Abstract
Context:
Type 1 diabetes mellitus (T1DM) is caused by an immune-mediated destruction of pancreatic beta cells. Other autoimmune diseases can be observed in association with T1DM. The screening for celiac disease (CD) and Hashimoto's thyroiditis is necessary due to the increased prevalence of these pathologies in T1DM patients.
Aims:
This study aimed to investigate the prevalence of autoimmune markers for pancreatitis, thyroiditis, and CD in racially admixtured children and adolescents with T1DM.
Settings and Design:
Cross-sectional clinic-based study.
Methods:
Seventy-one patients with T1DM (average: 11.6 ± 5.1 years). In all patients, the following antibodies were surveyed: Anti-glutamic acid decarboxylase (anti-GAD), immunoglobulin A (IgA) anti-transglutaminase (anti-tTG), Antithyroglobulin (AAT), anti-thyroid peroxidase (anti-TPO), and IgA.
Statistical Analysis Used:
The quantitative variables were expressed as a mean and standard deviation and the qualitative variables in contingency tables. Student's t-test and χ2 tests were used to assess the differences between the groups. The level of significance was established as P < 0.05.
Results:
The prevalence of anti-GAD antibodies was 5.9%; anti-tTG IgA, 7.4%; anti-TPO, 11.8%; and AAT, 11.8%.
Conclusions:
Children and adolescents with T1DM have increased the prevalence of antithyroid and CD-related antibodies. The positivity for anti-GAD and antithyroid antibodies was less frequent than in other studies. The prevalence of anti-tTG antibodies was similar to the literature.
Keywords: Autoimmunity, celiac disease, thyroiditis, type 1 diabetes
INTRODUCTION
The American Diabetes Association classifies type 1 diabetes mellitus (T1DM) into type 1A (T1DMA) and type 1B (T1DMB).[1] The T1DMA is caused by immune-mediated destruction of pancreatic beta cells and is associated with autoantibodies targeting components of insulin-producing cells: Anti-islet cell (ICA), anti-glutamic acid decarboxylase (anti-GAD), anti-tyrosine phosphatase (anti-IA2), and anti-zinc transporter 8 protein (ZnT8). The T1DMA has a genetic predisposition, particularly related to some antigens and human leukocyte antigen (HLA) haplotypes.[2] The T1DMB is a less common form of diabetes, where there are no markers of an autoimmune response against pancreatic beta cells or determined genetic predisposition.[3]
T1DMA is associated to several autoimmune diseases such as Graves’ disease, Hashimoto's thyroiditis, Addison's disease, celiac disease (CD), and pernicious anemia, which are more prevalent in this type of diabetes when compared to the healthy population.[4]
The association of T1DMA with autoantibodies specific for different organs and tissues remains clinically controversial. There is no exact definition of how and when to screen for autoimmune diseases in these patients and on the ethical aspects involved in the monitoring and treatment, in the case of positive laboratory results.[5] However, the study of demographic, immunologic, and genetic characteristics may contribute to prevent complications and provide future guidelines in the care to these patients.[4]
This study aimed to investigate the prevalence of autoimmune markers for pancreatitis, thyroiditis, and CDs in children and adolescents with T1DM, through the search of anti-GAD, antithyroglobulin, anti-thyroid peroxidase (anti-TPO), and immunoglobulin A (IgA) anti-transglutaminase (anti-tTG) antibodies.
METHODS
This study was conducted at the Pediatric Endocrinology Service of a Public University Hospital. The study design was a descriptive, cross-sectional type, assessing children and adolescents diagnosed with T1DM. The study was approved by the Research Ethics Committee of the institution, and informed consent was obtained from the patients’ parents.
Patients
Seventy-one patients, of racially admixture background, mostly Mulattoes from Brazil North-East Region, with T1DM were assessed, corresponding to 100% of the patients monitored in the study period. Patients with total IgA deficiency, neonatal diabetes, and other types of diabetes were excluded.
Anti-glutamic acid decarboxylase antibodies
The anti-GAD antibodies were determined by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Isletest-GAD, Biomerica Inc., Newport Beach, CA, USA). Values >1.05 U/mL were considered positive, lower than 1.0 U/mL were considered negative, and values between 1.0 and 1.05 U/mL were considered undetermined.
