Abstract
Despite the limited success of immunotherapies in solid malignancy, two human cancers, melanoma and renal cancer, have, for many years, responded to systemic administration of immune-targeted biologics and showed signals of response to certain therapeutic vaccines. These findings underpinned a long-held perception that melanoma and renal cancer were uniquely "immunogenic" but that virtually all other human cancers were not and thus would not respond to immune modulation. That notion has now been shattered by the significant and durable responses in non–small cell lung cancer induced by therapeutic treatment with antibodies blocking the PD-1 checkpoint. The immunotherapy success in lung cancer thus provides a gateway to development of treatments for multiple cancer types that were previously believed not accessible to immune-based therapies.
Introduction
Even with the approval of immune system-targeted biologics such as IFN, interleukin-2 (IL-2), and anti-CTLA-4 monoclonal antibody (mAb) in melanoma and kidney cancer, immunotherapy has, until recently, not had a significant impact on the treatment of lung cancer. Despite multiple trials of lung cancer vaccines, few objective responses were observed and none have yet shown a clear survival benefit in randomized trials (1); the most recent being a phase III trial of the Liposomal-BLP MUC-1 peptide vaccine given after definitive chemotherapy and radiotherapy in stage III non–small cell lung cancer (NSCLC; ref. 2). As more is learned about the biology of lung cancers and their immune microenvironment, a number of specific mechanisms of immune resistance have emerged that are particularly relevant to T-cell responses. Taken together, these insights, along with the clinical results from blockade of the programmed death-1 (PD-1) checkpoint (see below) suggest that a repertoire of tumor-specific or tumor-selective T cells indeed exists in many patients with lung cancer and this latent pool can be mobilized therapeutically once specific resistance mechanisms are blocked. While multiple immune effector mechanisms, both innate and adaptive, can be brought to bear against lung cancer, the focus of most translational efforts is directed at T cells. However, as will be discussed in the last section, opportunities to activate both innate and adaptive immune effector mechanisms in concert offer particular promise for the future.
Immune Resistance Mechanisms in Lung Cancer
Direct T-cell recognition of tumor cells requires the presentation of antigenic peptides by MHC molecules. These peptides are generated by proteasomal digestion and transported to the endoplasmic reticulum, where they are first loaded onto nascent MHC molecules, which ultimately transport them to the cell membrane. A significant proportion of lung cancers downregulates components of the antigen-presenting machinery such as the immunoproteasome subunits LMP2 and LMP7, the antigenic peptide transporters TAP1 and TAP2, and the MHC molecules. The downregulation is most commonly via epigenetic mechanisms but it can also involve mutation (3–5). These alterations represent fundamental "immune resistance" mechanisms that help explain how lung and other cancers evade detection and killing by T cells.
Suppression of the antigen-presenting machinery is likely a particularly important immune resistance mechanism for smoking- and pollution-associated lung cancers because these tumors possess among the highest density of missense mutations in expressed genes of any cancer type (roughly 12 mutations per megabase of expressed exonic sequence; ref. 6). These genetic alterations, together with activation of many genes due to epigenetic dysregulation (including induction of cancer-testes antigens that are otherwise only expressed on germ cells), endow lung cancer cells with huge numbers of tumor-specific and tumor-selective neoantigens that are able to be recognized by T cells. Restifo and colleagues showed that, in the majority of lung cancer cell lines, suppressed antigen-presenting molecules could be upregulated by IFN-γ (5). This finding is highly relevant to immunotherapy because it suggests that if T cells or NK cells (the two major producers of IFN-γ) could be activated within the tumor microenvironment, suppression of tumor antigen presentation can be reversed in the majority of lung cancers.
