Abstract
Context:
Anatomical localization of pituitary adenoma can be challenging in adrenocorticotropic hormone (ACTH)-dependent Cushing's syndrome, and bilateral inferior petrosal sinus sampling (BIPSS) is considered gold standard in this regard. Stimulation using corticotrophin-releasing hormone (CRH) improves the sensitivity of BIPSS, however, same is not easily available in India. Therefore, we undertook this study of BIPPS using vasopressin as agent for stimulation owing to its ability to stimulate V3 receptors present on corticotrophs.
Aims:
To study the tumor localization and lateralization in difficult to localize cases of ACTH-dependent Cushing's syndrome by bilateral inferior petrosal sinus sampling using vasopressin for corticotroph stimulation.
Settings and Design:
Prospective observational study.
Subjects and Methods:
Six patients (5 females) meeting inclusion criteria underwent BIPSS using vasopressin for stimulation.
Results:
All six patients had nonsuppressible overnight and low dose dexamethasone suppression test with elevated plasma ACTH levels suggestive of ACTH-dependent Cushing's syndrome. High dose dexamethasone suppression test showed suppressible cortisol in two cases, and microadenoma was seen in two patients on magnetic resonance imaging pituitary. Contrast enhanced computed tomography of the abdomen showed left adrenal hyperplasia in one case and anterior mediastinal mass with bilateral adrenal hyperplasia another. Using BIPSS four patients were classified as having Cushing's disease that was confirmed histopathologically following surgery. Of the remaining two, one had primary pigmented nodular adrenocortical disease, and another had thymic carcinoid with ectopic ACTH production as the cause of Cushing's syndrome. No serious adverse events were noted.
Conclusions:
Vasopressin may be used instead of CRH and desmopressin for stimulation in BIPSS.
Keywords: Bilateral inferior petrosal sinus sampling, Cushing's syndrome, vasopressin
INTRODUCTION
Adrenocorticotropin hormone (ACTH)-dependent Cushing's syndrome is caused by Cushing's disease (around 85%) and ectopic ACTH-secreting tumors (approximately 15%).[1] Subsequent to the diagnosis of ACTH-dependent Cushing's syndrome, the next step involves anatomical localization of the source of the ACTH secretion. Various biochemical and radiological techniques have been established to help in localization of the neoplastic lesion. Radiological techniques including computed tomography (CT)/magnetic resonance imaging (MRI) have poor sensitivity (around 60%).[2] Bilateral inferior petrosal sinus sampling (BIPSS) is considered the gold standard test for anatomical localization for Cushing's disease where radiology has been inconclusive.[3] In a meta-analysis of 21 studies, the overall sensitivity and specificity of BIPSS were found to be 96% and 100% respectively.[4] Various studies have utilized corticotropic releasing hormone (CRH) and desmopressin alone or in combination as ACTH secretagogues to increase the sensitivity and specificity of BIPSS.
Here, we report a series of six cases of ACTH-dependent Cushing's syndrome for localization of tumor by BIPSS using a novel agent vasopressin for stimulation, in whom we were previously unable to demonstrate it using biochemical and radiological investigations.
SUBJECTS AND METHODS
All the diagnosed cases of ACTH-dependent Cushing's syndrome with the inability to localize the tumor with biochemical and imaging studies presenting to our hospital in from April 2013 to June 2015 were included in this analysis.
A total of six patients underwent BIPSS procedure in last 2 years. BIPSS was done using catheterization of both inferior petrosal sinuses using percutaneous bilateral femoral vein approach. Verification of catheter position in the inferior petrosal sinuses was done using a small amount of nonionic iodinized contrast material. Blood was slowly withdrawn from both catheters simultaneously and from ipsilateral peripheral veins for ACTH measurement at 5 and 1 min prior to stimulation. Vasopressin (1.0 unit diluted in 10 ml saline)[5] was then infused into a peripheral vein slowly over 5 min, and samples were simultaneously obtained from both inferior petrosal sinuses and peripheral veins at 5 and 10 min after the administration of vasopressin and collected in cold test tubes.
Samples were transported in a cold environment and were cold centrifuged. ACTH assay was performed using immunoradiometric assay kit (Immunotech, Beckman Coulter Company) using Stratec SR300 automated radioimmunoassay system. The intra-assay and interassay coefficient of variation were below or equal to 9.1% and 9.6%, respectively.
