Structured Abstract
Background
Cognitive impairment is highly prevalent in patients with heart failure and is associated with adverse outcomes. However, whether specific cognitive abilities (e.g., memory versus executive function) are impaired in heart failure has not been fully examined. We investigated the prevalence of impairment in three cognitive domains in patients hospitalized with acute decompensated heart failure (ADHF) and the associations of impairment with demographic and clinical characteristics.
Methods
The sample included 744 patients hospitalized with ADHF (mean age = 72 years, 46% female) at 5 medical centers. Impairment was assessed in three cognitive domains (memory, processing speed, executive function) using standardized measures. Demographic and clinical characteristics were obtained from a structured interview and medical record review.
Results
A total of 593 of 744 (80%) patients were impaired in at least one cognitive domain; 32%, 31%, and 17% of patients were impaired in one, two, or all three cognitive domains, respectively. Patients impaired in more than one cognitive domain were significantly older, had less formal education, and had more non-cardiac comorbidities (all p’s < 0.05). In multivariable adjusted analyses, patients with older age and lower education had higher odds of impairment in two or more cognitive domains. Depressed patients had twice the odds of being impaired in all three cognitive domains (OR = 1.98, 95% CI: 1.08, 3.64).
Conclusion
Impairments in executive function, processing speed and memory are common among patients hospitalized for ADHF. Recognition of these prevalent cognitive deficits is critical for the clinical management of these high risk patients.
Introduction
Heart failure (HF) is a complex clinical syndrome that affects more than 5 million Americans and is a leading cause of hospital admissions among adults over age 65.1 Acute decompensated heart failure (ADHF) is characterized by progressively worsening symptoms that account for exacerbations, which frequently require hospitalization.1 Up to one quarter of patients with HF are readmitted within the first 30 days of hospital discharge, with ADHF accounting for the majority of rehospitalizations.2 There is currently a national focus to reduce HF associated readmissions through quality improvement initiatives.3 At least half of HF readmissions are considered preventable if patients follow recommended HF self-maintenance and management activities.4 They are instructed to monitor their condition regularly and to contact their healthcare provider in response to escalating symptoms and signs of possible HF.5
There is increasing attention being paid to the high rates of cognitive impairment (CI) among patients with cardiovascular disease. Multimorbidity in HF is common, and conditions accompanying HF, including atrial fibrillation6 and chronic kidney disease,7 are independently associated with cognitive decline. Cognitive function is impaired in up to three quarters of hospitalized patients with HF,8 with deficits in attention, memory, psychomotor speed and executive function being most pronounced.9, 10 Patients with HF, who are also cognitively impaired, are generally older, have less education, and have poorer clinical outcomes, including higher rates of mortality and rehospitalization than their non-impaired counterparts.11–14
CI has been implicated in inadequate self-care management, including the ability to recognize symptoms and to adhere to recommended self-monitoring and medication regimens.8, 15 Yet, CI is often under-recognized by healthcare providers.13 CI, when measured formally, could potentially improve HF teaching and reduce rehospitalizations, but measurements can be time consuming, and only recently has the National Institute of Neurological Disorders and Stroke (NINDS) recommended a standardized set of measures for bedside assessment of cognitive function in patients with cardiovascular disease.16 We administered three of these brief bedside measures to investigate the prevalence of impairment in memory, processing speed, and executive function in a large multicenter study of patients hospitalized with ADHF.
Methods
Patient Selection
Data were derived from an ancillary study to the Observational Study of Delay in Heart Failure, a prospective cohort study that has evaluated the extent of, and factors associated with, delays in hospital presentation among patients with ADHF.17, 18 Our ancillary study added measures of cognition and included 1,115 patients with ADHF who were enrolled between July 2007 and April, 2011 at five urban medical centers in Worcester (MA), Providence (RI), and Hamilton, Ontario. The study was approved by each institution’s Committee for the Protection of Human Subjects in Research.
