Abstract
Aims
The common MTNR1B genetic variant rs10830963 is associated with an increased risk of type 2 diabetes (T2D). To date, no experimental study has tested the effect of the MTNR1B variant on glucose metabolism in humans during exposure of the melatonin receptors to their ligand. The aim of this study was to investigate whether this MTNR1B variant influenced the effect of melatonin (5 mg) on glucose tolerance assessed by an oral glucose tolerance-test (OGTT; 75 g) at different times of the day (morning and evening) as compared to a placebo.
Methods
Seventeen normoglycemic women (24±6 y; BMI 23.0±3.3 kg/m2) completed the study (11 carriers of the risk allele [CG] and 6 noncarriers [CC]).
Results
The effect of melatonin on glucose tolerance depended on the genotype. In the morning, the effect of melatonin (melatonin-placebo) on the glucose area-under-the-curve (AUC) above baseline differed significantly (P=0.036) between the carriers and noncarriers. This effect of melatonin on the carriers was six times as large as that on the noncarriers. The MTNR1B SNP explained over one-quarter (26%) of the inter-individual differences in the effect of melatonin on glucose AUC. However, in the evening, the effect of melatonin on glucose AUC of the carriers and noncarriers did not differ significantly (P>0.05).
Conclusions
MTNR1B rs10830963 risk variant worsens the effect of melatonin on glucose tolerance, suggesting the importance of genotyping and personalized recommendations, especially in people consuming food when melatonin levels are elevated. Large-scale studies in vulnerable populations are necessary to translate these results into real-world, clinically-relevant recommendations.
Keywords: MTNR1B, type 2 diabetes, glucose tolerance, melatonin, genetic variation
Introduction
The discovery of MTNR1B as a novel type-2 diabetes (T2D) risk gene sparked great interest in the role of melatonin in glucose metabolism [1–5]. This gene encodes the melatonin type 2 receptor Mel1b (MT2), a G protein-coupled receptor that mediates the effects of melatonin and that is expressed in various tissues including pancreatic β-cells.
Genome-wide-association studies (GWAS) have found an association between MTNR1B rs10830963 and increased fasting plasma glucose levels and T2D risk [1, 2, 6]. Follow-up studies confirmed this association with T2D risk [7–9]. However, although the genetic association of rs10830963 with T2D risk is now established, the functional impact of this MTNR1B variant on glucose control is poorly characterized, because—to date—measures of glucose control, insulin secretion and insulin sensitivity have been performed during the daytime, when endogenous melatonin levels are very low and thus without the ligand of the receptor available to induce its effect.
Nevertheless, Mel1b is expressed in β-cells implying that MTNR1B rs10830963 might affect pancreatic glucose sensing and/or insulin release [1, 6]. Furthermore, Lyssenko et al. showed increased MTNR1B expression in pancreatic islets of carriers of the rs10830963 risk genotype compared to non-carriers and impaired insulin responses to oral and intravenous glucose, reporting a negative correlation between MTNR1B mRNA levels and insulin secretion [1]. Indeed, association of the rs10830963 risk allele with impaired insulin secretion is well-established and an inverse association with insulin sensitivity has also been reported [10, 11]. Importantly, among more than 60 variants associated with T2D and/or glycemic traits, the common MTNR1B risk variant rs10830963 had the most significant adverse influence on the disposition index, a product of both insulin secretion and insulin sensitivity, as estimated by an oral glucose tolerance test (OGTT) [12].
Despite GWAS have provided robust evidence for an association of the common MTNR1B risk variant with glycemic traits, the magnitude of its effect is small. Notably, all the metabolic assessments in these GWAS were performed during daytime hours when endogenous melatonin concentrations were very low or absent [13]. In addition, only a few functional studies have examined the impact of manipulating the melatonin concentration on glycemic measures [14, 15], and very few such studies have included humans. Functional studies in animal models are restricted mostly to nocturnal rodents, from which data cannot be directly translated to (diurnal) humans [16]. While in humans melatonin is produced when we are sleeping and fasting, in nocturnal rodents melatonin is produced when they are awake and eating [13]. To date no experimental study has been performed in humans in order to test the effect of the MTNR1B variant on glucose metabolism during exposure of the melatonin receptors to their ligand.
A new approach to investigate the role of melatonin and the MTNR1B diabetes risk variant in glucose metabolism could be to test the effect of exogenous melatonin administration during the daytime on glucose metabolism between carriers and non-carriers of the MTNR1B risk variant. The aim of the current study was to assess whether MTNR1B rs10830963 influences the magnitude of effect of exogenous melatonin administration on glucose tolerance at different times of the day (morning and evening), as compared to placebo administration.
