TO THE EDITOR
Regeneration of skin and hair follicles after wounding, a process known as wound-induced hair neogenesis (WIHN), is a rare example of adult organogenesis in mammals. WIHN is a phenomenon in which the skin, sebaceous glands, and hair follicles are regenerated after the occurrence of large, full-thickness wounds in mice or rabbits. It has been extensively characterized in histological and molecular detail (Breedis, 1954; Gay et al., 2013; Ito et al., 2007; Nelson et al., 2015). Hence, WIHN provides a unique model for deciphering mechanisms underlying mammalian regeneration. We recently identified TLR3 as an upstream regulator of the key signaling pathway IL-6/STAT3, which promotes WIHN (Nelson et al., 2015).
IL-6 activates target cells through IL-6 receptor alpha (IL-6Rα). The IL-6/IL-6Rα complex associates with the signal transducer gp130 (IL-6st), thus activating the STAT3 transcription factor. STAT3 is a transcriptional activator downstream of IL-6 and other IL-6–type family members and is involved in numerous cellular processes, including growth, apoptosis, and skin homeostasis.
The IL-6 pathway is important in the regeneration process in multiple organ systems. For example, IL-6 is up-regulated after spinal cord injury in mice, and neutralizing IL-6R retards nerve regeneration (Hirota et al., 1996). Mice with targeted disruption of IL-6 have impaired liver regeneration, which can be prevented with exogenous IL-6 protein (Cressman et al., 1996). Using IL-6Rα knockout (KO) mice and Stat3 KO mice separately, we demonstrated a requirement for the IL-6/STAT3 pathway for WIHN (Nelson et al., 2015).
We continued our investigations of the IL-6/STAT3 signaling axis in WIHN using IL-6 null mice (C57BL/6 background). Based on our previous findings, we hypothesized that WIHN would decrease in IL-6 KO mice compared to wild-type (WT) mice (C57BL/6).
All animal protocols were approved by the Johns Hopkins University Animal Care and Use Committee. We created 1-cm2 full-thickness wounds on the backs of 21-day-old male and female mice, as previously described, (see Supplementary Materials and Methods online) (Nelson et al., 2013). First, we verified KO of the IL-6 gene by the lack of IL-6 protein expression after wounding in IL-6 KO mice compared to WT mice (Figure 1a). IL-6–deficient mice have delayed wound healing (Gallucci et al., 2001); therefore, we measured wound size throughout our WIHN assay. IL-6 KO mice showed no delay in wound healing compared to WT mice (Figure 1b). This finding may be related to the larger wounds required in the WIHN assay. Re-epithelialization occurred about 10 days after wounding in both WT and IL-6 KO mice. Unexpectedly, we observed that IL-6 KO mice displayed a twofold increase (P = 0.001) in WIHN compared to WT mice (Figure 1c). When combined with our previous findings in IL-6Rα KO mice, this paradoxical increase in WIHN in IL-6 KO mice prompted us to consider the presence of possible compensatory mechanisms.
Figure 1. IL-6 knockout (KO) mice have increased wound-induced hair neogenesis (WIHN).
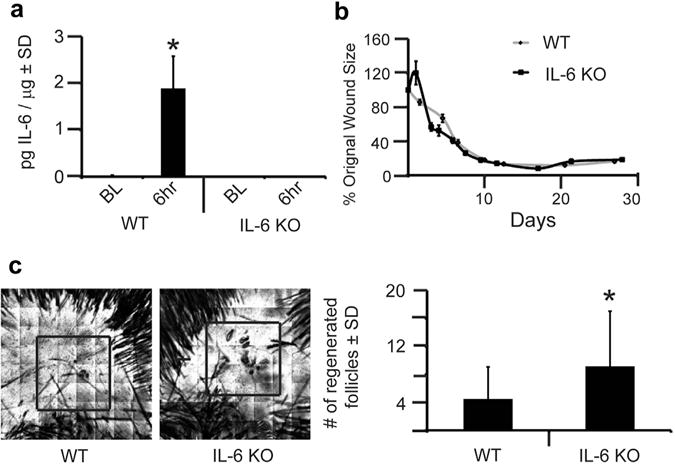
(a) Levels of IL-6 protein were measured by ELISA in wild-type (WT) and IL-6 KO mice, at baseline (BL) and 6 hours after wounding. n = 3. P < 0.05. (b) Full-excision skin wounding to the depth of skeletal muscle was performed in WT and IL-6 KO mice. At the time of wounding, we captured an image of the open wound. Subsequent images were captured every 1–2 days for 28 days. Using ImageJ computer software, wound size was calculated for each image, and data are shown as percentage of the original wound size (100%). Each datapoint is an average of 6–9 mice. (c) WIHN in IL-6 KO mice compared to WT control mice as measured by confocal scanning laser microscopy 20–24 days after wounding. Representative images for WT and IL-6 KO mice are shown. Area of WIHN is shown within box. Original image size is 4 mm2. n = 45. *P = 0.0004. SD, standard deviation.
