To the Editor
Recently, we successfully used everolimus to achieve a partial remission in a patient with advanced pancreatic cancer that was induced by Peutz-Jeghers syndrome (PJS). PJS has been associated with an increased risk of GI, gynecologic, breast, and pancreatic cancers. PJS is caused by a tumor-suppressor gene mutation in the serine threonine kinase gene 11 (STK11, also known as LKB1 gene), localized on chromosome 19p13.3.1,2 Up to 93% of patients with PJS develop some form of cancer; 11% to 36% develop pancreatic carcinoma.3,4 PJS-related cancer is thought to develop through loss of heterozygosity (LOH) of the remaining normal (wild type) STK11/LKB1 allele. Wild-type STK11/LKB1 activates adenine monophosphate–activated protein kinase, which is a regulator of cellular energy metabolism. Activation of adenine monophosphate–activated protein kinase leads to inhibition of the mammalian target of rapamycin 1 (mTOR1), a serine/threonine kinase with a key position in the regulation of cell growth.5–7 Loss of STK11/LKB1, therefore, leads to loss of suppression of mTOR signaling and results in increased cell size and mass.8 STK11/LKB1 is also somatically inactivated in approximately 30% of sporadic non–small-cell lung cancers and 20% of pancreatic ductal adenocarcinomas.8,9 Inhibition of mTOR can be achieved with rapamycin (sirolimus and its analogs, such as the registered anticancer drug everolimus and temsirolimus).10 In two PJS mouse-model studies, treatment of STK11/LKB1− and STK11/LKB1− mice with rapamycin resulted in a dramatic reduction in the number of polyps and a suppression of hypoxia-inducible factor-1-α and its target genes.7,11
In 2009, a 46-year-old man presented to our institution (Academic Medical Center, Amsterdam, the Netherlands) with hereditary PJS; he had been diagnosed with pancreatic cancer in 2008, when he was 45 years of age. The patient’s medical history included multiple polypectomies, hamartoma resections, liposarcoma, recurrent anemia caused by bleeding enteropolyps, pancreatitis, diabetes mellitus, and recurrent small bowel intussusceptions. The patient underwent an annual colonoscopy for resection of intercurrent large polyps. In 2008, a computed tomography (CT) scan revealed a pancreatic mass. In July 2008, a debulking resection of the pancreatic mass was macroscopically incomplete. The pancreatic mass revealed an acinar cell carcinoma of 15 cm extending into the splenic flexure. A period of observation followed, and in January 2009, a CT scan showed progression of a 9-cm residual mass in the pancreatic bed (Fig 1A). The mass was 11 cm when the patient was referred to our oncology department in May 2009 (Fig 1B). He was asymptomatic, with no specific abnormalities at physical examination beyond what his medical history would suggest. The most recent surveillance colonoscopy in November 2008 showed the customary multiple small and large colon polyps with long stalks, which had been removed. Treatment with everolimus was proposed to the patient and, after the patient signed an informed consent form, off-label treatment was commenced in June 2009 with a daily dose of 9 mg. The patient was seen once every 2 months on an outpatient basis for physical, blood, and radiologic investigations (CT scans). To evaluate the molecular response to mTOR inhibition, tumor and polyp biopsies were analyzed before the start of treatment and during treatment with everolimus. Tumor biopsies were performed after 1 and 4 weeks of treatment and again after 8 months of treatment. Surveillance colonoscopy with biopsies was performed 6 and 8 months after the start of treatment. The collected tissue was immediately fixated in formalin and paraffin embedded. For immunohistochemistry and biomarker investigations, standard procedures were used.12,13 Genotyping analysis of the STK11/LKB1 gene was performed in leukocyte DNA, polyps, and the tumor. LOH analysis of the 19p locus, where STK11/LKB1 is located, was performed by using four polymorphic markers (D19S565, D19S886, D19S883, and D19S814).