Given the plethora of potential antigenic targets in lung cancer, it has also been postulated that they can escape immune rejection by either "editing" out particularly immunogenic neoepitopes (7) or through the induction of antigen-specific tolerance (8, 9). These mechanisms are quite different: editing implies that T-cell recognition of a tumor neoantigen has resulted in selection for antigen-loss variants, whereas tolerance induction implies that tumor-specific T cells have been rendered incapable of attacking antigen-bearing cells. Evidence for both processes has been produced in a murine model of lung carcinogenesis created by pulmonary instillation of a replication-defective lentivirus encoding cre plus a foreign antigen into mice bearing an oncogenically mutated K-ras gene whose promoter contains a lox-stop-lox cassette. In this model, only infected pulmonary epithelial cells transform and express the foreign antigen as a tumor-specific neoantigen (10). Transfer of T cells specific for "neoantigens" into these mice early after transformation can induce editing, whereas T cells transferred later can slow tumor growth, but eventually the transferred T cells are rendered tolerant and ultimately deleted from the tumor microenvironment. The relative importance of editing versus tolerance induction in human lung cancer remains to be determined. A subset of small cell lung cancers does elicit CD4-dependent antibody and even CD8 T-cell responses against neuronal antigens ectopically expressed by this tumor type (11, 12). These antineuronal antigen responses are generally associated with paraneoplastic neurologic syndromes (PNS) that are thought to be mediated by the induced immune responses. The presence of PNS is frequently associated with limited disease, better prognosis, and rarely spontaneous regressions of this normally aggressive cancer type (13).
In NSCLC, the most therapeutically relevant mechanism for immune resistance is expression of immune inhibitory molecules in the tumor microenvironment. These molecules fall into a number of classes based on the nature of the inhibitory ligand: cytokines, membrane ligands, and metabolites. The two inhibitory cytokines commonly expressed in lung cancers are IL-10 and TGF-β. Among the membrane inhibitory ligands (so-called checkpoint ligands), PD-L1 has been the most studied in NSCLC, though PD-L2, B7-H3, and B7-H4 have also been reported as upregulated in lung cancer (14, 15). PD-L1 is expressed on tumor cells in roughly half of NSCLC but is sometimes expressed on myeloid cells in the stroma surrounding tumor nests. Grossly, increased numbers of CD4+ and CD8+ tumor-infiltrating T-lymphocytes (TIL) have been reported to be a good prognostic factor in lung cancer, whereas increased numbers of Foxp3+ TIL have been reported to be a poor prognostic factor (16, 17); these are relatively crude analyses and much more work needs to done to assess the biologic and therapeutic relevance of expression patterns of multiple inhibitory ligands as well as the distribution and expression pattern of their cognate receptors on TILs. Though not often considered, immune inhibitory metabolites are likely important players in local immune resistance in lung cancer. Concentrations of adenosine, which binds to the inhibitory G-protein-coupled A2a receptor expressed on lymphocytes, have been shown to be extremely high in NSCLC tissue. A2aR-triggering both inhibits effector T-cell function and drives the development of Tregs, another inhibitory component of the tumor microenvironment (18, 19).
Relevant to the immune inhibitory ligand-receptor interactions in the tumor microenvironment, a major turning point in cancer immunotherapy came with the clinical application of antibodies that block immune checkpoints. Of the many molecularly defined checkpoint ligands and receptors (reviewed in refs. 14, 15), blockers of only two pathways, CTLA-4 and PD-1, have been tested clinically to date. In the case of the PD-1 pathway, both PD-1 and one of its ligands, PD-L1, have been blocked. These two checkpoints seem to modulate very distinct components of T-cell immunity. CTLA-4 counterbalances the costimulatory signals delivered by CD28 during T-cell activation—both bind the B7 family ligands, B7.1 and B7.2 (reviewed in ref. 20; Fig. 1). PD-1 is also induced upon T-cell activation but seems to predominantly downmodulate T-cell responses in tissues. The PD-1 ligands, PD-L1 and PD-L2, are induced by distinct inflammatory cytokines—while PD-L1 expression can be induced on diverse epithelial and hematopoietic cell types, PD-L2 is predominantly expressed on dendritic cells (DC) and macrophages (21–24). Both CTLA-4 and PD-1 pathway-blocking antibodies have shown activity in lung cancer and, as mentioned above, the responses to anti-PD-1 and anti-PD-L1 monotherapy in NSCLC have garnered significant attention by the lung cancer community (reviewed in ref. 1).
