Abstract
PURPOSE
To assess the results of a single eye bank preparing a high volume of Descemet membrane endothelial keratoplasty (DMEK) tissues using multiple technicians to provide an overview of the experience and to identify possible risk factors for DMEK preparation failure.
DESIGN
Cross-sectional study.
METHODS
SETTING
Lions VisionGift and Wilmer Eye Institute at Johns Hopkins Hospital.
STUDY POPULATION
All 563 corneal tissues processed by technicians at Lions VisionGift for DMEK between October 2011 and May 2014 inclusive.
OBSERVATION PROCEDURES
Tissues were divided into 2 groups: DMEK preparation success and DMEK preparation failure.
MAIN OUTCOME MEASURES
We compared donor characteristics, including past medical history.
RESULTS
The overall tissue preparation failure rate was 5.2%. Univariate analysis showed diabetes mellitus (P = .000028) and its duration (P = .023), hypertension (P = .021), and hyperlipidemia or obesity (P = .0004) were more common in the failure group. Multivariate analysis showed diabetes mellitus (P = .0001) and hyperlipidemia or obesity (P = .0142) were more common in the failure group. Elimination of tissues from donors either with diabetes or with hyperlipidemia or obesity reduced the failure rate from 5.2% to 2.2%. Trends toward lower failure rates occurring with increased technician experience also were found.
CONCLUSIONS
Our work showed that tissues from donors with diabetes mellitus (especially with longer disease duration) and hyperlipidemia or obesity were associated with higher failure rates in DMEK preparation. Elimination of tissues from donors either with diabetes mellitus or with hyperlipidemia or obesity reduced the failure rate. In addition, our data may provide useful initial guidelines and benchmark values for eye banks seeking to establish and maintain DMEK programs.
Descemet stripping automated endothelial keratoplasty (DSAEK) and Descemet membrane endothelial keratoplasty (DMEK) have gained popularity over penetrating keratoplasty for the treatment of corneal endothelial diseases because of numerous inherent advantages.1-5 Thus, the demand for eye bank precut corneas for endothelial keratoplasty has increased dramatically in the United States. In 2013, the Eye Bank Association of America reported that 37% of corneas distributed for keratoplasty were for endothelial keratoplasty, representing a 12.4% increase from 2012.6 Despite studies showing better results over Descemet stripping endothelial keratoplasty or DSAEK, DMEK accounted for only about 6% of the endothelial tissues provided.6 Possible reasons include the challenges associated with preparing and handling the delicate graft tissue and lack of standardization of DMEK graft preparation by both surgeons and eye banks.7-9 Regarding the latter issue, knowledge of possible risk factors for failure in DMEK donor tissue preparation could provide both eye banks and corneal surgeons with information that would allow better selection of corneas to use for this procedure.7
Donor factors such as age and endothelial cell density have been shown to influence the properties of DMEK grafts, and thereby the duration of the surgical procedure.10 In a recent publication, Gorovoy and associates studied some risk factors for DMEK preparation, including donor age, gender, postmortem tissue time interval, contralateral eye data, peel time, and peel complications, in 116 consecutive DMEK donor tissues that were prepared by a single surgeon.11 They proposed that the major risk factor for DMEK preparation failure in the fellow eye is complications during peeling the first eye. Another risk factor proposed was donor age younger than 50 years. Recently, Greiner and associates also related diabetes mellitus as a risk factor for preparation failure.12
Given that eye banks are beginning to provide tissues prepared for DMEK and that interest in the procedure is evolving, it is likely that the volume of eye bank prepared tissue for this surgery will increase. Thus, the purpose of this study is to assess the results of a single eye bank preparing a high volume of DMEK tissues using multiple technicians to provide an overview of the experience and to identify possible risk factors for DMEK preparation failure. Such information may be important for eye banks seeking to develop DMEK preparation programs and for surgeons and eye bank personnel involved in the selection of donor tissues for this procedure.