Details of the study have been previously described.17, 18 Nurse and physician interviewers conducted daily reviews of computerized hospital admission data for patients with an admission diagnosis of possible HF (International Classification of Disease-9 code 428). Interviewers then performed a preliminary review of each patient’s medical record to determine if patients satisfied the Framingham criteria for HF.19 Patients were excluded if their HF occurred in the setting of a myocardial infarction, after an invasive procedure (e.g., coronary artery bypass surgery), or due to iatrogenic volume loading. Patients who developed HF secondary to admission for another disease, those with dementia documented in their medical chart, those who screened positive for delirium using a standardized assessment method (Confusion Assessment Method20), and those who spoke languages other than English were also excluded.
Each patient satisfying the Framingham criteria for HF was approached by interviewers within 72-hours of admission. Consenting patients completed a structured 30-minute interview during their hospital admission that included a history of the patient’s chief complaint(s), additional presenting symptoms, and confidence in recognizing health related changes and in seeking medical care (scored on 5-point Likert Scales from ‘not at all confident’ to extremely confident’). Depressive symptoms were assessed during the in-person interview using the self-report, short form of the Geriatric Depression Scale (GDS) and scores of ≥2 (on a 5-point scale) were classified as having high depressive symptomatology.21 Additional information was collected from hospital charts regarding each patient’s demographic and clinical characteristics and medical comorbidities. We created composite scores of each patient’s total number of cardiac comorbid conditions (HF, coronary artery disease, myocardial infarction, hypertension, diabetes, stroke, atrial fibrillation, and peripheral vascular disease) and non-cardiac comorbid conditions (alcohol dependency/abuse, anemia, asthma/chronic obstructive pulmonary disease (COPD), renal failure, and depression) based on classification of comorbidity in previous studies.22, 23
Assessment of Cognitive Function
Interviewers assessed each patient’s cognition using standardized assessments with established sensitivity to mild CI.10, 16 The National Institute of Neurological Disorders and Stroke (NINDS) recommended a five minute protocol encompassing memory and executive function tests, combined with a supplemental processing speed tool, for bedside screening of CI in patients with cardiovascular disease.16 On average, cognitive assessments occurred during the second day of hospitalization. Interviewers administered the 5-word immediate and delayed memory task, a subscale of the Montreal Cognitive Assessment Battery (MoCA; www.mocatest.org), to assess memory; a score ≤2 on the delayed memory task reflected memory impairment based on the MoCA scoring guidelines.24 To assess executive function, patients performed a modified Controlled Oral Word Association Test (COWA), in which they named as many words beginning with the letter “f,” in 60 seconds.25 According to the MoCA scoring algorithm, impairment was defined as generating fewer than 11 words during the 60-second period. The Digit Symbol Substitution Test (DSST),26 whereby patients translated a number to symbol code using a given key in 90 seconds, measured processing speed and attention; a total of less than 27 consecutive correct responses signaled impairment.27 CI was examined in two ways: (1) number of domains impaired (0–3); (2) impairment in specific domains of cognitive function.
Statistical Analysis
Participants with complete data on all cognitive tasks were included in the final study sample. Demographic and clinical characteristics were compared according to CI status (number of domains impaired versus no impairment) using chi-square tests for categorical variables and t-tests for continuous variables. Multinomial logistic regression models estimated the odds ratio (OR) and accompanying 95% confidence intervals (CI) for clinical and demographic factors, including age, gender, education level, depression, recent HF-related hospitalization, and number of comorbidities and number of domains impaired. All analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC, USA).
Funding
Funding from the National Institutes of Health (grant #s: R01HL077248; K01AG033643; K23HL101991; KL2RR031981 and U01HL105268) supported data collection and manuscript preparation. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Results
Study Sample
A total of 1,115 participants were enrolled in this study, including 744 patients with complete results on cognitive tests; 343 enrollees were missing data on processing speed, 104 enrollees were missing data on memory, and 98 study participants were missing data on executive function. Compared to the 744 patients with complete data, the 371 patients excluded due to incomplete cognitive assessments were older (mean age 75 versus 72 years), were more likely to be female (52% versus 45%), and were more likely to have less than a high school education (39% versus 24%), as well as a history of diabetes (42% versus 35%) and stroke (19% versus 13%) (all p-values < 0.05).