Research design and methods
Subjects
This study consisted of members of the female rugby team at the University of Murcia, Spain who fulfilled both inclusion and exclusion criteria and for whom blood for DNA extraction was available (n=17 from a total of n=21). Data from all the participants were collected within one month (April) to minimize any influence of seasonal effects. The population was a homogenous set of very healthy, young women. The exclusion criteria included endocrine, renal, hepatic, or psychiatric disorders; impaired glucose tolerance based on standard criteria; prescription drugs or other pharmacological treatment, except oral contraceptives; major weight fluctuations ±3 kg in the past 3 y; recent shift work (within the last 2 y); travel across more than one time zone (within the last 1 y); and sleep disorders.
Seventeen nondiabetic, nonobese, young women of European ancestry (mean±SD; 24±6 y; BMI: 23.0±3.3 kg/m2) completed the study (Table 1). Only two of the participants were smokers. All the participants had normal fasting glucose and insulin levels and normal glucose tolerance (<7.8 mmol/L, 2 h after 75 g OGTT; Table 1). There were no significant differences in the anthropometric, biochemical (including morning melatonin saliva concentrations), chronotype (morningness-eveningness scores), or habitual sleep characteristics of the carriers and noncarriers of the risk allele. The participants gave written informed consent, and the study was approved by the local Ethics Committee of the “Virgen de la Arrixaca” University Hospital in Murcia, Spain.
Table 1.
General characteristics of the population studied dependent on MTNR1B rs10830963 variant.
| Risk carriers (CG) |
Non-risk carriers (CC) |
|||
|---|---|---|---|---|
| n=11 | n=6 | |||
| Mean ± SD | Mean ± SD | P | P1 | |
| Age (y) | 24 ± 3 | 23 ± 5 | 0.828 | |
| Weight (kg) | 63.9 ± 12.6 | 59.5 ± 2.8 | 0.267 | 0.833 |
| Height (cm) | 163.50 ± 5.51 | 163.83 ± 9.99 | 0.928 | 0.987 |
| BMI (kg/m2) | 23.80 ± 3.90 | 22.32 ± 2.43 | 0.410 | |
| Body fat (%) | 27.3 ± 7.7 | 25.2 ± 2.9 | 0.412 | 0.982 |
| WC (cm) | 78.6 ± 9.5 | 78.0 ± 3.7 | 0.891 | 0.360 |
| Sagittal diameter (cm) | 17.1 ± 1.6 | 16.7 ± 0.8 | 0.546 | 0.901 |
| Coronal diameter (cm) | 30.2 ± 3.7 | 29.1 ± 2.2 | 0.512 | 0.967 |
| Tricipital skinfold (mm) | 22.4 ± 6.6 | 22.1 ± 5.8 | 0.906 | 0.539 |
| VA/SApredicted | 0.38 ± 0.16 | 0.40 ± 0.11 | 0.796 | 0.536 |
| Systolic blood pressure (mm Hg) | 109.1 ± 5.1 | 106.6 ± 5.1 | 0.346 | 0.378 |
| Diastolic blood pressure (mm Hg) | 67.5 ± 8.6 | 63.3 ± 10.3 | 0.379 | 0.427 |
| Fasting biochemical characteristics | ||||
| Glucose (mmol/L) | 4.30 ± 0.50 | 4.10 ± 0.39 | 0.421 | 0.372 |
| Insulin (µU/mL) | 7.85 ± 3.45 | 6.15 ± 3.25 | 0.337 | 0.323 |
| Cholesterol (mmol/L) | 4.24 ± 0.80 | 4.78 ± 1.22 | 0.285 | 0.348 |
| Triglycerides (mmol/L) | 0.92 ± 0.20 | 0.91 ± 0.45 | 0.930 | 0.989 |
| HDL-cholesterol (mmol/L) | 1.71 ± 0.34 | 2.26 ± 0.77 | 0.054 | 0.106 |
| LDL-cholesterol (mmol/L) | 2.10 ± 0.64 | 2.11 ± 1.08 | 0.993 | 0.875 |
| VLDL-cholesterol (mmol/L) | 0.43 ± 0.10 | 0.45 ± 0.20 | 0.830 | 0.843 |
| Endogenous melatonin (09:00) (pg/mL) | 14.38 ± 6.27 | 20.62 ± 13.70 | 0.336 | 0.369 |
| Morningness-eveningness Score | 42.80 ± 9.72 | 41 ± 7.15 | 0.699 | 0.596 |
| Sleep duration (hh:mm) ± (min) | 8:25 ± 24 | 8:01 ± 38 | 0.158 | 0.182 |
Data are presented as means ± SD. BMI: body mass index. WC: waist circumference. HC: hip circumference. VA/SApredicted: visceral area/subcutaneous areapredicted. HDL: high-density lipoprotein; LDL: low-density lipoprotein. P: unpaired t-test, P1:ANOVA adjusting by age and BMI.