METHODS
THE INSTITUTIONAL REVIEW BOARD AT LEGACY HEALTH Systems, (Portland, Oregon, USA) determined that approval was not required for cross-sectional data queries. This was a retrospective, cross-sectional study of electronic eye bank database records of tissue from donors prepared for DMEK at Lions VisionGift (Portland, Oregon, USA).
PROTOCOL
We retrospectively studied all 563 corneal tissues that were processed by trained technicians at Lions VisionGift for DMEK between October 2011 and May 2014 inclusive. The tissues were divided into 2 groups: DMEK preparation success and DMEK preparation failure (see “Tissue Preparation,” below). The following parameters were assessed: endothelial cell count before and after cutting; death to preservation time; donor age; donor past medical history (including diabetes mellitus, and its duration when available; hyperlipidemia, obesity, or both; hypertension; history of cancer; and tobacco and alcohol use); past ocular history, including superficial surgeries (eg, pterygium, refractive surgery), intraocular surgeries, and any other clinical ocular history (corneal or retinal diseases and glaucoma); technician experience; duration of tissue preparation (minutes); and days from death to tissue preparation. The influence of the learning curve for technicians was determined by the number of failures per each 50 consecutive procedures. We also reported the number of previous DSAEK tissues prepared by each technician (Galloway JS).
Tissue preparation
Corneas were prepared for DMEK per standard protocol of the eye bank. In brief, Descemet membranes were peeled under Optisol GS Bausch and Lomb (Rochester, New York) from the underlying stroma over 80% to 90% of their surface area, leaving a small hinge of residual attachment that facilitated storage and transportation in situ against the host donor corneoscleral tissue. Failure was defined as a tear that occurred while separating Descemet membrane from stroma that rendered the tissue unusable for transplantation. Endothelial cell counts before and after tissue preparation were performed using a standard protocol with an EB-10 eye bank specular microscope (Konan, Irvine, California, USA).
Technician training
The Lions VisionGift training program consists of 4 phases: observation, hands-on mentored training for basic competence, solo practice to solidify the new skill set, and finally a sequence of at least 10 consecutively observed and documented tissue preparation procedures in which the trainee is expected to perform at a level acceptable to the medical director of the eye bank or at a level comparable with that of previously approved technicians. Tissues included in this study were prepared by 3 technicians who had completed all 4 training stages.
STATISTICAL ANALYSES
Data are reported as mean ± standard deviation unless otherwise indicated. Welch 2-sample t tests were used to test for association between outcome and individual continuous variables such as age and duration of diabetes mellitus, whereas Fisher exact tests were used for factorial variables such as diabetes status and history of tobacco use. Logistic regression with the logit link function was used to construct a multivariate prediction model, evaluated by receiver operating characteristic (ROC) analysis to select optimal decision rules. To obtain unbiased estimates of predictive performance, logistic regression was applied within a leave-1-out cross-validation design wherein the model parameters were re-estimated within each iteration. Statistical analyses were carried out in the R program (www.cran.us.r-project.org) using standard functions for tests and logistic regression and customized functions for plotting ROC curves and calculating area under the curve. All tests were 2-tailed and P values of less than .05 were considered significant.
RESULTS
A TOTAL OF 563 TISSUE REPORTS WERE ANALYZED, WHICH included 534 successful and 29 failed procedures, representing a 5.2% failure rate for all attempts. The mean donor age (± standard deviation) was 64.0 ± 6.7 years (range, 43 to 73 years) in the failure group and 65.0 ± 6.8 years (range, 35 to 79 years) in the success group (P = .44).
The donor past medical and ocular history could be assessed in 488 cases, including 462 (87%) in the success group and 26 (90%) in the failure group. Diabetes mellitus was more than 3-fold more common in the failure group than in the success group (69% vs 24%; P = .000028). It was possible to assess diabetes mellitus duration in 16 donors (89% of the diabetics) in the failure group and in 85 donors (77% of the diabetics) in the success group, and the mean ± standard deviation durations were 13.9 ± 15.5 years in the failure group and 6.5 ± 8.4 years in the success group (P = .023). Higher rates of hypertension (85% vs 62%; P = .021) and hyperlipidemia, obesity, or both (85% vs 48%; P = .0004) also were found among the failure group. Tobacco use was nearly 2-fold more common (19% vs 10%) in the failure group (P = .175). The other clinical and ophthalmologic parameters studied were not significantly different and can be seen in Table 1. After Bonferroni multiple-test correction, only diabetes mellitus and hyperlipidemia, obesity, or both were significantly associated with donor preparation failure.
