Abstract
We investigated the neural correlates of remote traumatic reexperiencing in survivors of a life-threatening incident: the near crash of Air Transat (AT) Flight 236. Survivors' brain activity was monitored during video-cued recollection of the AT disaster, September 11th, 2001 (9/11), and a comparatively non-emotional (neutral) event. Passengers showed a robust memory enhancement effect for the AT incident relative to the 9/11 and neutral events. This traumatic memory enhancement was associated with activation in the amygdala, medial temporal lobe, anterior and posterior midline, and visual cortex in passengers. This brain-behavior relationship also held in relation to 9/11, which had elevated significance for passengers given its temporal proximity to the AT disaster. This pattern was not observed in a comparison group of non-traumatized individuals who were also scanned. These findings suggest that remote, traumatic memory is mediated by amygdalar activity, which likely enhances vividness via influences on hippocampal and ventral visual systems.
Introduction
Extensive evidence demonstrates that emotion enhances the expression of memory, an effect that is thought to be mediated by amygdalar influences on brain structures involved in the encoding, storage, and subsequent retrieval of episodic memories (see Buchanan, 2007; Holland & Kensinger, 2010; McGaugh, 2003 for review). Accordingly, functional neuroimaging studies demonstrate that emotional memories evoke greater engagement of the amygdala and medial temporal lobe structures relative to neutral memories (Dolcos, LaBar, & Cabeza, 2005; Kensinger & Schacter, 2007; Smith, Stephan, Rugg, & Dolan, 2006; also see Buchanan, 2007 for review). Much of this evidence is derived from the provision of laboratory stimuli to probe emotional memory. Although these studies have been instrumental to understanding emotion and memory, they fail to capture the full range of emotionality inherent to real-life experiences. Studies of autobiographical memory suggest greater amygdala involvement for negative relative to neutral experiences (Sharot, Martorella, Delgado, & Phelps, 2007) or parametric modulation of the amygdala with respect to the degree of emotional content or intensity of remembered events (Botzung, Rubin, Miles, Cabeza, & Labar, 2010; Daselaar et al., 2008; Muscatell, Addis, & Kensinger, 2010; Sharot et al., 2007). Yet other studies of emotional autobiographical memory do not report amygdalar engagement (e.g., see Damasio et al., 2000; Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003).
Even less is known about how extremely intense traumatic experiences are processed in memory and what brain regions support memory for such experiences. This type of research necessarily involves those exposed to real-life tragedies, including serious accidents, abuse, armed conflict, or natural disasters. Such studies have produced inconsistent findings, with evidence supporting both memory enhancement (e.g., McKinnon et al., 2014; Porter & Birt, 2001; Porter & Peace, 2007) and attenuation for traumatic events (e.g., Mechanic, Resick, & Griffin, 1998; van der Kolk & Fisler, 1995). Moreover, as in the studies of non-traumatic emotional memory (see above), amygdala engagement has been inconsistently reported during brain imaging of traumatic memory retrieval (Driessen et al., 2004; Fischer, Wik, & Fredrikson, 1996; Hayes, Hayes, & Mikedis, 2012; Lanius et al., 2001; Patel, Spreng, Shin, & Girard, 2012). Yet, these studies largely focus on symptom provocation in the context of posttraumatic stress disorder (PTSD) as opposed to exploring the effects of trauma on memory per se. Given these issues, the behavioral and neural mechanisms of traumatic memory remain controversial (Brewin, 2007).
Some of the inconsistencies reported may be attributable to inter-individual heterogeneity in event exposure, which can vary along many dimensions, including severity, repetition (single versus chronic trauma), or temporal proximity of trauma --factors known to have significant effects on how an event is processed in memory (Muscatell et al., 2010; Neisser et al., 1996; van Giezen, Arensman, Spinhoven, & Wolters, 2005). Moreover, there are individual differences associated with mnemonic responses to trauma: memory alterations (e.g., intrusiveness, forgetting) are a key feature in the diagnosis of PTSD (American Psychiatric Association, 2013), which is observed only in a subset of trauma-exposed individuals.
Using the Autobiographical Interview (Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002), we investigated traumatic autobiographical memory in a sample of survivors of a shared single-instance life-threatening event, the August 24, 2001 Air Transat (AT) disaster, in which passengers on a trans-Atlantic flight prepared for ditching in the Atlantic Ocean following a fuel leak, resulting in loss of engine power, then landed at an island military base (McKinnon et al., 2014). We observed a robust traumatic memory enhancement effect (i.e., an elevation of episodic details) for the AT incident relative to other types of autobiographical experiences in all AT passengers approximately 3.5 years after the event occurred. The presence of PTSD was associated with elevated external (i.e., non-episodic) details across all events probed, suggesting altered cognitive control over memory recall in PTSD.
