Abstract
OBJECT
Calcium phosphate cement provides a biomaterial that can be used for calvarial reconstruction in a retrosigmoid craniectomy for microvascular decompression (MVD). This study evaluates the outcomes of postoperative CSF leak and wound infection for patients undergoing a complete cranioplasty using calcium phosphate cement versus incomplete cranioplasty using polyethylene titanium mesh following a retrosigmoid craniectomy for MVD.
METHODS
The authors evaluated 211 cases involving patients who underwent first-time retrosigmoid craniectomies performed by a single attending surgeon for trigeminal neuralgia from October 2008 to June 2014. From this patient population, 111 patients underwent calvarial reconstruction after retrosigmoid craniectomy using polyethylene titanium mesh, and 100 patients had reconstructions using calcium phosphate cement. A Pearson’s chi-square test was used to compare postoperative complications of CSF leak and wound infection in these 2 types of cranioplasties.
RESULTS
The polyethylene titanium mesh group included 5 patients (4.5%) with postoperative CSF leak or pseudomeningocele and 3 patients (2.7%) with wound infections. In the calcium phosphate cement group, no patients had a CSF leak, and 2 patients (2%) had wound infections. This represented a statistically significant reduction of postoperative CSF leak in patients who underwent calcium phosphate reconstructions of their calvarial defect compared with those who underwent polyethylene titanium mesh reconstructions (p = 0.03). No significant difference was seen between the 2 groups in the number of patients with postoperative wound infections.
CONCLUSIONS
Calcium phosphate cement provides a viable alternative biomaterial for calvarial reconstruction of retrosigmoid craniectomy defects in patients who have an MVD. The application of this material provides a biocompatible barrier that reduces the incidence of postoperative CSF leaks. http://thejns.org/doi/abs/10.3171/2015.1.JNS142102
Keywords: calcium phosphate, titanium mesh, cranioplasty, microvascular decompression, retrosigmoid craniectomy, cerebrospinal fluid leak, diagnostic and operative techniques, infection
Microvascular decompression (MVD) is a surgical technique used for the treatment of cranial nerve neuralgias or spasm via alleviation of pathologic nerve compression using a retrosigmoid suboccipital approach.11 Trigeminal neuralgia and hemifacial spasm, as a result of compression of cranial nerves V and VII, respectively, account for the majority of cranial nerve diseases addressed with MVD.12 Despite progressive advances in surgical technique, complications with MVD still occur. CSF leaks are a serious postoperative complication. The incidence of CSF leak following a suboccipital craniotomy for multiple etiologies has been reported in the literature to range from 1.5% to 14.5%.3,6,8–11 To address this complication, various techniques in addition to creating a watertight dural closure have been developed.1,12 Complete cranioplasty is the replacement of the entire calvarial defect, using either the bone flap and a bone analog together or a bone analog alone to fill the entire defect. An incomplete cranioplasty involves partial reconstruction of the calvarial defect, using only a bone flap, a titanium mesh, or nothing at all.11 This retrospective study analyzes our experience with cranioplasties using calcium phosphate cement or polyethylene titanium mesh, following retrosigmoid suboccipital craniectomies for MVD for trigeminal neuralgia.
Methods
During the period from October 2008 to June 2014, 221 patients underwent a retrosigmoid craniectomy for MVD of the trigeminal nerve by a single surgeon (M.L.) at The Johns Hopkins Hospital. Patients included in the study were all undergoing MVD for the first time on the indicated side. Trigeminal neuralgia was diagnosed clinically by the senior surgeon and confirmed by T2-weighted MRI showing vascular compression of the trigeminal nerve. All patients were medically cleared for surgery by a certified anesthesiologist preoperatively. The Johns Hopkins Hospital Institutional Review Board approved this study.
Intraoperatively, following the decompression of cranial nerve V, the dura was closed primarily with interrupted 4-0 nonabsorbable braided nylon suture (Nurolon, Ethicon). If dural defects remained after attempting a primary closure, then a collagen dura substitute membrane (DuraMatrix, Stryker) was used to close the gaps by placing the dura substitute membrane over the dural opening and using a 4-0 nonabsorbable braided nylon suture to attach the membrane to the dura. A collagen matrix (Duragen, Integra) was placed over the dura, and fibrin sealant (Evicel, Ethicon) was applied over the collagen matrix.