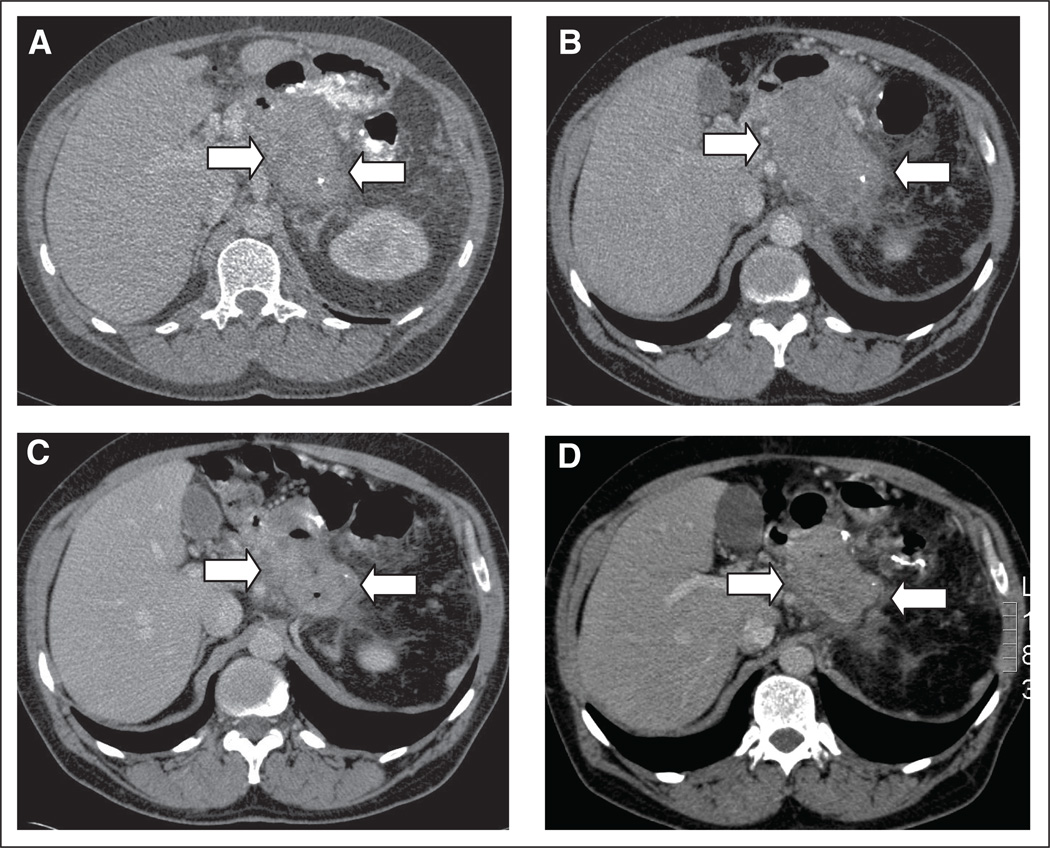
Fig 1.
Follow-up abdominal computed tomography (CT) scan after resection of pancreatic mass. Projections from CT scan with oral and intravenous contrast are shown. (A) A mass in the pancreatic bed with a diameter of 9 cm in January 2009, 6 months after resection (arrows). (B) Repeat CT scan during follow-up 10 months after resection shows a mass of 11 cm in maximal diameter (arrows). (C) Repeat CT scan after 3 months of treatment with everolimus shows a mass of 6 cm (arrows). (D) Repeat CT scan after 6 months of therapy shows a mass of 7 cm (arrows).
Treatment was well tolerated, with no adverse events except for grade 1 myalgia/arthralgia after 6 months of treatment. A partial response was confirmed by a CT scan 3 and 6 months after the start of treatment with everolimus. However, after 9 months of treatment, progressive disease was found (Figs 1A–1D). A colonoscopy 8 months after the start of treatment and 15 months after the last colonoscopy did not show any large (> 1 cm) colon polyps (in contrast to all previous colonoscopies).
Leukocyte DNA sequencing of the STK11/LKB1 gene showed a PJS-defining mutation c.582C>A, which led to the amino acid substitution p.Asp194Glu, D194E. Polyp DNA sequencing showed the same STK11/LKB1 mutation with retention of the wild-type allele. However, the DNA sequencing of the tumor showed a clear loss of the remaining wild-type allele. This was confirmed by LOH analysis of the four polymorphic markers surrounding the LKB1 locus, all of which showed LOH of the wild-type STK11/LKB1 allele in the tumor and retention of the mutated allele, which resulted in inactivation of both alleles (Figs 2A and 2B).
