Abstract
Background
The consequences of decentralizing prevention of mother-to-child HIV transmission and HIV-exposed infant services to antenatal care (ANC)/labor and delivery (L&D) sites from dedicated HIV care and treatment (C&T) centers remain unknown, particularly in low prevalence settings.
Methods
In a cohort of mother–infant pairs, we compared delivery of routine services at ANC/L&D and C&T facilities in Kinshasa, Democratic Republic of Congo from 2010–2013, using methods accounting for competing risks (eg, death). Women could opt to receive interventions at 90 decentralized ANC/L&D sites, or 2 affiliated C&T centers. Additionally, we assessed decentralization’s population-level impacts by comparing proportions of women and infants receiving interventions before (2009–2010) and after (2011–2013) decentralization.
Results
Among newly HIV-diagnosed women (N = 1482), the 14-week cumulative incidence of receiving the package of CD4 testing and zidovudine or antiretroviral therapy was less at ANC/L&D [66%; 95% confidence interval (CI): 63% to 69%] than at C&T (88%; 95% CI: 83% to 92%) sites (subdistribution hazard ratio, 0.62; 95% CI: 0.55 to 0.69). Delivery of cotrimoxazole and DNA polymerase chain reaction testing to HIV-exposed infants (N = 1182) was inferior at ANC/L&D sites (subdistribution hazard ratio, 0.84; 95% CI: 0.76 to 0.92); the 10-month cumulative incidence of the package at ANC/L&D sites was 89% (95% CI: 82% to 93%) versus 97% (95% CI: 93% to 99%) at C&T centers. Receipt of the pregnancy (20% of 1518, to 64% of 1405) and infant (16%–31%) packages improved post decentralization.
Conclusions
Services were delivered less efficiently at ANC/L&D sites than C&T centers. Although access improved with decentralization, its potential cannot be realized without sufficient and sustained support.
Keywords: prevention of mother to child transmission, vertical transmission, Democratic Republic of Congo, health services accessibility, delivery of health care, prenatal care/utilization, postnatal care/utilization
INTRODUCTION
Access to prevention of mother-to-child HIV transmission (PMTCT) services has improved over time–the proportion of HIV-positive pregnant women who received antiretroviral (ARV) prophylaxis in the 21 African priority countries of the UNAIDS Global Plan1 increased from 33% in 2009 to 68% in 2013.2 Despite this progress, this goal to reduce new pediatric HIV infections by 90% and halve AIDS-related maternal deaths by 2015 will not be achieved without improving the quality of service delivery and mitigating loss points throughout the PMTCT care continuum.3
After the identification of HIV infection during pregnancy, multiple steps are essential to minimize vertical transmission and assure long-term maternal and infant health.4,5 In resource-limited settings, barriers to effective HIV prevention and care often arise from deficiencies in the facilities where antenatal care (ANC), labor and delivery (L&D), and postpartum services are provided. For example, these sites regularly lack the CD4 assessment capacity6–8 needed to appropriately prescribe antiretroviral therapy (ART).9 As PMTCT programs are pregnancy-focused10 and frequently in distinct physical locations lacking linkages to HIV care and treatment (C&T),11–13 there are also postnatal barriers including low uptake of ARV prophylaxis by infants14,15 and unavailability of diagnostic DNA polymerase chain reaction (PCR) testing.16
One strategy to increase coverage levels of maternal and infant interventions is decentralization, a shifting of services to primary care centers.17 Decentralization to ANC sites has yielded promising results — in South Africa18 and Rwanda19, for example, ART initiation by pregnant women was equivalent at decentralized and centralized sites. Decentralization of services in the Democratic Republic of Congo (DRC) has been extremely limited, paralleling the scale-up of other HIV services that has lagged behind in most other countries7 in the wake of civil wars and decades of political, social, and economic difficulties that impeded the development of vital health care infrastructures.20,21 Few primary care clinics offer CD4 testing or efficacious ARV regimens for PMTCT22 recommended by the World Health Organization (WHO). This is reflected in the 35% of Congolese HIV-infected pregnant women who received appropriate ARVs in 201323 and the 2% who received a complete package of PMTCT services in 2012.22
The DRC, classified by UNAIDS as a focus country for pediatric HIV elimination,1 accounted for 3% of new HIV infections in children in 2013 despite low prevalence among young women (0.5%).2 This burden is largely unmet, as evidenced by our network of ANC/L&D sites in Kinshasa (the largest in this city of 10 million) identifying fewer than 15% of pregnancies among HIV-infected women in 2008.24 Decentralizing PMTCT and HIV-exposed infant services in the DRC thus has significant potential to alleviate both regional and global burdens of maternal and pediatric HIV, although it is incompletely explored if the delivery of interventions at decentralized sites not historically accustomed to such provision, is on par with that at experienced, specialized sites. Furthermore, the population-level impacts of decentralization in low HIV prevalence areas remain unknown.
To increase program scope and coverage, we decentralized an expanded package of PMTCT and HIV-exposed infant services to 90 high-volume ANC/L&D sites in the resource-deprived, low prevalence setting of Kinshasa. With the goal of informing program scale-up in similar implementation contexts, after rollout of new individual-level registers linking mother–infant pairs, this study had 2 aims. First, we assessed if intervention packages of CD4 testing and zidovudine (AZT) or ART for mothers, and DNA PCR testing and cotrimoxazole prophylaxis for infants were delivered as efficiently at decentralized ANC/L&D sites as at dedicated C&T centers. Secondly, we aimed to quantify the population-level impacts of decentralization by comparing the numbers and proportions of mothers and infants receiving the above intervention packages before and after their availability at ANC/L&D sites.