The goal of the present study was to examine the neural correlates of the observed traumatic memory enhancement effect. A partially overlapping sample of passengers from McKinnon et al. (2014) were scanned using functional magnetic resonance imaging (fMRI) while exposed to video footage from network news sources that faithfully replicated the AT disaster. Functional neuroimaging studies involving shared traumatic events are rare (Fischer et al., 1996); most studies have used mixed samples that mainly involve chronic trauma-exposed samples, typically those who experienced combat-based trauma or cases of repeated sexual or physical abuse. There are no published fMRI studies of a group of individuals who were threatened with death from a single shared event, which permits the examination of traumatic memory, while circumventing error variance associated with employing mixed samples.
We used a multivariate statistical technique, partial least squares (PLS; Krishnan, Williams, McIntosh, & Abdi, 2011), to explore patterns of neural activity that relate to the AT event in comparison to non-traumatic autobiographical experiences (see Methods). We predicted that activity in the amygdala and hippocampus would be associated with recall of the AT disaster, extending a functional neuroanatomical model of emotional enhancement of memory via amygdalar influences on the hippocampus (e.g., McGaugh, 2003; Phelps, 2004; Sharot, Delgado, & Phelps, 2004) to retrieval of a remote, life-threatening trauma.
Method
Participants
Participants included eight passengers from Air Transat (AT) Flight 236 (42.0 ± 14.3 years old; 15.1 ± 4.5 years of education). Of those eight passengers, four had participated in our previous study, which occurred approximately 3.5 years after the trauma (McKinnon et al., 2014). Scans took place 8.9 years after the trauma (range=8.2-10.4, median=8.7). Passengers were administered the Structured Clinical Interview for Diagnosis (SCID) of the DSM-IV (First, Spitzer, Gibbon, & Williams, 1996) to examine a diagnosis of PTSD or other co-morbidities. The SCID revealed a diagnosis of active PTSD and alcohol abuse in one passenger (in remission from major depressive disorder; MDD), and a diagnosis of active MDD in a second passenger. A third passenger had a diagnosis of active non-alcoholic substance abuse with a history of MDD and alcohol dependency. All other passengers were healthy, with two additional passengers in remission from PTSD. Passengers were also administered the Impact of Event Scale-Revised (IES-R), a 22-item self-report measure of one's subjective response to a specific traumatic event (Weiss & Marmar, 1997). The IES-R provides a current (within the last 7 days) continuous measure of PTSD symptoms (mean score = 21.9; SD: 9.8; range=9-40). Passengers were compared to 10 healthy age- and education-matched participants (39.4 ± 14.0 years old; 16.8 ± 3.9 years of education) with no history of psychopathology. The study was approved by the Research Ethics Board at Baycrest Health Sciences.
Scanning
Passengers were scanned (Siemens Magnetom Trio Tim, Numaris/4Syngo MR B13; see Supplementary Materials) during recall of three different events: A traumatic event (the AT near-disaster; hereafter referred to as AT), a negative non-traumatic event (the terrorist attacks of September 11, 2001; hereafter referred to as 9/11) and a comparatively non-emotional event that also occurred around 2001 (i.e., a road trip, hereafter referred to as NEUTRAL). Examples of NEUTRAL events included road trips to visit out-of-city family or a university campus (selected by participants immediately prior to scanning). In response to video cues depicting each event (see Supplementary Materials), participants were instructed to recollect what happened to them, including thoughts, feelings, visual images, physical sensations and sounds that may come to mind. Participants were told to remember events that happened to them directly, rather than thinking about something they may have learned about through the media or by talking to other people. In addition to the three memory conditions, an odd number detection task (ODD/EVEN) served as a ‘baseline’ (Stark & Squire, 2001), where subjects noted whether a digit was odd or even; the ODD/EVEN condition was not used in the present analyses.
All scanning conditions were identical for the passengers and the comparison group, with the exception of the AT event: In lieu of recalling the AT disaster, the comparison group imagined themselves on the plane experiencing the incident, enabling parallel stimulus exposure across the two groups. While this limits conclusions drawn from comparison of AT passengers to the comparison group for the AT condition, it does allow for inter-group comparison for the 9/11 and NEUTRAL conditions to determine whether or not trauma exposure in the passengers altered the recollection of other events.