IL-6 and other IL-6–type cytokine family members, such as oncostatin M and IL-11, share gp130, resulting in Stat3 activation (Heinrich et al., 2003). We asked whether these factors were elevated in IL-6 KO mice. Oncostatin M levels immediately increased after wounding in both IL-6 KO and WT mice; however, oncostatin M levels were approximately threefold greater in IL-6 KO mice (Figure 2a). In addition, IL-11 expression was significantly elevated 6 hours after wounding in IL-6 KO mice compared to WT mice; however, the overall levels of IL-11 remained relatively low. These data suggest that in the absence of IL-6, other IL-6–type cytokines are elevated, which may lead to enhanced STAT3 activation in the absence of IL-6. To directly test this, phosphorylated STAT3 protein levels were measured in both WT and IL-6 KO mice. In unwounded skin, the baseline level of phosphorylated STAT3 was increased 10-fold in IL-6 KO mice compared to WT mice (Figure 2b), implying that increased STAT3 activation promoted WIHN in IL-6 KO mice.
Figure 2. IL-6 knockout (KO) mice exhibit increased and necessary phosphorylated Stat3 (P-Stat3) signaling for wound-induced hair neogenesis.
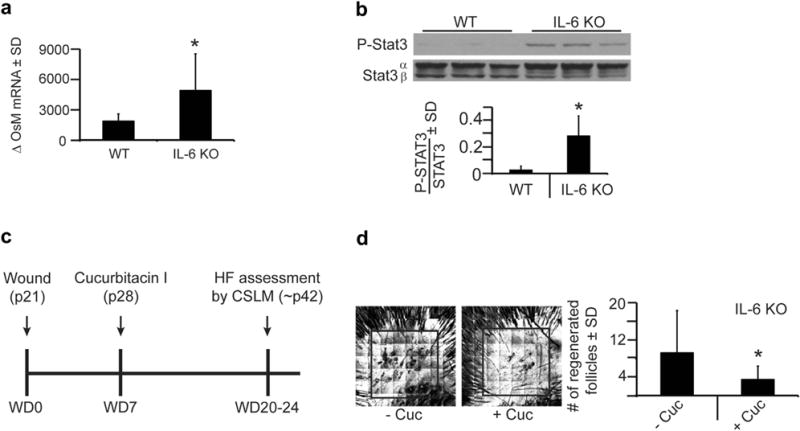
(a) Quantitative real-time PCR of oncostatin M (OsM) levels 6 hours after wounding in wild-type (WT) and IL-6 KO mice. Shown as fold change relative to baseline. n = 5–6. *P = 0.01. (b) Levels of P-Stat3 protein expression in WT and IL-6 KO mice in normal, nonwounded skin (baseline) as measured by western blot. Bar graph depicts ratio of P-Stat3/Stat 3 in arbitrary units as measured by Image J software. n = 3. *P = 0.02. (c) Full-excision skin wounding to the depth of skeletal muscle performed in WT and IL-6 KO mice, as in Figure 1c, and 7 days after wounding (WD7), cucurbitacin I (2 mg/kg), or control (phosphate buffered saline) was injected into the healing wound. Hair follicles (HF) were assessed by CSLM ~ 2 weeks later. (d) Number of regenerated hair follicles within the scar, as assessed in Figure 1c, in IL-6 KO mice with cucurbitacin I (+Cuc) compared to vehicle (−Cuc). Representative confocal scanning laser microscopy images are shown. Area of wound-induced hair neogenesis shown within box. Original image size is 4 mm2. n = 11–13. *P < 0.05. SD, standard deviation.