METHODS
Study Setting, Interventions, and Data Sources
In 2003, the University of North Carolina at Chapel Hill (UNC) initiated its HIV prevention, care, and treatment programs in the DRC (UNC-DRC) by strengthening Kinshasa ANC/L&D sites through the introduction of a minimum package of maternal and infant services, including HIV counseling and testing and PMTCT prophylaxis.25 This package, instituted in June 2010 in 37 of the city’s highest volume facilities, has been described previously.24–26
In April 2010, we began rolling out an expanded package to these sites, and 53 others (for a total of 90) were added by September 2012 to cover 32/35 provincial health zones. The prior standard of care was bolstered by training personnel on the 2010 WHO PMTCT guidelines27 that includes Option A, empathetic counseling skills, co-located delivery care, and the importance of patient retention, paying delivery fees to encourage safe birth, and providing (1) CD4 testing, cotrimoxazole, and AZT for mothers, and (2) cotrimoxazole, extended nevirapine, and DNA PCR testing for infants. Transportation costs to the first visit at either of 2 affiliated (but separately located) HIV C&T centers were provided to promote long-term care uptake; however, once the new interventions were available at ANC/L&D sites, women could opt to remain there for PMTCT and HIV-exposed infant services. At 45 sites, volunteer HIV-infected “mother-mentors” worked as clinic assistants, helping women to navigate care while encouraging C&T uptake. Generally, women with CD4 counts ≤350 could access ART at C&T centers only. Details on the ANC/L&D sites are outlined in Table S1 (see Supplemental Digital Content, http://links.lww.com/QAI/A726).
An essential expanded package component was a new paper register that allowed for mothers and their infants to be linked and longitudinally tracked. Service delivery and patient dispositions were recorded in the registers by the ANC/L&D site providers and UNC-DRC personnel, who made supportive supervisory monitoring visits monthly as part of the expanded package. Data were recorded between a woman’s presentation for ANC or L&D, until her infant was confirmed as HIV-infected or ruled out as HIV-uninfected. Details on the populations of mothers and infants tracked in the registers are presented in Table S2 (see Supplemental Digital Content, http://links.lww.com/QAI/A726).
The study sites included the 90 ANC/L&D sites and 2 UNC-DRC C&T centers, Kalembe Lembe Pediatric Hospital and Bomoi Healthcare Center.28,29 The data sources were the registers at the ANC/L&D sites, which were entered into an Epi Info database, the real-time Epi Info database at the C&T centers,30 and an aggregate service delivery database from the ANC/L&D sites used to provide program context and quantify decentralization’s population-level impacts. The Epi Info databases contained unique patient identifiers to link mother–infant pairs and were linked to each other, as the ANC/L&D database included C&T codes and the C&T database included ANC/L&D codes. This facilitated tracking of individuals between facility types. All database records as of July 2013, when data collection ended, were included in analyses.
Measures and Analyses
In the service delivery aim, we calculated the cumulative incidence of 2 outcomes between 2010 and 2013: (1) receipt of CD4 testing, and AZT or ART (pregnancy package), by women newly diagnosed with HIV before L&D, and (2) receipt of DNA PCR testing and cotrimoxazole (infant package) by HIV-exposed infants, at 2 facility types: (1) ANC/L&D site, providing decentralized services, and (2) C&T center, specialized to deliver these services. In the analysis of women, follow-up began at C&T enrollment if the pregnancy was registered at a site before it began offering CD4 testing and AZT; otherwise, follow-up began at ANC presentation. Follow-up ended at the earliest of (1) the outcome, (2) censoring (loss to follow-up, transfer, move, voluntary withdrawal, or end of study), or (3) a competing risk (delivery, miscarriage, or death). In the infant analysis, follow-up began on the date of first visit at ≥1 month of age (when cotrimoxazole is indicated31) at a facility offering DNA PCR testing and cotrimoxazole. Additionally, reaching 18 months of age (the end of the early infant diagnosis period, when HIV can be serologically confirmed) was an additional censoring event, and competing risks were limited to death and confirmation of HIV-negative status.
As individuals could opt to receive services at a C&T center, exposure could switch from “decentralized” to “specialized” (but not vice-versa) if this transfer occurred before either component of the intervention package was received; if 1 of the outcome services was received, followup was censored on the transfer date (date of C&T enrollment). To allow for this late entry, we used the counting process configuration as applied by Geskus.32 Individuals who received the intervention package on their enrollment date and those lost after a single visit33 were assigned a follow-up time of 1 day. For others censored, if deemed lost to follow-up after a missed visit, follow-up ended on the last visit date. Only newly HIV-diagnosed women were eligible because the outcome included ARV receipt, and many previously diagnosed women were already on ART; additionally, time in care was likely a confounder and modifier of the associations of interest. Infants were eligible for the infant analysis even if their mothers were not in the analysis of women. DNA PCR testing was defined as specimen collected, not also receipt of result.
Competing risks are events such as death that preclude occurrence of the outcome and can create bias.34 To account for competing risks in our data, the SAS macro %PSHREG was used to obtain competing risk cumulative incidence functions stratified by facility type (decentralized versus specialized) and to quantify the effects of facility type on delivery of the intervention packages.35 Crude and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were generated using the Fine and Gray subdistribution hazards model,36 which produces an effect measure due to both the association of the exposure on the event of interest and the possibly differential impact of competing events on exposed and unexposed individuals.37 We also employed the cause-specific Cox proportional hazards model, which treats competing events as censored, and results in biased effect estimates if its inherent assumption of independence between the outcome and competing events is incorrect.37
Causal diagrams were used to identify confounders for adjusted models.38 In the analysis of women, covariates were age and gestational age at registration along with CD4 count during pregnancy; infant analysis covariates were age at first visit and 3 maternal factors: gestational age, HIV status (undiagnosed or previously diagnosed) at registration, and CD4 count during pregnancy. We employed multiple imputation with fully conditional specification methods39 to account for missing covariate data; 5 imputed datasets were generated. To relax the linearity assumption in modeling continuous variables, we used Stone and Koo’s additive splines constrained to be linear in the tails, with knots at the fifth, 35th, 65th, and 95th percentiles. χ2 or Cochran-Armitage trend tests were used to compare proportions, with medians compared by the Mann–Whitney test.40
In the aim to quantify decentralization’s population-level impacts, we calculated the proportions of HIV-infected women who received the pregnancy package pre-decentralization (at affiliated C&T centers only) versus post-decentralization (at either the C&T centers or ANC/L&D sites). Similarly, proportions of their HIV-exposed infants who received the infant package, pre- and post-decentralization, were calculated. All analyses were completed in SAS 9.3 (SAS Institute Inc., Cary, NC).