All stimuli were presented in blocked format, with two blocks per condition for a total of 8 blocks (2 for each of the three memory conditions and 2 for ODD/EVEN). For the three memory conditions, each block contained five trials, which consisted of a unique 30 s video clip with no sound for each of the memory conditions. Each 30 s video cue was followed by a rating of reexperiencing (or imagination for the comparison participants in lieu of the AT event) on a scale of 1-10, with a higher rating reflecting greater reexperiencing (see Supplementary Materials for ratings results).
Post Scan Autobiographical Recall
Immediately following the scan, participants were administered the free recall portion of the Autobiographical Interview (AI; Levine et al., 2002): Here, participants described everything they could remember about each of the events. In lieu of the AT event, the comparison group described what they imagined in the scanner during the AT event. Participants' responses were recorded, transcribed, and scored according to the AI protocol. Briefly, the text was segmented and categorized into: (1) “internal” episodic details that conveyed a sense of reexperiencing due to recall of event, time, place, perceptual or emotional information, or (2) “external” non-episodic details that consisted of semantic (factual or temporally-extended) information, repetitions, metacognitive or editorial statements, or details about other events (see Supplementary Materials).
Data Analyses
Analysis of Functional NeuroImages software (AFNI; Cox & Hyde, 1997) was used to pre-process the neuroimaging data (see Supplementary Materials). PLS, a flexible multivariate technique, was used to analyze the functional neuroimaging data. In PLS, relationships between patterns of whole brain activity and variables of interest are expressed as latent variables (LV) that represent similarities and differences in patterns of activation in relation to the selected variables of interest, such as experimental conditions or group (see Krishnan et al., 2011). The LVs are computed using singular value decomposition (analogous to eigenvectors in principal components analysis; see Supplementary Materials). Following our hypotheses, interpretation was focused on the amygdala and hippocampus, although whole brain data are presented given the strength of PLS analysis in the specification of brain-wide networks in association with these emotional and mnemonic hubs.
First, a task PLS analysis was used to assess patterns of whole brain activation within each of the three memory conditions in each group. However, to directly examine the emotional enhancement effect per se, we examined areas of the brain that correlated with episodic (internal) detail generation within each condition (behavioral PLS). Since only passengers actually experienced the AT disaster, this analysis was restricted to passengers. For completeness, we repeated this analysis with comparison participants included (see Supplementary Materials). As with the analysis of the passengers, this included each participants' episodic detail count for 9/11 and NEUTRAL only. In contrast, for the AT condition comparison participants' imagined internal details were used.
Results
Behavioral Measures
Post Scan Autobiographical Recall
Corresponding with passengers' enhanced rated reexperiencing for the AT condition in the scanner, there was a marked emotionally-enhanced memory effect for the AT condition on the AI in the passengers (Figure 1A; see Supplementary Materials), which is in accordance with our previous study (McKinnon et al., 2014).
Figure 1.
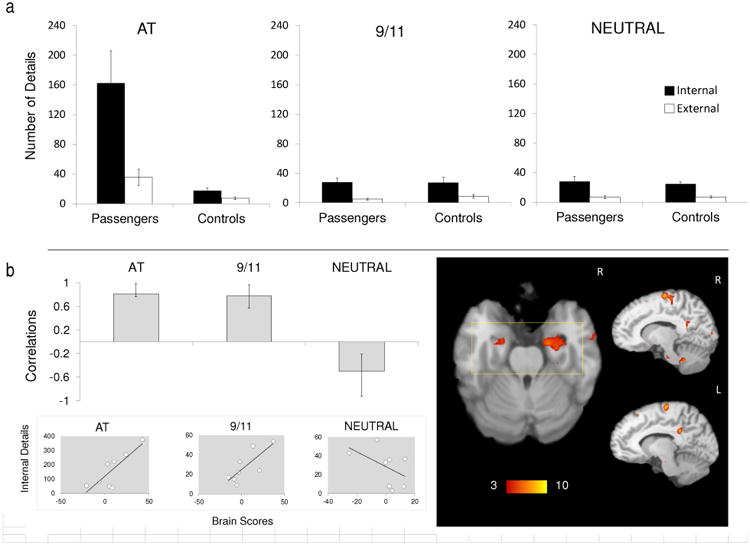
(a) Mean number of internal (episodic) and external (non-episodic) details recalled for the AT disaster (AT), September 11, 2001 (9/11), and a neutral event (NEUTRAL), for the passengers and comparison participants (controls). Error bars represent standard error of the mean. (b) The graph displays correlations from the significant latent variable (LV) from the partial least squares (PLS) analysis in passengers for all three memory conditions (also see Figure S2). Correlations express how strongly internal (episodic) detail generation was correlated with the pattern of brain regions (i.e., brain scores) that were reliable for the LV (no reliable pattern was observed for external details; see main text). Error bars indicate confidence intervals, with condition differences denoted by non-overlapping error bars. The mean correlation for each condition reliably contributes to the LV if the error bar does not cross zero. The bootstrap ratio map is overlaid on a standard template (Talairach & Tournoux, 1988), which demonstrates clusters with positive bootstrap ratios that were associated with positive mean correlations. A rectangular overlay indicates significant bootstrap ratios in the amygdalae and hippocampi (also see Table 1). Below the PLS graph, scatterplots are displayed to illustrate the individual distribution of brain scores versus internal details. R, right; L, left.