Immunoglobulin A anti-transglutaminase antibodies
Anti-tTG antibodies were screened through time-resolved fluorimetry technique using a commercial kit (PerkinElmer Life and Analytical Sciences Inc., Turku, Finland). Values lower than 8.0 AU were considered negative, between 8.0 and 22.0 AU were considered undetermined, and positive those >22.0 AU.
Serum immunoglobulin A
The concentrations of serum IgA were determined by immunoturbidimetry (IGAII 375 test, Siemens, Tarrytown, New York, USA). IgA deficiency was established in patients with serum IgA levels below normal, according to the age range.
Antithyroglobulin antibodies
These antibodies were detected by solid-phase sequential immunometric assay with luminous substances in the specific commercial kit (EURO/DPC Ltd., Llanberis, United Kingdom). Levels >1/200 were considered positive.
Anti-thyroid peroxidase antibodies
Anti-TPO antibodies were detected by solid-phase sequential immunometric assay with luminous substances in the specific commercial kit (EURO/DPC Ltd., Llanberis, United Kingdom). Levels higher than 35 IU/ml were defined as positive.
Statistical analysis
The data were analyzed by Statistical Program for Social Science software (SPSS, Chicago, IL, USA), version 12.0. The quantitative variables were expressed as a mean and standard deviation and the qualitative variables in contingency tables. Student's t-test and χ2 tests were used to assess the differences between the groups. The level of significance was established as P < 0.05.
RESULTS
The study sample consisted of 68 patients (51.5%/35 were male). Three patients with IgA deficiency were excluded [Table 1]. The patient's age ranged from 2 to 22 years (11.6 ± 5.1 years), and age at diagnosis of T1DM ranged from 1.6 to 20.7 years (7.78 ± 4.35). The duration of diabetes at the time of data collection was 0.02–9.83 years (3.01 ± 2.57 years).
Table 1.
Profile of the study patients

The prevalence of antibodies against autoimmune diseases was anti-GAD (5.9%), anti-tTG IgA (7.4%), anti-TPO (11.8%), and AAT (11.8%) [Table 2]. Concomitant positivity of anti-TPO and AAT was found in six patients (8.82%) (P < 0.05). One patient had positive anti-GAD and anti-TPO antibodies, and two patients had positive anti-GAD and AAT antibodies. There was no concomitant positivity between anti-tTG and other antibodies. Of the individuals with positive anti-TPO and AAT, three had hypothyroidism (P < 0.05).
Table 2.
Prevalence of antibodies by gender
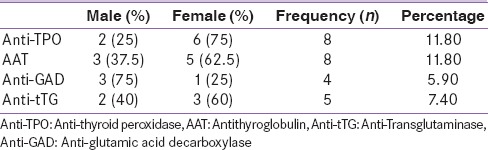
Anti-TPO and AAT antibodies were predominant among females (75% and 62.5%) [Table 2]. The anti-GAD antibody was more prevalent in males (75%). There was no difference in the positivity of anti-tTG associated to gender.
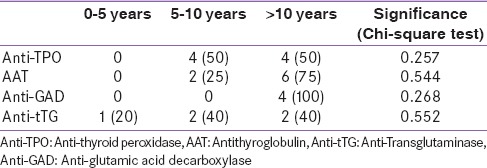
The positivity of anti-GAD and AAT antibodies was more prevalent in the age group of 10–15 years [Table 3]. All subjects positive for anti-GAD were older than 10 years. Half of the subjects with positive anti-TPO antibodies were aged 5–10 years. There was no age-related change in anti-tTG.
Table 3.
Prevalence of antibodies by age range
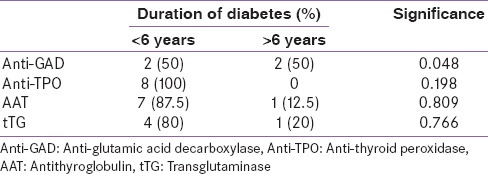
The positivity of antibodies was more prevalent in patients with less than six years of disease, except for anti-GAD antibodies [Table 4].
Table 4.