Sample Characteristics
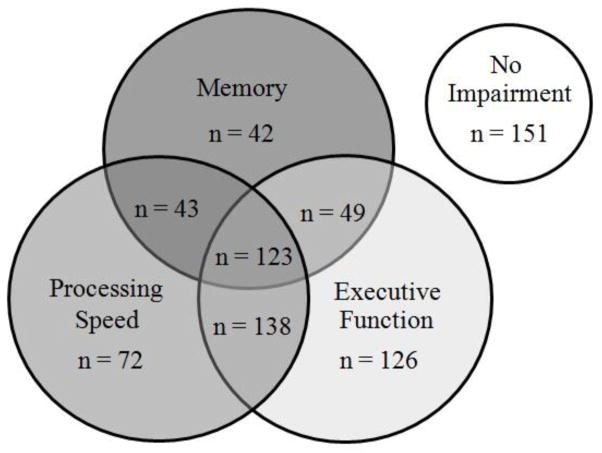
Of the 744 individuals in the study sample, their average age was 72 years, 45% were female, and 88% self-identified as Caucasian. More than three quarters of patients (80%) had impairment in at least one domain of cognitive function (Figure 1). Thirty five percent of patients were impaired in memory, 51% were impaired in processing speed, and 59% were impaired in executive function. Many patients were impaired in more than one domain. 32% of patients were impaired in one domain only, 31% were impaired in two domains and 17% were impaired in all three domains.
Figure 1.
Patients Categorized by Impairment in Cognitive Domains
Characteristics Associated with Severity of Cognitive Impairment
Patients with more domains impaired were significantly older (effects size = .31), had less formal education (effect size = .11), and had more non-cardiac comorbidities previously diagnosed (effect size = .10) (all p’s < 0.05) (Table 1). With increasing number of domains impaired, patients were less likely to report feeling confident in recognizing either general changes in their health (effect size = .13; p = 0.006) or HF symptoms requiring medical attention (effect size = .13; p = 0.004). The clinical histories and physical features did not vary by severity number of domains impaired, with the exception that pulmonary rales on lung exam were more common in patients with any, as compared to no, cognitive domains impaired (Table 2).
Table 1.
Sample Characteristics by Severity of Cognitive Impairment (CI)
| Characteristic | Number of Cognitive Domains Impaired | P-value | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| N= 151 | N=240 | N=230 | N=123 | ||
| Age, years, mean (SD) | 65 (13) | 70 (13) | 75 (12) | 77 (11) | <0.001 |
| Age, years, % | |||||
| <65 | 41 | 35 | 18 | 11 | |
| 65–74 | 30 | 22 | 24 | 20 | |
| 75–84 | 22 | 30 | 37 | 42 | |
| ≥85 | 7 | 13 | 21 | 27 | <0.001 |
| Female, % | 54 | 46 | 44 | 35 | 0.02 |
| White race, % | 87 | 91 | 88 | 82 | 0.11 |
| Education, % | |||||
| < High school | 9 | 16 | 33 | 43 | |
| High school/some college | 32 | 44 | 37 | 38 | |
| ≥ College graduate | 60 | 40 | 30 | 19 | <0.001 |
| Depression, % | 31 | 39 | 39 | 45 | 0.11 |
| Current smoker, % | 11 | 17 | 7 | 11 | 0.01 |
| Hospitalized previously for HF, % | 49 | 57 | 55 | 49 | 0.63 |
| Hospitalized w/in 3 months for HF, % | 20 | 29 | 24 | 26 | 0.09 |
| Attended HF Outpatient Clinic, % | 30 | 31 | 31 | 24 | 0.62 |
| Attended HF Outpatient Clinic w/in 3 months, % | 20 | 23 | 25 | 18 | 0.37 |
| Confidence recognizing changes in health, very/extremely confident, % | 65 | 54 | 57 | 45 | 0.006 |