Functional Neuroimaging
The Task PLS revealed one significant LV that explained 61.53% of the cross-block covariance (p<0.001, Figure S2). This LV dissociated the AT condition from the NEUTRAL condition –a pattern that was demonstrated to the same extent in passengers and comparison participants. The pattern of brain activity associated with 9/11 was not statistically reliable in passengers; for comparison participants, this condition showed a similar pattern to that of the NEUTRAL condition. This LV indicated increased BOLD response associated with the AT condition in bilateral middle occipital gyri (peak located on the right) extending bilaterally across the ventral visual system into the fusiform and lingual gyri and parahippocampal gyrus/hippocampus, and thalamus. Peaks were also observed bilaterally in the precuneus and the medial and inferior prefrontal cortex (PFC). Critically, bilateral amygdalar activity was observed. Although peak activation was not observed in the right amygdala, statistically reliable activity was observed in this region as part of a larger cluster (bootstrap ratio = 4.73; 28, -8, -12) and comparable in magnitude to that of the left amygdala peak (bootstrap ratio = 4.72; -24, -8, -12; Table S2).
When internal details (indexing episodic detail recall) were entered into a Behavioral PLS analysis (passengers only), one significant LV emerged that explained 53.57% of the cross-block covariance; p = .03; Figure 1B). Production of episodic details in both the 9/11 and AT condition was positively correlated with bilateral anterior medial temporal activity (parahippocampal gyrus, hippocampus and amygdala), bilateral midline posterior activity (posterior cingulate and cuneus), high midline PFC activity (precentral gyrus), as well as activity in the right middle temporal gyrus and insula, the left middle occipital gyrus, the right pons and bilateral cerebellum. The AT and 9/11 conditions dissociated from that of NEUTRAL: In the NEUTRAL condition, internal details were negatively correlated with the specified pattern. To determine the specificity of this effect, we also ran a behavioral PLS using external details (indexing non-episodic recall). No significant pattern of brain activation in relation to external details emerged (p = .12). As shown in Supplementary Figure S3 (see Table S3), when this same analysis was rerun including the comparison group, the pattern of activation for passengers was similar; by contrast, the details generated by comparison participants in association with the AT condition were negatively correlated with this pattern, confirming that this memory enhancement effect was specific to passengers; internal details associated with the 9/11 and NEUTRAL conditions did not significantly contribute to the pattern.
Discussion
We examined the neural correlates of emotional enhancement for a single-blow, life-threatening event, the AT disaster. As in our prior behavioral study (McKinnon et al., 2014), emotional enhancement was quantified by an excess of internal details indexing greater episodic recollection in association with traumatic recall. The behavioral PLS analysis most directly addressed the emotional enhancement effect by interrogating brain regions specifically associated with the degree of mnemonic reexperiencing in each condition per se. Here, we observed a restricted network of brain regions that was positively correlated with episodic memory performance for both the AT and 9/11 conditions in passengers only. Following our hypothesis, this pattern was observed bilaterally in midline structures, including anterior parahippocampal regions (including the hippocampus) that extended into the amygdala and other paralimbic regions as well as visual regions.
The Task PLS also demonstrated preferential activation of the amygdala and hippocampus as well as a wider and robust network of regions in the canonical autobiographical memory network (Svoboda, McKinnon, & Levine, 2006) during the AT condition. Yet this same pattern was observed in the comparison group, who only imagined the traumatic event while watching the videos. It is possible that group differences may have been observed with a larger sample. Nonetheless, it is important to consider that the shared activation patterns across groups likely reflects perceptual activity in association with the rich retrieval cues (Todd, Talmi, Schmitz, Susskind, & Anderson, 2012) as well as overlap in activation patterns for remembered and imagined autobiographical events (Rabin, Gilboa, Stuss, Mar, & Rosenbaum, 2010). Accordingly, patterns of similar or differential activity should not be interpreted solely on the basis of neural responses to the videos, but also in light of how this pattern of brain activity relates to measures of traumatic reexperiencing.