Relationship between positivity of antibodies and duration of type 1 diabetes mellitus
DISCUSSION
Pancreatic autoimmunity
The immune destruction of pancreatic beta cells is associated with various antigens. Antibodies against some of these antigens are used in clinical practice to assist in the diagnosis and classification of diabetes type, as well predictors of the disease.[6] These include anti-GAD, ICA, anti-tyrosine phosphatase (anti-IA2), anti-insulin (IAA), anti-antigen 2 associated to insulinoma (IA-2), and ZnT8 antibody.[6,7] The ICA is characteristic of the onset of T1DM[8] and its serum levels decrease each year after diagnosis.[9] The ZnT8 comes later than the anti-GAD and IAA.[6] IAA has a little value after onset of insulin therapy.[8,9]
Although it is not a genetic marker specific for diabetes, being positive in other diseases,[7] the anti-GAD is considered the ideal marker for patients who have T1DMA for a long time and are treated with insulin, because it remains positive for many years after diagnosis.[8,9] The prevalence of anti-GAD increases is higher in older children and with some HLA genotypes.[6,10] The cell lysis associated with T1DM increases the release of GAD. This may explain the later appearance of anti-GAD compared to ICA.[8] The presence of anti-GAD 1 month after diagnosis of T1DM is related to the quicker loss of beta cell function.[11] The persistent positivity of anti-GAD can be used to predict other autoimmune diseases in children with T1DM.[12]
A study with Brazilian children with T1DM showed the anti-GAD prevalence of 70–80% in newly diagnosed patients and 54.1% in patients more than 12 months of diagnosis.[13] A more recent study found no difference in anti-GAD prevalence (45.8%) in a comparison of Brazilian children with new-onset or diabetes of longer duration.[14] Moreira et al.[15] described a prevalence of anti-GAD of 63.8% in patients with T1DM in Southern Brazil. Our study, in contrast with the literature, obtained an anti-GAD prevalence of 5.9%. One explanation for this low value may be the fact that the study population is young, with an average age of 11.64 ± 5.1 years, unlike the population studied by Moreira et al.,[15] where the average age was 27 ± 1.7 years. Another explanation could be the low age range of diagnosis (67.7% of the individuals were under 10 years of age at the time of diagnosis). These controversial data may also be due to the different cutoff values established between laboratories or to other environmental factors that impact the pathogenesis of the disease.[16] As in our study, Moreira et al.[15] used ELISA assay to determine the levels of anti-GAD, but the biochemical kits and cutoff values were different. Moreover, the study of Moreira et al.[15] was conducted in Southern Brazil where the population is characterized by large European ancestry, which may explain the divergence in prevalence since anti-GAD frequency can vary between the different ethnic groups.
Thyroid autoimmunity
The prevalence for thyroid autoantibodies in individuals with T1DM varies in different countries and ethnic groups.[17] It is not known whether these antibodies are directly responsible for the pathogenesis of thyroid disease or are the result of destruction mediated by T-cell infiltration in the thyroid.[18] However, Ardestani et al.[19] observed that patients with an increase of thyroid-stimulating hormone and autoantibodies progressed more frequently to thyroid disease.
In international studies, the prevalence of anti-TPO and/or AAT in patients with T1DM ranged from de 20–30%.[12,20,21] Jonsdottir et al.[18] obtained a prevalence of 12.3% for anti-TPO, AAT, or both, shortly after the diagnosis of T1DM. This prevalence is higher in diabetic patients with HLA DQA1*0301 – DQB1*0302[22] and in women.[21] However, more recent studies did not find this association between individuals with the haplotype HLA DQA1*0301–DQB1*0302 and autoimmune thyroid disease.[18]
In their study of Brazilians with T1DM, Araujo et al.[23] found a prevalence of anti-TPO of 25.2%, predominantly among females. Rodrigues et al.[24] found prevalence rates for anti-TPO and AAT in 31% and 16% of the patients, respectively. In the study of Nunes et al.,[25] anti-TPO was positive in 25% of the patients.
Our study found prevalence rates of 11.8% for anti-TPO and AAT. The titers of these antibodies were lower than those reported in the literature, which probably occurs due to the wide variation of these antibodies, the low age range of the study population and the short duration of the disease. The comparison of the values of anti-TPO according to gender, regardless of whether these values were above the threshold of positivity, showed higher values for females.