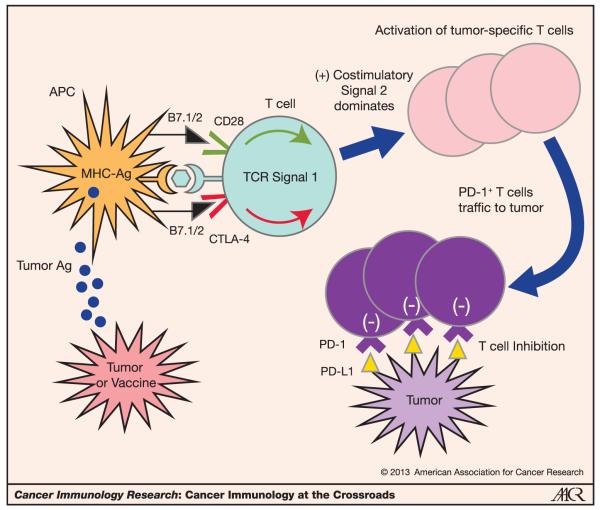
Figure 1.
Distinct roles of CTLA-4 and PD-1 in the regulation of antitumor T-cell responses. CD28 is the master costimulatory receptor expressed on T cells and enhances T-cell activation upon antigen recognition when the antigen presenting cell (APC) expresses its ligands, B7-1 and B7-2. Tumor or tumor vaccine is the source of tumor antigens that must be processed and presented by the MHC complex to activate T cells. CTLA-4 is rapidly expressed on T cells once antigen is recognized, and it binds the same ligands (B7.1/2) as CD28 but at higher affinity, thereby counterbalancing the costimulatory effects of CD28 on T-cell activation. Tumor-specific T-cell activation leads to proliferations and effector function, but also the upregulation of PD-1. After trafficking to the tumor microenvironment, PD-1+ T cells might encounter PD-1 ligands, which can inhibit them from mediating their killing function. Thus, the CTLA-4 and PD-1 pathways provide complementary mechanisms to regulate antitumor effector T cells, and blocking each one may prove to be synergistic.
CTLA-4 Inhibitors in Lung Cancer
In contrast with melanoma (25), ipilimumab, an anti-CTLA-4 blocking mAb has virtually no effect as a single agent in lung cancer (26); however, it does seem to provide modest benefit in NSCLC as well as small cell lung cancer (SCLC) when tested in combination with chemotherapy. A randomized phase II trial of two different schedules of combining ipilimumab with standard chemotherapy compared with standard combination chemotherapy (paclitaxel and carboplatin) alone was reported recently. Patients with advanced NSCLC and SCLC were randomized to paclitaxel and carboplatin versus a "phased" schedule of chemotherapy alone for 2 cycles followed by ipilimumab combined with the chemotherapy regimen for 4 additional cycles versus concurrent administration of chemotherapy with ipilimumab upfront for 4 cycles followed by 2 cycles of chemotherapy alone (27). If patients had stable or responding disease, patients were given ipilimumab "maintenance" once every 12 weeks until progression.
Evaluation of patient responses and progression-free survival (PFS) used immune-related response criteria, which take into account tumor regression in the face of new lesions—a unique pattern of response that can be seen with checkpoint blockade. The study showed that addition of ipilimumab improved immune-related PFS (ir-PFS) in patients with advanced NSCLC. On the basis of the immune mechanism of action of ipilimumab, the immune-related grades 3 and 4 side effects were increased in the two ipilimumab-containing arms; however, the overall side effects were not significantly different. The phased schedule significantly improved the ir-PFS compared with that of the control arm (median 5.7 months vs. 4.6 months respectively, HR 0.72; P = 0.05), whereas the concurrent schedule did not improve the ir-PFS or overall survival (OS). The overall survival for the phased arm was 12.2 months compared with 8.3 months in the control arm but this difference did not achieve statistical significance. Interestingly, in the phased schedule group, the ir-PFS was significantly improved in patients with squamous cell histology and not in patients with nonsquamous histology (mostly adenocarcinoma). The OS was only improved in the patients with squamous histology. Alternatively, for the nonsquamous histology group, the phased schedule resulted in worse OS compared with standard chemotherapy [HR = 1.17; 95% confidence interval (CI), 0.74–1.86]. On the basis of these phase II data, patients with squamous cell histology are being recruited for a phase III trial of the phased schedule combining ipilimumab with chemotherapy versus chemotherapy alone (paclitaxel and carboplatin) for first-line treatment.