TABLE 1.
Past Medical History and Past Ocular History among Descemet Membrane Endothelial Keratoplasty Donor Preparation Failure and Success Groups
| Outcome Group | Diabetes | Diabetes Duration (y) |
Hypertension | Hyperlipidemia, Obesity, or Both |
Tobacco | Cancer | Alcohol | Intraocular Surgery |
Superficial Surgery |
Other Ocular Disorders |
|---|---|---|---|---|---|---|---|---|---|---|
| Failure (n = 26) | 18 (69) | 13.9 ± 15.5 | 22 (85) | 22 (85) | 5 (19) | 11 (42) | 3 (12) | 1 (4) | 1 (4) | 2 (8) |
| Success (n = 462) | 110 (24) | 6.5 ± 8.4 | 287 (62) | 224 (48) | 46 (10) | 181 (39) | 75 (16) | 24 (5) | 28 (6) | 43 (9) |
| P value | .000028 | .023 | .021 | .0004 | .175 | .84 | .78 | 1.00 | 1.00 | 1.00 |
SD = standard deviation.
Data are no. (%) or mean ± standard deviation, unless otherwise indicated.
Before attempting to peel Descemet membrane, the mean endothelial cell count among success and failure groups were, respectively, 2763 ± 293 cells/mm2 and 2753 ± 318 cells/mm2 (P = .86). After preparation, it was 2768 ± 248 cells/mm2 in the success group and could not be determined in the failure group.
The mean death to preservation time was 09 hours and 58 minutes in the success group and 10 hours and 29 minutes in the failure group (P = .61). The mean duration of tissue preparation was 25 ± 7 minutes in the success group and 25 ± 6 minutes in the failure group (P = 1.0). The mean death to tissue preparation time was 3.6 ± 1.7 days in the success group and 4.2 ± 2.0 in the failure group (P = .058).
Next, we fit a multivariate logistic regression model to the data using the logit link function. Results are shown in Table 2. In addition to diabetes mellitus and hyperlipidemia, obesity, or both, which were the only significant predictors of failure in univariate analysis after Bonferroni multiple test correction, we also included donor age because of a previously published association, with success (Table 2).10 Diabetes mellitus remained the most significant risk factor in the multivariate model, with diabetic donors having nearly 6 times the risk of failure of nondiabetic individuals. Hyperlipidemia, obesity, or both likewise was statistically significant in the multivariate model, with an odds ratio of 4, suggesting that both diabetes mellitus and hyperlipidemia, obesity or both have independent predictive value after adjusting for the effects of each other. Although donor age was not statistically significant, the expected trend, decreased risk of failure in older patients, was observed with an approximate 5% reduction in risk per year over the age range of donors included in this study.
TABLE 2.
Multivariate Logistic Regression Analysis of Risk Factors for Descemet Membrane Endothelial Keratoplasty Donor Preparation Failure
| Variable | Odds Ratio for Failure |
95% CI | P Value |
|---|---|---|---|
| Diabetes mellitus | 5.80 | 2.35 to 14.35 | .0001 |
| Hyperlipidemia, obesity, or both |
4.07 | 1.32 to 12.49 | .0142 |
| Age | 0.94 | 0.89 to 1.00 | .0588 |
CI = confidence interval.