ACTH values were used to calculate the ratio of ACTH between the right or left inferior petrosal sinus and the concentration in the peripheral blood (IPS: P ratio) and the maximal ratio (right or left) was calculated. Sampling giving the highest value of ACTH (5 or 10 min after the injection of vasopressin) was used to determine the ratio.
A diagnosis of Cushing's disease was rendered if basal central to peripheral ACTH ratio was more than two and/or postvasopressin stimulation ratio of more than threes. For lateralization to a given side, a ratio of ≥1.5 was used. In case, the ratio was less than these values, and then a diagnosis of ectopic Cushing's syndrome was made.
RESULTS
A total of six patients (five females) of Cushing's syndrome meeting inclusion criteria underwent BIPSS in this hospital during mentioned time period. Mean age of the patients was 28.3 years (range: 19–33 years), mean body mass index was 28.5 kg/m2 (range: 24.4–34.7 kg/m2) and mean lag period was 26.3 months (range: 2–84 months). Presenting features and baseline characteristics of all six patients are enumerated in Tables 1 and 2.
Table 1.
Demographic profile of patients
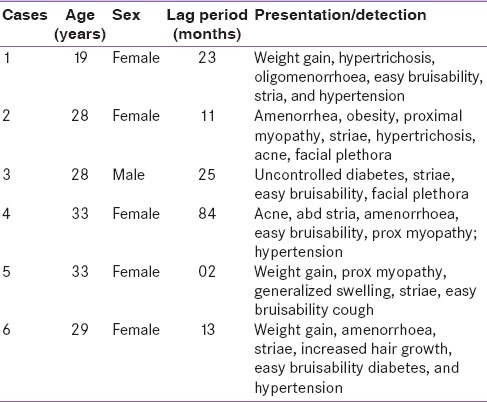
Table 2.
Body mass index, blood pressure, and biochemical profile
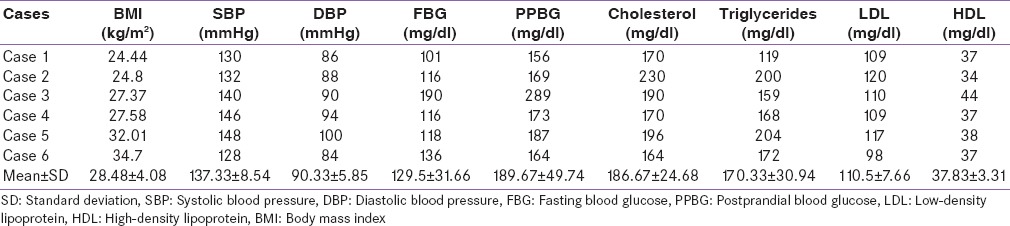
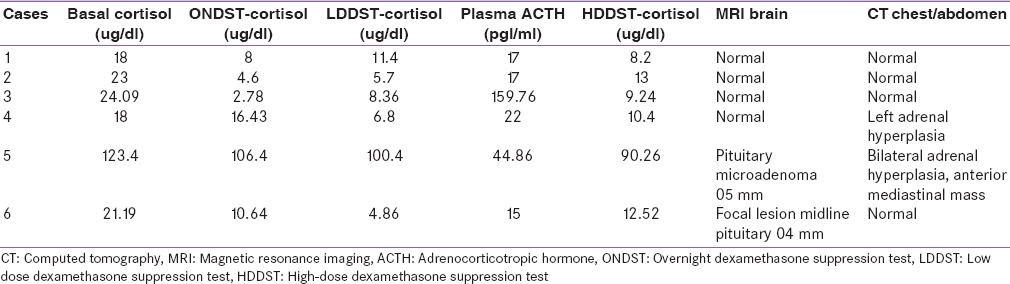
All patients demonstrated hypercortisolism with nonsuppressible serum cortisol levels on overnight dexamethasone suppression test and low dose dexamethasone suppression test. ACTH levels were elevated in all the cases suggestive of ACTH-dependent Cushing's syndrome. Only two cases (cases 1 and 3) demonstrated more than 50% drop in cortisol levels with high-dose dexamethasone suppression test (HDDST). MRI sella demonstrated pituitary microadenoma in two cases (cases 5 and 6) and contrast enhanced CT (CECT) chest and abdomen demonstrated left adrenal hyperplasia in case 4 and anterior mediastinal mass with bilateral adrenal hyperplasia in case 5 [Table 3].
Table 3.
