Abstract
IMPORTANCE
A genetic polymorphism affecting FUT2 secretor status in approximately one-quarter of humans of European descent affects the expression of histo-blood group antigens on the mucosal epithelia of human respiratory, genitourinary, and digestive tracts. These histo-blood group antigens serve as host receptor sites necessary for attachment and infection of some pathogens, including norovirus.
OBJECTIVE
We investigated whether an association exists between FUT2 secretor status and laboratory-confirmed rotavirus infections in US children.
DESIGN, SETTING, AND PARTICIPANTS
Multicenter case-control observational study involving active surveillance at 6 US pediatric medical institutions in the inpatient and emergency department clinical settings. We enrolled 1564 children younger than 5 years with acute gastroenteritis (diarrhea and/or vomiting) and 818 healthy controls frequency matched by age and month, from December 1, 2011, through March 31, 2013.
MAIN OUTCOMES AND MEASURES
Paired fecal-saliva specimens were tested for rotavirus and for secretor status. Comparisons were made between rotavirus test–positive cases and healthy controls stratified by ethnicity and vaccination status. Adjusted multivariable analyses assessed the preventive association of secretor status against severe rotavirus gastroenteritis.
RESULTS
One (0.5%) of 189 rotavirus test–positive cases was a nonsecretor, compared with 188 (23%) of 818 healthy control participants (P < .001). Healthy control participants of Hispanic ethnicity were significantly less likely to be nonsecretors (13%) compared with healthy children who were not of Hispanic ethnicity (25%) (P < .001). After controlling for vaccination and other factors, children with the nonsecretor FUT2 polymorphism appeared statistically protected (98% [95% CI, 84%–100%]) against severe rotavirus gastroenteritis.
CONCLUSIONS AND RELEVANCE
Severe rotavirus gastroenteritis was virtually absent among US children who had a genetic polymorphism that inactivates FUT2 expression on the intestinal epithelium. We observed a strong epidemiologic association among children with rotavirus gastroenteritis compared with healthy control participants. The exact cellular mechanism behind this epidemiologic association remains unclear, but evidence suggests that it may be rotavirus genotype specific. The lower prevalence of nonsecretors among Hispanic children may translate to an enhanced burden of rotavirus gastroenteritis among this group. Our findings may have bearing on our full understanding of rotavirus infections and the effects of vaccination in diverse populations.
Histo-blood group antigens (HBGAs) are carbohydrates expressed on the mucosal epithelia of human respiratory, genitourinary, and digestive tracts that serve as host receptor sites necessary for bacterial or viral attachment and cellular entry and, therefore, infection. Their production is encoded by gene families expressing the ABO (A/B enzymes), secretor (α [1,2]-fucosyltransferase 2, or FUT2), and Lewis-type (FUT3) antigens. Single-nucleotide polymorphisms (SNPs) can inactivate the expression of these products. Individuals with genetically inactivated FUT2 production are termed nonsecretors (eFigure 1 in the Supplement).
Relationships between HBGA functionality and enteric infections have been suggested for norovirus,1 Helicobacter pylori,2 and cholera,3 but this relationship is less well described for rotavirus infections. Correlations between rotavirus infections and secretor status have been observed in Vietnamese4 and French5 children hospitalized with acute gastroenteritis (AGE), each with nearly perfect correlation between HGBA polymorphisms and the absence of rotavirus gastroenteritis, but evidence from US children is lacking. Rotaviruses of P[8] type (viral protein, VP-4) have been demonstrated to exclusively infect children of Lewis-positive and secretor phenotypes in Burkina Faso and Nicaragua; these findings support the important effect of both FUT2 and Lewis type on rotavirus susceptibility.6 Therefore, we sought to determine the robustness and generalizability of an association between FUT2 secretor/nonsecretor status and laboratory-confirmed rotavirus infections in an ethnically diverse, large sample of US children under active surveillance for AGE, compared with healthy controls.