A separate ongoing study is comparing the phased schedule of ipilimumab combined with cisplatin and etoposide in the first-line treatment setting for SCLC. Ipilimumab's phase III study in SCLC was based on the same trial above but in an extensive stage SCLC population (28). The trial endpoint of ir-PFS was improved in the phased schedule arm with a median of 6.4 months compared with chemotherapy alone with a median of 5.3 months (HR = 0.64; 95% CI, 0.40–1.02; P = 0.03), although OS was not improved. The immune-related side effects were increased in the two ipilimumab arms but the discontinuation rate based on treatment-related side effects was similar across the arms.
Anti-PD-1 Inhibitors
While the anti-CTLA-4 antibodies have not resulted in significant single-agent responses, PD-1 checkpoint blockade with blocking antibodies targeted to either the receptor or its major ligand, PD-L1, have resulted in significant single-agent activity in advanced, heavily pretreated patients with NSCLC, in terms of OR, stable disease (SD), and associated long-term survival. Several antibodies have been developed to either block the PD-1 receptor or its ligand PD-L1. The anti-PD-1 antibodies [Nivolumab (BMS-936558/MDX-1106/ONO-4538) or Lambrolizumab (MK3475)] block the binding of PD-1 receptor to its 2 ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC).
The first-in-human, single dose phase I study of Nivolumab in refractory solid tumors revealed no maximum tolerated dose (MTD) and initial durable activity in solid tumors (29). A two-week dosing schedule was explored in the next phase I trial (30). Because of initial signs of activity in patients with NSCLC in the dose-escalation phase of the trial, expansion cohorts of patients with lung cancer were randomized to one of three doses, 1, 3, 10 mg/kg once every two weeks. 129 patients with NSCLC were enrolled in the trial of which 54% had received ≥3 previous therapies. The most common side effects in the lung cancer cohort were fatigue (24%), decreased appetite (12%), and diarrhea (10%), which were similar to the full study population. Grade 3 or 4 treatment-related select adverse events were seen in 14% of patients with NSCLC. Eight patients (6%) with NSCLC developed pneumonitis, 3 of which were grade 3 or 4. Two patients with NSCLC died from pneumonitis on this trial. On the basis of the role of the PD-1 pathway in downmodulating tissue inflammation, it is generally believed that organ-specific immune toxicities observed in patients receiving blockers of this pathway reflect underlying subclinical inflammation that is exacerbated upon initiation of therapy. The lung is a major organ site in which immunity must be carefully modulated, as excessive tissue destructive inflammation from responses to inhaled microbes will compromise oxygen exchange. Because NSCLC is commonly associated with chronic (chronic obstructive pulmonary disease) and acute (i.e., post-obstructive) pneumonia, oncologists must pay special attention to this toxicity in this patient population.
Of the 122 patients with NSCLC evaluable for response on this trial, 22 (17%) achieved a partial response (PR) based on RECIST 1.0 criteria and 10% had stable disease at ≥24 weeks. Unconventional immune-related responses occurred in an additional 5% of patients. While the adenocarcinoma subset of NSCLC shows reasonable initial responses to both chemotherapy and targeted tyrosine kinase inhibitors (TKI; when matched to appropriate driver oncogene mutations), squamous cell carcinoma is highly refractory to virtually all chemotherapies and TKIs. In contrast, Nivolumab activity in both NSCLC histologies is similar, with antitumor effects observed particularly at the 3 mg/kg dose level. The response rate was 28% (5/18) for nonsquamous histology and 27% (4/15) for squamous histology at this dose.
Beyond the significant response and disease stabilization rate in advanced chemotherapy-refractory patients, the durability of responses was unprecedented. Median duration of response was 74 weeks, compared with 4–8 months for chemotherapy regimens and oncogene-targeted TKI. Even stable disease was durable—roughly half of the patients with stable disease ≥24 weeks maintained stable disease beyond 48 weeks at the time of most recent evaluation. These durable responses were associated with improved survival outcomes relative to reports of other salvage therapies applied to this population of advanced inoperable and multiply pretreated patients: 1 year and 2 year overall survival was 42% and 14%, respectively (31). Taken together, the durable responses, unique to immunotherapy, support the long held concept within the cancer immunology community that immune modulators may reset the patient's endogenous tumor-specific T-cell immunity in a fashion that allows it to adapt to the evolution of the tumors genetics, something that tumor-targeted drug therapies cannot do.