Cortisol suppression tests, adrenocorticotropic hormone values and imaging studies
All six patients underwent BIPSS procedure. ACTH values (basal and postvasopressin stimulation) in both inferior petrosal sinuses, both femoral veins are and central to peripheral ACTH ratios and inter-petrosal gradients are shown in Tables 4 and 5.
Table 4.
Adrenocorticotropic hormone values basal and postvasopressin stimulation from both petrosal sinuses and femoral
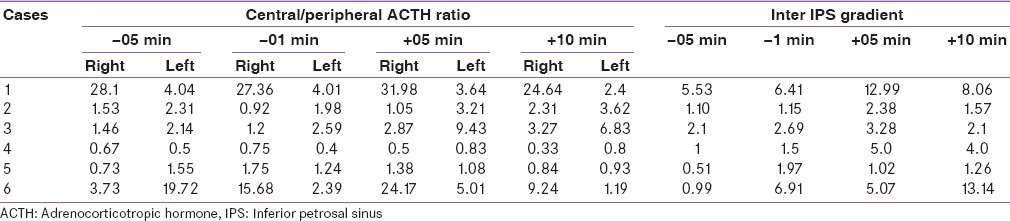
Table 5.
Basal and postvasopressin stimulation highest central to peripheral adrenocorticotropic hormone ratio and inter-petrosal adrenocorticotropic hormone gradient
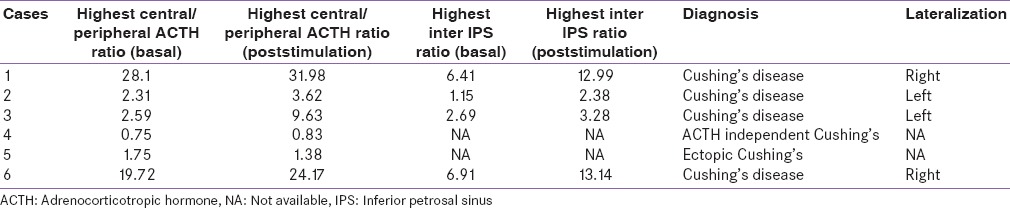
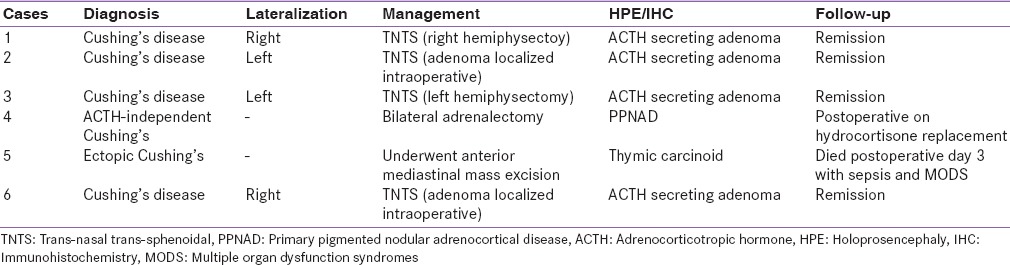
Based on BIPSS cases 1, 2, 3, and 6 were diagnosed as Cushing's disease with lateralization to left or right side in two cases each side. All four patients underwent pituitary surgery via trans-nasal trans-sphenoidal approach, adenoma could be localized intraoperatively in two cases (cases 2 and 4) and adenomectomy was done, and rest two cases adenoma could not be localized, and hemihypophysectomy was done. Histopathological examination in all four cases revealed ACTH secreting corticotroph adenoma. All four cases achieved clinical and biochemical remission on follow-up. Case 4 showed low levels of ACTH on BIPPS and had left adrenal hyperplasia on imaging. Hence, a diagnosis of ACTH-independent Cushing's syndrome was arrived at was subjected to adrenal surgery. During surgery, left adrenal was found to have features suggestive of primary pigmented nodular adrenal disease (PPNAD) and bilateral adrenalectomy was done. The histopathological examination also confirmed the diagnosis of PPNAD. Case 5 with ectopic Cushing's syndrome and anterior mediastinal mass, underwent anterior mediastinal mass excision via thoracotomy, had stormy postoperative course and died on the 3rd postoperative day of sepsis and multiorgan dysfunction. Histopathological examination showed thymic carcinoid [Table 6].
Table 6.
Final diagnosis, management, and follow-up
Complications
Two patients had transient nausea and vomiting, lasting for <6 h. Procedures were uneventful in rest of the cases. No major complication was noted in any of the cases.