Methods
The New Vaccine Surveillance Network7,8 is a multisite, active prospective surveillance system of US medical institutions. We included specimens and data from the following geographically diverse medical institutions: Vanderbilt University Medical Center (Nashville, Tennessee), the University of Rochester Medical Center (Rochester, New York), Cincinnati Children’s Hospital Medical Center (Cincinnati, Ohio), Seattle Children’s Hospital (Seattle, Washington), Texas Children’s Hospital (Houston), and Children’s Mercy Hospitals and Clinics (Kansas City, Missouri). Institutional review board approvals were obtained from the CDC and from each study site. Written consent was obtained from the parent or guardian of each enrolled child at the time of enrollment.
Enrollment
Children between 15 days and 5 years of age who were hospitalized or visited the emergency department with AGE (diarrhea [≥3 episodes within 24 hours] and/or vomiting [≥1 episode within 24 hours]) were enrolled during a 16-month period from December 1, 2011, through March 31, 2013. Children were ineligible if they had a history of immunodeficiency, were previously enrolled for the same AGE episode within 3 days, or were transferred from another hospital.
Healthy controls between 15 days and 5 years of age were enrolled during scheduled well-child visits at the medical institution–affiliated clinics. They were excluded if they had diarrhea or vomiting within 14 days of enrollment, immunodeficiency, or symptoms of acute respiratory infection in the 3 days prior to enrollment. We used a frequency-matched enrollment strategy, actively attempting to enroll healthy children each month in a proportion similar to the age and seasonality of our study participants with AGE.
Data Collection
Demographic and clinical information were collected for each enrolled child in the AGE and healthy control groups. Medical records and rotavirus vaccination data were reviewed from health care institutions and electronic vaccine registries.
Rotavirus Laboratory Testing
Fecal samples were obtained within 14 days of symptom onset, with 94% of specimens obtained within 7 days. Testing for rotavirus was performed using the commercial enzyme immunoassay Rotaclone (Meridian Bioscience) at each surveillance site. Rotavirus strains were characterized by genotyping using reverse transcription polymerase chain reaction (RT-PCR) and nucleotide sequencing at the Centers for Disease Control and Prevention (CDC).9–12 Specimens without rotavirus amplification by RT-PCR were retested by enzyme immunoassay at the CDC to confirm positive results.
FUT2 Genotyping
Paired saliva samples were obtained for each enrolled participant in the AGE and healthy control groups at the time of enrollment using Oragene-Dx-575 assisted saliva collection and DNA stabilization kits (DNA Genotek).
Human DNA from saliva was genotyped using both RT-PCR and the Immunochip (Illumina Infinium).13 The Immunochip was selected for its coverage of the FUT2 locus. Following Illumina protocols, samples having SNPs with call rates above 95% were retained. All samples with incomplete Immunochip analysis, as well as 10% of the overall sample to ensure quality control, were analyzed at the FUT2 428 position using RT-PCR (TaqMan, Life Technologies).
Samples were checked for agreement with self-identified sex and autosomal heterozygosity less than 0.4. FUT2 SNPs rs601338 (428G>A nonsense mutation) and rs1800030 (rare 849G>A nonsense mutation) were directly genotyped from the Immunochip. Additional nonsecretor mutations 385 t>G and 571C>T were imputed from Immunochip data using the program IMPUTE2 to calculate the genotype of unobserved SNPs with greater than 0.9 probability. IMPUTE2 compares highly associated observed SNPs to a database of haplotypes constructed from the 1000 Genomes Project.14
Statistical Analysis
Categorical comparisons between secretor and nonsecretor results for rotavirus test–positive and healthy control participants, as well as bivariate analyses with rotavirus test–negative participants, were conducted by χ2 and Fisher exact test. Logistic regression models were performed on participants with AGE to generate adjusted, multivariable estimates for the effect of secretor status on severe rotavirus gastroenteritis accounting for rotavirus vaccination, age, ethnicity, and health insurance status as a proxy for socioeconomic status. Any enrolled children were excluded from these analyses if they did not have a rotavirus test result, secretor status test results, or documented race/ethnicity, rotavirus vaccination, or insurance data. Rotavirus vaccination effectiveness analyses included a full course of both licensed rotavirus vaccines, RotaTeq (RV5; Merck and Company) and Rotarix (RV1; GlaxoSmithKline Biologicals).