Although numerous functional neuroimaging studies have demonstrated a role of the amygdala in emotional memory (see Buchanan, 2007), as noted earlier, this finding is less consistently observed in emotional autobiographical memory or even in trauma studies. Dolcos and colleagues (2012) allude that less consistent activation of the amygdala during emotional autobiographical memory relative to laboratory events may relate to differences in the retention interval, speculating that with the increased retention interval in autobiographical memory, vividness and the role of the amygdala may decline and effortful processes divert neural resources away from retrieving emotional content. It is possible that traumatic or very highly negative memories are unique for their persistence, retaining their vivid sensory quality over time, while less significant emotional experiences resemble neutral autobiographical experiences with the passage of time. In the present study, the persistence of the emotional enhancement effect nearly a decade post-trauma is consistent with the combination of the life-threatening nature of this trauma and the specificity of the cues. Future research is required to assess the effects of more recent exposure in response to such cues and to compare recent and remote trauma within the same study.
The precise role played by the amygdala in the retrieval of traumatic or highly emotional memories is unknown. It may be involved in reinstating the content of the original encoding context, in turn promoting the retrieval of other sensory and contextual details supported by neocortical areas, particularly those in ventral visual areas that are then bound by the hippocampus (Eldridge, Knowlton, Furmanski, Bookheimer, & Engel, 2000). Accordingly, we observed robust hippocampal and posterior visual cortical engagement as part of the network correlated with internal detail generation for traumatic and negative emotional memory. This recapitulation of highly emotional memory may occur via direct connections of the amygdala with the hippocampus (Pitkänen, 2000), or via connections with visual cortices (McDonald, 1998). It is additionally possible that the recollection of deeply encoded sensory details may subsequently facilitate the retrieval of emotional content supported by the amygdala. Although either interpretation is consistent with our findings, some evidence suggests that amygdalar activity peaks during earlier phases of autobiographical memory retrieval (i.e., during memory access) for more emotionally intense events (Daselaar et al., 2008).
Despite the fact that the observed behavioral emotional enhancement effect was restricted to AT, both the 9/11 and the AT condition similarly contributed to the observed brain-behavior correlations in the PLS analysis in passengers. This dissociation between the AT and 9/11 conditions and the NEUTRAL one could not be accounted for by a restricted range of internal details in the NEUTRAL condition, where the mean and standard error were similar to the 9/11 condition (Figure 1A). As the terrorist attacks of 9/11 occurred three weeks after the AT disaster, this event likely had elevated significance for the passengers, which has been associated with greater amygdala activation previously (Sharot et al., 2007). Indeed, we also did not observe a similar correlation in the comparison group for 9/11, further suggesting that memory for 9/11 is qualitatively different in passengers. In light of the similarity in brain-behavioral correlations in the passengers for AT and 9/11, it is intriguing that we did not observe a behavioral memory enhancement effect for 9/11 relative to the NEUTRAL condition in passengers per se. Inconsistencies in the brain-behavior relationship associated with the 9/11 condition in passengers and the comparison group may also be due to greater error variance attributable to individual differences in media exposure.
As noted in the introduction, studies of traumatic memory have inconsistently reported amygdalar activation, but these studies largely focus on PTSD effects (Driessen et al., 2004; Fischer et al., 1996; Hayes et al., 2012; Lanius et al., 2001; Patel et al., 2012). In the present study, we were unable to explore hypotheses related to PTSD status versus trauma exposure per se, given the restricted range of PTSD symptomology across subjects and the lack of individuals with an active diagnosis. Nonetheless, our findings of a medial temporal lobe amydalar-hippocampal system (along with additional paralimbic, medial PFC, and visual regions) in association with traumatic episodic memory enhancement would be expected to hold in PTSD as the behavioral memory enhancement effect was common to passengers with and without PTSD (McKinnon et al., 2014). Our prior finding of elevated external (non-episodic) details only in passengers with PTSD (across all autobiographical events) due to impaired cognitive control would support a prediction of altered functioning of the frontoparietal control network (or altered coupling of this network with the limbic-salience network) during memory retrieval in PTSD (Seeley et al., 2007; Vincent, Kahn, Snyder, Raichle, & Buckner, 2008).