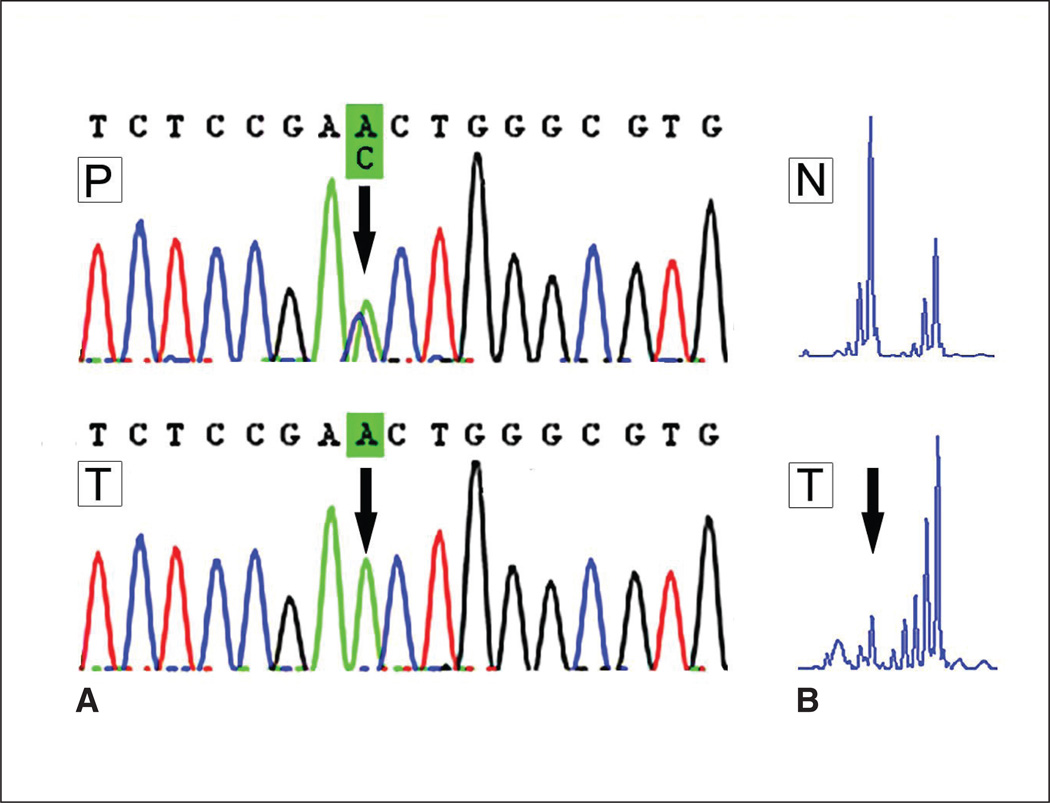
Fig 2.
(A) DNA sequence analysis of STK11/LKB1 gene. The black arrow marks the location of the mutation in exon 4 (D194E). In the upper sequence, the polyp (P), a heterozygous mutation, is shown; both the wild-type C allele (blue peak) and mutated A allele (green peak) are visible at the arrow. In the lower sequence, which shows the tumor (T), only the mutated A allele (green peak) remains; this is indicative of loss of the wild-type allele. (B) Loss of heterozygosity analysis of marker D19S565 at chromosome 19p, where STK11/LKB1 is located. Note that in the normal tissue (N), both STK11/LKB1 alleles are visible, whereas in the tumor (T), the small allele is missing (left peak, at the arrow), which clearly indicates loss of heterozygosity.
In line with the complete absence of STK11/LKB1, the mTOR pathway seemed to be activated in the primary tumor before and after treatment, as evident from strong cytoplasmic staining for phospho-S6 ribosomal protein (Ser235/236) (data not shown), which is not observed in normal pancreatic tissue.14 Biomarker investigations of the polyps were performed before and during treatment, and a clear increase of the apoptosis marker cleaved caspase-3 was observed 6 and 8 months after the start of treatment with everolimus (Figs 3A–3C).
