Abstract
Extracelluar matrix undergoes constant remodeling, cell–cell, and cell–matrix interactions during chicken ovarian follicle growth, which is coordinated by matrix metalloproteinases (MMPs), and their associated endogenous inhibitors (TIMPs). Transcriptome analysis revealed upregulation of MMP13 in sexually mature chicken ovaries. In this study, we found that the expression of MMP13 in chicken ovary was stably elevated from 60 d to 159 d, and was significantly higher at 159 d than at the other three developmental stages (P < 0.05). The expression of MMP13 mRNA increased from SW (small white follicles) to F5 (fifth largest follicles), then decreased to F1 (first largest follicles), and dramatically increased again in POF1 (newly postovulatory follicles) follicles (P < 0.05). The MMP13 protein was localized in stroma cells and primordial follicles of sexually immature chicken ovaries, in the theca cell layers of all sized follicles of sexually mature chicken ovaries. Furthermore, we identified a positive element (positions –1863 to –1036) controlling chicken MMP13 transcription, and, in this region, six single nucleotide polymorphisms were found and genotyped in chicken populations. In the White Recessive Rock population, hens with A–1356-C–1079/A–1356-C–1079 genotype had earlier “age at first laying” than those with G–1356-T–1079/G–1356-T–1079 genotype (P < 0.05), and exhibited significantly lower transcriptional activity (P < 0.01). Collectively, chicken MMP13 plays an important role in ovarian follicle growth and regression, and polymorphisms in its promoter region could be used as molecular markers for improving the trait “age at first laying” in chicken breeding.
Keywords: chicken, MMP13, follicle, polymorphism, age at first laying
The ovary represents a truly dynamic organ system characterized by structuring and restructuring events during the development of the ovarian follicle for ovulation. Follicle development is characterized as continued growth from the initial primordial follicle to a mature follicle that is approximately 400-fold larger. During this process, the extracellular matrix (ECM) requires constant remodeling, and cell–cell and cell–matrix interactions to control the wide range of cellular functions, which is coordinated by matrix metalloproteinases (MMPs) and their associated endogenous inhibitors (TIMPs) (Curry and Osteen 2003; Rodgers et al. 2003; Ohnishi et al. 2005; Curry and Smith 2006).The exquisite control of the MMP system has been postulated to regulate many of the cyclic changes, such as the follicular development, ovulation, the formation or destruction of vascularized ovarian structure (Fata et al. 2000; Curry and Osteen 2001; Ny et al. 2002; Smith et al. 2002; Page-McCaw et al. 2007), and therefore is essential for the proper follicular function in the ovary while simultaneously preserving ovarian integrity.
Unlike mammals, the domestic fowl has only a single left ovary, containing follicles of various sizes and developmental stages, including primordial follicles, primary follicles, prehierarchal follicles, such as small white follicles (SW) and small yellow follicles (SY), hierarchal follicles of F5 to F1, and postovulatory follicles (POFs); the POF in birds fails to form a functional corpus luteum (CL) and rapidly regresses. To maintain this hierarchy and daily ovulation, a follicle is selected from the pool of 6–8 mm SY follicles, and grows into a 40 mm preovulatory follicle in just 5–9 d (Gilbert et al. 1983). Follicular maturation also requires yolk deposition, which involves the process of transport, transformation, and deposition of yolk prerequisite material that is conveyed to the oocyte via the vascular system. In addition, the POFs regress shortly after ovulation, and are almost entirely regressed by cellular apoptosis accompanied by proteolysis and dissolution of the ECM within 4–6 d after ovulation (Sudaresan et al. 2008). These extensive cyclic changes in the follicular ECM throughout each reproductive cycle are postulated to occur via the action of a cascade of proteolytic events involving matrix metalloproteinases (MMPs) activity (Zhu et al. 2014).