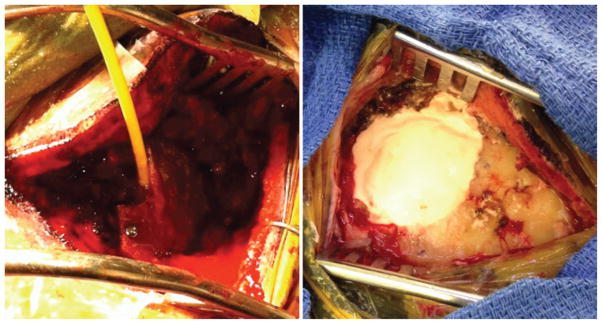
The term complete cranioplasty was assigned to retrosigmoid craniectomy calvarial defects that were completely filled using calcium phosphate cement (HydroSet, Stryker). The calcium phosphate cement was mixed to a consistent thickness, according to the manufacturer’s instructions, and was applied over a 1–2-minute interval to the craniectomy defect, filling the cranial defect by shaping it to replicate the removed bone and allowing it to dry (Fig. 1).
Fig. 1.
Representative intraoperative images of the closure techniques. Porous polyethylene implant with embedded titanium mesh applied to craniotomy defect with insertion of fibrin sealant (left) and calcium phosphate cement applied to craniotomy defect (right). Figure is available in color online only.
Incomplete cranioplasty was the term designated to retrosigmoid craniectomies that were reconstructed by placing a biocompatible, porous polyethylene implant with embedded titanium mesh (Medpor Titan, Stryker) over the top of the calvarial defect. The cavity between the dura and the mesh was then filled with fibrin sealant (Evicel, Ethicon), and the titanium mesh was secured on the bone with titanium screws (Fig. 1).
In both procedures, the area was then irrigated with normal saline, and a layered closure was performed using 0-0 and 3-0 synthetic, absorbable, copolymer (VICRYL, Ethicon) suture, with a running 3-0 nylon suture used to close the superficial skin layer. The closed wound was then washed with a saline-soaked sponge, dried, and dressed in a sterile fashion.
Patients were evaluated in the outpatient clinic for signs of CSF leak or infection. CSF leakage was reported at follow-up visits in patients with clinical evidence of postoperative otorrhea and/or incisional fluid drainage. A pseudomeningocele was documented when a significant collection of fluid was found beneath the operative incision without any incision fluid leaks or otorrhea. Infections were determined if there was purulent drainage from the incision, persistent erythema, swelling, or dehiscence at the incision.
A 2-tailed t-test was used to compare the baseline characteristics of the calcium phosphate and the titanium mesh study groups, while Pearson’s chi-square test was used to compare the occurrence of CSF leaks and infections in the study populations.
Results
Over a 69-month period, 221 patients underwent a retrosigmoid craniectomy for MVD for trigeminal neuralgia, with 105 patients undergoing a complete cranioplasty reconstruction with calcium phosphate cement (from March 2013 to June 2014) and 116 patients receiving a polyethylene titanium mesh for incomplete cranioplasty (from October 2008 to June 2013). One hundred (92.5%) of the 105 patients in the calcium phosphate group and 111 (95.7%) of the 116 patients in the polyethylene titanium mesh group maintained follow-up. The mean age for the calcium phosphate group and the titanium mesh group was 52.5 years (range 16–76 years) and 51.2 years (range 24–86 years), respectively (p = 0.44).
The mean postoperative follow-up time for the calcium phosphate and titanium mesh study group was 2.29 month and 2.35 months, respectively (p = 0.83). For the calcium phosphate group, no patient was found to have a CSF leak at the follow-up visit. Five patients (4.5%) from the polyethylene titanium mesh group had CSF leakage through either the incision (4 patients) or in the form of a pseudomeningocele (1 patient) (p = 0.03). The 5 cases involved a pseudomeningocele in 1 patient and a CSF leak in 1 patient whose incision was partially oversewn in the emergency department; both patients were treated with acetazolamide. In the other 3 cases, 2 patients were treated with a temporary lumbar drain, and 1 patient was treated with surgical repair in the operating room.
Two patients (2%) in the calcium phosphate group developed postoperative wound infections, with 1 patient requiring surgical debridement, and the other patient undergoing intravenous antibiotic treatment. Three patients (2.7%) from the polyethylene titanium mesh group developed wound infections (p = 0.31) (Table 1). Two of these 3 patients underwent surgical debridement, and 1 patient was treated with oral antibiotics.
TABLE 1.
Demographic characteristics and postoperative outcomes for patients who underwent cranioplasty
| Variable | Polyethylene Titanium Mesh | Calcium Phosphate Cement | p Value |
|---|---|---|---|
| Total no. of patients w/ FU | 111 | 100 | |
| Mean age (yrs) | 51.2 | 52.5 | 0.44* |
| Mean outpatient FU time (mos) | 2.35 | 2.29 | 0.83* |
| CSF leak/pseudomeningocele | 4.5% (n = 5) | 0% (n = 0) | 0.03† |
| Wound infection | 2.7% (n = 3) | 2% (n = 2) | 0.31† |
FU = follow-up.
Two-tailed t-test.
Pearson’s chi-square test.