Results
A total of 1564 children with AGE in the inpatient or emergency department clinical settings, and 818 healthy controls, were enrolled with paired fecal-saliva specimens from December 1, 2011, through March 31, 2013. Forty-eight rotavirus test–positive cases were enrolled from December 1, 2011, through November 30, 2012, and 141 rotavirus test–positive cases were enrolled from December 1, 2012, through March 31, 2013 (eFigure 2 in the Supplement).
The median age of children with AGE was 16 months, compared with 12 months for healthy controls (P < .001). A greater proportion of Hispanic children was observed among the participants with AGE (29%) compared with healthy controls (18%) (P < .001), and differences by insurance status occurred between these groups (P = .03) (Table 1). The FUT2 genotype frequency in healthy controls was similar to the known expected population value for persons of European descent of approximately 25%, and this frequency is static.15 None of the healthy controls tested positive for rotavirus.
Table 1.
Descriptive Comparison of Participants With Acute Gastroenteritis and Healthy Controlsa
| Characteristic | No. (%)
|
χ2 P Value | |
|---|---|---|---|
| Acute Gastroenteritis (n = 1564) | Healthy Controls (n = 818) | ||
| Age (median), mo | 16 | 12 | <.001b |
|
| |||
| Sex | |||
|
| |||
| Male | 838 (54) | 421 (51) | .33 |
|
| |||
| Female | 726 (46) | 397 (49) | |
|
| |||
| Race/ethnicity | |||
|
| |||
| White, non-Hispanic | 416 (27) | 225 (28) | <.001 |
|
| |||
| Black, non-Hispanic | 548 (35) | 364 (45) | |
|
| |||
| Hispanic | 450 (29) | 145 (18) | |
|
| |||
| Other | 150 (10) | 84 (10) | |
|
| |||
| Insurance | |||
|
| |||
| Public | 1224 (78) | 625 (76) | .03 |
|
| |||
| Private | 283 (18) | 175 (21) | |
|
| |||
| None | 57 (4) | 18 (2) | |
|
| |||
| Study site | |||
|
| |||
| Nashville | 348 (22) | 155 (19) | <.001 |
|
| |||
| Rochester | 58 (4) | 58 (7) | |
|
| |||
| Cincinnati | 330 (21) | 208 (25) | |
|
| |||
| Seattle | 97 (6) | 51 (6) | |
|
| |||
| Houston | 185 (12) | 106 (13) | |
|
| |||
| Kansas City | 546 (35) | 240 (29) | |
Data are reported as number (percentage) unless otherwise specified.
Wilcoxon 2-sample test.
Of the 189 rotavirus test–positive cases, 188 (99%) were characterized as secretors, and 1 (0.5%) was a nonsecretor. In comparison, 630 (77%) of the 818 healthy controls were secretors and 188 (23%) were nonsecretors (P < .001). Nonsecretors were less frequent among healthy controls characterized as being of Hispanic ethnicity (13%) compared with non-Hispanic children (25%) (P < .001) (Table 2).
Table 2.
Nonsecretors by Hispanic Ethnicity Among Rotavirus Test–Positive Cases With Acute Gastroenteritis and Healthy Controls
| Ethnicity | Nonsecretors, Proportion (%)
|
Fisher Exact P Value | |
|---|---|---|---|
| Cases | Controls | ||
| Non-Hispanic | 1/128 (1) | 169/673 (25) | <.001 |
|
| |||
| Hispanic | 0/61 | 19/145 (13) | .001 |
|
| |||
| Total | 1/189 (1) | 188/818 (23) | <.001 |
The rotavirus genotype proportions among the 188 rotavirus test–positive secretors were G12,P[8] (72%), G3,P[8] (16%), and G2,P[4] (9%), with G9,P[8], G2,P[NT], and G1,P[8] representing 1% each. The single nonsecretor rotavirus-positive case among these vaccinated children was typed a G3,P[8] rotavirus (Figure).
Figure.