MMP13, also known as collagenase-3, initiates the breakdown of the fibrillar collagens that form a key structural element of membranes. In chicken, MMP13 is involved in embryonic membrane remodeling, and corneal development related biochemical and molecular changes (Lei et al. 1999; Huh et al. 2007). In the angiogenesis system of the chorioallantoic membrane, MMP13 is the only enzyme associated with collagen remodeling (Zijlstra et al. 2004). Periodic changes of collagenase-3 have been detected in the rat ovary during the ovulatory process (Balbin et al. 1996); however, the role of MMP13 in chicken follicle growth, ovulation, and ovary function is unclear. In this study, we investigated the expression pattern and cellular localization of MMP13 during the reproductive cycle of hens. We also analyzed the regulatory elements of the chicken MMP13 promoter, and identified six single nucleotide polymorphisms (SNPs) that are associated with egg production traits in a White Recessive Rock population.
Materials and Methods
Birds, sample collection, and sample preparation
Hy-line Brown commercial hens at the ages of 60 d, 90 d, 123 d (sexually immature), and 159 d (sexually mature) were used for analyzing the expression pattern of the chicken MMP13 gene. All birds had free access to water and feed. The hens were housed in separate cages under a daily light period of 14 hr. Hens were killed by cervical dislocation, then ovary and follicles at different stages of development were collected and stored in liquid nitrogen immediately. The whole ovary was harvested for RNA or protein extraction for comparing ovarian MMP13 expression between hens of different developmental stages. Follicles with varied sizes, including SW, F5, F3, F1, and POF1 follicles, were manually dissected out for RNA or protein extractions for comparing chicken MMP13 expression between different follicles.
Chicken populations of White Recessive Rock (n = 510), Hy-line brown (n = 45), Wenchang (n = 53), Wenshang Barred (n = 46), and Jining Bairi (n = 37), which were randomly sampled from their respective breeding populations, were used for polymorphism analysis. The White Recessive Rock population (n = 510) was also used for association analysis of diplotype and egg production traits. Egg production traits, including age at first laying, and egg number at 32 wk, were recorded individually. Sampling was carried out by collecting blood from the wing vein, and storing it at –20° until delivered to the laboratory. Genomic DNA was extracted from the blood sample using a DNA Extraction mini kit (Tiangen, Beijing, China), and stored in TE (pH 8.0) at –20°. The birds were handled and treated according to the Animal Care and Use Committee of Shandong Agricultural University.
Total RNA extraction and real-time quantitative PCR
Total RNA was extracted from all tissues using an RNA extraction kit DP419 (Tiangen). The amount and integrity of isolated total RNA were measured using a spectrophotometer (Eppendorf, Hamburg, Germany), and checked by loading total RNA onto a 1% agarose gel that was stained with ethidium bromide. The cDNA was synthesized using a PrimeScript RT reagent kit with a gDNA Eraser (TaKaRa, Dalian, China) from 1 μg of total RNA mixed with 1 μl of oligo (dT)18 primer, 1 μl of PrimerScript RT Enzyme Mix I, 4 μl of 5× Prime Script Buffer, and RNase-free ddH2O up to 20 μl, and incubated at 37° for 15 min and 85° for 5 sec. The resultant cDNA was stored at –20° for mRNA expression analysis. Real-time quantitative RT-PCR (qRT-PCR) was conducted on an MX3000p (Stratagene, La Jolla, CA) in a 15-μl volume containing 7.5 μl of 2× SYBR Premix Ex Taq II (TaKaRa, Dalian, China), 0.3 μl of 50× Rox Reference Dy II, 0.2 μl of each forward and reverse primers (10 μM, β-actin-F/R, MMP13-F/R, and VEGFA-F/R in Table 1), and 1.5 μl of cDNA at a dilution of 1:8 according to the following program: 95° for 30 sec to activate the reaction, and 95° for 5 sec, 56° for 20 sec, and 72° for 15 sec for 40 cycles. Melting curves were used to confirm the specificity of each product, and the efficiency of the PCR was determined by analysis of twofold serial dilutions of cDNA. The PCR efficiency was close to 100%, allowing the use of the 2–ΔΔCt method for the calculation of relative gene expression (Livak and Schmittgen 2001). All qRT-PCRs were carried out with negative controls.