Given that the PD-1 pathway seems to mediate tumor immune resistance at the level of the tumor microenvironment, expression within the tumor of the major PD-1 ligand, PD-L1 has been explored as a potential biomarker for response to blockade of this pathway. As part of the initial phase I trial and the expanded trial reported in 2012, PD-L1 expression by tumor cells was assessed in a subset of the patients. Among all histologies tested in the second trial, 0 of 17 patients whose tumors were PD-L1(−) and 11 of 25 patients whose tumors were PD-L1+ (defined as >5% of tumor cells displaying a clear membrane pattern of expression on immunohistochemistry), showed responses to anti-PD-1. Of these cases, 10 were NSCLC. Of the 10 NSCLC patients evaluated, 0 of 5 patients whose tumors were PD-L1(−) and 1 of 5 patients whose tumors were PD-L1+ showed responses to anti-PD-1 (30). Preliminary results correlating PD-L1 expression in NSCLC with response to the anti-PD-L1 antibody MPDL3280A (see below, refs. 32–34) showed that 4 of 4 PD-L1+ tumors showed an objective response in contrast to only 4 of 26 PD-L1(−) tumors. Taken together, the results of the two studies suggest that PD-L1 expression in lung cancer may be predictive of response to blockade of this pathway but these numbersare clearly too small to draw any definitive conclusions. There is ongoing analysis of both PD-L1 and PD-L2 expression in follow-up trials to determine their suitability as biomarkers for patient selection in NSCLC.
The encouraging results of the reported clinical trials have led to two separate phase III trials of Nivolumab as a single agent compared with docetaxel (35). One trial is enrolling only patients with nonsquamous histology in the second- or third-line treatment setting with a goal to improve OS. In the other trial with coprimary endpoints of OS and response rate, patients with squamous cell histology after one prior platinum combination chemotherapy are being enrolled. In addition to these pivotal phase III trials, a single-arm study of Nivolumab in the third-line treatment setting and beyond in only squamous histology patients has recently finished recruitment. In addition to single-agent trials, multiple treatment-arm phase I trials in NSCLC combining Nivolumab with various chemotherapy regimens as well as with ipilimumab have been launched. The combination of Nivolumab and ipilimumab makes sense given the distinct roles of the CTLA-4 and PD-1 pathways in regulating the initiation and execution of immune responses within tumors; in additon, a recent phase I trial testing this combination in patients with melanoma was highly encouraging (36). A second anti-PD-1 monoclonal antibody (Lambrolizumab) was recently reported to manifest substantial antitumor effects in patients with advanced melanoma (37); testing is underway in NSCLC and the results should be of great interest.
Anti-PD-L1 Antibodies
Three blocking anti-PD-L1 antibodies, BMS-936559, MPDL3280A, and MedI-4736 have been or are being evaluated in NSCLC. There has been much debate about the relative merits of antibodies directed at PD-1 versus PD-L1. Antibodies directed to PD-1 block its binding to both known PD-1 ligands, PD-L1, and PD-L2, whereas anti-PD-L1 antibodies only block the PD-1:PD-L1 interaction. However, another unexpected inhibitory interaction between PD-L1 and B7.1 (with B7.1 acting as an inhibitory receptor on T cells) has been described; this interaction is blocked by anti-PD-L1 antibodies but not anti-PD-1 antibodies. While the PD-L1:PD-1 interaction is considered the most important mediator of tumor immune resistance, the importance of the PD-L2:PD-1 and PD-L1:B7.1 interactions in human cancers is not well studied and could play a role in distinguishing the clinical activities of anti-PD-1 versus anti-PD-L1 antibodies. It is also possible that these additional interactions may be important in organ-specific immune modulation, which could impact relative immune toxicities of the two classes of antibody.