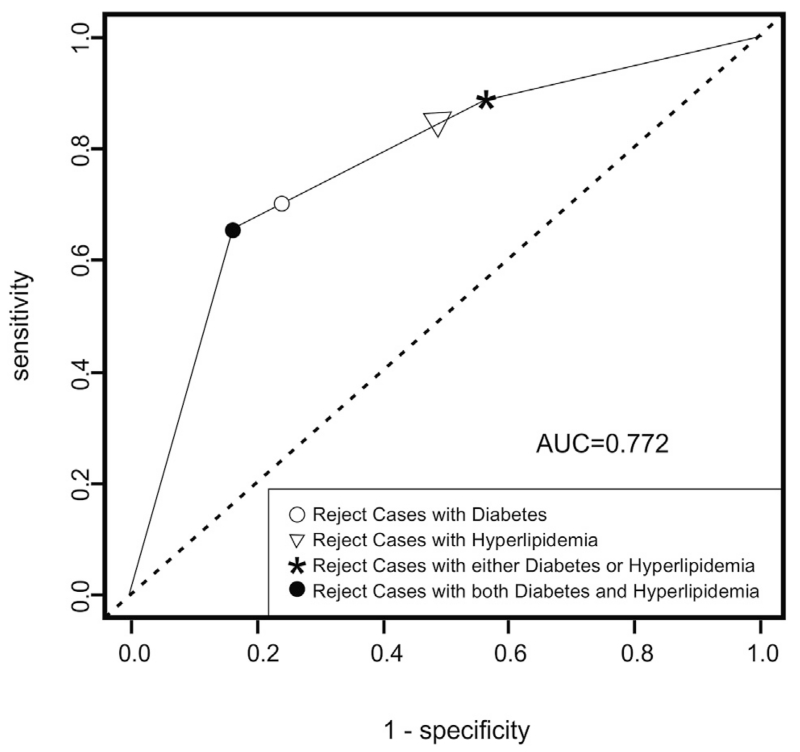
We used the logistic regression model to predict which cases were most likely to fail, using ROC analysis to help select optimal prediction rules and evaluate performance. The area under the ROC curve was 0.772 (95% confidence interval, 0.68 to 0.86; Figure), with maximum predictive power at a sensitivity of 65% and specificity of 84%. Because both of the risk factors in the model are binary, the selection rule corresponding to this decision point is simple to describe: potential donors may have either (1) diabetes mellitus or (2) hyperlipidemia, obesity, or both, but not both (1) and (2). Applying this rule to the full donor pool (n = 563) and assuming the full donor pool has the same rates of diabetes mellitus and hyperlipidemia, obesity, or both as in the evaluable samples (n = 488), 82% (460/563) of donors included in this study would have been eligible, with a failure rate of only 2.2% (10/460), compared with 5.2% in the full donor group (29/563). Eliminating all cases of diabetes mellitus (with or without hyperlipidemia, obesity, or both) resulted in a similar failure rate (2.2%), but further diminished the donor pool so that only 74% of those included in this study were eligible. The lowest failure rate was achieved by eliminating all donors with either diabetes mellitus or hyperlipidemia, obesity, or both (1.5%), but at great cost to the available donor pool: only 236 in 563 (42%) subjects in this study would have been eligible donors under these criteria. Eliminating hyperlipidemic or obese patients (with or without diabetes mellitus; failure rate, 1.7%; eligible donor pool, 50%) gave comparable results to eliminating donors with either diabetes mellitus or hyperlipidemia, obesity, or both.
FIGURE.
Receiver operating characteristic analysis of the risk of Descemet membrane endothelial keratoplasty preparation failure related to diabetes mellitus and hyperlipidemia, obesity, or both. Area under the ROC curve (AUC) was 0.772 with maximum predictive power at a sensitivity of 65% and specificity of 84% (solid circle). This point represents rejection of highest-risk donors with both diabetes mellitus and hyperlipidemia, obesity, or both, which results in exclusion of 18% of the donor pool, for a reduction in failure rate from 5.2% to 2.2%. The asterisk represents the predictive power of rejecting medium-risk donors either with diabetes mellitus or with hyperlipidemia, obesity, or both, which results in a failure rate of only 1.5%, but which excludes 58% of the donor pool. Excluding those with hyperlipidemia, obesity, or both (with or without diabetes mellitus; triangle) removes 50% of the donor pool and results in a failure rate of 1.7%, whereas excluding those with diabetes mellitus (with or without hyperlipidemia, obesity, or both; open circle) removes 26% of the donor pool at a failure rate of 2.2%.