Table 1. Primers used for real-time quantitative RT-PCR and polymorphism analyses and plasmid construction of chicken MMP13 gene.
| Primer Name | Sequence (5′–3′) | Annealing Temperature (°C) | Size (bp) |
|---|---|---|---|
| MMP13-F | TTTGGATTAGAGGTGACGG | 56 | 253 |
| MMP13-R | CCACTTCGTATTCTGGTGA | ||
| β-actin-F | TGGATGATGATATTGCTGC | 56 | 251 |
| β-actin-R | ATCTTCTCCATATCATCCC | ||
| VEGFA-F | GGAAGCCCAACGAAGTTA | 55 | 231 |
| VEGFA-R | CGCTATGTGCTGACTCTGAT | ||
| snpMMP13-F | ACAATCCCAGTTCCCTCAG | 58 | 807 |
| snpMMP13-R | GCCCAGGCTTGTATCACT | ||
| pGL3-MMP13-1F | CGACGCGTGTGCTGTGCTGTAAGTTGTCTT | ||
| pGL3-MMP13-2F | CGACGCGT CACTTCTCCCATTCTTCCAT | ||
| pGL3-MMP13-3F | CGACGCGT ACAATCCCAGTTCCCTCAG | ||
| pGL3-MMP13-4F | CGACGCGT AGTGATACAAGCCTGGGC | ||
| pGL3-MMP13-5F | CGACGCGT GTCATGTCCTATTATGTACTCC | ||
| pGL3-MMP13-R | GGAGATCTTTTGTGAAGTCTGAAGCCC | ||
All forward primers contained an MluI site at their 5′-ends, and the reverse primers had an added BglII site at their 5′-ends (underlined).
Western blotting
Total protein was extracted from homogenized ovaries and different developing follicles using the Cell Lysis Reagent (Fermentas). Protein concentration was determined by the bicinchoninic acid assay (BCA Protein Array kit, Tiangen). The mouse anti-rat MMP13 monoclonal antibody was produced by Chemicon (Temecula, CA). An equal amount of protein was separated by 10% SDS gel electrophoresis under denaturing and nonreducing conditions, after which the proteins were transferred to a polyvinylidene fluoride membrane, and then incubated (1 hr, 37°) with the MMP13 (1:250) antibodies in a 5% bovine serum albumin/PBS solution. After washing in PBST (1000 ml PBS:500 μl Tween-20), the blots were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G antibody (1:1000; Abcam) in a 5% bovine serum albumin/PBS solution (1 hr, 37°). Specific binding was visualized using diaminobenzidine (Tiangen).
Immunohistochemistry
Ovaries and follicles were collected from 45-d-old (sexually immature) and 159-d-old (sexually mature) hens. Tissues were fixed in 10% buffered formalin and paraffin-embedded, and then cut into 5 μm tissue sections. All the sections were deparaffinized, rehydrated through a graded ethanol series, boiled in 10 mM sodium citrate buffer, quenched in 3% hydrogen peroxide, and blocked with 10% goat serum for 20 min. Next, the slides were incubated with mouse anti-rat MMP13 monoclonal antibody (1:50) for 2 hr at 37°. Then, the sections were incubated with the biotinylated secondary antibody and avidin-biotin-peroxidase complex for 0.5 hr according to the Histostain-plus kit instructions (Zhongshan Golden Bridge Biotechnology, China). Finally, immunoprecipitates were visualized by incubation with a diaminobenzidine kit (Zhongshan Golden Bridge Biotechnology). The sections were counterstained with hematoxylin after immunostaining, dehydrated, and covered. Negative control staining was performed using PBS instead of primary antibody. No specific staining was found on the control slides.