| Confidence recognizing symptoms of HF, very/extremely confident, % | 60 | 57 | 57 | 45 | 0.07 |
| Confidence recognizing symptoms requiring medical contact, very/extremely confident, % | 60 | 58 | 54 | 40 | 0.004 |
| Confidence recognizing symptoms requiring 911, very/extremely confident, % | 72 | 74 | 66 | 58 | 0.006 |
| Medical History, % | |||||
| Heart failure | 66 | 70 | 71 | 81 | 0.03 |
| Coronary artery disease | 56 | 54 | 57 | 59 | 0.78 |
| Myocardial infarction | 36 | 30 | 32 | 30 | 0.61 |
| Peripheral vascular disease | 17 | 21 | 17 | 21 | 0.49 |
| Hypertension | 79 | 77 | 83 | 79 | 0.44 |
| Diabetes | 36 | 32 | 36 | 38 | 0.61 |
| Stroke | 8 | 10 | 16 | 19 | 0.15 |
| Atrial fibrillation | 38 | 43 | 50 | 47 | 0.12 |
| Alcohol abuse/dependency | 7 | 3 | 6 | 8 | 0.69 |
| Anemia | 18 | 18 | 25 | 19 | 0.18 |
| Asthma/COPD | 23 | 36 | 28 | 31 | 0.06 |
| Renal failure | 27 | 34 | 38 | 39 | 0.11 |
| Implantable defribrillator | 13 | 8 | 12 | 15 | 0.25 |
| Pacemaker | 20 | 17 | 22 | 19 | 0.63 |
| Total cardiac comorbid conditions, mean (SD) | 3.4 (1.5) | 3.4 (1.6) | 3.6 (1.5) | 3.7 (1.6) | 0.06 |
| Total non-cardiac comorbid conditions, mean (SD) | 0.9 (1.0) | 1.1 (0.9) | 1.1 (1.0) | 1.1 (0.9) | 0.04 |
Table 2.
Vital Signs and Clinical Exam Findings on Admission by Severity of Cognitive Impairment (CI)
| Characteristic | Number of Domains Impaired | P-value | |||
|---|---|---|---|---|---|
| None | 1 | 2 | 3 | ||
| N= 151 | N=240 | N=230 | N=123 | ||
| Vital signs, mean (SD) | |||||
| Diastolic blood pressure | 77 (20) | 75 (20) | 76 (20) | 75 (20) | 0.78 |
| Systolic blood pressure | 140 (29) | 142 (31) | 140 (30) | 140 (31) | 0.91 |
| Heart rate, bpm | 87 (22) | 84 (21) | 85 (21) | 84 (22) | 0.68 |
| Respiratory rate | 22 (5) | 22 (5) | 22 (5) | 22 (5) | 0.37 |
| Clinical exam findings, % | |||||
| Lower extremity edema | 71 | 78 | 77 | 80 | 0.31 |
| Jugular venous distention | 52 | 50 | 51 | 50 | 0.97 |
| Dyspnea | 71 | 64 | 70 | 65 | 0.31 |
| Pulmonary edema | 25 | 25 | 25 | 23 | 0.95 |
| Pulmonary rales | 77 | 87 | 84 | 85 | 0.04 |
| S3 gallop | 6 | 5 | 3 | 2 | 0.24 |
| S4 gallop | 1 | 3 | 1 | 1 | 0.26 |
In multinomial logistic regression models (Table 3), there was a dose-response relationship between age and the number of cognitive domains impaired; persons 65–84 years old had a significantly higher odds of having 2 or 3 domains impaired compared to those <65 years, and those 85 and older had a greater odds of having any impairment. Patients with higher formal education had a lower odds of having 2 or 3 domains impaired, and individuals with depression had twice the odds of impairment in all three domains assessed (OR = 1.98, 95% CI: 1.08, 3.64).
Table 3.