The small sample size of the present study is an important caveat that limits the generalizability of our results. Nonetheless, the sample size here reflects a tradeoff inherent in this unprecedented examination of the neural correlates of memory for a shared, life-threatening traumatic event, which enabled the use of highly specific retrieval cues. By capitalizing on this shared traumatic experience, we were able to test a hypothesis that originated in animal research (e.g., McGaugh, 2003), where conditions can be highly controlled in a manner not possible in human studies. In this sense, our findings bridge basic neuroscience and clinical science to illuminate neural mechanisms of traumatic memory enhancement. Future studies of shared trauma, involving larger sample sizes, are required in order to enhance our knowledge of the effects of trauma exposure on the neural correlates of traumatic and non-traumatic autobiographical memory as well as how this network may be altered as a key feature of trauma-related disorders. To the extent that this type of design accounts for much external variance, it may be fruitful for examining internal (individual differences) factors in relation to the neural correlates of traumatic autobiographical memory, such as personality (McKinnon et al., 2014) or genetics.
Supplementary Material
Figure S1. Task and run overview for the scanning session. Participants were scanned during video cueing of three events: the AT disaster (AT), September 11, 2001 (9/11), and a neutral event (NEUTRAL) with an interleaved counting (ODD/EVEN) task for a total of 8 runs. The two AT runs always appeared last, whereas the presentation of the 9/11 and NEUTRAL run pairs were counterbalanced across participants. Each run consisted of five 30 s video clips (or 30 s of ODD/EVEN), followed by a rating of reexperiencing.
Figure S2. Brain scores from the significant latent variable (LV) from the task partial least squares (PLS) analysis in passengers and the comparison group (controls) for all three memory conditions: the AT disaster (AT), September 11, 2001 (9/11), and a neutral event (NEUTRAL). Brain scores express how strongly each individual expresses the pattern produced by the LV. Error bars indicate confidence intervals with condition differences denoted by non-overlapping error bars. The mean brain scores for each condition in each group reliably contribute to the LV if the error bar does not cross zero. The bootstrap ratio map is overlaid on an anatomical template in Talairach space (Talairach & Tournoux, 1988). The brain image demonstrates clusters with positive bootstrap ratios, which were preferentially more active in group and task conditions with positive brain scores. There were no brain regions that were reliably more active for conditions with negative mean brain scores. A rectangular overlay indicates significant bootstrap ratios in the amygdalae and hippocampi. R, right.
Figure S3. Correlations from the significant latent variable (LV) from the behavioral partial least squares (PLS) analysis in passengers and the comparison group (controls) for all three memory conditions: the AT disaster (AT), September 11, 2001 (9/11), and a neutral event (NEUTRAL). Correlations express how strongly internal (episodic) detail generation was correlated with the pattern of brain regions (i.e., brain scores) that were reliable for the LV (no reliable pattern was observed for external details). Error bars indicate confidence intervals, with condition differences denoted by non-overlapping error bars. The mean correlation for each condition for each group reliably contributes to the LV if the error bar does not cross zero. The bootstrap ratio map is overlaid on an anatomical template in Talairach space (Talairach & Tournoux, 1988), which demonstrates clusters with positive bootstrap ratios that were associated with positive mean correlations. A rectangular overlay indicates significant bootstrap ratios in the amygdalae and hippocampi. R, right.
Table S1. In-scanner reexperiencing ratings
Table S2. Results of task partial least squares (PLS) analysis (passengers and comparison group)
Table S3. Results of behavioral partial least squares (PLS) analysis (passengers and comparison group)
Table 1. Results of Behavioral PLS analysis (passengers).
Brain regions associated with the behavioral PLS in passengers. BA, Brodmann areas, R, right; L, left. Talairach coordinates indicate voxel coordinates in Talairach space (Talairach & Tournoux, 1988). Size refers to the number of contiguous voxels in the cluster. Bootstrap ratio is an index of reliability for each cluster.
| Side | Anatomy | BA | Talairach coordinates | Size | Bootstrap Ratio | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| L | Precentral gyrus | 6 | -12 | -20 | 64 | 111 | 7.04 |
| R | Pons | 8 | -20 | -32 | 19 | 6.40 | |
| R | Superior frontal gyrus | 6 | 4 | 20 | 56 | 21 | 6.14 |
| L | Cingulate gyrus | 31 | -8 | -44 | 28 | 15 | 6.09 |
| R | Temporal lobe (subgyral)/parahippocampal gyrusa | 20 | 40 | -12 | -16 | 49 | 5.91 |
| R | Cerebellar tonsil | 12 | -48 | -36 | 37 | 5.82 | |
| L | Middle occipital gyrus | 18 | -28 | -80 | 4 | 19 | 5.38 |
| R | Cuneus | 17 | 12 | -92 | 8 | 16 | 5.20 |
| L | Cerebellar tonsil | -24 | -52 | -36 | 28 | 5.01 | |
| R | Cerebellar culmen | 20 | -56 | -8 | 31 | 4.90 | |
| R | Posterior cingulate | 31 | 12 | -52 | 20 | 16 | 4.60 |
| R | Insula | 13 | 44 | -36 | 20 | 11 | 4.58 |
| R | Cerebellar inferior semilunar lobule | 16 | -76 | -52 | 13 | 4.38 | |
| R | Middle temporal gyrus | 21 | 64 | -48 | 0 | 10 | 4.24 |
| L | Parahippocampal gyrusa | 35 | -20 | -8 | -24 | 14 | 4.07 |
Extends into the hippocampus and amygdala
Acknowledgments
The authors thank Namita Kumar, Priya Kumar, Nivethika Jeyakumar, Aggie Bacopulos, Robert S.C. Amaral, Wayne Khuu, Fred Tam, and Norman Farb for assistance. This research was supported by the Canadian Institute of Health Research, MOP-104209; and National Institutes of Mental Health, R01 MH076067 [two grants] awarded to B.L.