Ethical Approval
The study was approved by the Kinshasa School of Public Health Ethics Committee and the UNC Institutional Review Board. Consent was obtained at C&T enrollment; activities at the ANC/L&D sites met criteria for waiver of consent.
RESULTS
Aim 1: Service Delivery
Between 2010 and 2013, in our cohort of linked mother–infant pairs, we identified 1482 newly HIV-diagnosed women eligible for the analysis of CD4 testing and ARV regimen delivery during pregnancy, as well as 1142 HIV-exposed infants eligible for the analysis of DNA PCR testing and cotrimoxazole delivery. Their characteristics and outcomes are described in Table 1. Women at a C&T center did not differ from those at only a ANC/L&D site in terms of age (P = 0.88) or gestational age (P = 0.05) at registration. The cumulative incidence of receiving the pregnancy package at 14 weeks was less at the ANC/L&D sites (66%; 95% CI: 63% to 69%) than that at the C&T centers (88%; 95% CI: 83% to 92%) (Fig. 1), and both the unadjusted (0.59; 95% CI: 0.51 to 0.69) and adjusted (0.62; 95% CI: 0.55 to 0.69) subdistribution HRs indicate inferior delivery of the package at the ANC/L&D sites compared with the C&T centers (Table 2). No marked changes in delivery of the package over time at either facility type were noted.
TABLE 1.
Characteristics and Outcomes of HIV-Infected Women and Their HIV-Exposed Infants Included in Cumulative Incidence Analyses, Kinshasa, Democratic Republic of Congo, 2010–2013*
| Women Newly Diagnosed With HIV During Pregnancy (If ANC/L&D Site Person-Time, Pregnancy Registered While Decentralized CD4 Testing and AZT Available) |
At ANC/L&D Site Only (N = 1222) | At Affiliated HIV C&T Center for All or Part of Follow-up† N = 260) | Total (N = 1482) |
|---|---|---|---|
| Year of registration, n (%) | |||
| 2010 | 0 (0.0) | 77 (29.6) | 77 (5.2) |
| 2011 | 151 (12.4) | 176 (67.7) | 327 (22.1) |
| 2012 | 677 (55.4) | 6 (2.3) | 683 (46.1) |
| 2013 | 394 (32.2) | 1 (0.4) | 395 (26.7) |
| Age at registration, median (IQR),‡ yrs | 29 (25–34) | 30 (25–34) | 29 (25–34) |
| Gestational age at registration, median (IQR),§ wks | 22 (18–28) | 22 (17–26) | 22 (18–28) |
| WHO HIV clinical stage at registration, n (%) | |||
| 1 | 403 (33.0) | 16 (6.2) | 419 (28.3) |
| 2 | 63 (5.2) | 0 (0.0) | 63 (4.3) |
| 3 | 13 (1.1) | 0 (0.0) | 13 (0.9) |
| 4 | 3 (0.2) | 0 (0.0) | 3 (0.2) |
| Not assessed | 740 (60.6) | 244 (93.8) | 984 (66.4) |
| Referred to affiliated HIV C&T center, n (%) | |||
| No | 988 (80.9) | 0 (0.0) | 988 (66.7) |
| Yes | 234 (19.1) | 260 (100.0) | 494 (33.3) |
| CD4 test during pregnancy, n (%) | |||
| No | 407 (33.3) | 6 (2.3) | 413 (27.9) |
| Yes | 815 (66.7) | 254 (97.7) | 1069 (72.1) |
| ≤350∥ | 397 (50.7) | 108 (45.6) | 505 (49.5) |
| >350 | 386 (49.3) | 129 (54.4) | 515 (50.5) |
| CD4 count during pregnancy, median (IQR)¶ | 347 (223–506) | 379 (235–577) | 353 (227–519) |
| Antiretroviral regimen during pregnancy, n (%) | |||
| None | 188 (15.4) | 21 (8.1) | 209 (14.1) |
| AZT | 927 (75.9) | 142 (54.6) | 1069 (72.1) |
| Triple-drug ART | 107 (8.8) | 97 (37.3) | 204 (13.8) |
| Outcome,# n (%) | |||
| CD4 testing, and AZT or ART | 730 (59.7) | 234 (90.0) | 964 (65.0) |
| Delivered** | 197 (16.1) | 23 (8.8) | 220 (14.8) |
| Lost to follow-up | 231 (18.9) | 1 (0.4) | 232 (15.7) |
| Miscarriage or died | 6 (0.5) | 2 (0.8) | 8 (0.5) |
| Transferred, moved, or voluntarily withdrew | 43 (3.5) | 0 (0.0) | 43 (2.9) |
| Pregnant at study end | 15 (1.2) | 0 (0.0) | 15 (1.0) |
|
| |||
|
HIV-Exposed Infants (If ANC/L&D Site Person-Time, First Visit at ≥ 1 mo of Age While Decentralized Cotrimoxazole and DNA PCR Testing Available) |
At ANC/L&D Site Only (N = 718) | At Affiliated HIV C&T Center for All or Part of Follow-up†† (N = 424) | Total (N = 1142) |
|
| |||
| Year of first visit at ≥1 mo of age, n (%) | |||
| 2010 | 0 (0.0) | 17 (4.0) | 17 (1.5) |
| 2011 | 234 (32.6) | 223 (52.6) | 457 (40.0) |
| 2012 | 320 (44.6) | 134 (31.6) | 454 (39.8) |
| 2013 | 164 (22.8) | 50 (11.8) | 214 (18.7) |
| Age at first visit at ≥1 mo of age, median (IQR), wks | 6.7 (6.4–7.4) | 6.6 (6.0–7.3) | 6.7 (6.3–7.3) |