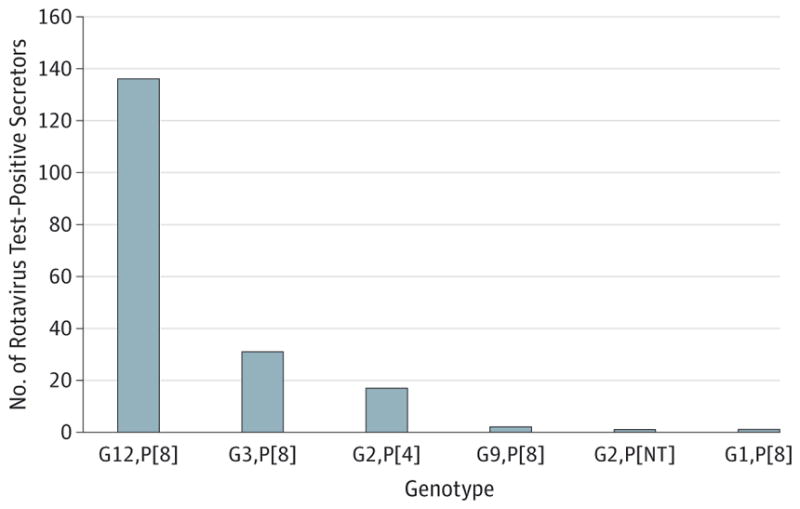
Distribution of Rotavirus Genotypes Among 188 Secretors
Among the 1214 children with AGE further stratified by having received any dose of rotavirus vaccine, a statistically significant difference in secretor status was observed by rotavirus test result: nonsecretors made up 1% of the rotavirus test–positive vaccinees compared with rotavirus test–negative participants (18%) who received any dose of rotavirus vaccine (P < .001) (Table 3).
Table 3.
Secretor Status Among Rotavirus-Vaccinated Patients With Acute Gastroenteritis
| Patients | No. (%)
|
P Value | |
|---|---|---|---|
| Secretor | Nonsecretor | ||
| Rotavirus positive (n = 110) | 109 (99) | 1 (1) | < .001 |
|
| |||
| Rotavirus negative (n = 1104) | 905 (82) | 199 (18) | |
Last, we conducted a multivariable analysis for the rotavirus test–positive and rotavirus AGE test–negative participants, adjusted for participant secretor status, rotavirus vaccination status, age group, ethnicity, and insurance status. We found that the nonsecretor FUT2 polymorphism was associated with 98% protection (95% CI, 84%–100%) against hospitalized and emergency department–attended rotavirus gastroenteritis. Using the same adjusted methodology in this sample, we estimated rotavirus vaccination effectiveness at 77% (95% CI, 66%–84%).
Discussion
In our large, ethnically diverse US sample of 1564 participants with AGE and 818 healthy controls, just 1 (0.5%) of the 189 rotavirus test–positive patients was classified as a FUT2 nonsecretor, compared with 23% of the healthy controls. This significant epidemiological association suggests that nonsecretors who do not express functional FUT2 on their intestinal mucosal epithelium appear to be innately protected from rotavirus infection. Our epidemiologic findings are consistent with similar associations reported from 4 continents,4–6 and we provide, to our knowledge, the first evidence through an adjusted, multivariable analysis that children with the nonsecretor phenotype had a statistically significant degree of protection (98%) against severe rotavirus gastroenteritis that was even greater than that afforded by a complete course of rotavirus vaccination.
Molecular evidence suggests that the viral protein 8 (VP-8) distal region of human rotavirus spike proteins recognizes HBGAs16 and that this binding pattern may be specific by rotavirus VP-4 type,17 and the implication that secretors are susceptible to genotype P[8] infection but nonsecretors are not. For our US participants, as well as those from Nicaragua,6 Burkina Faso,6 and Vietnam,4 among whom rotavirus P[8] infections were virtually absent among nonsecretors, these findings have potential effects on our understandings of rotavirus immunity, evolution, and genotypic predominance.