Discussion
Previous studies have looked at operative variations for cranioplasty following retrosigmoid craniectomy for MVD that aim to reduce the incidence of CSF leak. Stoker et al.11 evaluated both incomplete and complete cranioplasties of retrosigmoid calvarial defects, comparing the use of the original bone flap, mesh plating, methyl methacrylate (MMA), or nothing. This study suggested that complete cranioplasty with MMA had a lower incidence of CSF leak (6%) when compared with mesh plating (25%) or with putting the original bone flap back on (21%), but the rate was not statistically significant. Calcium phosphate, which converts to hydroxyapatite, provides an alternative material that can be used for complete cranioplasty. The crystalline structure and porosity of calcium phosphate make it an effective osteoconductive and osteointegrative material with good biocompatibility.2,4 Our study provided a comparison between calcium phosphate cement for complete cranioplasty and polyethylene titanium mesh plating for incomplete cranioplasty of retrosigmoid craniectomies, evaluating a larger patient population with longer term outpatient follow-up than had previously been reported.4 No patient evaluated in the calcium phosphate study group experienced a CSF leak, a finding significantly different from the 4.5% of patients in the polyethylene titanium mesh group with a CSF leak. The rates of wound infections were similar in both groups. Of note, a chi-square test performed in this study with 4 CSF leaks included was significant with a p value of 0.05.
Although this study did not address dural closure, which also contributes to CSF leak prevention, the reconstruction of the retrosigmoid calvarial defects using calcium phosphate creates a biocompatible and watertight barrier above the dura that provides an additional protective layer that prevents CSF leakage.8 In incomplete cranioplasty, using mesh plating or the original bone flap and titanium plates, open space remaining between the dura and muscle layers could allow CSF to leak from an incomplete dural closure through the surgical incision. Although performing a craniotomy and replacing the original bone flap is perfectly valid, our group prefers craniectomy because of concern for sinus injury that may occur in a craniotomy. Additionally, our preference is to drill as laterally as possible, and as a result, in our surgical technique, larger gaps would be created with a craniotomy, hence our institution’s preference not to perform craniotomies for MVDs.
In this study, the surgeon used titanium mesh plating for cranioplasties from 2008 to 2013 and switched to calcium phosphate cement reconstructions starting in 2013. The surgeon had performed over 100 MVDs using a titanium mesh prior to the use of the porous implant and calcium phosphate. Although increased surgeon experience could be a confounder in the rates of CSF leak in each group, specifically because the titanium mesh reconstruction was performed first, MVDs are one of the most routine cases performed by this surgeon, and we feel that the technique for these cohorts has been the most consistent. The techniques used for dural and myocutaneous closure for the MVD operation remained the same in all cases, with the only changing variable being the type of material used for the bony reconstruction.
Limited comparisons between MMA and calcium phosphate use in calvarial reconstruction exist in the literature. Lee et al.7 reported that there was no significant difference in postoperative complication rates between using MMA only or calcium phosphate only for calvarial reconstruction. This study showed minimal complications in patients seen at a median follow-up time of 2.5 months. Previous studies suggested that complications with calcium phosphate cement for skull reconstruction can take longer to develop. Afifi et al.1 reported that complications such as infections, seromas, and foreign body reactions can take 17.5–89 months to present. Zinn et al.13 and Gilardino et al.5 reported on calcium phosphate cement complications presenting 6–7.5 years after skull reconstructions.
Despite this study, which presents the largest patient group and longest clinic follow-up for calcium phosphate cranioplasties for retrosigmoid craniectomies, longer follow-up would be warranted to observe if there is degradation of the cranioplasty construct over several years, resulting in additional complications. In addition, other causes of poor wound healing that may progress to CSF leak in MVD patients, including diabetes, steroid use, or malnutrition, could also be evaluated.
Calcium phosphate cement provides a quick method to fill a calvarial defect, having malleable properties that allow for good reconstructive cosmesis. The cost of the cement, however, may limit its use to smaller craniectomies. Two patients from the titanium mesh study group required removal of their mesh plate secondary to persistent pain from protruding screws. These mesh plates were replaced with calcium phosphate cement cranioplasties. Further research is needed to determine if calcium phosphate cranioplasty reduces the incidence of postoperative incision pain in the retrosigmoid craniectomy patients.
Conclusions
Several cranioplasty techniques exist for the reconstruction of a retrosigmoid craniectomy for MVD. Our retrospective study provides evidence suggesting that calcium phosphate cement is a viable, alternative biomaterial that may reduce the incidence of postoperative CSF leak in MVD operations. A randomized controlled trial would be warranted to better analyze whether calcium phosphate cement cranioplasty reduces postoperative CSF leak compared with polyethylene titanium mesh plating. By providing biocompatibility and malleability calcium phosphate cement offers a viable alternative that creates an additional barrier against CSF leak when reconstructing retrosigmoid calvarial defects.