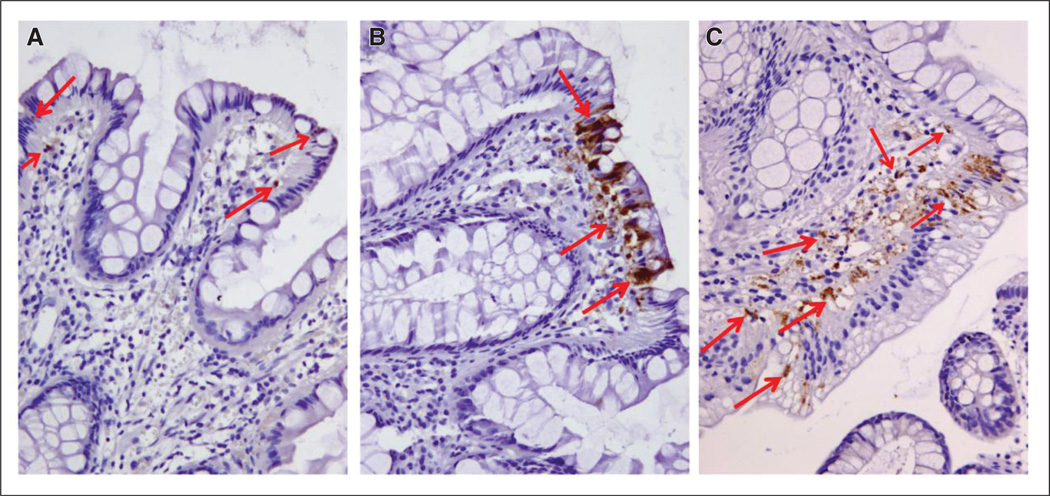
Fig 3.
Caspase-3 activity in polyps in response to treatment with everolimus. Polyps collected before and after treatment were immunohistochemically stained for cleaved caspase-3 (active capsase-3) and counterstained with hematoxylin (in blue). The red arrows point to some of the cleaved caspase-3 positive cells (in brown). (A) Polyp tissue collected before treatment. (B) Polyps obtained after 6 months. (C) Polyps obtained after 8 months. Original magnification, ×20
With everolimus treatment, our patient with PJS experienced a partial response of an acinar cell carcinoma of the pancreas and concomitant clearance of large colon polyps. Our hypothesized mechanism of cancer development via LOH of the remaining nonmutated STK11/LKB1 gene was retrospectively confirmed by gene analysis. Targeting the activated mTOR pathway with everolimus resulted in the initial good response of the tumor to the treatment. Biomarker investigations showed hyperactivation of the mTOR pathway signaling within the tumor, and this confirmed that mTOR was an appropriate target for anticancer therapy. Interestingly, phospho-S6 ribosomal protein levels, as markers of mTOR pathway activity, did not change during everolimus treatment (data not shown). Whether this is consistent with the hypothesis that cells with inhibited mTOR pathways had already died because of apoptosis remains unclear.
Treatment-induced apoptosis in the polyps was specifically detected in epithelial cells on the top of the crypt or in cells just beneath the epithelial layer, which suggests that proliferation and survival of the epithelium in the polyp is dpendent on the mTOR pathway, whereas proliferation and survival of stromal cells are not.13,15 The induced apoptosis in the polyps confirms the findings of mTOR inhibition in tumors of mouse models.14 Endoscopically, no new large polyps developed in our patient during the treatment period of 9 months.
We were able to induce shrinkage of the tumor while avoiding classic chemotherapy adverse effects, but speculate that the observed progressive disease, which occurred after 9 months of mTOR inhibition, was a result of either development of treatment-resistant cancer cells with other pathway mutations as the main driver of proliferation, or was caused by hyperactivation of an alternative pathway such as the AKT pathway. In future trials in this patient group, it needs to be confirmed that mTOR inhibition is a promising new targeted treatment for advanced malignancies. Because of the low incidence of this condition, a comparative trial does not seem feasible, but sporadic cancers with mutated STK11/LKB1 may result in an alternative group of patients without PJS who can be evaluated.8,9 To our knowledge, this is the first report about regression of pancreatic cancer and apoptosis in colonic polyps by mTOR inhibition in a patient with PJS. This has important implications for the much larger group of patients with pancreatic cancer and STK11/LKB1 mutations because they would be expected to benefit from this type of personalized treatment.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory
Role: None Stock Ownership: None Honoraria: None Research
Funding: Dirk J. Richel, Novartis Expert Testimony: None Other
Remuneration: None
Contributor Information
Heinz-Josef Klümpen, Academic Medical Center, Amsterdam, the Netherlands.