Indeed, an analogous host-pathogen association is acknowledged between FUT2 secretor status and norovirus infection,18,19 and this epidemiologic relationship also appears to be norovirus genotype specific.20 This relationship is so well recognized for norovirus that challenge studies routinely screen for nonsecretor status among volunteers21 who appear to lack innate susceptibility to norovirus infection. As recently suggested for norovirus,22 it is possible that interactions with other HBGA-expressing commensal enteric modalities are involved.23
Interestingly, children of Hispanic ethnicity in our US sample were significantly less likely to be nonsecretors (13%) compared with non-Hispanic children (25%), consistent with previous observations of Central American children.24 Therefore, our epidemiological association potentially indicates enhanced rotavirus gastroenteritis infection risk among Hispanic children compared with non- Hispanic children. This is substantiated by studies conducted prior to rotavirus vaccine licensure, which illustrate a higher burden of rotavirus among Hispanic or Native American populations, particularly among young age groups.25,26 Historically, such findings have been explained as stemming from the influences of lower socioeconomic status. Whereas socioeconomic status and other related factors remain important, genetics may also influence differential rates of severe rotavirus gastroenteritis by Hispanic ethnicity.
Nordgren et al6 hypothesized that these genetic characteristics could help explain differential immunologic responses to the VP-4 vaccine component of both live, attenuated rotavirus vaccines. Our US data did not provide a sufficient non-P[8] sample to ascertain whether this is accurate. However, published clinical trial results in 11 Latin American countries and Finland suggested lower vaccine protection against a non-P[8] rotavirus strain, G2,P[4].27 In addition, in a Nicaraguan postlicensure evaluation, wherein 85% of the rotavirus test–positive participants had G2,P[4] strains, a lower overall vaccination effectiveness was observed compared with the vaccine efficacy demonstrated in highly developed countries.28 On the other hand, vaccine effectiveness in a less-developed region of Mexico during a G9,P[4] outbreak was comparable to that in other middle-income settings.29 Of note, large reductions in postlicensure rotavirus gastroenteritis hospitalizations and mortality have been observed among Latin American children.30 Efforts to protect children of Hispanic ethnicity via rotavirus vaccination will likely continue to have positive effects, as US postlicensure rotavirus vaccination effectiveness assessments show both vaccines to perform well among children of Hispanic and non-Hispanic ethnicity.31,32
Our study is limited by a lack of Lewis-type characterization, which has been hypothesized to play a further role in this association. In addition, some differences in age between participants with rotavirus and healthy controls persisted, despite our attempt to limit selection bias by frequency matching our healthy controls by age and month in a proportion similar to that of our case enrollment.
Conclusions
Our analysis of a large sample of US children with AGE compared with healthy controls demonstrates an epidemiological association between secretor status and laboratory confirmed rotavirus gastroenteritis, which is notably disproportionate by Hispanic ethnicity. The adjusted effect of the nonsecretor FUT2 polymorphism on protection against rotavirus gastroenteritis is even greater than the effect from rotavirus vaccination. Practical public health and epidemiological implications of these results include fostering improved explanations of disease burden for diverse populations, inspiring novel therapeutic developments designed to block HBGA binding sites,33 the development of better-informed rotavirus transmission modeling, and the contribution to a more complete epidemiological understanding of rotavirus infection susceptibility and prevention.
Supplementary Material
At a Glance.
We investigated whether an association exists between a FUT2 genetic polymorphism affecting the expression of histo-blood group antigens on the intestinal epithelia and laboratory-confirmed rotavirus infections in US children.
Of 1564 children younger than 5 years with acute gastroenteritis, we confirmed that just 1 (0.5%) of 189 rotavirus test–positive cases was a nonsecretor, compared with 188 (23%) of 818 healthy controls (P < .001).
Healthy control participants of Hispanic ethnicity were significantly less likely to be nonsecretors (13%) compared with healthy children who were not of Hispanic ethnicity (25%) (P < .001).
Severe rotavirus gastroenteritis was virtually absent among US children who had a genetic polymorphism that inactivates FUT2 expression on the intestinal epithelium.
The lower prevalence of nonsecretors among Hispanic children may translate to an enhanced burden of rotavirus gastroenteritis among individuals of this ethnicity.