Abbreviations
- MMA
methyl methacrylate
- MVD
microvascular decompression
Footnotes
Disclosure
Dr. Lim reports being a consultant for and receiving clinical or research support from Stryker.
Author Contributions
Conception and design: Lim, Eseonu, Goodwin, Zhou, Theodros, Bender, Bettegowda. Acquisition of data: Lim, Eseonu, Goodwin, Zhou, Theodros, Bender, Mathios. Analysis and interpretation of data: Lim, Eseonu, Goodwin, Zhou, Theodros. Drafting the article: Lim, Eseonu, Goodwin, Zhou. Critically revising the article: Lim, Eseonu, Goodwin, Zhou, Theodros, Bettegowda. Reviewed submitted version of manuscript: all authors. Statistical analysis: Eseonu. Administrative/technical/material support: Lim, Eseonu, Goodwin, Mathios. Study supervision: Lim.
References
- 1.Afifi AM, Gordon CR, Pryor LS, Sweeney W, Papay FA, Zins JE. Calcium phosphate cements in skull reconstruction: a meta-analysis. Plast Reconstr Surg. 2010;126:1300–1309. doi: 10.1097/PRS.0b013e3181ead057. [DOI] [PubMed] [Google Scholar]
- 2.Chow LC, Takagi S. A natural bone cement—a laboratory novelty led to the development of revolutionary new biomaterials. J Res Natl Stand Technol. 2001;106:1029–1033. doi: 10.6028/jres.106.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey A, Sung WS, Shaya M, Patwardhan R, Willis B, Smith D, et al. Complications of posterior cranial fossa surgery— an institutional experience of 500 patients. Surg Neurol. 2009;72:369–375. doi: 10.1016/j.surneu.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Frederickson AM, Sekula RF., Jr The utility of calcium phosphate cement in cranioplasty following retromastoid craniectomy for cranial neuralgias. Br J Neurosurg. 2013;27:808–811. doi: 10.3109/02688697.2013.776670. [DOI] [PubMed] [Google Scholar]
- 5.Gilardino MS, Cabiling DS, Bartlett SP. Long-term followup experience with carbonated calcium phosphate cement (Norian) for cranioplasty in children and adults. Plast Reconstr Surg. 2009;123:983–994. doi: 10.1097/PRS.0b013e318199f6ad. [DOI] [PubMed] [Google Scholar]
- 6.Huh R, Han IB, Moon JY, Chang JW, Chung SS. Microvascular decompression for hemifacial spasm: analyses of operative complications in 1582 consecutive patients. Surg Neurol. 2008;69:153–157. doi: 10.1016/j.surneu.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Lee EI, Chao AH, Skoracki RJ, Yu P, DeMonte F, Hanasono MM. Outcomes of calvarial reconstruction in cancer patients. Plast Reconstr Surg. 2014;133:675–682. doi: 10.1097/01.prs.0000438061.46290.33. [DOI] [PubMed] [Google Scholar]
- 8.Park JS, Kong DS, Lee JA, Park K. Intraoperative management to prevent cerebrospinal fluid leakage after microvascular decompression: dural closure with a “plugging muscle” method. Neurosurg Rev. 2007;30:139–142. doi: 10.1007/s10143-006-0060-6. [DOI] [PubMed] [Google Scholar]
- 9.Samii M, Günther T, Iaconetta G, Muehling M, Vorkapic P, Samii A. Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery. 2002;50:712–719. doi: 10.1097/00006123-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Sarsam Z, Garcia-Fiñana M, Nurmikko TJ, Varma TR, Eldridge P. The long-term outcome of microvascular decompression for trigeminal neuralgia. Br J Neurosurg. 2010;24:18–25. doi: 10.3109/02688690903370289. [DOI] [PubMed] [Google Scholar]
- 11.Stoker MA, Forbes JA, Hanif R, Cooper C, Nian H, Konrad PE, et al. Decreased rate of CSF leakage associated with complete reconstruction of suboccipital cranial defects. J Neurol Surg B Skull Base. 2012;73:281–286. doi: 10.1055/s-0032-1312709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong J, Zhu J, Sun H, Dou NN, Wang YN, Ying TT, et al. Microvascular decompression surgery: surgical principles and technical nuances based on 4000 cases. Neurol Res. 2014;36:882–893. doi: 10.1179/1743132814Y.0000000344. [DOI] [PubMed] [Google Scholar]
- 13.Zins JE, Moreira-Gonzalez A, Papay FA. Use of calcium-based bone cements in the repair of large, full-thickness cranial defects: a caution. Plast Reconstr Surg. 2007;120:1332–1342. doi: 10.1097/01.prs.0000279557.29134.cd. [DOI] [PubMed] [Google Scholar]