Footnotes
Author Contributions: B.L., M.C.M., and A.K.A. designed the research, D.J.P. collected the data, D.J.P. analyzed the data with input from B.L. and A.R.M., D.J.P. wrote the paper with input from all co-authors.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Botzung A, Rubin DC, Miles A, Cabeza R, Labar KS. Mental hoop diaries: emotional memories of a college basketball game in rival fans. J Neurosci. 2010;30(6):2130–2137. doi: 10.1523/JNEUROSCI.2481-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin CR. Autobiographical memory for trauma: update on four controversies. Memory. 2007;15(3):227–248. doi: 10.1080/09658210701256423. [DOI] [PubMed] [Google Scholar]
- Buchanan TW. Retrieval of emotional memories. Psychol Bull. 2007;133(5):761–779. doi: 10.1037/0033-2909.133.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10(4-5):171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LL, Parvizi J, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18(1):217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Denkova E, Dolcos S. Neural Correlates of Emotional Memories: A Review of Evidence from Brain Imaging Studies. Psychologia. 2012;55:80–111. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci U S A. 2005;102(7):2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen M, Beblo T, Mertens M, Piefke M, Rullkoetter N, Silva-Saavedra A, et al. Posttraumatic stress disorder and fMRI activation patterns of traumatic memory in patients with borderline personality disorder. Biol Psychiatry. 2004;55(6):603–611. doi: 10.1016/j.biopsych.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nat Neurosci. 2000;3(11):1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C.: American Psychiatric Press; 1996. [Google Scholar]
- Fischer H, Wik G, Fredrikson M. Functional neuroanatomy of robbery re-experience: affective memories studied with PET. Neuroreport. 1996;7(13):2081–2086. doi: 10.1097/00001756-199609020-00005. [DOI] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2(1):9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AC, Kensinger EA. Emotion and autobiographical memory. Phys Life Rev. 2010;7(1):88–131. doi: 10.1016/j.plrev.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Remembering the specific visual details of presented objects: neuroimaging evidence for effects of emotion. Neuropsychologia. 2007;45(13):2951–2962. doi: 10.1016/j.neuropsychologia.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56(2):455–475. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA, Neufeld RW, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry. 2001;158(11):1920–1922. doi: 10.1176/appi.ajp.158.11.1920. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55(3):257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory and emotion: The making of lasting memories Maps of the mind. New York, NY: Columbia University Press; 2003. [Google Scholar]
- McKinnon MC, Palombo DJ, Nazarov A, Kumar N, Khuu W, Levine B. Threat of death and autobiographical memory: a study of passengers from Flight AT236. Clinical Psychological Science. 2014 doi: 10.1177/2167702614542280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic MB, Resick PA, Griffin MG. A comparison of normal forgetting, psychopathology, and information-processing models of reported amnesia for recent sexual trauma. J Consult Clin Psychol. 1998;66(6):948–957. doi: 10.1037//0022-006x.66.6.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Addis DR, Kensinger EA. Self-involvement modulates the effective connectivity of the autobiographical memory network. Soc Cogn Affect Neurosci. 2010;5(1):68–76. doi: 10.1093/scan/nsp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser U, Winograd E, Bergman ET, Schreiber CA, Palmer SE, Weldon MS. Remembering the earthquake: direct experience vs. hearing the news. Memory. 1996;4(4):337–357. doi: 10.1080/096582196388898. [DOI] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14(2):198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala: A functional analysis. Oxford, UK: Oxford University Press; 2000. pp. 31–115. [Google Scholar]
- Porter S, Birt AR. Is traumatic memory special? A comparison of traumatic memory characteristics with memory for other emotional life experiences. Applied Cognitive Psychology. 2001;15(7):S101–S117. [Google Scholar]
- Porter S, Peace KA. The scars of memory: a prospective, longitudinal investigation of the consistency of traumatic and positive emotional memories in adulthood. Psychol Sci. 2007;18(5):435–441. doi: 10.1111/j.1467-9280.2007.01918.x. [DOI] [PubMed] [Google Scholar]