| Maternal HIV status at registration, n (%) | |||
| Previously diagnosed | 208 (29.0) | 156 (36.8) | 364 (31.9) |
| Undiagnosed | 510 (71.0) | 268 (63.2) | 778 (68.1) |
| Maternal pregnancy stage at registration,§ n (%) | |||
| ≤28 wks | 430 (59.9) | 330 (77.8) | 760 (66.5) |
| >28 wks, before labor and delivery | 143 (19.9) | 54 (12.7) | 197 (17.3) |
| Unknown, before labor and delivery | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Labor and delivery | 145 (20.2) | 39 (9.2) | 184 (16.1) |
| Maternal gestational age at registration, median (IQR),§ wks | 26 (20–36) | 24 (18–28) | 24 (20–32) |
| Maternal WHO HIV clinical stage at registration, n (%) | |||
| 1 | 133 (18.5) | 79 (18.6) | 212 (18.6) |
| 2 | 29 (4.0) | 26 (6.1) | 55 (4.8) |
| 3 | 5 (0.7) | 16 (3.8) | 21 (1.8) |
| 4 | 1 (0.1) | 2 (0.5) | 3 (0.3) |
| Not assessed | 550 (76.6) | 301 (71.0) | 851 (74.5) |
| Maternal CD4 test during pregnancy, n (%) | |||
| No | 421 (58.6) | 115 (27.1) | 536 (46.9) |
| Yes | 297 (41.4) | 309 (72.9) | 606 (53.1) |
| ≤350∥ | 102 (35.5) | 157 (53.2) | 259 (44.5) |
| >350 | 185 (64.5) | 138 (46.8) | 323 (55.5) |
| Maternal CD4 count during pregnancy, median (IQR)¶ | 421 (284–559) | 336 (218–504) | 378 (248–545) |
| Maternal antiretroviral regimen during pregnancy, n (%) | |||
| None | 254 (33.4) | 49 (11.6) | 303 (26.5) |
| AZT | 318 (44.3) | 143 (33.7) | 461 (40.4) |
| Triple-drug ART | 146 (20.3) | 232 (54.7) | 378 (33.1) |
| Extended nevirapine, n (%) | |||
| No | 39 (5.4) | 26 (6.1) | 65 (5.7) |
| Yes | 679 (94.6) | 398 (93.9) | 1077 (94.3) |
| Cotrimoxazole, n (%) | |||
| No | 109 (15.2) | 22 (5.1) | 131 (11.4) |
| Yes | 609 (84.8) | 409 (94.9) | 1018 (88.6) |
| DNA PCR test, n (%) | |||
| No | 131 (18.2) | 22 (5.6) | 153 (13.3) |
| Yes | 587 (81.8) | 409 (94.4) | 996 (86.7) |
| Positive‡‡ | 20 (3.8) | 23 (5.7) | 43 (4.6) |
| Negative | 503 (96.2) | 379 (94.3) | 882 (95.4) |
| Outcome,# n (%) | |||
| Cotrimoxazole and DNA PCR test | 562 (78.3) | 398 (93.9) | 960 (84.1) |
| Reached 18 mo of age | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Lost to follow-up | 144 (20.1) | 3 (0.7) | 147 (12.9) |
| Died | 0 (0.0) | 3 (0.7) | 3 (0.3) |
| Transferred, moved, or voluntarily withdrew | 9 (1.3) | 2 (0.5) | 11 (1.0) |
| Under follow-up at study end | 3 (0.4) | 17 (4.0) | 20 (1.8) |
Data from ANC/L&D sites (2 of 90 had zero registered pregnancies) and 2 affiliated HIV C&T centers.
Twelve women contributed person-time at both an ANC/L&D site and an HIV C&T center.
Among 1336 pregnancies where the woman’s date of birth was recorded.
Among pregnancies with actual or projected gestational age. If gestational age at registration was not recorded, it was estimated using projected date of delivery and otherwise date of delivery (if available).
Denominator: Non-missing CD4 results.
Among non-missing CD4 results.
Proportions, not cumulative incidences.
Includes 16 women without a recorded delivery date who were projected, at study end, to have delivered.
Eleven infants contributed person-time at both an ANC/L&D site and an HIV C&T center.
Denominator: Non-missing DNA PCR results.
IQR, interquartile range.
FIGURE 1.
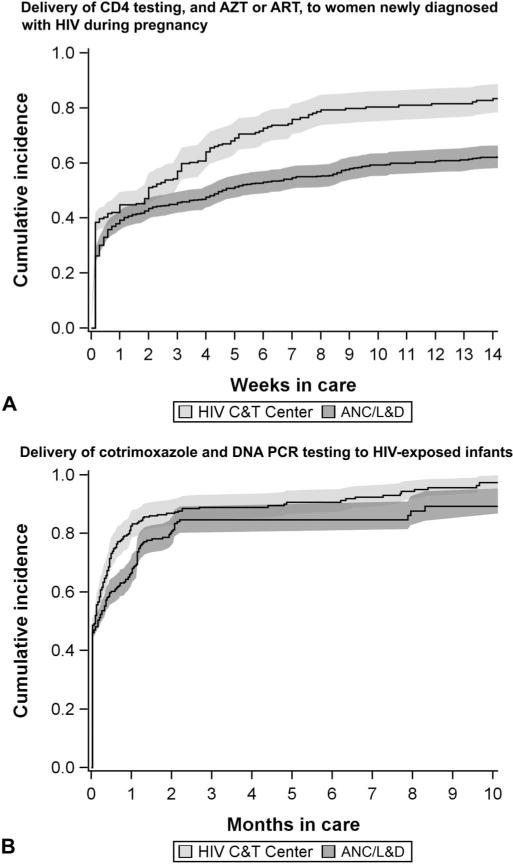
Cumulative incidence of delivery of CD4 testing, and AZT or ART, to women newly diagnosed with HIV during pregnancy (A) and cotrimoxazole and DNA PCR testing to HIV-exposed infants (B), by facility type, Kinshasa, Democratic Republic of Congo, 2010–2013. Bands represent 95% CIs. A, The cumulative incidence function is estimated at the mean values of (1) age at registration, (2) gestational age at registration, and (3) CD4 count during pregnancy. B, The cumulative incidence function is estimated at the mean values of (1) age at first visit at ≥1 month of age, (2) maternal gestational age at registration, and (3) maternal CD4 count during pregnancy (a proxy for HIV disease progression), as well as the reference level for maternal HIV status (undiagnosed, rather than previously diagnosed). If gestational age at registration was not recorded, it was estimated using projected date of delivery and otherwise date of delivery (if available).