OGTT
To determine the effects of melatonin administration on glucose tolerance, each participant underwent four OGTTs after a 10 h fast on four nonconsecutive days, two after a placebo and two after melatonin (5 mg) administration, as previously described [15]. The placebo was administered once in the morning (9 A.M.) and once in the evening (9 P.M.). The same was true for melatonin administration. For each of the four OGTTs, an oral glucose load of 75 g was given 15 min after the administration of the placebo or melatonin. During each OGTT, blood samples were obtained immediately prior to the administration of the melatonin/placebo and 30, 60, 90, and 120 min after glucose administration. The 2 h area under the curve (AUC) above baseline was calculated. Plasma glucose and insulin levels were measured by automated chemical analysis (IL ILAB 600 Chemistry Analyzer of Instrumentation Laboratory, MA, USA) and a solid-phase, 2-site chemiluminescent immunometric assay (IMMULITE 2000 Insulin, DPC, USA), respectively.
Genotyping
Genotyping in all participants was performed by the same operator who was blinded to their clinical characteristics. DNA was isolated from blood samples using routine DNA isolation kits (Qiagen, Hilden, Germany). MTNR1B rs10830963 genotypes were identified by standard Sanger sequencing of a 354bp PCR amplification product surrounding the variant (forward primer GAATTGGCATTTCTGGGG and reverse primer AGCATCATTTGCTGTCTC). Hardy–Weinberg equilibrium was fulfilled (P=0.1). Of the 17 participants, 11 were carriers of the risk allele (CG), and six were noncarriers (CC). The allele frequency of the variant in this population was (11G/(34 G+C))*100 = 32.4%, similar to the frequency of 30% in HapMap European populations.
Statistical analysis and calculations
The general characteristics of the population studied are presented as means ± SD (Table 1). Responses of glucose and insulin observed during OGTT were examined as area under the curve above baseline across 120 minutes following the oral glucose load (AUC), calculated by the trapezoidal method [17]. Differences between carriers (CG) and non-carriers (CC) in the effect of melatonin (melatonin-placebo) on AUC for glucose and insulin in the morning and in the evening were analyzed by unpaired t-test and further analyses were performed by ANCOVA in which effects were adjusted for age and BMI (Table 1). In addition, we assessed the effect of melatonin on each of the genotypes at each time point of the OGTT (TF, time fasting; T30, 60, 90, and 120) by a two-way repeated measures ANOVA, including the time and treatment condition as the two factors. A paired t-test was used to evaluate any differences between the individual time points.
Genetic association analyses were performed using linear regression models in PLINK, which also provided an estimate of the trait variance explained by the SNP (r2; Supplemental Table) [18]. The genetic variant effect size was calculated with Cohen’s d according to the formula . For Cohen’s d, an effect size of 0.2–0.3 is considered a “small” effect size, around 0.5 is considered a “medium” effect size, and 0.8 to infinity signifies a “large” effect size [19]. To assess whether melatonin influenced insulin sensitivity, we used the Insulin Sensitivity Index (ISI: 10,000/√[fasting glucose × fasting insulin × mean OGTT glucose × mean OGTT insulin]; mean OGTT across fasting-120 min) [20]. To estimate the effect of melatonin on β cell function, we determined the corrected insulin response(CIR: 100 × insulin at 30 min/[glucose at 30 min × glucose 30min-3.89]) [21]. Finally, the disposition index (DI) was calculated as a measure of insulin secretion in relation to insulin sensitivity (DI = CIR × ISI) [22].
All other statistical analyses were carried out using SPSS for windows 19.0 (SPSS Inc., Chicago, IL). The level of significance for all the statistical tests and hypotheses was set at P<0.05.