DISCUSSION
Cushing's syndrome is usually easy to diagnose clinically owing to its characteristic features. However, anatomical localization of source of excess ACTH Cushing's disease can be quite challenging. Sometimes, it becomes very difficult to differentiate ectopic ACTH source from Cushing's disease.[6,7] Definite treatment of Cushing's syndrome is surgical removal of ACTH-secreting lesion and prerequisite for this surgical management requires localization of ACTH-secreting source.[8]
ACTH-dependent Cushing's syndrome in most of the cases is caused by Cushing's disease and in approximately 15% of the cases, ectopic ACTH secretion is the cause.[1] In 90% of the cases, pituitary microadenoma is the etiological cause in Cushing's disease. Pituitary MRI can localize microadenoma in only 50–60% of the cases as the size of the lesion is very small.[9] Although, dynamic studies have improved the sensitivity of MRI, it still fails to localize the adenoma in many cases.[10] Even approximately 10% of general population harbor pituitary incidentaloma, and may result in false positivity and failed surgery.[6] In our series, out of four cases of Cushing's disease MRI sella could localize pituitary microadenoma in only one case and one case with ectopic ACTH secreting Cushing's was found to have pituitary microadenoma on MRI pituitary.
HDDST has low sensitivity (65%) and specificity (60%) in predicting Cushing's disease.[2] In our series, out of four cases of Cushing's disease, HDDST could localize Cushing's disease in only two cases (50%). Two cases with ectopic and adrenal Cushing's syndrome had <50% suppression of cortisol on HDDST, as expected.
All six patients had discordant results with HDDST and imaging findings and underwent BIPSS. In two cases (case 1 and case 3), where HDDST was suggestive of Cushing's disease, however, MRI failed to localize any lesion in the pituitary. In one case (case 2), HDDST was suggestive of ectopic Cushing's syndrome. However, MRI pituitary and imaging of the chest and abdomen, could not localize any tumor or lesion. In one case (case 6), HDDST was suggestive of the ectopic source of ACTH but, there was suspicious midline lesion (04 mm) in the pituitary while imaging of the chest and abdomen was normal. In one case (case 4), there was left adrenal hyperplasia on CECT abdomen, HDDST was suggestive of the ectopic source of ACTH and MRI sella was normal. In one case (case 5), clinical and biochemical profile was suggestive of ectopic Cushing's syndrome, and there was an anterior mediastinal mass on CECT chest. However, MRI sella also showed pituitary adenoma 05 mm in size.
BIPSS depicts central to the peripheral gradient of ACTH in basal conditions that becomes even more pronounced after corticotrophs are stimulated for the production of ACTH by an exogenous agent. BIPSS series has shown the sensitivity of 88–100% and specificity of 67–100% in the localization of the Cushing's disease.[11] In our case series, BIPSS could accurately localize the source of ACTH production in all the cases, four cases had Cushing's disease, one case had ectopic Cushing's syndrome, and one case had adrenal Cushing's syndrome.
The prediction BIPSS of for lateralization of the lesion in Cushing's disease has been questioned, with accuracies ranging from 50% to 100%.[4] In our series, all four cases with Cushing's disease, we could lateralize the lesion using BIPSS technique that was confirmed by histopathological examination in all and led to remission of disease postoperatively.
In most of the studies with BIPSS, CRH has been used to stimulate corticotrophs. Besides CRH, injection desmopressin in combination with CRH or alone has also been used alone for stimulation of corticotrophs during BIPSS with satisfactory results.[12,13] Neither CRH nor intravenous desmopressin is available in India. Hence, we vasopressin for the corticotroph stimulation in our cases. Vasopressin acts on V3 (arginine vasopressin receptor 1B) receptors present on corticotrophic cells in the anterior pituitary and has been shown to have weak ACTH secretogogues effect. Acting synergistically with CRH, vasopressin causes significant secretion of ACTH.[14] In our series, all four cases with Cushing's disease, we could demonstrate significant increment in ACTH values after vasopressin injection and also could localize and lateralize the lesion in all the cases.
Here, we report a series of six cases of Cushing's syndrome where we performed bilateral IPSS with vasopressin stimulation and found satisfying results for the first time. Limitations of this study are its small sample size and use of the same cutoff for diagnosis as used in previous studies with CRH and vasopressin.