TABLE 2.
Estimated Effects of Facility Type on the Delivery of Maternal PMTCT and HIV-Exposed Infant Services, Kinshasa, Democratic Republic of Congo, 2010–2013
| Outcome | Facility Type | Cox Proportional Hazards Model, Unadjusted
|
Fine and Gray Model, Unadjusted
|
Fine and Gray Model, Covariate-Adjusted, Multiple Imputation*
|
|||
|---|---|---|---|---|---|---|---|
| Cause-specific HR | 95% CI | Subdistribution HR | 95% CI | Subdistribution HR | 95% CI | ||
| Receipt of CD4 testing, and AZT or ART, during pregnancy by women newly diagnosed with HIV | ANC/L&D site | 0.59 | 0.51 to 0.69 | 0.59 | 0.51 to 0.69 | 0.62 | 0.53 to 0.71 |
| HIV C&T center | 1 | 1 | 1 | ||||
| Receipt of cotrimoxazole and DNA PCR testing by HIV-exposed infants | ANC/L&D site | 0.85 | 0.75 to 0.97 | 0.85 | 0.75 to 0.97 | 0.84 | 0.73 to 0.96 |
| HIV C&T center | 1 | 1 | 1 | ||||
The estimate for HIV-exposed infants is adjusted for age at first visit at ≥1 month of age, along with 3 maternal factors: HIV status (undiagnosed or previously diagnosed) and gestational age at registration, as well as CD4 count during pregnancy (a proxy for HIV disease progression). If gestational age at registration was not recorded, it was estimated using projected date of delivery and otherwise date of delivery (if available). Missing data were imputed (5 datasets); data were missing on at least 1 adjustment variable for 37% of newly diagnosed women and 49% of HIV-exposed infants.
The estimate for newly diagnosed women is adjusted for age and gestational age at registration, as well as CD4 count during pregnancy.
Compared with infants at a C&T center, those only at an ANC/L&D site were older at first visit (P < 0.01) and more likely had mothers who were previously HIV undiagnosed (P = 0.01), of greater gestational age (P < 0.01) at registration, healthier as reflected by pregnancy CD4 count (P < 0.01), and did not receive AZT or ART (P < 0.01). The cumulative incidences of receiving the infant package at 10 months (Fig. 1) at the ANC/L&D sites (89%; 95% CI: 82% to 93%) and the C&T centers (97%; 95% CI: 93% to 99%), as well as the unadjusted (0.85; 95% CI: 0.75 to 0.97) and adjusted (0.84; 95% CI: 0.76 to 0.92) subdistribution HRs (Table 2), suggest that delivery of the package at the ANC/L&D sites was poorer than that at the C&T centers. No marked changes in delivery of the package over time at either facility type were noted. Using age rather than months in care as the timescale, the median age at package receipt at the ANC/L&D sites was 7.3 weeks (interquartile range, 6.6–11.7), similar to that at the C&T sites (7.0 weeks; interquartile range, 6.4–8.9).
Only 14.2% of newly diagnosed cases remaining at the ANC/L&D sites received all 4 components of the pregnancy and infant packages, markedly lower than the 53.5% observed among cases at the C&T centers (Fig. 2). Altogether, just 21.1% of total newly diagnosed cases received all 4 components of the packages, which emphasizes that overall impact was driven more by the ANC/L&D sites where most individuals remained for care.
FIGURE 2.
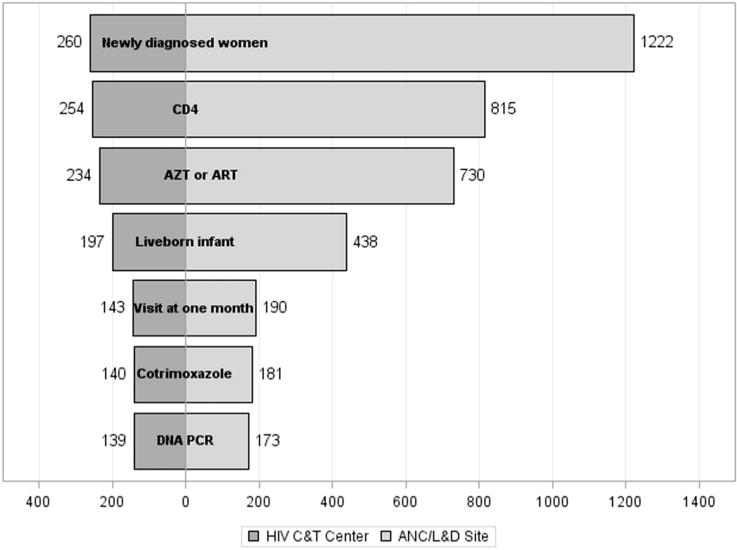
Cascade of PMTCT and HIV-exposed infant services by facility type, Kinshasa, Democratic Republic of Congo, 2010–2013. The percentages represent the counts of infants who have received a DNA PCR test divided by the total number of newly diagnosed women (eg, 173/1222 = 14.2%), not cumulative incidences.
Aim 2: Population-Level Impacts of Decentralization
During the pre-decentralization period of 2009–2010, considering all HIV-infected pregnant women identified at ANC/L&D sites including those not presenting to C&T (N = 1518), package uptake at affiliated C&T centers was approximately 20% (pregnancy) and 16% (infant). In 2011–2013 when services were decentralized (N = 1405), uptake across facilities increased to about 64% (pregnancy) and 31% (infant). These increases are depicted in Fig. 3. During the period of decentralization, 1260 women and 634 infants received at least 1 intervention at an ANC/L&D site; no temporal changes in pregnancy and infant package uptake were noted. Greater proportions of women (P < 0.01) and infants (P < 0.01) remained at ANC/L&D sites in later years (Table 1), reflecting the increased dissemination of decentralized services over time.
FIGURE 3.