Results
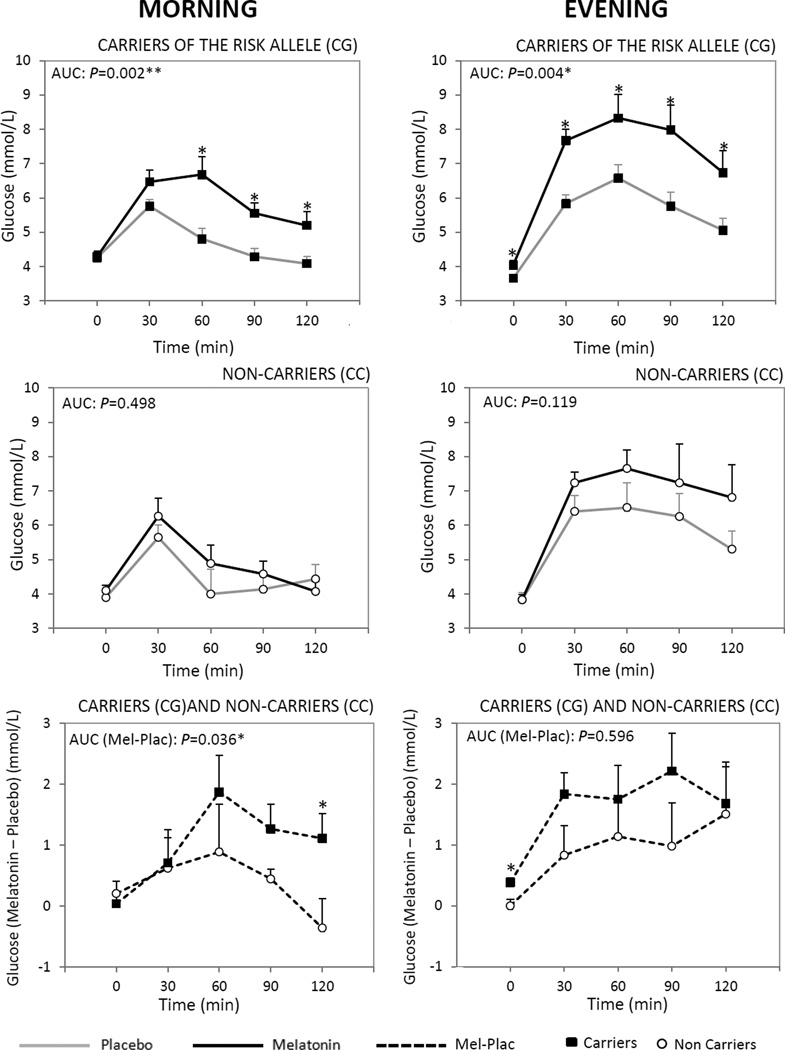
The effect of melatonin on glucose tolerance depended on rs10830963. In the morning, the effect of melatonin (melatonin-placebo) glucose AUC differed significantly between the carriers and noncarriers (P=0.036; unpaired t-test; Fig. 1 and Table 2). Similar results were found after adjusting for age and BMI (P=0.046). The MTNR1B SNP explained over one-quarter (26%) of the inter-individual variance in the effect of melatonin on the glucose AUC (Supplemental Table 1). The effect size per risk allele was large (1.17) for the influence of the genotype. Remarkably, the effect of melatonin was six times greater in the carriers of the risk allele than in the noncarriers (2.41 vs. 0.38 µU/mL × h; Table 2). Moreover, the effect of melatonin on glucose concentration 120 min after the start of the OGTT (C120) differed significantly between carriers and noncarriers (P=0.040; Table 2). Similar results, with a higher significance (P=0.017), were found after adjusting for age and BMI.
Figure 1.
Comparison between the effects of exogenous placebo and melatonin administrations on plasma glucose concentrations in response to an oral load of glucose in the morning and evening in carriers of the risk allele (CG) (top panels) and in non-carriers (CC) (middle panels). Responses of glucose during OGTT were examined as area under the curve above baseline across 120 minutes following the oral glucose load (AUC), calculated by the trapezoidal method. Furthermore, the time-dependent effects were tested by two-way ANOVA for time and treatment effects with repeated measurements. paired t-test was used to evaluate times in which variations were different. *Different from placebo at that time, P<0.05.
Differences between carriers of the MTNR1B risk variant (CG) and non-carriers (CC) in the effect of melatonin (melatonin-placebo) on AUC for glucose in the morning and in the evening (lower panels) were analyzed by unpaired t-test and further analyses were performed by ANCOVA in which effects were adjusted for age and BMI. Data are represented as mean ± SEM. TF, time fasting; T30, 60, 90, 120, time after OGTT (min).
Table 2.