Autoimmunity related to celiac disease
CD is an autoimmune enteropathy caused by ingestion of gluten in genetically susceptible individuals, which may range from asymptomatic presentation to active intestinal malabsorption syndromes.[26,27] Several studies demonstrated the greater prevalence of the disease in patients with T1DM.[4,28] T1DM is usually diagnosed before CD. Only in 10–25% of the cases CD is diagnosed before T1DM.[4,5,29]
The presence of antibodies for CD is associated with younger age at diagnosis and longer duration of T1DM.[4,30] The screening for CD is performed by the research of anti-endomysial, anti-gliadin, or anti-tTG IgA human (Ac anti-tTG) antibodies.[5,26] The anti-tTG antibody has good sensitivity and specificity, occurring in more than 95% of patients with biopsy-proven CD.[28] The survey of the anti-endomysial antibody has the same accuracy of anti-tTG but is more expensive and observer-dependent, while the research of anti-gliadin antibodies, because they are less sensitive and specific, is not recommended for the screening of CD.[5] It is advisable to determine the level of serum IgA.[31] In individuals with deficiency of IgA, survey of anti-tTG IgG, anti-endomysial IgG, or anti-gliadin IGg antibodies can be done.[32] The patients who have suggestive serological screening should be referred for a biopsy of the small intestine to confirm the diagnosis of CD.[4,27,31]
There is no consensus in the literature on the management of screening for CD in patients with T1DM.[5,26] However, since diabetic patients have generally few or no symptoms associated to CD, some studies suggest the screening for CD at the time of diagnosis of T1DM, annually in the first 4 years of the diagnosis and every 2 years in the successive 6 years of follow-up.[4,27,29]
Van den Driessche et al.[22] described a prevalence of 8–12% for anti-tTG. Djurić et al.[33] showed that 7.4% of the patients with T1DM had anti-tTG. In the studies of Bhadada et al.[34] and Tiberti et al.,[30] the prevalence rates for anti-tTG were 9.2% and 12.8%, respectively.
In Brazilian children with T1DMA, the prevalence of anti-tTG ranged from 2.5% to 15.8%.[32,35,36,37] A recent Brazilian study reported a prevalence of 3.1% of CD in patients diagnosed with T1DM.[5] Consistent with these studies, the prevalence of anti-tTG IgA human antibodies in our study was 7%. The diagnosis of CD was confirmed in 4 of the patients with positive anti-tTG antibodies.
Some limitations of our study were the nonpossibility of correlation of serology with clinical findings such as goiter, anthropometry, dyspepsia, and duodenal biopsy findings; and the relationship of antibodies titers with the age of onset and duration of illness.
CONCLUSIONS
Brazilian children and adolescents, from a racially admixtured background, mostly Mulattoes have increased the prevalence of antithyroid and CD-related antibodies. The positivity for anti-GAD and antithyroid antibodies was less frequent than in other studies. The prevalence of anti-tTG antibodies was similar to the literature.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.American Diabetes Association. Standards of medical care in diabetes-2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Alves C, Toralles MB, Carvalho GC. HLA class II polymorphism in patients with type 1 diabetes mellitus from a Brazilian racially admixtured population. Ethn Dis. 2009;19:420–4. [PubMed] [Google Scholar]
- 3.Michels AW, Eisenbarth GS. Immunologic endocrine disorders. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S226–37. doi: 10.1016/j.jaci.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camarca ME, Mozzillo E, Nugnes R, Zito E, Falco M, Fattorusso V, et al. Celiac disease in type 1 diabetes mellitus. Ital J Pediatr. 2012;38:10. doi: 10.1186/1824-7288-38-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonçalves CB, Silva IN, Tanure MG, Bahia M. Study of prevalence of celiac disease in children with type 1 diabetes mellitus: Result of 10 years of follow-up. Arq Bras Endocrinol Metabol. 2013;57:375–80. doi: 10.1590/s0004-27302013000500007. [DOI] [PubMed] [Google Scholar]