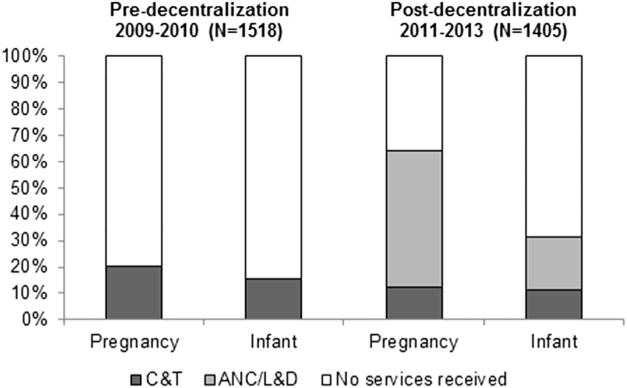
Population-level impacts of decentralization on uptake of pregnancy and infant packages, Kinshasa, Democratic Republic of Congo, 2009–2013. The pregnancy package is CD4 testing and AZT or ART; the infant package is cotrimoxazole and DNA PCR testing.
DISCUSSION
This study, by using a novel system to link and track mother–infant pairs in Kinshasa, DRC, revealed that decentralized primary care facilities focused on ANC/L&D delivered PMTCT and HIV-exposed infant services less efficiently than specialized HIV C&T centers. The finding held true for the pregnancy package of CD4 testing and AZT or ART (HR, 0.62; 95% CI: 0.55 to 0.69) as well as the infant package of DNA PCR testing and cotrimoxazole (HR, 0.84; 95% CI: 0.76 to 0.92), and provides unique evidence on integration called “urgently needed” in a recent systematic review.41 As PMTCT and HIV-exposed infant services are increasingly incorporated into primary care in conjunction with the accelerating global scale-up of WHO Options B/B+,42 our work provides a caution to program implementers that even with sustained commitment to training, capacity, and monitoring and evaluation (M&E), optimal service delivery may be harder to attain at decentralized sites.
Despite the noted discrepancies between facility types, performance at the ANC/L&D sites was encouraging. At these sites, the cumulative incidences of the pregnancy and infant packages were 66% and 89%, respectively, and substantial proportions of the populations received the interventions on their first day in care. Uptake of interventions by both mothers and infants increased dramatically post-decentralization, resulting in greater absolute numbers of individuals reached, as observed in Nigeria.43 There were specific areas where the C&T centers appeared to outperform, including CD4 testing (98% of women versus 67%), as observed in Rwanda,19 and provision of services at visits during later follow-up, consistent with personnel at dedicated sites having a deeper understanding of the need for HIV-related care. These differences inform the selection of strategies to improve service delivery in other contexts, for example point-of-care assays44,45 (which were unavailable at the ANC/L&D sites) and integrating HIV interventions into routine clinical encounters such as infant immunization visits.46
The new register and data systems were instrumental in providing a detailed, individual-level picture of our populations for the first time. Notably, these low-tech tools were successfully implemented in a resource-constrained environment, responding to calls for strengthening of PMTCT systems to improve health,47–49 targeting areas for improvement, and demonstrating the feasibility of such innovations in similar settings. Despite rigorous M&E and retention efforts, there was frequent loss to follow-up of mothers and infants, particularly at ANC/L&D sites (Table 1; Fig. 2), and between delivery and first infant visit (Fig. 2; see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A726). Programs decentralizing services must actively combat this phenomenon, which negatively impacts outcomes and their assessment. Accordingly, an inadequate percentage of women ultimately had their infants DNA PCR tested–Fig. 2 shows high attrition, as commonly noted,50 regardless of facility type. Programs experiencing attrition should employ a multifocal approach with tactics such as peer support,51 cash transfers,52 and maternal ART provision,53 to mitigate loss across the cascade.
Study strengths include examining relevant outcomes (eg, CD4 testing; maternal immunological assessment will occur in an Option B context upon completion of breastfeeding, and in an Option B+ context during ART follow-up), high generalizability because evaluated ANC/L&D sites were locally administered and were not research facilities, and intensive M&E and training that likely resulted in superior data quality and improved service delivery. We believe that stronger magnitudes of effect favoring the C&T centers would have been noted without our strong caliber of technical support to the ANC/L&D sites, and that decentralized sites lacking such support would underperform relative to the ANC/L&D sites in this study. As the study was in a low HIV prevalence context, it provides valuable information for programs in declining prevalence settings while demonstrating that low prevalence does not imply low unmet need. An additional strength is the competing risks methodology, although in this application, Fine and Gray models yielded effect measures not appreciably different than those from traditional Cox Proportional Hazards models, due to the timing and low frequency of competing events. The presence of competing risks in implementation settings and aim to affect policy37 informed the chosen methodological approach, which precluded questions of possible bias had competing risks not been taken into account.
Study limitations include (1) inconsistent recording of extended nevirapine initiation, which prevented its inclusion in the infant package, (2) rarely assessing WHO HIV clinical stage, precluding its use as a covariate, and (3) not collecting the data necessary to see if there were sociodemographic differences between women at ANC/L&D and C&T sites. We were not able to evaluate if effects were heterogeneous across ANC/L&D sites due to limited sample size, and we also lacked data on receipt of DNA PCR test result, an important PMTCT indicator. Although it is possible that women who presented at a C&T center or were in contact with a “mother-mentor” were more motivated to follow through with care for themselves and their infants, thus potentially impacting results, we believe that service delivery was primarily provider-driven. Relatedly, we speculated that a woman’s health status (as reflected by her CD4 count) might influence a provider’s likelihood of delivering services, and hence this factor was included in adjusted analyses.
In conclusion, decentralization of PMTCT and HIV-exposed infant services is a structural intervention with great promise to help meet global benchmarks to reduce pediatric HIV, as evidenced by the observed increase in package uptake post-decentralization and the encouraging performance by ANC/L&D sites in delivering key interventions. However, our results also suggest that if the approach is to be maximally effective, efforts to integrate such services into primary care must be accompanied by training and support that are sufficient and sustained, as well as requisite systems for the M&E of impact. This message should be considered in decision-making on resource allocation and program implementation.
Supplementary Material
Acknowledgments
The authors are grateful for the patient care, data entry, and program administration, coordination, and support contributions of all UNC-DRC personnel.