Differences of the effect of melatonin (melatonin minus placebo) on glucose metabolism between MTNR1B risk allele carriers (CG) and non-risk carriers (CC).
| Glucose | Morning | Evening | ||||
|---|---|---|---|---|---|---|
| Risk carriers (CG) | Non-risk carriers (CC) | P | Risk carriers (CG) | Non-risk carriers (CC) | P | |
| AUC (Mel-Plac; mmol/L × h) | 2.41 ± 0.57 | 0.38 ± 0.53 | 0.036* | 2.97 ± 0.784 | 2.23 ± 1.18 | 0.596 |
| Cmax (Mel-Plac; mmol/L) | 2.36 ± 0.41 | 0.48 ± 0.56 | 0.237 | 2.32 ± 0.44 | 1.34 ± 0.593 | 0.183 |
| C120(Mel-Plac; mmol/L) | 1.11± 0.40 | −0.36± 0.47 | 0.040* | 1.68 ± 0.581 | 1.51 ± 0.787 | 0.870 |
| Insulin | Morning | Evening | ||||
|---|---|---|---|---|---|---|
| Risk carriers (CG) | Non-risk carriers (CC) | P | Risk carriers (CG) | Non-risk carriers (CC) | P | |
| AUC (Mel-Plac; µU/mL × h) | 22.99 ± 18.66 | −8.99 ± 16.11 | 0.274 | 36.64±5.95 | 52.23 ± 22.01 | 0.395 |
| Cmax (Mel-Plac; µU/mL) | 9.75 ± 15.00 | −2.07 ± 10.90 | 0.600 | 16.21 ± 8.13 | 45.53 ± 20.99 | 0.139 |
| C120(Mel-Plac; µU/mL) | 10.46 ± 9.73 | −1.71 ± 10.05 | 0.436 | 37.75 ± 12.68 | 32.30 ± 17.17 | 0.837 |
Mel: Melatonin; Plac: Placebo.
Data are represented as Mean ± SEM
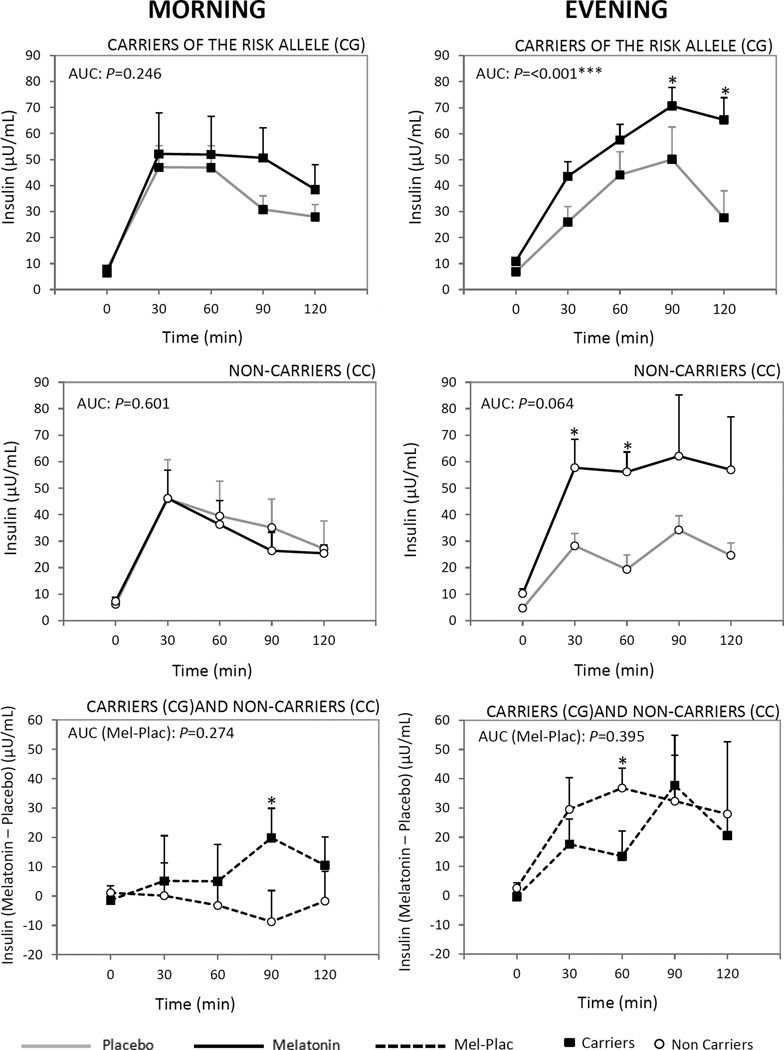
However, in the evening, no significant differences were found between the carriers and noncarriers in the effect of melatonin on glucose AUC (P=0.596) and on insulin measures (P=0.395) (Table 2, Fig.1 and Fig. 2).