- 6.Nokoff N, Rewers M. Pathogenesis of type 1 diabetes: Lessons from natural history studies of high-risk individuals. Ann N Y Acad Sci. 2013;1281:1–15. doi: 10.1111/nyas.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boitard C. Pancreatic islet autoimmunity. Presse Med. 2012;41(12P2):e636–50. doi: 10.1016/j.lpm.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Tsirogianni A, Pipi E, Soufleros K. Specificity of islet cell autoantibodies and coexistence with other organ specific autoantibodies in type 1 diabetes mellitus. Autoimmun Rev. 2009;8:687–91. doi: 10.1016/j.autrev.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem. 2011;57:168–75. doi: 10.1373/clinchem.2010.148205. [DOI] [PubMed] [Google Scholar]
- 10.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev. 2008;7:550–7. doi: 10.1016/j.autrev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Mortensen HB, Swift PG, Holl RW, Hougaard P, Hansen L, Bjoerndalen H, et al. Multinational study in children and adolescents with newly diagnosed type 1 diabetes: Association of age, ketoacidosis, HLA status, and autoantibodies on residual beta-cell function and glycemic control 12 months after diagnosis. Pediatr Diabetes. 2010;11:218–26. doi: 10.1111/j.1399-5448.2009.00566.x. [DOI] [PubMed] [Google Scholar]
- 12.Karavanaki K, Kakleas K, Paschali E, Kefalas N, Konstantopoulos I, Petrou V, et al. Screening for associated autoimmunity in children and adolescents with type 1 diabetes mellitus (T1DM) Horm Res. 2009;71:201–6. doi: 10.1159/000201108. [DOI] [PubMed] [Google Scholar]
- 13.Pardini VC, Mourao DM, Nascimento PD, Vívolo MA, Ferreira SR, Pardini H. Frequency of islet cell autoantibodies (IA-2 and GAD) in young Brazilian type 1 diabetes patients. Braz J Med Biol Res. 1999;32:1195–8. doi: 10.1590/s0100-879x1999001000003. [DOI] [PubMed] [Google Scholar]
- 14.Rodacki M, Zajdenverg L, Albernaz MS, Bencke-Gonçalves MR, Milech A, Oliveira JE. Relationship between the prevalence of anti-glutamic acid decarboxylase autoantibodies and duration of type 1 diabetes mellitus in Brazilian patients. Braz J Med Biol Res. 2004;37:1645–50. doi: 10.1590/s0100-879x2004001100008. [DOI] [PubMed] [Google Scholar]
- 15.Moreira MC, Lara GM, Linden R, Feksa LR, Tavares RG, Almeida SE, et al. Frequency of the anti-glutamic acid decarboxylase immunological marker in patients with diabetes duration longer than three years in southern Brazil. Sao Paulo Med J. 2011;129:130–3. doi: 10.1590/S1516-31802011000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mezher IA, Al-Khalidy NT, Nsiaf AS. Study of the prevalence of anti-glutamic acid decarboxylase antibody in Iraqi children and adolescent with type 1 diabetes mellitus. AJPS. 2011;10:114–22. [Google Scholar]
- 17.Hage M, Zantout MS, Azar ST. Thyroid disorders and diabetes mellitus. J Thyroid Res 2011. 2011:439463. doi: 10.4061/2011/439463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsdottir B, Andersson C, Carlsson A, Delli A, Forsander G, Ludvigsson J, et al. Thyroid autoimmunity in relation to islet autoantibodies and HLA-DQ genotype in newly diagnosed type 1 diabetes in children and adolescents. Diabetologia. 2013;56:1735–42. doi: 10.1007/s00125-013-2934-9. [DOI] [PubMed] [Google Scholar]
- 19.Ardestani SK, Keshteli AH, Khalili N, Hashemipour M, Barekatain R. Thyroid disorders in children and adolescents with type 1 diabetes mellitus in isfahan, iran. Iran J Pediatr. 2011;21:502–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Triolo TM, Armstrong TK, McFann K, Yu L, Rewers MJ, Klingensmith GJ, et al. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care. 2011;34:1211–3. doi: 10.2337/dc10-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kota SK, Meher LK, Jammula S, Kota SK, Modi KD. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metab Syndr. 2012;6:70–6. doi: 10.1016/j.dsx.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Van den Driessche A, Eenkhoorn V, Van Gaal L, De Block C. Type 1 diabetes and autoimmune polyglandular syndrome: A clinical review. Neth J Med. 2009;67:376–87. [PubMed] [Google Scholar]