The UNC-DRC program, conducted in collaboration with the Kinshasa School of Public Health and the National AIDS Control Program, was funded by the US Centers for Disease Control and Prevention Global AIDS Program (Grant Number U62/CCU422422) and the President’s Emergency Plan for AIDS Relief (Grant Number 5U2GPS001179-01), with additional support from the Elizabeth Glaser Pediatric AIDS Foundation, the Belgian Development Cooperation, the William J. Clinton Foundation, the United Nations Children’s Fund, and the Global Fund to Fight AIDS, Tuberculosis, and Malaria. The UNC Center for AIDS Research Social and Behavioral Sciences Research Core (NIH P30-AI50410) also provided program assistance.
Footnotes
Presented in part at the 19th International AIDS Conference, July 22–27, 2012, Washington, DC (abstract THAE0103).
The authors have no conflicts of interest to disclose, but it was declared that money was paid to their institution, in grants, for the HIV program that provided the data for this study (grants were not for this data analysis; rather, they were for the overall program).
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
References
- 1.UNAIDS. Global Plan Towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2011. [Google Scholar]
- 2.UNAIDS. The Gap Report. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014. [Google Scholar]
- 3.Chi BH, Bolton-Moore C, Holmes CB. Prevention of mother-to-child HIV transmission within the continuum of maternal, newborn, and child health services. Curr Opin HIV AIDS. 2013;8:498–503. doi: 10.1097/COH.0b013e3283637f7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems’ performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:e45–e48. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- 5.Stringer EM, Chi BH, Chintu N, et al. Monitoring effectiveness of programmes to prevent mother-to-child HIV transmission in lower-income countries. Bull World Health Organ. 2008;86:57–62. doi: 10.2471/BLT.07.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palombi L, Nielsen-Saines K, Giuliano M, et al. Easier said than done: World Health Organization recommendations for prevention of mother-to-child transmission of HIV-areas of concern. AIDS Res Hum Retroviruses. 2011;27:807–808. doi: 10.1089/AID.2010.0296. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress towards Universal Access: Progress Report 2011. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 8.Zolfo M, De Weggheleire A, Schouten E, et al. Time for “test and treat” in prevention of mother-to-child transmission programs in low-and middle-income countries. J Acquir Immune Defic Syndr. 2010;55:287–289. doi: 10.1097/QAI.0b013e3181eef3da. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Programmatic Update. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 10.Kak L, Chitsike I, Luo C, et al. Prevention of Mother-to-child Transmission of HIV/AIDS Programmes. Opportunities for Africa’s Newborns: Practical Data, Policy and Programmatic Support for Newborn Care in Africa. Geneva, Switzerland: WHO on behalf of The Partnership for Maternal Newborn and Child Health; 2006. [Google Scholar]
- 11.Mofenson LM. Prevention in neglected subpopulations: prevention of mother-to-child transmission of HIV infection. Clin Infect Dis. 2010;50(suppl 3):S130–S148. doi: 10.1086/651484. [DOI] [PubMed] [Google Scholar]
- 12.Otieno PA, Kohler PK, Bosire RK, et al. Determinants of failure to access care in mothers referred to HIV treatment programs in Nairobi, Kenya. AIDS Care. 2010;22:729–736. doi: 10.1080/09540120903373565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Merwe K, Chersich MF, Technau K, et al. Integration of antiretroviral treatment within antenatal care in Gauteng Province, South Africa. J Acquir Immune Defic Syndr. 2006;43:577–581. doi: 10.1097/01.qai.0000243099.72770.d2. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa N, Shimbo T, Miyano S, et al. Field effectiveness of WHO PMTCT guidelines in preventing postnatal HIV transmission in resource-limited settings: operational barriers and complexities related to the implementation of extended infant prophylaxis [TUPE177]. Presented at: 19th International AIDS Conference; July 22–27, 2012; Washington, DC.. [Google Scholar]
- 15.Walakira M, Sripipatana T, Mirembe Kunya B, et al. The challenge of translating policy into practice: the impact of revised prevention of mother-to-child transmission of HIV guideline implementation on uptake of infant antiretroviral prophylaxis in South-Western Uganda [MOPE609]. Presented at: 19th International AIDS Conference; July 22–27, 2012; Washington, DC.. [Google Scholar]
- 16.IeDEA Pediatric Working Group. A survey of paediatric HIV programmatic and clinical management practices in Asia and sub-Saharan Africa–the International epidemiologic Databases to Evaluate AIDS (IeDEA) J Int AIDS Soc. 2013;16:17998. doi: 10.7448/IAS.16.1.17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedelu M, Ford N, Hilderbrand K, et al. Implementing antiretroviral therapy in rural communities: the Lusikisiki model of decentralized HIV/AIDS care. J Infect Dis. 2007;196(suppl 3):S464–S468. doi: 10.1086/521114. [DOI] [PubMed] [Google Scholar]
- 18.Stinson K, Boulle A, Coetzee D, et al. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15:825–832. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsague L, Tsiouris FO, Carter RJ, et al. Comparing two service delivery models for the prevention of mother-to-child transmission (PMTCT) of HIV during transition from single-dose nevirapine to multi-drug antiretroviral regimens. BMC Public Health. 2010;10:753. doi: 10.1186/1471-2458-10-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey SE, Mitchell KT, Amisi IM, et al. Use of facility assessment data to improve reproductive health service delivery in the Democratic Republic of the Congo. Confl Health. 2009;3:12. doi: 10.1186/1752-1505-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarocostas J. Some 45,000 people die each month in Congo as result of collapsed health service, says UN official. BMJ. 2009;338:b2652. doi: 10.1136/bmj.b2652. [DOI] [PubMed] [Google Scholar]
- 22.PEPFAR. Democratic Republic of the Congo Operational Plan Report FY 2012. 2013 Available at: http://www.pepfar.gov/documents/organization/212141.pdf. Accessed June 1, 2014.