Overall, the DMEK preparation failure rate ranged from 2.6% to 11.2% among the 3 technicians (Galloway JS) included in this study. As shown in Table 3, we observed a trend toward decreasing numbers of DMEK failures with increasing numbers of attempts for technician 1, with diabetes mellitus being the most significant risk factor for failure (P = .056). For technician 2, the number of failures was higher in procedure numbers 101 through 150, and donor age was found to be the most important risk factor in these failures (P = .095). For technician 3, there was 1 more failure in procedure numbers 50 through 100 than in procedure numbers 0 through 50. Age (P = .027), diabetes mellitus (P = .035), and hyperlipidemia, obesity, or both (P = .032) were risk factors in the later failures for this technician. Similarly, a possible relationship was observed between DMEK failure rates and amount of DSAEK tissue preparation experience. Our data showed that the technician having the least experience in DSAEK preparation had more than twice the number and almost 3 times the percent of DMEK failures compared with the technician with the greatest DSAEK experience.
TABLE 3.
Failures by Technician According to Number of Attempted Descemet Membrane Endothelial Keratoplasty Preparations and Number of Prior Descemet Stripping Automated Endothelial Keratoplasty Preparations
| No. of Attempted DMEK Tissue Preparations |
Technician 1 Failures (395 Previous DSAEK Preparations) |
Technician 2 Failures (2745 Previous DSAEK Preparations) |
Technician 3 Failures (137 Previous DSAEK Preparations) | Total Failures for Technicians 1, 2 and 3 |
|---|---|---|---|---|
| 0 to 50 | 5 (diabetes mellitus was a possible risk factor for failure; P = .056) |
0 | 6 | 11 |
| 51 to 100 | 1 | 1 | 7 (age [P = .027], diabetes mellitus [P = .035], and hyperlipidemia [P = .032] were possible risk factors for failure) |
9 |
| 101 to 150 | 0 | 5 (age was a possible risk factor for failure; P = .095) |
2 | 7 |
| 151 to 200 | 0 | 1 | – | 1 |
| 201 to 250 | 1 | – | – | 1 |
| 251 to 267 | 0 | – | – | 0 |
| Total failures for each technician |
7 (in 267 DMEK attempts; 2.6%) | 7 (in 162 DMEK attempts; 4.3%) | 15 (in 134 DMEK attempts; 11.2%) | 29 (in 563 DMEK attempts; 5.2%) |
DMEK = Descemet membrane endothelial keratoplasty; DSAEK = Descemet stripping automated endothelial keratoplasty.
DISCUSSION
VARIOUS ASPECTS OF EYE BANK DMEK PREPARATION, including endothelial cell loss, have been published.13,14 However, risk factors for failure in DMEK graft preparation and, consequently, for a higher endothelial cell loss or tissue wastage, have not been addressed in a large-volume, multitechnician eye bank setting and have been limited to surgeon preparation of donor tissue. In this latter context, difficulty in graft peeling for the first eye has been shown to be the most important factor for failure in the fellow eye.11,15 Also, ultrastructural abnormalities (peg-like interlockings) or biochemical abnormalities (increased staining intensities for adhesive glycoproteins) along the Descemet membrane–stroma interface have been proposed as the main cause of tears during unsuccessful preparation.16 A recognized association for this is young donor age, which represents a risk factor for DMEK graft preparation failure.10,11,16
To date, besides young donor age, the only donor factor that has been proposed to increase the failure rate is diabetes mellitus.12 Potential explanations for this finding include glycation products resulting from chronic hyperglycemia, which form and deposit between the posterior stroma and Descemet membrane, creating stronger adhesion, and wide-spaced collagen present in the Descemet membrane of diabetic corneas, which may have a deleterious effect on the tensile strength of Descemet membrane.17,18