Factors Associated with Severity of Cognitive Impairment (CI)
| Characteristic | Number of Domains Impaired | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| N=224 | N=215 | N=114 | |
|
| |||
| OR (95% CI) | |||
| Age | |||
| <65 | (Referent)† | (Referent) | (Referent) |
| 65–74 | 0.83 (0.46–1.52) | 2.56 (1.30–5.03) | 4.34 (1.71–10.98) |
| 75–84 | 1.50 (0.80–2.82) | 5.77 (2.85–11.68) | 12.22 (4.89–30.58) |
| ≥85 | 3.82 (1.48–9.88) | 22.44 (8.36–60.21) | 65.82 (20.22–214.28) |
| Female | 0.51 (0.31–0.82) | 0.31 (0.18 – 0.51) | 0.18 (0.10 – 0.33) |
| White race | 1.43 (0.68–3.02) | 0.89 (0.41–1.96) | 0.48 (0.20–1.15) |
| Education | |||
| < High school | (Referent) | (Referent) | (Referent) |
| High school/some college | 0.68 (0.31–1.48) | 0.23 (0.10–0.49) | 0.18 (0.08–0.41) |
| ≥ College graduate | 0.31 (0.15–0.67) | 0.10 (0.05–0.21) | 0.04 (0.02–0.11) |
| Depression | 1.56 (0.96–2.55) | 1.52 (0.91–2.55) | 1.98 (1.08–3.64) |
| Hospitalized w/in 3 months for HF | 0.69 (0.42–1.14) | 0.99 (0.59–1.65) | 0.84 (0.46–1.53) |
| Confidence recognizing changes in health, very/extremely confident | 0.66 (0.39–1.16) | 0.87 (0.49–1.54) | 0.75 (0.38–1.47) |
| Confidence recognizing symptoms of HF, very/extremely confident | 0.79 (0.60–1.03) | 1.01 (0.76–1.34) | 0.88 (0.62–1.26) |
| Confidence recognizing symptoms requiring medical contact, very/extremely confident§ | 0.93 (0.52–1.65) | 0.89 (0.49–1.64) | 0.55 (0.27–1.12) |
| Confidence recognizing symptoms requiring 911, very/extremely confident | 1.66 (0.90–3.08) | 1.17 (0.62–2.22) | 1.25 (0.61–2.58) |
| Total cardiac comorbid conditions | 0.97 (0.83–1.13) | 1.00 (0.85–1.18) | 1.00 (0.82–1.22) |
| Total non-cardiac comorbid conditions | 1.36 (1.06–1.75) | 1.27 (0.98–1.66) | 1.16 (0.84–1.60) |
| Clinical exam findings | |||
| Pulmonary rales | 1.70 (0.95–3.03) | 1.45 (0.78–2.68) | 1.41 (0.66–3.03) |
Referent group consists of those patients with no cognitive impairment
Discussion
The current study examined the prevalence of CI in a large sample of older patients hospitalized with ADHF. More than three quarters exhibited impairment in at least one cognitive domain. There was also considerable overlap in impairment among domains, with one third of patients impaired in two domains and more that 15% impaired in three domains. Patients with impairment in more domains tended to be older, male, have less formal education, and screen positive for depression.
The rate of cognitive impairment, not dementia (CIND) in a nationally representative sample of persons 72 and older the Aging, Demographic, and Memory Study (ADAMS) was 60.4 cases per 1,000 person years.28 The frequency of impairment in any cognitive domain in our sample was 80%, nearly twice the documented prevalence of mild CI in patients with either a history of stroke29 or moderate to severe COPD.30 The rate of CI in patients hospitalized with ADHF varies widely from 25–75% in the literature.8, 11, 13 The primary reason for this discrepancy is likely related to the variation in cognitive assessments used, with most studies employing tests of global cognitive function (e.g., the Mini-Mental State Exam (MMSE)) in their study designs.11, 13, 31 These tools have limitations in detecting CI associated with cardiovascular disease due to their emphasis on memory over executive function and processing speed, the latter of which decline earlier in the disease course.16, 29
Our study extends the current literature on CI in hospitalized patients with HF by administering three brief cognitive instruments recommended by the NINDS for detection of CI in patients with underlying vascular disease rather than a test of global cognitive function.16 These highly sensitive tests, administered within five minutes, examine the cognitive domains of executive function, processing speed, and memory that are commonly impaired in patients with HF.9, 10, 16 Deficits in these cognitive domains have been implicated in increased HF severity10 and cardiovascular related mortality.9 Our results support previous findings, which showed relatively preserved memory in HF patients with mild CI.10 Only one third of our study participants exhibited deficits in delayed recall, compared to over one half of individuals with impairment in either executive function or processing speed. In addition, when memory was impaired, it was accompanied by deficits in a second cognitive domain 83% of the time, highlighting that memory may decline later than processing speed and executive function in patients with HF.