Karla C.S. Queiroz, Center for Experimental and Molecular Medicine; Academic Medical Center, Amsterdam, the Netherlands
C. Arnold Spek, Center for Experimental and Molecular Medicine; Academic Medical Center, Amsterdam, the Netherlands.
Carel J.M. van Noesel, Academic Medical Center, Amsterdam, the Netherlands
Helmy C. Brink, Academic Medical Center, Amsterdam, the Netherlands
Wendy W.J. de Leng, University Medical Center Utrecht, Utrecht, the Netherlands
Roeland F. de Wilde, University Medical Center Utrecht, Utrecht, the Netherlands
Elisabeth M.H. Mathus-Vliegen, Academic Medical Centre Amsterdam, Amsterdam, the Netherlands
G. Johan A. Offerhaus, University Medical Center Utrecht, Utrecht, the Netherlands
Maarten A. Alleman, Isala Hospitals, Zwolle, the Netherlands
Anneke M. Westermann, Academic Medical Center, Amsterdam, the Netherlands
Dirk J. Richel, Academic Medical Center, Amsterdam, the Netherlands
REFERENCES
- 1.Jenne DE, Reimann H, Nezu J, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 2.Hezel AF, Bardeesy N. LKB1: Linking cell structure and tumor suppression. Oncogene. 2008;27:6908–6919. doi: 10.1038/onc.2008.342. [DOI] [PubMed] [Google Scholar]
- 3.van Lier MG, Wagner A, Mathus-Vliegen EM, et al. High cancer risk in Peutz-Jeghers syndrome: A systematic review and surveillance recommendations. Am J Gastroenterol. 2010;105:1258–1264. doi: 10.1038/ajg.2009.725. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 5.Hardie DG. AMP-activated/SNF1 protein kinases: Conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 6.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 7.Shackelford DB, Vasquez DS, Corbeil J, et al. mTOR and HIF-1alpha-mediated tumor metabolism in an LKB1 mouse model of Peutz-Jeghers syndrome. Proc Natl Acad Sci U S A. 2009;106:11137–11142. doi: 10.1073/pnas.0900465106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahoney CL, Choudhury B, Davies H, et al. LKB1/KRAS mutant lung cancers constitute a genetic subset of NSCLC with increased sensitivity to MAPK and mTOR signalling inhibition. Br J Cancer. 2009;100:370–375. doi: 10.1038/sj.bjc.6604886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton JP, Jamieson NB, Karim SA, et al. LKB1 haploinsufficiency cooperates with Kras to promote pancreatic cancer through suppression of p21-dependent growth arrest. Gastroenterology. 2010;139:586–597. doi: 10.1053/j.gastro.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol. 2009;20:1674–1681. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 11.Wei C, Amos CI, Zhang N, et al. Suppression of Peutz-Jeghers polyposis by targeting mammalian target of rapamycin signaling. Clin Cancer Res. 2008;14:1167–1171. doi: 10.1158/1078-0432.CCR-07-4007. [DOI] [PubMed] [Google Scholar]
- 12.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: Targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28:835–840. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katajisto P, Vaahtomeri K, Ekman N, et al. LKB1 signaling in mesenchymal cells required for suppression of gastrointestinal polyposis. Nat Genet. 2008;40:455–459. doi: 10.1038/ng.98. [DOI] [PubMed] [Google Scholar]
- 14.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Vaahtomeri K, Ventelä E, Laajanen K, et al. Lkb1 is required for TGFbeta-mediated myofibroblast differentiation. J Cell Sci. 2008;121:3531–3540. doi: 10.1242/jcs.032706. [DOI] [PubMed] [Google Scholar]