Acknowledgments
Funding/Support: The design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication was through a cooperative agreement by the US Centers for Disease Control and Prevention (funding for IP11010 awarded to each medical institution affiliated with this study). Genetic analyses were performed with support from a National Institute of Child Health and Human Development grant (HD13021 to A.L.M.), and a National Institutes of Health, Ruth L. Kirschstein National Research Service Award (AI 109893 to R.L.C.) from the National Institute of Allergy and Infectious Diseases.
Footnotes
Role of the Funder/Sponsor: The funders participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author Contributions: Dr Payne had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Payne, Staat, Sahni, Selvarangan, Englund, Weinberg, Harrison, Morrow, Parashar.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Payne, Szilagyi.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Payne, Currier, Szilagyi, Klein, Wikswo, Morrow.
Obtained funding: Payne, Staat, Halasa, Englund, Weinberg, Morrow.
Administrative, technical, or material support: Payne, Staat, Sahni, Selvarangan, Englund, Weinberg, Boom, Harrison, Davidson, Moffatt, Wikswo, Bowen, Morrow, Parashar.
Study supervision: Payne, Staat, Sahni, Selvarangan, Weinberg, Boom, Harrison, Davidson, Moffatt, Morrow, Parashar.
Conflict of Interest Disclosures: Dr Staat has received grant support from GlaxoSmithKline for epidemiologic studies of acute respiratory illness and acute gastroenteritis including postlicensure vaccine effectiveness studies for rotavirus and influenza vaccines (2009–2012). No other disclosures are reported.
References
- 1.Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis. 2002;185(9):1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 2.Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 3.Glass RI, Holmgren J, Haley CE, et al. Predisposition for cholera of individuals with O blood group: possible evolutionary significance. Am J Epidemiol. 1985;121(6):791–796. doi: 10.1093/oxfordjournals.aje.a114050. [DOI] [PubMed] [Google Scholar]
- 4.Van Trang N, Vu HT, Le NT, Huang P, Jiang X, Anh DD. Association between norovirus and rotavirus infection and histo-blood group antigen types in Vietnamese children. J Clin Microbiol. 2014;52(5):1366–1374. doi: 10.1128/JCM.02927-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imbert-Marcille BM, Barbé L, Dupé M, et al. A FUT2 gene common polymorphism determines resistance to rotavirus A of the P[8] genotype. J Infect Dis. 2014;209(8):1227–1230. doi: 10.1093/infdis/jit655. [DOI] [PubMed] [Google Scholar]
- 6.Nordgren J, Sharma S, Bucardo F, et al. Both Lewis and secretor status mediate susceptibility to rotavirus infections in a rotavirus genotype-dependent manner. Clin Infect Dis. 2014;59(11):1567–1573. doi: 10.1093/cid/ciu633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne DC, Staat MA, Edwards KM, et al. Active, population-based surveillance for severe rotavirus gastroenteritis in children in the United States. Pediatrics. 2008;122(6):1235–1243. doi: 10.1542/peds.2007-3378. [DOI] [PubMed] [Google Scholar]
- 8.Payne DC, Szilagyi PG, Staat MA, et al. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr Infect Dis J. 2009;28(11):948–953. doi: 10.1097/INF.0b013e3181a6ad6e. [DOI] [PubMed] [Google Scholar]
- 9.Das BK, Gentsch JR, Cicirello HG, et al. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32(7):1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30(6):1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouvea V, Glass RI, Woods P, et al. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin DD, Nakagomi T, Hoshino Y, et al. National Rotavirus Surveillance System. Characterization of nontypeable rotavirus strains from the United States: identification of a new rotavirus reassortant (P2A[6],G12) and rare P3[9] strains related to bovine rotaviruses. Virology. 2002;294(2):256–269. doi: 10.1006/viro.2001.1333. [DOI] [PubMed] [Google Scholar]
- 13.Cortes A, Brown MA. Promise and pitfalls of the Immunochip. Arthritis Res Ther. 2011;13(1):101. doi: 10.1186/ar3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5(6):e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrer-Admetlla A, Sikora M, Laayouni H, et al. A natural history of FUT2 polymorphism in humans. Mol Biol Evol. 2009;26(9):1993–2003. doi: 10.1093/molbev/msp108. [DOI] [PubMed] [Google Scholar]