The first-in-human trial of BMS-936559 resulted in no MTD being identified, and in general the antibody was well tolerated (38). Only 6% of patients stopped treatment due to drug-related side effects and there were no drug-related deaths. Initial activity was seen in melanoma, renal cell carcinoma, ovarian cancer, and NSCLC. Of the 49 patients with lung cancer that were evaluable at the time of reporting, 5 patients (10%) had a response to therapy. The duration of response was long lasting (2.3 months up to 16.6 months and ongoing). The stable disease rate after 6 months of treatment was 12%. Results from 37 patients with NSCLC (both squamous and non-squamous histologies included) treated with MPDL3280A and evaluable for response showed a 24% response rate with additional immune-related responses (progression before regression or new lesions in the face of overall decrease in tumor burden; ref. 32). However, results with the two anti-PD-L1 antibodies cannot be directly compared as the patient population in the MPDL3280A trial was partially enriched for tumor expression of PD-L1. Given that multiple anti-PD-1 and anti-PD-L1 antibodies are under development, an important question is whether there are fundamental efficacy and toxicity differences between antibodies targeting the ligand versus the receptor. While Nivolumab and BMS-936559 (no longer in development) were never compared in a randomized trial, the similarities in patient characteristics between the two trials reported simultaneously in 2012 suggest that anti-PD-L1 is somewhat less active than anti-PD-1 but may also be associated with slightly lower toxicity (30, 38). Ongoing studies with the anti-PD-L1 antibodies MPDL3280A and MedI-4736 as well as the anti-PD-1 antibodies Nivolumab and Lambrolizumab will ultimately shed light on whether there are target-specific differences in activity or toxicity in NSCLC.
The Future: Beyond Single-Agent Immunotherapy
Now that the door for immunotherapy in lung cancer has been opened, the future lies in application of immunotherapies at earlier stages of lung cancer, introduction of modulatory antibodies and drugs against additional targets (both costimulatory pathway agonists and coinhibitory pathway antagonists), and finally, development of combinatorial therapy. Ultimately, profiling of the tumor microenvironment will provide biomarkers to guide precision immunotherapy. Multiple studies are trying to capitalize on the single-agent activity of checkpoint blockers, particularly PD-1 pathway blockers, by combining these antibodies either with each other, vaccines, tyrosine kinase inhibitors, or even standard chemotherapy (Fig. 2). One could envision checkpoint blockade being an option alone or in combination at any stage of the treatment algorithm, though care in scheduling driven by preclinical modeling and treatment science will be critical. Even though vaccines have yet to show activity in lung cancer, new vaccine formulations have been shown in animal models to display increased potency and combinations of vaccines and checkpoint inhibitors show the most potent synergy of all in multiple preclinical models. It is likely that the lack of activity of vaccines used as single agents in lung cancer is related to the inability of vaccine-enhanced T-cell responses to overcome intratumoral checkpoints—this notion underpins the value of vaccine-checkpoint inhibitor combinations.
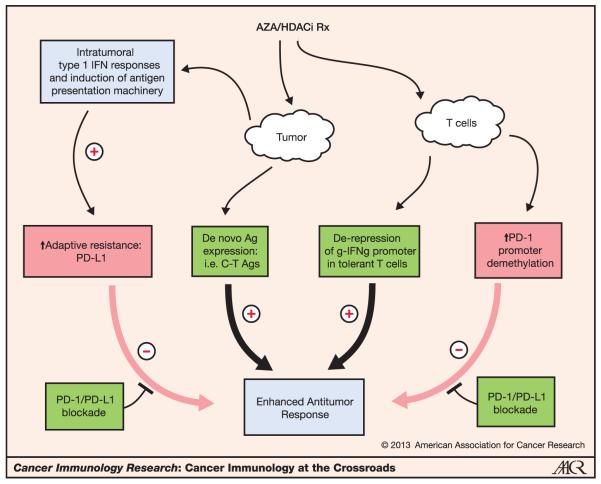
Figure 2.