Figure 2.
Comparison between the effects of exogenous placebo and melatonin administrations on plasma insulin concentrations in response to an oral load of insulin in the morning and evening in carriers of the risk allele (CG) (top panels) and in non-carriers (CC) (middle panels). Responses of glucose during OGTT were examined as area under the curve above baseline across 120 minutes following the oral insulin load (AUC), calculated by the trapezoidal method. Furthermore, the time-dependent effects were tested by two-way ANOVA for time and treatment effects with repeated measurements. Paired t-test was used to evaluate times in which variations were different. *Different from placebo at that time, P<0.05.
Differences between carriers of the MTNR1B risk variant (CG) and non-carriers (CC) in the effect of melatonin (melatonin-placebo) on AUC for glucose in the morning and in the evening (lower panels) were analyzed by unpaired t-test and further analyses were performed by ANCOVA in which effects were adjusted for age and BMI. Data are represented as mean ± SEM. TF, time fasting; T30, 60, 90, 120, time after OGTT (min).
A subsequent analysis at the different time points revealed significant differences between the effect of melatonin and the placebo on plasma glucose 60, 90, and 120 min after the start of the OGTT both in the morning and evening in the carriers of the risk allele (CG) (Fig. 1). However, no significant differences were found in the noncarriers (Fig. 1).
Genetic association analysis
The genetic association analysis confirmed a significant association between the MTNR1B SNP and the effect of melatonin on both glucose AUC (P=0.036) and glucose 120 min after the start of the OGTT (P=0.040), whereas no significant association was found for the derived measures (CIR, ISI, and DI; supplemental Table 1).
Discussion
We identified a significant effect of the common T2D risk variant MTNR1B on melatonin-induced impairment of glucose tolerance. The decrease in glucose tolerance induced by exogenous melatonin was observed only in the carriers of the risk variant and not in the noncarriers.
These results support the existence of inter-individual variation in the interaction between melatonin and the Mel1b receptor, with consequences for glucose tolerance. It is well known that variants affecting receptor function or receptor expression levels can lead to gain- or loss-of-function phenotypes. Previous results have shown increased MTNR1B expression in carriers of the rs10830963 risk genotype [1]. This would imply that the risk allele carriers have a gain-of-function phenotype, a hypothesis supported by the current results in which risk carriers showed an intensification of the deleterious effect of melatonin on glucose tolerance.
Previous data from our group has already demonstrated that daytime administration of melatonin diminishes glucose tolerance and increases estimates of insulin resistance in humans, both in the morning and in the evening [15]. The novel finding from the current work showing the differential effect of melatonin across genotypes at the MTNR1B locus on glucose tolerance extends our previous finding of the deleterious role of melatonin in glucose tolerance. The current data revealed a common variant that increased this deleterious effect of melatonin on glucose AUC by 6 fold as compared to that in the non-carriers.The relative risk of the genetic variant in glucose AUC was exceptionally large for genetic studies. These findings further support the integral link between melatonin and glucose tolerance.
A major limitation of all previous GWAS into the role of MTNR1B is that the assessments of glucose metabolism are based on daytime measurements when endogenous plasma melatonin concentrations are at their lowest. Plasma melatonin concentration has one of the most robust day/night rhythms of all hormones, with high levels during the night and near-undetectable levels during the day [13]. Thus, these daytime assessments cannot detect any direct effects of melatonin on glucose tolerance, or they may measure only the proverbial tip of the iceberg.
Our data suggest that when raising plasma melatonin concentrations to or above physiological nighttime levels, the MTNR1B risk variant causes substantially larger changes in glucose tolerance in carriers compared to noncarriers. Another interesting outcome from the present data is that the differences across genotypes for glucose tolerance were obtained even in normal-weight and nondiabetic participants. The rs10830963-G allele has recently been implicated in the transition of normoglycemia to a state of impaired fasting glucose [23]. It has been postulated that this particular variant promotes the progression from a normal to a prediabetic state rather than the progression from a prediabetic to a diabetic state [23]. Further interventional studies in shift workers will help to determine whether changing the timing of food intake to avoid concurrent exposure to melatonin can improve glucose tolerance.
We found no significant differences in the glucose and insulin AUC in the placebo condition between genotypes which suggests that during the time of assessment (9 A.M. and 9 P.M.) endogenous melatonin did not account for the differential effects between both genotypes with melatonin administration.