Plasmid construction
The 5′-regulatory region of chicken MMP13 gene was inserted upstream of the firefly luciferase gene of the pGL3-Basic vector (Promega, Madison, WI) to generate reporter plasmids. To construct serial deletion promoter reporters, five forward primers (from pGL3-MMP13-1F to pGL3-MMP13-5F, Table 1), and one reverse primer (pGL3-MMP13-R, Table 1) located downstream of the transcription start site of chicken MMP13 gene were synthesized. All forward primers contained an MluI site at their 5′-ends, and the reverse primers had an added BglII site at their 5′-ends. The PCR amplification of the chicken MMP13 promoter was carried out with high-fidelity Taq DNA polymerase PrimeStar (TaKaRa, Dalian, China) using the chicken genome as template, and all PCR fragments were inserted into pGL3-Basic between the MluI and BglII restriction sites to generate 5′ serially deleted promoter constructs. The PCR products and pGL3-Basic vector were separately digested with the same restriction enzyme for 0.5 hr at 37°, and then the two purified products were ligated for 30 min at 22°. To evaluate the effect of the SNPs (g.–1356 G > A, g.–1128 A > G, g.–1094 C > A, g.–1079 T > C) on MMP13 promoter activity, two reporter plasmids (wt-MMP13 and mut-MMP13 constructs), which encompassed the core promoter region, were constructed with individuals of G-1356A-1128C-1094T-1079/G-1356A-1128C-1094T-1079 genotype and A-1356G-1128A-1094C-1079/A-1356G-1128A-1094C-1079 genotype as a template, respectively. PCR amplification was performed in 20 μl volumes containing 4 μl of 5× prime STAR HS Buffer, 1.6 μl (2.5 mM) of dNTPs (TaKaRa, Dalian, China), 0.2 μl of prime STAR HS polymerase (5 U/μl) (TaKaRa, Dalian, China), 0.5 μl of each forward (pGL3-MMP13-3F) and reverse (pGL3-MMP13-R, Table 1) primers (10 μM), 1 µl genomic DNA(50–100 ng), and 12.7 μl of nuclease ddH2O, and run on a Mastercycler gradient (Eppendorf, Germany) according to the following program: 98° for 10 min, 35 cycles of 98° for 10 sec, annealing at 58° for 30 sec, and 72° for 1 kb/min, and final extension at 72° for 5 min. All constructs were sequenced in both directions to confirm the authenticity of the sequences. Plasmids were reproduced in Escherichia coli DH5α, and purified using the Endo-Free Plasmid Purification Kit (Tiangen).
Cell culture and luciferase assay
The F1, F2, and F3 follicles of the ovaries of egg-laying hens were separated from the ovaries and placed in PBS (HyClone). Theca cells were isolated according to the protocol described in Gilbert et al. (1977). The theca cells were dispersed by treatment with 0.1% collagenase II at 37° for 25 min with gentle agitation in a flask. After centrifugation, the cells were suspended in culture medium (M199 with 10% fetal bovine serum, and 1% penicillin/streptomycin), and subsequently seeded in 24-well culture plates at a density of 1 × 106/well. The number of viable cells (90%) was estimated using Trypan blue. Cells were cultured at 38.5° in a water-saturated atmosphere of 95% air and 5% CO2 for 24 hr.
Theca cells grown to 80% confluency were transfected with plasmids using NanoFectin Transfection Reagent (Excell Biology, Shanghai, China) following the supplier’s protocol. Different promoter–reporter fusion plasmids (800 ng), and 25 ng of the pGL4.74 control vector (Promega, Madison, WI) were cotransfected into theca cells; 12 hr later, the cells were maintained in M199 medium without fetal bovine serum. The cells were then washed twice in phosphate-buffered saline and lysed in 1× Passive Lysis Buffer. Luciferase activity was measured in supernatant extracts with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI). Cotransfection with pGL4.74 plasmid DNA was carried out to normalize transfection efficiencies. The transfections were performed at least in triplicate for each construct.