The present study assessed other systemic conditions that also could affect DMEK preparation failure. Our general rate of failure (5.2%) was consistent with that in the literature.19,20 The mean donor age showed no statistically significant difference, in contrast to previously reported results,10,11,16 which can be explained by the fact that 99% of the tissues used in this study were older than 50 years. Our database shared information with 286 donors who also were included in Greiner and associates’ work collected at the same eye bank.12 The conservation of their findings after the inclusion of 277 additional donors confirms the role of diabetes as an important risk factor for DMEK preparation failure. Additionally, we also showed that the time of known diagnosis of diabetes also is an important risk factor and could be explained possibly by the prolonged exposure time to the same pathophysiologic factors previously proposed.17,18
Interestingly, hyperlipidemia, obesity, or both and hypertension also were associated with higher rates of DMEK preparation failure in our study, and tobacco use, although not statistically significant because of the overall low number of tobacco smokers, also showed a trend toward a higher failure rate. However, tobacco use history is not collected as rigorously as other parameters because of lack of interest by transplant surgeons and need for documentation by eye banks. We hypothesize that, similar to diabetes, tobacco smoking represents a source of advanced glycation end-products, and moreover, by-products of cigarette smoke, such as nitrogen oxides, nitrite, and formaldehyde, induce cross-links between collagen fibers.21 Concerning hyperlipidemia, obesity, or both, the possible mechanisms are speculative at best, but could involve oxidative stress or other effects similar to those occurring in diabetes mellitus and tobacco use.22,23 An overall limitation of our study was that the data did not allow stratification of these positive conditions by severity to assess further their influence on the risk of failure. Cancer and alcohol use were not associated with DMEK preparation failure in our study.
A multivariate logistic regression model using diabetes mellitus; hyperlipidemia, obesity, or both; and age also was carried out. This analysis showed that exclusion of donors with both diabetes mellitus and hyperlipidemia, obesity, or both reduced the failure rate from 5.2% to 2.2%. Such a finding may be a valuable guide to lower the number of tissues lost in preparation and allow better distribution of donor material to appropriate surgical applications such as DSAEK, penetrating keratoplasty, or anterior lamellar keratoplasty.
Previous work showed that early failed attempts at DSAEK tissue cutting were likely the result of the initial learning curve of the technicians.24 Although our cohort was not large enough for definitive conclusions, we observed similar possible trends with lower failure rates occurring with increased experience with DMEK and DSAEK preparations. For technicians 2 and 3, the higher number of failures in a later stage of the learning curve can be explained by the significantly higher age (both of them) and amount of diabetes mellitus and hyperlipidemia, obesity, or both (technician 3) found in these donors.
In summary, our work showed that tissues from donors with diabetes mellitus (especially in longer duration cases) and hyperlipidemia, obesity, or both are more likely to have failures during DMEK preparation in an eye bank setting. Cases with associated diabetes and hyperlipidemia, obesity, or both should be avoided when possible for this purpose. Furthermore, an overall failure rate of 5.2% or less with an apparent reduction in failures occurring after 100 to 150 preparations as indicated by our data may provide useful initial guidelines and may contribute to benchmark values for eye banks seeking to establish and maintain DMEK programs.
Biographies

Lucas M M Vianna, MD, graduated from Medical School at Federal University of Juiz de Fora, Brazil, in 2006. He completed his residency and fellowship in Cornea, Cataract, and Refractive Surgery at the Department of Ophthalmology, Federal University of São Paulo (UNIFESP), São Paulo, Brazil, in 2013. Currently, he is resuming his PhD program at Johns Hopkins University in association with UNIFESP and is also a surgical instructor at State University of Rio de Janeiro.