Future studies are required to test whether the magnitude of the effects of physiological nighttime concentrations of melatonin would be similar to those of exogenous melatonin administration. While it remains a question to what degree the Mel1b receptor plays a role in the sleep and circadian effects of melatonin, it will be of clinical importance to develop specific melatonin agonists that do not act on the Mel1b receptor and thereby prevent the melatonin-induced adverse metabolic effects.
The main strength of our study was the novel approach of testing the influence of the MTNR1B genotype on glucose control in the presence of the ligand of the MT2 receptor (i.e., melatonin) at different times of day (morning and evening). To date no such experimental study had been performed in humans to test the effect of the MTNR1B variant on glucose metabolism. One limitation is the low number of subjects. Future studies with a larger number of subjects are needed to confirm these results. Furthermore, our study population consisted of normal-weight and nondiabetic individuals. Further interventional studies in shift workers will help to determine whether changes the timing of food intake, to avoid concurrent exposure to melatonin, will improve glucose tolerance.
The strong effect and high prevalence of this genetic variant (MAF: ~30%; thus, 51% are carriers among a population of European ancestry), as well as the common use of melatonin supplementation (over-the-counter or by prescription), make our findings particularly relevant for clinical practice, not only for treatment but also for preventive purposes. Regarding the use of exogenous melatonin, identifying individuals with the rs10830963-G allele may be helpful to provide personalized recommendations about the relative timing of food consumption and melatonin administration.
In conclusion, we show that the MTNR1B risk variant worsens the effect of melatonin on glucose tolerance in humans. The detrimental effect of melatonin is particularly relevant in carriers of the risk allele in the morning. This novel finding points to the importance of genotyping the type 2 diabetes risk variant at MTNR1B to personalize the timing of melatonin administration and food intake with the aim of improving glucose tolerance. Further large-scale studies will be necessary to investigate the mechanistic contribution of pancreatic β-cell function and insulin sensitivity to the gene-ligand interaction and to translate these results into real-world clinically relevant recommendations.
Supplementary Material
Highlights.
-
-
Genetic link of MTNR1B variant rs10830963 with type 2 diabetes has been demonstrated.
-
-
The functional impact of this MTNR1B variant on metabolic physiology is poorly characterized.
-
-
To date no experimental study has been performed in humans in this respect.
-
-
In US 5–12 million adults are treating their sleep-related problems with melatonin.
-
-
We show in humans MTNR1B variant worsens the effect of melatonin on glucose tolerance.
Acknowledgments
Funding: This study was supported by grants from the Spanish Government of Science and Innovation (BFU2011-24720); SAF2014-52480-R, the Séneca Foundation from the Government of Murcia (15123/PI/10), and the NIH (R21 DK089378) to Richa Saxena and Frank AJL Scheer and NIH (R01 DK102696) to Frank AJL Scheer and Richa Saxena.
Abbreviations
- AUC
area under the curve
- CIR
corrected insulin response
- C120
concentration 120 min after the start of the OGTT
- DI
disposition index
- GWAS
Genome-wide-association studies
- ISI
Insulin Sensitivity Index
- MAF
minor allele frequency
- MT2
melatonin type-2 receptor Mel1b
- OGTT
oral glucose tolerance test
- SNP
single Nucleotide Polymorphism
- T2D
type-2 diabetes
- TF
time fasting
Footnotes
Disclosure statement: The authors have declared that no competing interests exist.
Contribution statement: Marta Garaulet designed the research, wrote the paper, and had primary responsibility for the final content. Purificación Gómez-Abellán collected and analyzed the data. Patricia Rubio-Sastre collected and analyzed the data and recruited the study participants. Juan A. Madrid designed the research. Richa Saxena designed the research, wrote the paper, and performed the genotyping. Frank AJL Scheer designed the research, wrote the paper, and had primary responsibility for the final content. All the authors have read and approved the final manuscript.
Contributor Information
Marta Garaulet, Email: garaulet@um.es.
Purificación Gómez-Abellán, Email: puriki4@hotmail.com.
Patricia Rubio-Sastre, Email: patrirru@hotmail.com.
Juan A Madrid, Email: jamadrid@um.es.
Frank AJL Scheer, Email: fscheer@bwh.harvard.edu.