Whereas cognitive decline is a well-known phenomenon in ambulatory patients with HF,32 CI may be under-recognized in hospitalized patients with ADHF. In a recent study of 282 hospitalized elderly patients with ADHF, approximately 50% of patients demonstrated CI based on standardized tests, but only 20% of these individuals had documentation of CI at the time of hospital discharge.13 Patients with under-reported CI had a higher likelihood of dying or being rehospitalized at six months of discharge. These findings illustrate the importance of recognition and documentation of CI before discharge, when patients receive preliminary HF management instructions. Brief screening tools, like those administered in this current study, may enhance the feasibility of screening for CI in the inpatient population.
To date, there has been mixed success with regards to HF management programs, with some studies failing to demonstrate long-term reductions in mortality and frequency of rehospitalization.33, 34 In response to clinical deterioration, patients are advised to restrict fluid and salt intake, modify their diuretic dose, and seek medical attention.5 However, adherence to these guidelines is suboptimal, even following hospitalization, when patients receive self-care management education.35, 36 CI may predispose patients to readmission through impaired learning and implementation of self-monitoring strategies.12, 37 CI could also contribute to difficulties in appreciating, interpreting, and promptly responding to worsening signs and symptoms.8, 37 Interestingly, cognitively impaired patients in our study reported less confidence in recognizing changes in their general health or HF symptoms requiring medical attention.
The collective findings suggest that cognitive status may be an important mediator of HF disease exacerbation and self-care behavior. Although there is considerable controversy regarding the benefit of cognitive screening for treatment decision making and clinical outcomes,38, 39 there is also acknowledgement of variability in cognitive function around the time of an acute illness or hospitalization, which has been described as part of the post-hospital syndrome.40, 41 Our findings suggest that there is a high degree of cognitive impairment in older patients hospitalized with ADHF that could impact uptake of discharge instructions and education on self-management. Regular cognitive assessments, combined with increased surveillance and tailored education in patients who are cognitively impaired may enhance clinical outcomes in these high risk patients.
Study Strengths and Limitations
The strengths of the present study include the large number of patients with ADHF who were interviewed using standardized data collection instruments. Assessment of domains of CI, examined using validated instruments amenable to bedside administration, is also unique in this context. We were unable to systematically characterize the severity of ADHF due to missing data on ejection fraction and New York Heart Association (NYHA) Class. Due to the logistical complexities of administration of the paper and pencil DSST, we had a high degree of missing data on this test; we also excluded patients with dementia, delirium, severe sensory impairments and those who were aphasic, reducing the generalizability of our findings and potentially underestimating the prevalence of cognitive impairment. In addition, since our cognitive measures were (by design) brief screening instruments we were unable to examine degree of impairment within domain and rather examined impaired vs. non-impaired. Although all tests in the screening battery have been validated and were recommended as a bedside screener,16 the tests have not been validated together as a screening instrument. Although we screened for delirium using the CAM, it is possible that we enrolled patients with subsyndromal delirium or those who developed delirium after our study interview and these patients would be more likely to be cognitively impaired. In addition, our sample consisted of patients hospitalized with ADHF, and our conclusions cannot be extrapolated to patients who seek treatment exclusively at outpatient facilities. Another limitation was the large amount of missing data for results of the Digit Symbol Substitution Test, the measure used to assess processing speed. Nevertheless, compared to patients with complete data, those with missing data on the DSST were not significantly different with respect to most demographic and clinical characteristics. We also do not know if the CI observed in our sample is a temporary phenomenon or if it persists after discharge, although inpatient interventions that enhance cardiac performance have been shown to improve cognitive function.42
Conclusions
The current study demonstrates that impairment in 3 important cognitive domains: memory, processing speed, and executive function, is common in patients admitted with ADHF. CI may impact a patient’s ability to recognize and respond to changes in their underlying symptoms and to seek care in a timely manner. Consequently, the findings have direct clinical relevance to the management of these high risk and medically complex patients.