Potential mechanisms of therapeutic synergy between epigenetic modulation and PD-1 pathway blockade. Epigenetic modulation, originally tested in lung cancer based on its capacity to induce expression of epigenetically silenced tumor suppressor genes, also has significant immunologic activity. Treatment of lung cancer cells with the DNA-demethylating agent 5-azacytidine (AZA) can induce the type I IFN pathway as well as multiple components of antigen presentation, thereby enhancing intratumoral inflammatory responses. However, PD-L1 is also induced on tumor cells, which could blunt immunity. Concomitant blockade of the PD-1 pathway would shift the balance such that the immune-enhancing effects of epigenetic modulation would dominate. DNA demethylation also induces expression of cancer–testes (C-T) antigens, which are known targets for tumor-specific T cells. Finally, epigenetic modulation can activate silenced effector cytokine genes in anergized T cells and induce PD-1 expression. Again, concomitant PD-1 pathway blockade would favor the immune-enhancing effects of epigenetic modulators on T cells. These potential mechanisms of synergy may account for recent preliminary clinical observations in 5 patients with NSCLC treated with either anti-PD-1 or anti-PD-L1 antibodies after receiving a combination of 5-azacytodine and entinostat (a class 1-specific HDACi); durable objective responses were observed in 3 patients and stable disease for more than 6months in the other two patients. This combination approach is a potential example of leveraging the immunologic effects of "nonimmunologic" therapies.
Another highly promising opportunity in lung cancer comes from the significant proportion of adenocarcinomas that possess oncogene mutations that confer susceptibility to targeted TKI. Four mutations—EGFR, ALK, BRAF, and ROS1, are druggable and collectively comprise 25%–30% of adenocarcinomas. While the response rate to targeted TKI in patients whose tumors bear these mutations is rapid and frequent, resistance also develops rapidly. Given that the mutant oncogene-specific TKIs would not be expected to inhibit T-cell responses (this has been shown for the BRAF inhibitors), it makes sense that the initial tumor lysis driven by the TKI would provide a large release of tumor antigen that could prime immune responses, and that could kill residual tumor when checkpoints are simultaneously blocked.
Opportunities to leverage immune effects of many therapeutic strategies abound. One such example in development in lung cancer is using epigenetic therapy (i.e., DNA-demethylating agents and HDACi) before Nivolumab treatment to prime the tumor to become more responsive to immunotherapy. This concept is based on both preclinical findings as well as the recent clinical observation that patients with lung cancer that first received a combination of 5-Aza-CR (a DNMT inhibitor that induces gene expression via demethylation of promoter regions) and an HDACi followed by either anti-PD-1 or anti-PD-L1 had significant benefit from the checkpoint blockade. Of the 5 patients treated this way, 3 had durable PRs ongoing for over a year and the other 2 patients had stable disease on therapy for at least 6 months. Preclinical data in a set of lung cancer lines show that the mechanism by which epigenetic priming of immunotherapy could improve outcomes may involve upregulation of a broad but related set of genes associated with IFN-signaling, antigen processing, and presentation as well as genes encoding cancer–testes antigens. In addition, epigenetic treatment of these lines increased expression of PD-L1 (39). Thus, a combination of this epigenetic therapy together with PD-1 blockade may shift the balance toward enhanced adaptive and innate immune responses within the tumor microenvironment, a hypothesis being tested prospectively in a combination clinical trial.
Results from the past 3 years have not only validated the clinical potential for immunotherapy but also the sense that we are just scratching the surface. Lung cancer, the number one cancer killer in the United States and worldwide, has been the frontier for immunotherapy's leap beyond the melanoma and renal cancer and will continue to provide vistas for future innovation in this burgeoning field.
Footnotes
Disclosure of Potential Conflicts of Interest
J.R. Brahmer has a commercial research grant from Bristol Myers Squibb and is a consultant/advisory board member of Bristol Myers Squibb, Merck, and Eli Lilly. D. Pardoll receives no monetary remuneration or equity from companies mentioned in this article. He is coinventor of patents covering therapies mentioned in this article.