Albert S. Jun, MD, PhD, is Associate Professor of Ophthalmology at the Wilmer Eye Institute, Johns Hopkins Medical Institutions. Dr Jun’s clinical and scientific interests include keratoplasty and pathophysiology of corneal diseases. His clinical and research activities have been recognized with grants and awards from numerous organizations including the National Institutes of Health, the Heed Ophthalmic Foundation, the Eye Bank Association of America, Research to Prevent Blindness, and the Association of University Professors of Ophthalmology.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and the following were reported. Dr Terry has received grants from Moria (Doylestown, Pennsylvania, USA) and Bausch and Lomb Surgical (Rochester, New York, USA) for educational breakfast meetings and royalties from Bausch and Lomb Surgical for instruments he designed. Dr Jun has received research grants from the National Institutes of Health (Bethesda, Maryland, USA, number EY019874) paid to his institution for unrelated work. Supported by the J. Willard and Alice S. Marriott Foundation, Bethesda, Maryland, USA; Edward Colburn; Richard Dianich; Barbara Freeman; Mary Finegan; Stanley Friedler, MD; Herbert Kasoff; Diane Kemker; James Lamson; Jean Mattison; Florenz Ourisman; Lee Silverman; and Norman Tunkel, PhD (all to A.S.J.); the Brazilian government CAPES Foundation (Brasilia, DF, Brazil [L.M.M.V.]); and the State University of Rio de Janeiro (Rio de Janeiro, RJ, Brazil [L.M.M.V.]). Involved in Design of study (L.M.M.V., A.S.J.); Conduct of study (L.M.M.V., A.S.J.); Data collection (L.M.M.V., C.G.S., J.D.G., M.T., A.S.J.); Data management (L.M.M.V., C.G.S., J.D.G., M.T., L.C., A.S.J.); Data analysis (L.M.M.V., C.G.S., J.D.G., M.T., L.C., A.S.J.): Data interpretation (L.M.M.V., C.G.S., J.D.G., M.T., L.C., R.B., A.S.J.); Preparation of manuscript (L.M.M.V., A.S.J.); Review of manuscript (L.M.M.V., C.G.S., J.D.G., M.T., L.C., R.B., A.S.J.); and Final approval of manuscript (L.M.M.V., C.G.S., J.D.G., M.T., L.C., R.B., A.S.J.).
REFERENCES
- 1.Melles GRJ, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK) Cornea. 2006;25(8):987–990. doi: 10.1097/01.ico.0000248385.16896.34. [DOI] [PubMed] [Google Scholar]
- 2.Bahar I, Kaiserman I, Levinger E, Sansanayudh W, Slomovic AR, Rootman DS. Retrospective contralateral study comparing Descemet stripping automated endothelial keratoplasty with penetrating keratoplasty. Cornea. 2009;28(5):485–488. doi: 10.1097/ICO.0b013e3181901df4. [DOI] [PubMed] [Google Scholar]
- 3.Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. 2011;30(12):1382–1386. doi: 10.1097/ICO.0b013e31821ddd25. [DOI] [PubMed] [Google Scholar]
- 4.Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012;153(6):1082–1090. doi: 10.1016/j.ajo.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Dapena I, Ham L, Melles GR. Endothelial keratoplasty: DSEK/DSAEK or DMEK—the thinner the better? Curr Opin Ophthalmol. 2009;20(4):299–307. doi: 10.1097/ICU.0b013e32832b8d18. [DOI] [PubMed] [Google Scholar]
- 6. [Accessed August 15, 2014];Eye Bank Association of America Statistical Report 2013. Available at, http://www.restoresight.org/wp-content/uploads/2014/04/2013_Statistical_Report-FINAL.pdf.