Acknowledgments
Funding Sources
This study was supported by NIH grant R01 HL077248 (Dr. Goldberg) and NIH grant K23 HL101991 (Dr. Darling). Drs. Goldberg, McManus and Saczynski also receive funding support from NIH1U01 HL105268. Dr. Saczynski is supported by award number K01AG033643 from the National Institute on Aging, and Dr. McManus receives additional support from NIH grant KL2RR031981.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL. Epidemiology of heart failure. Circ Res. 2013 Aug 30;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013 Jan 23;309(4):355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan J, Kang S, Dolacky S, Ingrassia J, Ganeshan R. Change in readmissions and follow-up visits as part of a heart failure readmission quality improvement initiative. Am J Med. 2013 Nov;126(11):989–994. e981. doi: 10.1016/j.amjmed.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998 Nov;80(5):437–441. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lainscak M, Blue L, Clark AL, et al. Self-care management of heart failure: practical recommendations from the Patient Care Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2011 Feb;13(2):115–126. doi: 10.1093/eurjhf/hfq219. [DOI] [PubMed] [Google Scholar]
- 6.Thacker EL, McKnight B, Psaty BM, et al. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013 Jul 9;81(2):119–125. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013 Feb;24(3):353–363. doi: 10.1681/ASN.2012050536. [DOI] [PubMed] [Google Scholar]
- 8.Cameron J, Worrall-Carter L, Page K, Riegel B, Lo SK, Stewart S. Does cognitive impairment predict poor self-care in patients with heart failure? Eur J Heart Fail. 2010 May;12(5):508–515. doi: 10.1093/eurjhf/hfq042. [DOI] [PubMed] [Google Scholar]
- 9.Pressler SJ, Kim J, Riley P, Ronis DL, Gradus-Pizlo I. Memory dysfunction, psychomotor slowing, and decreased executive function predict mortality in patients with heart failure and low ejection fraction. J Card Fail. 2010 Sep;16(9):750–760. doi: 10.1016/j.cardfail.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harkness K, Demers C, Heckman GA, McKelvie RS. Screening for cognitive deficits using the Montreal cognitive assessment tool in outpatients >/=65 years of age with heart failure. Am J Cardiol. 2011 Apr 15;107(8):1203–1207. doi: 10.1016/j.amjcard.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Zuccala G, Pedone C, Cesari M, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. Am J Med. 2003 Aug 1;115(2):97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 12.McLennan SN, Pearson SA, Cameron J, Stewart S. Prognostic importance of cognitive impairment in chronic heart failure patients: does specialist management make a difference? Eur J Heart Fail. 2006 Aug;8(5):494–501. doi: 10.1016/j.ejheart.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. Am J Med. 2013 Feb;126(2):120–126. doi: 10.1016/j.amjmed.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kindermann I, Fischer D, Karbach J, et al. Cognitive function in patients with decompensated heart failure: the Cognitive Impairment in Heart Failure (CogImpair-HF) study. European Journal of Heart Failure. 2012;14(4):404–413. doi: 10.1093/eurjhf/hfs015. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins LA, Kilian S, Firek A, Kashner TM, Firek CJ, Silvet H. Cognitive impairment and medication adherence in outpatients with heart failure. Heart Lung. 2012 Nov-Dec;41(6):572–582. doi: 10.1016/j.hrtlng.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006 Sep;37(9):2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg RJ, Goldberg JH, Pruell S, et al. Delays in seeking medical care in hospitalized patients with decompensated heart failure. Am J Med. 2008 Mar;121(3):212–218. doi: 10.1016/j.amjmed.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darling C, Saczynski JS, McManus DD, Lessard D, Spencer FA, Goldberg RJ. Delayed hospital presentation in acute decompensated heart failure: clinical and patient reported factors. Heart Lung. 2013 Jul-Aug;42(4):281–286. doi: 10.1016/j.hrtlng.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993 Jul;88(1):107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001 Dec 5;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003 May;51(5):694–698. doi: 10.1034/j.1600-0579.2003.00216.x. [DOI] [PubMed] [Google Scholar]
