103/2230-8210.131184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unnikrishnan R, Anjana RM, Mohan V. Diabetes in South Asians: Is the phenotype different? Diabetes. 2014;63:53–5. doi: 10.2337/db13-1592. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, et al. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012;35:1232–8. doi: 10.2337/dc11-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, et al. Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55-80 years with type 2 diabetes. Diabetes Obes Metab. 2015;17:294–303. doi: 10.1111/dom.12428. [DOI] [PubMed] [Google Scholar]
- 10.Stenlöf K, Cefalu WT, Kim KA, Jodar E, Alba M, Edwards R, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: Findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163–75. doi: 10.1185/03007995.2013.850066. [DOI] [PubMed] [Google Scholar]
- 11.Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: A randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355–64. doi: 10.2337/dc13-2762. [DOI] [PubMed] [Google Scholar]
- 12.Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–27. doi: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 13.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–82. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–50. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 15.Lavalle-González FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia. 2013;56:2582–92. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: A 52-week randomized trial. Diabetes Care. 2013;36:2508–15. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilding JP, Charpentier G, Hollander P, González-Gálvez G, Mathieu C, Vercruysse F, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: A randomised trial. Int J Clin Pract. 2013;67:1267–82. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–77. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463–73. doi: 10.1111/dom.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode B, Stenlöf K, Sullivan D, Fung A, Usiskin K. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: A randomized trial. Hosp Pract (1995) 2013;41:72–84. doi: 10.3810/hp.2013.04.1020. [DOI] [PubMed] [Google Scholar]
- 21.Sha S, Devineni D, Ghosh A, Polidori D, Hompesch M, Arnolds S, et al. Pharmacodynamic effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS One. 2014;9:e105638. doi: 10.1371/journal.pone.0105638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devineni D, Curtin CR, Polidori D, Gutierrez MJ, Murphy J, Rusch S, et al. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co-transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2013;53:601–10. doi: 10.1002/jcph.88. [DOI] [PubMed] [Google Scholar]
- 23.Polidori D, Sha S, Ghosh A, Plum-Mörschel L, Heise T, Rothenberg P. Validation of a novel method for determining the renal threshold for glucose excretion in untreated and canagliflozin-treated subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:E867–71. doi: 10.1210/jc.2012-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, et al. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012;14:539–45. doi: 10.1111/j.1463-1326.2012.01558.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar KM, Mohan V, Sethi B, Gandhi P, Bantwal G, Xie J, et al. Singapore: Suntec; Efficacy and Safety of Canagliflozin in Patients with Type 2 Diabetes Mellitus from India. Poster Presented at: The 10th International Diabetes Federation Western Pacific Region (IDF-WPR) Congress; 21-24 November 2014. [Google Scholar]
- 26.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS) – A randomized placebo-controlled trial. Am Heart J. 2013;166:217–223.e11. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Ji L, Han P, Liu Y, Yang G, Dieu Van NK, Vijapurkar U, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17:23–31. doi: 10.1111/dom.12385. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15:1136–45. doi: 10.1111/dom.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: A 24-week, randomized, double-blind, placebo-controlled, phase III study. Expert Opin Pharmacother. 2014;15:1501–15. doi: 10.1517/14656566.2014.935764. [DOI] [PubMed] [Google Scholar]
- 30.Inagaki N, Kondo K, Yoshinari T, Kuki H. Efficacy and safety of canagliflozin alone or as add-on to other oral antihyperglycemic drugs in Japanese patients with type 2 diabetes: A 52-week open-label study. J Diabetes Investig. 2015;6:210–8. doi: 10.1111/jdi.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma RC, Chan JC. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell RK, Cobble ME, Reid TS, Shomali ME. Distinguishing among incretin-based therapies. Pathophysiology of type 2 diabetes mellitus: Potential role of incretin-based therapies. J Fam Pract. 2010;59(9 Suppl 1):S5–9. [PubMed] [Google Scholar]
- 33.Mohan V, Yang W, Son HY, Xu L, Noble L, Langdon RB, et al. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes Res Clin Pract. 2009;83:106–16. doi: 10.1016/j.diabres.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Pan C, Xing X, Han P, Zheng S, Ma J, Liu J, et al. Efficacy and tolerability of vildagliptin as add-on therapy to metformin in Chinese patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:737–44. doi: 10.1111/j.1463-1326.2012.01593.x. [DOI] [PubMed] [Google Scholar]
- 35.Pan CY, Yang W, Tou C, Gause-Nilsson I, Zhao J. Efficacy and safety of saxagliptin in drug-naïve Asian patients with type 2 diabetes mellitus: A randomized controlled trial. Diabetes Metab Res Rev. 2012;28:268–75. doi: 10.1002/dmrr.1306. [DOI] [PubMed] [Google Scholar]
- 36.Yang W, Pan CY, Tou C, Zhao J, Gause-Nilsson I. Efficacy and safety of saxagliptin added to metformin in Asian people with type 2 diabetes mellitus: A randomized controlled trial. Diabetes Res Clin Pract. 2011;94:217–24. doi: 10.1016/j.diabres.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Yang W, Guan Y, Shentu Y, Li Z, Johnson-Levonas AO, Engel SS, et al. The addition of sitagliptin to ongoing metformin therapy significantly improves glycemic control in Chinese patients with type 2 diabetes. J Diabetes. 2012;4:227–37. doi: 10.1111/j.1753-0407.2012.00213.x. [DOI] [PubMed] [Google Scholar]
- 38.Polidori D, Mari A, Ferrannini E. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, improves model-based indices of beta cell function in patients with type 2 diabetes. Diabetologia. 2014;57:891–901. doi: 10.1007/s00125-014-3196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji L, Ma J, Li H, Mansfield TA, T’joen CL, Iqbal N, et al. Dapagliflozin as monotherapy in drug-naive Asian patients with type 2 diabetes mellitus: A randomized, blinded, prospective phase III study. Clin Ther. 2014;36:84–100.e9. doi: 10.1016/j.clinthera.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Kaku K, Inoue S, Matsuoka O, Kiyosue A, Azuma H, Hayashi N, et al. Efficacy and safety of dapagliflozin as a monotherapy for type 2 diabetes mellitus in Japanese patients with inadequate glycaemic control: A phase II multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2013;15:432–40. doi: 10.1111/dom.12047. [DOI] [PubMed] [Google Scholar]
- 41.Kaku K, Watada H, Iwamoto Y, Utsunomiya K, Terauchi Y, Tobe K, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: A combined phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol. 2014;13:65. doi: 10.1186/1475-2840-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of documented hypoglycemia episodes in the Indian subgroup (Population 2)*, †