- 7.Boynton GE, Woodward MA. Eye-bank preparation of endothelial tissue. Curr Opin Ophthalmol. 2014;25(4):319–324. doi: 10.1097/ICU.0000000000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terry MA. Endothelial keratoplasty: why aren’t we all doing Descemet membrane endothelial keratoplasty? Cornea. 2012;31(5):469–471. doi: 10.1097/ICO.0b013e31823f8ee2. [DOI] [PubMed] [Google Scholar]
- 9.Melles GR, Ong TS, Ververs B, van der Wess J. Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2008;145(2):222–227. doi: 10.1016/j.ajo.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 10.Heinzelmann S, Hüther S, Böhringer D, Eberwein P, Reinhard T, Maier P. Influence of donor characteristics on descemet membrane endothelial keratoplasty. Cornea. 2014;33(6):644–648. doi: 10.1097/ICO.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 11.Gorovoy IR, Cui QN, Gorovoy MS. Donor tissue characteristics in preparation of DMEK grafts. Cornea. 2014;33(7):683–685. doi: 10.1097/ICO.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 12.Greiner MA, Rixen JJ, Wagoner MD, et al. Diabetes mellitus increases risk of unsuccessful graft preparation in descemet membrane endothelial keratoplasty: a multicenter study. Cornea. 2014;33(11):1129–1133. doi: 10.1097/ICO.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 13.Jardine GJ, Holiman JD, Stoeger CG, Chamberlain WD. Imaging and quantification of endothelial cell loss in eye bank prepared DMEK grafts using trainable segmentation software. Curr Eye Res. 2014;39(9):894–901. doi: 10.3109/02713683.2014.887120. [DOI] [PubMed] [Google Scholar]
- 14.Krabcova I, Studeny P, Jirsova K. Endothelial quality of pre-cut posterior corneal lamellae for Descemet membrane endothelial keratoplasty with a stromal rim (DMEK-S): two-year outcome of manual preparation in an ocular tissue bank. Cell Tissue Bank. 2013;14(2):325–331. doi: 10.1007/s10561-012-9327-z. [DOI] [PubMed] [Google Scholar]
- 15.Tenkman LR, Price FW, Price MO. Descemet membrane endothelial keratoplasty donor preparation: navigating challenges and improving efficiency. Cornea. 2014;33(3):319–325. doi: 10.1097/ICO.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 16.Schlötzer-Schrehardt U, Bachmann BO, Tourtas T, et al. Reproducibility of graft preparations in Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2013;120(9):1769–1777. doi: 10.1016/j.ophtha.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Akimoto Y, Sawada H, Ohara-Imaizumi M, Nagamatsu S, Kawakami H. Change in long-spacing collagen in Descemet’s membrane of diabetic Goto-Kakizaki rats and its suppression by antidiabetic agents. Exp Diabetes Res. 2008;2008:818341. doi: 10.1155/2008/818341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latour G, Kowalczuk L, Savoldelli M, et al. Hyperglycemia-induced abnormalities in rat and human corneas: the potential of second harmonic generation microscopy. PLoS One. 2012;7(11):e48388. doi: 10.1371/journal.pone.0048388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price MO, Giebel AW, Fairchild KM, et al. Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116(12):2361–2368. doi: 10.1016/j.ophtha.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 20.Guerra FP, Anshu A, Price MO, et al. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118(12):2368–2373. doi: 10.1016/j.ophtha.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Sady C, Khosrof S, Nagaraj R. Advanced Maillard reaction and crosslinking of corneal collagen in diabetes. Biochem Biophys Res Commun. 1995;214(3):793–797. doi: 10.1006/bbrc.1995.2356. [DOI] [PubMed] [Google Scholar]
- 22.Gaens KH, Stehouwer CD, Schalkwijk CG. Advanced glycation endproducts and its receptor for advanced glycation endproducts in obesity. Curr Opin Lipidol. 2013;24(1):4–11. doi: 10.1097/MOL.0b013e32835aea13. [DOI] [PubMed] [Google Scholar]
- 23.Barchiesi BJ, Eckel RH, Ellis PP. The cornea and disorders of lipid metabolism. Surv Ophthalmol. 1991;36(1):1–22. doi: 10.1016/0039-6257(91)90205-t. erratum in Surv Ophthalmol 1992;36(4):324. [DOI] [PubMed] [Google Scholar]
- 24.Kelliher C, Engler C, Speck C, Ward D, Farazdaghi S, Jun AS. A comprehensive analysis of eye bank-prepared posterior lamellar corneal tissue for use in endothelial keratoplasty. Cornea. 2009;28(9):966–970. doi: 10.1097/ICO.0b013e31819c4fcf. [DOI] [PubMed] [Google Scholar]