CONCLUSIONS
At present, there is not a single test, or series of tests, to definitively localize the tumor source in ACTH-dependent Cushing's syndrome with 100% accuracy. BIPSS is considered the gold standard in differentiating Cushing's disease from ectopic Cushing's syndrome. Vasopressin may be used instead of CRH and desmopressin for stimulation in BIPSS. However, further larger studies are required to assess its efficacy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stewart PM, Krone NP. 12th ed. Philadelphia: Elsevier Saunders; 2011. The Adrenal Cortex. Williams Text Book of Endocrinology; pp. 479–544. [Google Scholar]
- 2.Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: The limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. J Clin Endocrinol Metab. 1997;82:1780–5. doi: 10.1210/jcem.82.6.3991. [DOI] [PubMed] [Google Scholar]
- 3.Lad SP, Patil CG, Laws ER Jr, Katznelson L. The role of inferior petrosal sinus sampling in the diagnostic localization of Cushing's disease. Neurosurg Focus. 2007;23:E2. doi: 10.3171/foc.2007.23.3.3. [DOI] [PubMed] [Google Scholar]
- 4.Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing's syndrome and pseudo-Cushing's states. Endocr Rev. 1998;19:647–72. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 5.Kharb S, Gundgurthi A, Pandit A, Garg MK, Brar KS, Singh A, et al. Inferior petrosal sinus sampling: Final solution to a riddle called “Cushing's syndrome”. Med J Armed Forces India. 2013;69:74–7. doi: 10.1016/j.mjafi.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: A systematic review. Cancer. 2004;101:613–9. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 7.Utz A, Biller BM. The role of bilateral inferior petrosal sinus sampling in the diagnosis of Cushing's syndrome. Arq Bras Endocrinol Metabol. 2007;51:1329–38. doi: 10.1590/s0004-27302007000800019. [DOI] [PubMed] [Google Scholar]
- 8.Katznelson L, Bogan JS, Trob JR, Schoenfeld DA, Hedley-Whyte ET, Hsu DW, et al. Biochemical assessment of Cushing's disease in patients with corticotroph macroadenomas. J Clin Endocrinol Metab. 1998;83:1619–23. doi: 10.1210/jcem.83.5.4845. [DOI] [PubMed] [Google Scholar]
- 9.Hall WA, Luciano MG, Doppman JL, Patronas NJ, Oldfield EH. Pituitary magnetic resonance imaging in normal human volunteers: Occult adenomas in the general population. Ann Intern Med. 1994;120:817–20. doi: 10.7326/0003-4819-120-10-199405150-00001. [DOI] [PubMed] [Google Scholar]
- 10.Kaskarelis IS, Tsatalou EG, Benakis SV, Malagari K, Komninos I, Vassiliadi D, et al. Bilateral inferior petrosal sinuses sampling in the routine investigation of Cushing's syndrome: A comparison with MRI. AJR Am J Roentgenol. 2006;187:562–70. doi: 10.2214/ajr.05.0557. [DOI] [PubMed] [Google Scholar]
- 11.Swearingen B, Katznelson L, Miller K, Grinspoon S, Waltman A, Dorer DJ, et al. Diagnostic errors after inferior petrosal sinus sampling. J Clin Endocrinol Metab. 2004;89:3752–63. doi: 10.1210/jc.2003-032249. [DOI] [PubMed] [Google Scholar]
- 12.Machado MC, de Sa SV, Domenice S, Fragoso MC, Puglia P, Jr, Pereira MA, et al. The role of desmopressin in bilateral and simultaneous inferior petrosal sinus sampling for differential diagnosis of ACTH-dependent Cushing's syndrome. Clin Endocrinol (Oxf) 2007;66:136–42. doi: 10.1111/j.1365-2265.2006.02700.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsagarakis S, Vassiliadi D, Kaskarelis IS, Komninos J, Souvatzoglou E, Thalassinos N. The application of the combined corticotropin-releasing hormone plus desmopressin stimulation during petrosal sinus sampling is both sensitive and specific in differentiating patients with Cushing's disease from patients with the occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 2007;92:2080–6. doi: 10.1210/jc.2006-2691. [DOI] [PubMed] [Google Scholar]
- 14.Carmody D, Hannon MJ, Thompson C. Vasopressin, diabetes insipid and the syndrome of inappropriate ADH secretion. In: Jameson JL, DeGroot LJ, editors. Endocrinology Adult and Pediatrics. 6th ed. Philadelphia: Elsevier Saunders; 2010. pp. 386–99. [Google Scholar]