References
- 1.Lyssenko V, Nagorny CL, Erdos MR, Wierup N, Jonsson A, Spegel P, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nature Genetics. 2009;41:82–88. doi: 10.1038/ng.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. Variants in MTNR1B influence fasting glucose levels. Nature Genetics. 2009;41:77–81. doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soranzo N, Sanna S, Wheeler E, Gieger C, Radke D, Dupuis J, et al. Common variants at 10 genomic loci influence hemoglobin A(1)(C) levels via glycemic and nonglycemic pathways. Diabetes. 2010;59:3229–3239. doi: 10.2337/db10-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnefond A, Clement N, Fawcett K, Yengo L, Vaillant E, Guillaume JL, et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nature Genetics. 2012;44:297–301. doi: 10.1038/ng.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dashti HS, Follis JL, Smith CE, Tanaka T, Garaulet M, Gottlieb DJ, et al. Gene-Environment interactions of circadian-related genes for cardiometabolic traits. Diabetes Care. 2015 doi: 10.2337/dc14-2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouatia-Naji N, Bonnefond A, Cavalcanti-Proenca C, Sparso T, Holmkvist J, Marchand M, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nature Genetics. 2009;41:89–94. doi: 10.1038/ng.277. [DOI] [PubMed] [Google Scholar]
- 7.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segre AV, Steinthorsdottir V, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nature Genetics. 2012;44:981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nature Genetics. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Q, Chen ZX, Wang YC, Ma YS, Zhang F, Che W, et al. Association between the melatonin receptor 1B gene polymorphism on the risk of type 2 diabetes, impaired glucose regulation: a meta-analysis. PloS one. 2012;7:e50107. doi: 10.1371/journal.pone.0050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparso T, Bonnefond A, Andersson E, Bouatia-Naji N, Holmkvist J, Wegner L, et al. G-allele of intronic rs10830963 in MTNR1B confers increased risk of impaired fasting glycemia and type 2 diabetes through an impaired glucose-stimulated insulin release: studies involving 19,605 Europeans. Diabetes. 2009;58:1450–1456. doi: 10.2337/db08-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimas AS, Lagou V, Barker A, Knowles JW, Magi R, Hivert MF, et al. Impact of type 2 diabetes susceptibility variants on quantitative glycemic traits reveals mechanistic heterogeneity. 2013 Dec 2; doi: 10.2337/db13-0949. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonsson A, Ladenvall C, Ahluwalia TS, Kravic J, Krus U, Taneera J, et al. Effects of common genetic variants associated with type 2 diabetes and glycemic traits on alpha- and beta-cell function and insulin action in humans. Diabetes. 2013;62:2978–2983. doi: 10.2337/db12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheer FA, Czeisler CA. Melatonin, sleep, and circadian rhythms. Sleep Medicine Reviews. 2005;9:5–9. doi: 10.1016/j.smrv.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Cagnacci A, Arangino S, Renzi A, Paoletti AM, Melis GB, Cagnacci P, et al. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clinical Endocrinology. 2001;54:339–346. doi: 10.1046/j.1365-2265.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 15.Garaulet M, Madrid JA. Methods for monitoring the functional status of the circadian system in dietary surveys studies: application criteria and interpretation of results. Nutricion Hospitalaria: Organo Oficial de la Sociedad Espanola de Nutricion Parenteral y Enteral. 2015;31(Suppl 3):279–289. doi: 10.3305/nh.2015.31.sup3.8776. [DOI] [PubMed] [Google Scholar]
- 16.Karamitri A, Renault N, Clement N, Guillaume JL, Jockers R. Minireview: Toward the establishment of a link between melatonin and glucose homeostasis: association of melatonin MT2 receptor variants with type 2 diabetes. Molecular Endocrinology (Baltimore, Md) 2013;27:1217–1233. doi: 10.1210/me.2013-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18:245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 18.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American Journal of Human Genetics. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. Routledge. 2013 [Google Scholar]
- 20.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 21.Sluiter WJ, Erkelens DW, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach I. Assay of the beta-cell response after oral glucose loading. Diabetes. 1976;25:241–244. doi: 10.2337/diab.25.4.241. [DOI] [PubMed] [Google Scholar]
- 22.Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. American Journal of Epidemiology. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 23.Walford GA, Green T, Neale B, Isakova T, Rotter JI, Grant SF, et al. Common genetic variants differentially influence the transition from clinically defined states of fasting glucose metabolism. Diabetologia. 2012;55:331–339. doi: 10.1007/s00125-011-2353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.She M, Laudon M, Yin W. Melatonin receptors in diabetes: a potential new therapeutical target? European Journal of Pharmacology. 2014;744:220–223. doi: 10.1016/j.ejphar.2014.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.