STAT3 is involved in hair follicle morphogenesis, hair cycling, wound repair, and WIHN (Nelson et al., 2015; Sano et al., 1999). Increased WIHN in IL-6 KOs is likely due to STAT3 activity. To functionally test the role of STAT3 signaling in these mice, we used cucurbitacin I, a selective JAK2/STAT3 pharmacological inhibitor (Blaskovich et al., 2003). A single intradermal injection of cucurbitacin I, 7 days after wounding but before complete re-epithelialization, was sufficient to inhibit phosphorylation of STAT3 by 85% (Figure 2c) (Nelson et al., 2015). Cucurbitacin I significantly reduced the number of regenerated hair follicles (approximately threefold) in IL-6 KO mice compared to vehicle (Figure 2d). Together these data suggest that although IL-6 expression is lacking within these IL-6 KO mice, the STAT3 signaling pathway is still present and is functionally important for WIHN.
There are several implications for this work. Because we had expected to see decreased WIHN in IL-6 KO mice, our paradoxical finding led us to carefully characterize our murine model. We demonstrate that in the absence of IL-6, other IL-6–type family members are elevated and that the activity of the downstream transcription factor STAT3 is significantly enhanced. Our results highlight the need for caution when interpreting results from IL-6 KO mice as related to the IL-6 signaling pathway and the general complexities of ligand compensation. For example, the use of bodywide constitutive KOs, such as the IL-6 KO murine model, seems to allow for more compensation than previously used conditional tissue-specific null models (Nelson et al, 2015).
STAT3 is functionally required for WIHN and is a critical regulator of regeneration in many systems. Exogenous addition of IL-6 during wound healing does not augment WIHN in these IL-6 KO mice (data not shown) as it does in WT mice (Nelson et al., 2015), suggesting that STAT3 pathway activation is already sufficient for WIHN and WIHN cannot be enhanced further. Dimerization with gp130 to elicit activation of the JAK/STAT3 pathway is common to IL-6 family members. Ciliary neurotrophic factor and leukemia inhibitory factor have also been linked to tissue regeneration, suggesting that the activity of gp130 is the critical key player in this pathway (Heinrich et al., 2003).
Supplementary Material
Acknowledgments
Research reported in this article was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number F32AR062932 to AMN and R01AR064297 to LAG. This work was also supported by the Department of Defense, Armed Forces Institute of Regenerative Medicine, Extremities Regeneration (AFIRM2-ER11), Northrop Grumman Electronic Systems, Alliance for Veterans Support, Inc. (Veteran/Amputee Skin Regeneration Program Initiative), and the Thomas Provost, MD Young Faculty Development Fund of Johns Hopkins Dermatology to LAG.
Abbreviations
- IL-6Rα
IL-6 receptor alpha
- KO
knockout
- WIHN
wound-induced hair neogenesis
- WT
wild type
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.doi.org/10.1016/j.jid.2015.12.043.
References
- Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–9. [PubMed] [Google Scholar]
- Breedis C. Regeneration of hair follicles and sebaceous glands from the epithelium of scars in the rabbit. Cancer Res. 1954;14:575–9. [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379–83. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- Gallucci RM, Sugawara T, Yucesoy B, Berryann K, Simeonova PP, Matheson JM, et al. Interleukin-6 treatment augments cutaneous wound healing in immunosuppressed mice. J Interferon Cytokine Res. 2001;21:603–9. doi: 10.1089/10799900152547867. [DOI] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–23. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota H, Kiyama H, Kishimoto T, Taga T. Accelerated nerve regeneration in mice by upregulated expression of interleukin (IL) 6 and IL-6 receptor after trauma. J Exp Med. 1996;183:2627–34. doi: 10.1084/jem.183.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Nelson AM, Loy DE, Lawson JA, Katseff AS, Fitzgerald GA, Garza LA. Prostaglandin D(2) inhibits wound-induced hair follicle neogenesis through the receptor, Gpr44. J invest Dermatol. 2013;133:881–9. doi: 10.1038/jid.2012.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AM, Reddy SK, Ratliff TS, Hossain MZ, Katseff AS, Zhu AS, et al. dsRNA released by tissue damage activates TLR3 to drive skin regeneration. Cell Stem Cell. 2015;17:139–51. doi: 10.1016/j.stem.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 1999;18:4657–68. doi: 10.1093/emboj/18.17.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