Authors' Contributions
Conception and design: J.R. Brahmer, D. Pardoll
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): J.R. Brahmer, D. Pardoll
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J.R. Brahmer
Writing, review, and/or revision of the manuscript: D.M. Pardoll, J.R. Brahmer
References
- 1.Brahmer JR. Harnessing the immune system for the treatment of non-small-cell lung cancer. J Clin Oncol. 2013;31:1021–8. doi: 10.1200/JCO.2012.45.8703. [DOI] [PubMed] [Google Scholar]
- 2.Butts C. START: a phase III study of L-BLP25 cancer immunotherapy for unresectable stage III non-small cell lung cancer. J Clin Oncol. 2013;31 (suppl; abstr 7500) [Google Scholar]
- 3.Ramnath N, Tan D, Li Q, Hylander BL, Bogner P, Ryes L, et al. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006;55:891–9. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 5.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, et al. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:265–72. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pleasance ED, Stephens PJ, O'Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–90. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 8.Bogen B. Peripheral T cell tolerance as a tumor escape mechanism: deletion of CD4+ T cells specific for a monoclonal immunoglobulin idiotype secreted by a plasmacytoma. Eur J Immunol. 1996;26:2671–9. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 9.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, et al. Induction of antigen-specific T cell anergy: An early event in the course of tumor progression. Proc Natl Acad Sci USA. 1998;95:1178–83. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DuPage M, Mazumdar C, Schmidt LM, Cheung AF, Jacks T. Expression of tumour-specific antigens underlies cancer immunoediting. Nature. 2012;482:405–9. doi: 10.1038/nature10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts WK, Deluca IJ, Thomas A, Fak J, Williams T, Buckley N, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8 +T cells. J Clin Invest. 2009;119:2042–51. doi: 10.1172/JCI36131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darnell RB, Posner JB. Paraneoplastic syndromes affecting the nervous system. Semin Oncol. 2006;33:270–98. doi: 10.1053/j.seminoncol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Manley GT, Smitt PS, Dalmau J, Posner JB. Hu antigens: reactivity with Hu antibodies, tumor expression, and major immunogenic sites. Ann Neurol. 1995;38:102–10. doi: 10.1002/ana.410380117. [DOI] [PubMed] [Google Scholar]
- 14.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 15.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–80. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer. 2012;75:95–101. doi: 10.1016/j.lungcan.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–9. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fishman P, Bar-Yehuda S, Synowitz M, Powell JD, Klotz KN, Gessi S, et al. Adenosine receptors and cancer. Handb Exp Pharmacol. 2009;193:399–441. doi: 10.1007/978-3-540-89615-9_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 21.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 22.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 25.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zatloukal P, Heo DS, Park K, Kang J, Butts C, Bradford D, et al. Randomized phase II clinical trial comparing tremelimumab (CP-675, 206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2009;27:15s. (suppl; abstr 8071) [Google Scholar]
- 27.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 28.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 29.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brahmer JR. Survival and long-term follow-up of the phase I trial of nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with previously treated advanced non-small cell lung cancer. Proc Am Soc of Clin Oncol. 2013;31 [Google Scholar]
- 32.Herbst RS, Gordon MS, Fine GD, Sosman JA, Soria J, Hamid O, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced metastatic tumors. J Clin Oncol. 2013;31 (suppl; abstr 3000) [Google Scholar]
- 33.Powderly JD, Koeppen H, Hodi FS, Sosman JA, Gettinger SN, Desai R, et al. Biomarkers and associations with the clinical activity of PD-L1 blockade in a MPDL3280A study. J Clin Oncol. 2013;31 (suppl; abstr 3001) [Google Scholar]
- 34.Spigel DR, Gettinger SN, Horn L, Herbst RS, Gandhi L, Gordon MS, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cance (NSCLC) J Clin Oncol. 2013;31 (suppl; abstr 8008) [Google Scholar]
- 35.Rizvi N. A phase 1 study of nivolumab (anti-PD-1; BMS-936558; ONO-4538) +platinum-based doublet chemotherapy (Pt-doublet) in Chemotherapy-naïve non-small cell lung cancer patients. J Clin Oncol. 2013;31 (suppl; abstr 8072) [Google Scholar]
- 36.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013 Jun 2; doi: 10.1056/NEJMoa1302369. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013 Jun 2; doi: 10.1056/NEJMoa1305133. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrangle J, Wang W, Koch A, Easwaran H, Mohammad H, Parsana P, et al. Epigenetic therapy and sensitization of lung cancer to immunotherapy. AACR Annual Meeting; Apr, 2013. Abstract 4819. [Google Scholar]