- Rabin JS, Gilboa A, Stuss DT, Mar RA, Rosenbaum RS. Common and unique neural correlates of autobiographical memory and theory of mind. J Cogn Neurosci. 2010;22(6):1095–1111. doi: 10.1162/jocn.2009.21344. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, Delgado MR, Phelps EA. How emotion enhances the feeling of remembering. Nat Neurosci. 2004;7(12):1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- Sharot T, Martorella EA, Delgado MR, Phelps EA. How personal experience modulates the neural circuitry of memories of September 11. Proc Natl Acad Sci U S A. 2007;104(1):389–394. doi: 10.1073/pnas.0609230103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49(4):631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Science USA. 2001;98:12760–6. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Stuttgart/New York: Thieme; 1988. [Google Scholar]
- Todd RM, Talmi D, Schmitz TW, Susskind J, Anderson AK. Psychophysical and neural evidence for emotion-enhanced perceptual vividness. J Neurosci. 2012;32(33):11201–11212. doi: 10.1523/JNEUROSCI.0155-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk BA, Fisler R. Dissociation and the fragmentary nature of traumatic memories: overview and exploratory study. J Trauma Stress. 1995;8(4):505–525. doi: 10.1007/BF02102887. [DOI] [PubMed] [Google Scholar]
- van Giezen AE, Arensman E, Spinhoven P, Wolters G. Consistency of memory for emotionally arousing events: a review of prospective and experimental studies. Clin Psychol Rev. 2005;25(7):935–953. doi: 10.1016/j.cpr.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, Marmar CR. Assessing Psychological Trauma and PTSD: A Practitioner's Handbook. New York, N.Y.: Guilford Press; 1997. The Impact of Event Scale-Revised; pp. 399–411. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Task and run overview for the scanning session. Participants were scanned during video cueing of three events: the AT disaster (AT), September 11, 2001 (9/11), and a neutral event (NEUTRAL) with an interleaved counting (ODD/EVEN) task for a total of 8 runs. The two AT runs always appeared last, whereas the presentation of the 9/11 and NEUTRAL run pairs were counterbalanced across participants. Each run consisted of five 30 s video clips (or 30 s of ODD/EVEN), followed by a rating of reexperiencing.
Figure S2. Brain scores from the significant latent variable (LV) from the task partial least squares (PLS) analysis in passengers and the comparison group (controls) for all three memory conditions: the AT disaster (AT), September 11, 2001 (9/11), and a neutral event (NEUTRAL). Brain scores express how strongly each individual expresses the pattern produced by the LV. Error bars indicate confidence intervals with condition differences denoted by non-overlapping error bars. The mean brain scores for each condition in each group reliably contribute to the LV if the error bar does not cross zero. The bootstrap ratio map is overlaid on an anatomical template in Talairach space (Talairach & Tournoux, 1988). The brain image demonstrates clusters with positive bootstrap ratios, which were preferentially more active in group and task conditions with positive brain scores. There were no brain regions that were reliably more active for conditions with negative mean brain scores. A rectangular overlay indicates significant bootstrap ratios in the amygdalae and hippocampi. R, right.
Figure S3. Correlations from the significant latent variable (LV) from the behavioral partial least squares (PLS) analysis in passengers and the comparison group (controls) for all three memory conditions: the AT disaster (AT), September 11, 2001 (9/11), and a neutral event (NEUTRAL). Correlations express how strongly internal (episodic) detail generation was correlated with the pattern of brain regions (i.e., brain scores) that were reliable for the LV (no reliable pattern was observed for external details). Error bars indicate confidence intervals, with condition differences denoted by non-overlapping error bars. The mean correlation for each condition for each group reliably contributes to the LV if the error bar does not cross zero. The bootstrap ratio map is overlaid on an anatomical template in Talairach space (Talairach & Tournoux, 1988), which demonstrates clusters with positive bootstrap ratios that were associated with positive mean correlations. A rectangular overlay indicates significant bootstrap ratios in the amygdalae and hippocampi. R, right.
Table S1. In-scanner reexperiencing ratings
Table S2. Results of task partial least squares (PLS) analysis (passengers and comparison group)
Table S3. Results of behavioral partial least squares (PLS) analysis (passengers and comparison group)