- 23.Araujo J, Brandão LA, Guimarães RL, Santos S, Falcão EA, Milanese M, et al. Prevalence of autoimmune thyroid disease and thyroid dysfunction in young Brazilian patients with type 1 diabetes. Pediatr Diabetes. 2008;9(4 Pt 1):272–6. doi: 10.1111/j.1399-5448.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues R, Gonçalves FT, Jorge PT. Prevalence of thyroid dysfunction and antithyroid antibodies in type 1 diabetic mellitus patients and their first degree relatives. Arq Bras Endocrinol Metabol. 2008;52:985–93. doi: 10.1590/s0004-27302008000600009. [DOI] [PubMed] [Google Scholar]
- 25.Nunes RC, Almeida MH, Rodacki M, Noé RA, Bencke MR, Oliveira JE, et al. Prevalence of anti-thyroid peroxidase and anti-adrenal 21-hidroxylase in type 1 diabetes patients. Arq Bras Endocrinol Metabol. 2009;53:461–5. doi: 10.1590/s0004-27302009000400012. [DOI] [PubMed] [Google Scholar]
- 26.Simpson SM, Ciaccio EJ, Case S, Jaffe N, Mahadov S, Lebwohl B, et al. Celiac disease in patients with type 1 diabetes: Screening and diagnostic practices. Diabetes Educ. 2013;39:532–40. doi: 10.1177/0145721713487998. [DOI] [PubMed] [Google Scholar]
- 27.Volta U, Tovoli F, Caio G. Clinical and immunological features of celiac disease in patients with Type 1 diabetes mellitus. Expert Rev Gastroenterol Hepatol. 2011;5:479–87. doi: 10.1586/egh.11.38. [DOI] [PubMed] [Google Scholar]
- 28.Popp A, Mihu M, Munteanu M, Ene A, Dutescu M, Colcer F, et al. Prospective antibody case finding of coeliac disease in type-1 diabetes children: Need of biopsy revisited. Acta Paediatr. 2013;102:e102–6. doi: 10.1111/apa.12117. [DOI] [PubMed] [Google Scholar]
- 29.Greco D, Pisciotta M, Gambina F, Maggio F. Celiac disease in subjects with type 1 diabetes mellitus: A prevalence study in western Sicily (Italy) Endocrine. 2013;43:108–11. doi: 10.1007/s12020-012-9718-8. [DOI] [PubMed] [Google Scholar]
- 30.Tiberti C, Panimolle F, Bonamico M, Shashaj B, Filardi T, Lucantoni F, et al. IgA anti-transglutaminase autoantibodies at type 1 diabetes onset are less frequent in adult patients and are associated with a general celiac-specific lower immune response in comparison with nondiabetic celiac patients at diagnosis. Diabetes Care. 2012;35:2083–5. doi: 10.2337/dc11-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miskiewicz P, Kepczynska-Nyk A, Bednarczuk T. Coeliac disease in endocrine diseases of autoimmune origin. Endokrynol Pol. 2012;63:240–9. [PubMed] [Google Scholar]
- 32.Brandt KG, Silva GA, Antunes MM. Celiac disease in a group of children and adolescents with type 1 diabetes mellitus. Arq Bras Endocrinol Metabol. 2004;48:823–7. doi: 10.1590/s0004-27302004000600007. [DOI] [PubMed] [Google Scholar]
- 33.Djuric Z, Stamenkovic H, Stankovic T, Milicevic R, Brankovic L, Ciric V, et al. Celiac disease prevalence in children and adolescents with type 1 diabetes from Serbia. Pediatr Int. 2010;52:579–83. doi: 10.1111/j.1442-200X.2010.03085.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhadada SK, Kochhar R, Bhansali A, Dutta U, Kumar PR, Poornachandra KS, et al. Prevalence and clinical profile of celiac disease in type 1 diabetes mellitus in north India. J Gastroenterol Hepatol. 2011;26:378–81. doi: 10.1111/j.1440-1746.2010.06508.x. [DOI] [PubMed] [Google Scholar]
- 35.Baptista ML, Koda YK, Mitsunori R, Nisihara, Ioshii SO. Prevalence of celiac disease in Brazilian children and adolescents with type 1 diabetes mellitus. J Pediatr Gastroenterol Nutr. 2005;41:621–4. doi: 10.1097/01.mpg.0000181400.57884.c3. [DOI] [PubMed] [Google Scholar]
- 36.Tanure MG, Silva IN, Bahia M, Penna FJ. Prevalence of celiac disease in Brazilian children with type 1 diabetes mellitus. J Pediatr Gastroenterol Nutr. 2006;42:155–9. doi: 10.1097/01.mpg.0000189338.15763.4a. [DOI] [PubMed] [Google Scholar]
- 37.Mont-Serrat C, Hoineff C, Meirelles RM, Kupfer R. Diabetes and autoimmune diseases: Prevalence of celiac disease in children and adolescents with type 1 diabetes. Arq Bras Endocrinol Metabol. 2008;52:1461–5. doi: 10.1590/s0004-27302008000900009. [DOI] [PubMed] [Google Scholar]