- 16.Hu L, Crawford SE, Czako R, et al. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012;485(7397):256–259. doi: 10.1038/nature10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Huang P, Tan M, et al. Rotavirus VP8*: phylogeny, host range, and interaction with histo-blood group antigens. J Virol. 2012;86(18):9899–9910. doi: 10.1128/JVI.00979-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindesmith L, Moe C, Marionneau S, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9(5):548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 19.Carlsson B, Kindberg E, Buesa J, et al. The G428A nonsense mutation in FUT2 provides strong but not absolute protection against symptomatic GII.4 norovirus infection. PLoS One. 2009;4(5):e5593. doi: 10.1371/journal.pone.0005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Currier RL, Payne DC, Staat MA, et al. Innate susceptibility to norovirus infections influenced by FUT2 genotype in a United States pediatric population. Clin Infect Dis. 2015;60(11):1631–1638. doi: 10.1093/cid/civ165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atmar RL, Opekun AR, Gilger MA, et al. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis. 2014;209(7):1016–1022. doi: 10.1093/infdis/jit620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones MK, Watanabe M, Zhu S, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346(6210):755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böhm R, Fleming FE, Maggioni A, et al. Revisiting the role of histo-blood group antigens in rotavirus host-cell invasion. Nat Commun. 2015;6:5907. doi: 10.1038/ncomms6907. [DOI] [PubMed] [Google Scholar]
- 24.Bucardo F, Kindberg E, Paniagua M, et al. Genetic susceptibility to symptomatic norovirus infection in Nicaragua [published correction appears in J Med Virol. 2009;81(6):1129] J Med Virol. 2009;81(4):728–735. doi: 10.1002/jmv.21426. [DOI] [PubMed] [Google Scholar]
- 25.Yen C, Steiner CA, Barrett M, et al. Racial disparities in diarrhea-associated hospitalizations among children in five US States, before and after introduction of rotavirus vaccine. Vaccine. 2010;28(46):7423–7426. doi: 10.1016/j.vaccine.2010.08.094. [DOI] [PubMed] [Google Scholar]
- 26.Holman RC, Parashar UD, Clarke MJ, Kaufman SF, Glass RI. Trends in diarrhea-associated hospitalizations among American Indian and Alaska native children, 1980–1995. Pediatrics. 1999;103(1):E11. doi: 10.1542/peds.103.1.e11. [DOI] [PubMed] [Google Scholar]
- 27.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Human Rotavirus Vaccine Study Group. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371(9619):1181–1189. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 28.Patel M, Pedreira C, De Oliveira LH, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA. 2009;301(21):2243–2251. doi: 10.1001/jama.2009.756. [DOI] [PubMed] [Google Scholar]
- 29.Yen C, Figueroa JR, Uribe ES, et al. Monovalent rotavirus vaccine provides protection against an emerging fully heterotypic G9P[4] rotavirus strain in Mexico. J Infect Dis. 2011;204(5):783–786. doi: 10.1093/infdis/jir390. [DOI] [PubMed] [Google Scholar]
- 30.Desai R, Oliveira LH, Parashar UD, Lopman B, Tate JE, Patel MM. Reduction in morbidity and mortality from childhood diarrhoeal disease after species A rotavirus vaccine introduction in Latin America—a review. Mem Inst Oswaldo Cruz. 2011;106(8):907–911. doi: 10.1590/s0074-02762011000800002. [DOI] [PubMed] [Google Scholar]
- 31.Payne DC, Boom JA, Staat MA, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children <5 years of age, 2009–2011. Clin Infect Dis. 2013;57(1):13–20. doi: 10.1093/cid/cit164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics. 2010;125(2):e199–e207. doi: 10.1542/peds.2009-1021. [DOI] [PubMed] [Google Scholar]
- 33.Morrow AL, Ruiz-Palacios GM, Jiang X, Newburg DS. Human-milk glycans that inhibit pathogen binding protect breast-feeding infants against infectious diarrhea. J Nutr. 2005;135(5):1304–1307. doi: 10.1093/jn/135.5.1304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