- 23.WHO. Global Update on HIV Treatment 2013: Results, Impact and Opportunities: WHO Report in Partnership with UNICEF and UNAIDS. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 24.Behets F, Edmonds A, Kitenge F, et al. Heterogeneous and decreasing HIV prevalence among women seeking antenatal care in Kinshasa, Democratic Republic of Congo. Int J Epidemiol. 2010;39:1066–1073. doi: 10.1093/ije/dyq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behets FM, Matendo R, Vaz LM, et al. Preventing vertical transmission of HIV in Kinshasa, Democratic Republic of the Congo: a baseline survey of 18 antenatal clinics. Bull World Health Organ. 2006;84:969–975. doi: 10.2471/blt.05.028217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behets F, Mutombo GM, Edmonds A, et al. Reducing vertical HIV transmission in Kinshasa, Democratic Republic of Congo: trends in HIV prevalence and service delivery. AIDS Care. 2009;21:583–590. doi: 10.1080/09540120802385595. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants: Recommendations for a Public Health Approach—2010 Version. Geneva, Switzerland: World Health Organization; 2010. [PubMed] [Google Scholar]
- 28.Edmonds A, Yotebieng M, Lusiama J, et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med. 2011;8:e1001044. doi: 10.1371/journal.pmed.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feinstein L, Edmonds A, Chalachala JL, et al. Temporal changes in the outcomes of HIV-exposed infants in Kinshasa, Democratic Republic of Congo during a period of rapidly evolving guidelines for care (2007–2013) AIDS. 2014;28(suppl 3):S301–S311. doi: 10.1097/QAD.0000000000000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edmonds A, Thompson D, Kleckner D, et al. Improving HIV prevention, care, and treatment in severely resource-deprived settings through real-time data use - development and implementation of a user-friendly data management system. Presented at: 6th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; July 17–20, 2011; Rome, Italy. [Google Scholar]
- 31.WHO. Co-trimoxazole Prophylaxis for HIV-exposed and HIV-infected Infants and Children: Practical Approaches to Implementation and Scale up. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 32.Geskus RB. Cause-specific cumulative incidence estimation and the fine and gray model under both left truncation and right censoring. Biometrics. 2011;67:39–49. doi: 10.1111/j.1541-0420.2010.01420.x. [DOI] [PubMed] [Google Scholar]
- 33.Grimsrud AT, Cornell M, Egger M, et al. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol. 2013;66:1006–1013. doi: 10.1016/j.jclinepi.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20:555–561. doi: 10.1097/EDE.0b013e3181a39056. [DOI] [PubMed] [Google Scholar]
- 35.Kohl M, Heinze G. PSHREG: A SAS Macro for Proportional and Nonproportional Substribution Hazards Regression with Competing Risk Data. Technical Report 08/2012, Center for Medical Statistics, Informatics and Intelligent Systems. Vienna, Austria: Medical University of Vienna, Center for Medical Statistics, Informatics and Intelligent Systems, Section for Clinical Biometrics; 2012. [Google Scholar]
- 36.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 37.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 39.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 40.Stone CJ, Koo C-Y. Additive splines in statistics. Proc Stat Comp Sect Am Statist Assoc. 1985;27:45–48. [Google Scholar]
- 41.Tudor Car L, Brusamento S, Elmoniry H, et al. The uptake of integrated perinatal prevention of mother-to-child HIV transmission programs in low- and middle-income countries: a systematic review. PLoS One. 2013;8:e56550. doi: 10.1371/journal.pone.0056550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson LJ, Beusenberg M, Habiyambere V, et al. Adoption of national recommendations related to use of antiretroviral therapy before and shortly following the launch of the 2013 WHO consolidated guidelines. AIDS. 2014;28(suppl 2):S217–S224. doi: 10.1097/QAD.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 43.Akinseye M, Adenusi O, Adebiyi O, et al. Expansion of access to PMTCT services in Nigeria through the decentralization of services to PHCs: the Hygeia Foundation experience [TULBPE37]. Presented at: 7th IAS Conference on HIV Pathogenesis, Treatment, and Prevention; June 30 – July 3, 2013; Kuala Lumpur, Malaysia. [Google Scholar]
- 44.Myer L, Daskilewicz K, McIntyre J, et al. Comparison of point-of-care versus laboratory-based CD4 cell enumeration in HIV-positive pregnant women. J Int AIDS Soc. 2013;16:18649. doi: 10.7448/IAS.16.1.18649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wynberg E, Cooke G, Shroufi A, et al. Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc. 2014;17:18809. doi: 10.7448/IAS.17.1.18809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]
- 47.Larsson EC, Thorson A, Pariyo G, et al. Opt-out HIV testing during antenatal care: experiences of pregnant women in rural Uganda. Health Policy Plan. 2012;27:69–75. doi: 10.1093/heapol/czr009. [DOI] [PubMed] [Google Scholar]
- 48.Mate KS, Bennett B, Mphatswe W, et al. Challenges for routine health system data management in a large public programme to prevent mother-to-child HIV transmission in South Africa. PLoS One. 2009;4:e5483. doi: 10.1371/journal.pone.0005483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Youngleson MS, Nkurunziza P, Jennings K, et al. Improving a mother to child HIV transmission programme through health system redesign: quality improvement, protocol adjustment and resource addition. PLoS One. 2010;5:e13891. doi: 10.1371/journal.pone.0013891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO. A Short Guide on Methods: Measuring the Impact of National PMTCT Programmes: Towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 51.Shroufi A, Mafara E, Saint-Sauveur JF, et al. Mother to Mother (M2M) peer support for women in Prevention of Mother to Child Transmission (PMTCT) programmes: a qualitative study. PLoS One. 2013;8:e64717. doi: 10.1371/journal.pone.0064717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor NK, Buttenheim AM. Improving utilization of and retention in PMTCT services: can behavioral economics help? BMC Health Serv Res. 2013;13:406. doi: 10.1186/1472-6963-13-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feinstein L, Edmonds A, Okitolonda V, et al. Maternal combination antiretroviral therapy is associated with improved retention of HIV-exposed infants in Kinshasa, Democratic Republic of Congo. J Acquir Immune Defic Syndr. 2015;69:e93–99. doi: 10.1097/QAI.0000000000000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