- 22.Saczynski JS, Go AS, Magid DJ, et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc. 2013 Jan;61(1):26–33. doi: 10.1111/jgs.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McManus DD, Nguyen HL, Saczynski JS, Tisminetzky M, Bourell P, Goldberg RJ. Multiple cardiovascular comorbidities and acute myocardial infarction: temporal trends (1990–2007) and impact on death rates at 30 days and 1 year. Clin Epidemiol. 2012;4:115–123. doi: 10.2147/CLEP.S30883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005 Apr;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 25.Benton AL. Development of a multilingual aphasia battery. Progress and problems. J Neurol Sci. 1969 Jul-Aug;9(1):39–48. doi: 10.1016/0022-510x(69)90057-4. [DOI] [PubMed] [Google Scholar]
- 26.Joy S, Fein D, Kaplan E, Freedman M. Quantifying qualitative features of Block Design performance among healthy older adults. Arch Clin Neuropsychol. 2001 Feb;16(2):157–170. [PubMed] [Google Scholar]
- 27.Madureira S, Verdelho A, Ferro J, et al. Development of a neuropsychological battery for the Leukoaraiosis and Disability in the Elderly Study (LADIS): experience and baseline data. Neuroepidemiology. 2006;27(2):101–116. doi: 10.1159/000095381. [DOI] [PubMed] [Google Scholar]
- 28.Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the united states. Annals of Neurology. 2011;70(3):418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke. 2012 Feb;43(2):464–469. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villeneuve S, Pepin V, Rahayel S, et al. Mild cognitive impairment in moderate to severe COPD: a preliminary study. Chest. 2012 Dec;142(6):1516–1523. doi: 10.1378/chest.11-3035. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Pascual C, Vilches-Moraga A, Paredes-Galan E, Ferrero-Marinez AI, Torrente-Carballido M, Rodriguez-Artalejo F. Comprehensive geriatric assessment and hospital mortality among older adults with decompensated heart failure. Am Heart J. 2012 Nov;164(5):756–762. doi: 10.1016/j.ahj.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Gure TR, Blaum CS, Giordani B, et al. Prevalence of cognitive impairment in older adults with heart failure. J Am Geriatr Soc. 2012 Sep;60(9):1724–1729. doi: 10.1111/j.1532-5415.2012.04097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaarsma T, van der Wal MH, Lesman-Leegte I, et al. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Arch Intern Med. 2008 Feb 11;168(3):316–324. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 34.Angermann CE, Stork S, Gelbrich G, et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail. 2012 Jan;5(1):25–35. doi: 10.1161/CIRCHEARTFAILURE.111.962969. [DOI] [PubMed] [Google Scholar]
- 35.Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005 Nov;150(5):984. doi: 10.1016/j.ahj.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 36.Artinian NT, Magnan M, Sloan M, Lange MP. Self-care behaviors among patients with heart failure. Heart Lung. 2002 May-Jun;31(3):161–172. doi: 10.1067/mhl.2002.123672. [DOI] [PubMed] [Google Scholar]
- 37.Hajduk AM, Lemon SC, McManus DD, et al. Cognitive impairment and self-care in heart failure. Clin Epidemiol. 2013;5:407–416. doi: 10.2147/CLEP.S44560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boustani M, Campbell N, Khan B, et al. Enhancing Care for Hospitalized Older Adults with Cognitive Impairment: A Randomized Controlled Trial. Journal of General Internal Medicine. 2012;27(5):561–567. doi: 10.1007/s11606-012-1994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moyer VA. Screening for Cognitive Impairment in Older Adults: U.S. Preventive Services Task Force Recommendation StatementScreening for Cognitive Impairment in Older Adults. Annals of Internal Medicine. 2014;160(11):791–797. doi: 10.7326/M14-0496. [DOI] [PubMed] [Google Scholar]
- 40.Inouye SK, Zhang Y, Han L, Leo-Summers L, Jones R, Marcantonio E. Recoverable cognitive dysfunction at hospital admission in older persons during acute illness. J Gen Intern Med. 2006 Dec;21(12):1276–1281. doi: 10.1111/j.1525-1497.2006.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krumholz HM. Post-Hospital Syndrome — An Acquired, Transient Condition of Generalized Risk. New England Journal of Medicine. 2013;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajduk AM, Kiefe CI, Person SD, Gore JG, Saczynski JS. Cognitive change in heart failure: a systematic review. Circ Cardiovasc Qual Outcomes. 2013 Jul;6(4):451–460. doi: 10.1161/CIRCOUTCOMES.113.000121. [DOI] [PMC free article] [PubMed] [Google Scholar]