Polymorphism analysis
PCR amplification was performed in 20 µl volumes containing 2 µl of 10× Ex-buffer, 1.6 μl (2.5 mM) of dNTPs (TaKaRa, Dalian, China), 0.1 μl (5 U/μl) of Ex-Taq DNA polymerase (TaKaRa, Dalian, China), 0.4 μl of each forward and reverse primers (10 μM, snpMMP13-F/R in Table 1) designed according to the chicken MMP13 gene (GenBank accession No. NC_006088.3), 1 µl genomic DNA pools (50–100 ng), and 14.5 μl of nuclease ddH2O, and run on a Mastercycler gradient (Eppendorf, Germany) according to the following program: 94° for 4 min, 35 cycles of 94° for 30 sec, annealing at 58° for 30 sec, and 72° for 45 sec, and final extension at 72° for 5 min. Fragments of 807 bp were resolved by electrophoresis on a 1% agarose gel, purified with AxyPrep DNA Gel Extraction Kit (Axygen, Union City, CA), and sequenced using forward primer. Sequences were aligned with the reference sequence (GenBank accession No. NC_006088.3) to identify nucleotide changes using the DNAMAN program.
Statistical analysis
The SHEsis software (http://analysis.bio-x.cn) was used to analyze the pairwise linkage disequilibrium and haplotype frequency. The genotype and allelic frequencies, Hardy-Weinberg equilibrium χ2 test, polymorphism information contents (PIC), heterozygosities (He), and the effective population of the allele (Ne), were calculated using Tools for Population Genetic Analyses software (http://www.marksgeneticsoftware.net/tfpga.htm). Haplotypes were constructed in chicken populations using the PHASE v2.0 program. The associations of MMP13 genotypes with egg production traits including age at first laying, and egg number at 32 wk, were analyzed in White Recessive Rock hen populations that were derived from the same chicken farm using the General Linear Model of SAS (version 9.2; Cary, NC). The linear model is represented as follows: Yij = μ + Gi + eij, where Yij is the phenotypic value of traits, μ is the population mean, Gi is the fixed effect of genotype, and eij is the random error effect. For qRT-PCR analysis, western blotting analysis, and luciferase assay, differences between the experimental groups were evaluated by ANOVA, followed by Duncan’s multiple range test (P < 0.05) using the General Linear Model procedure of SAS (SAS version 9.2; Cary, NC). All the expressions were repeated at least three times, and all data were presented as the mean ± SEM.
Data availability
Supplementary file File S1 contains genotype and phenotype data of chicken individuals used for diplotype and egg production trait association analysis. Sequence data are available at GenBank and the accession number is 1971458461 for SNP g. -1356G>A.
Results
Expression of MMP13 in chicken ovaries of different developmental stages
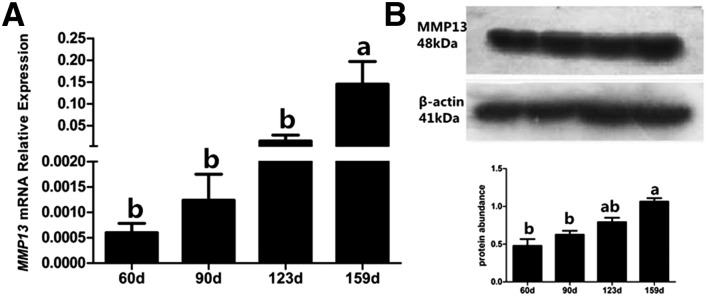
Illumina/solexa sequencing revealed that the expression of chicken MMP13 was significantly higher in the ovaries of laying hens than in prepubertal hens (Zhu et al. 2014). This expression dynamic was also validated in Jining Bairi and Hy-line hens (Supplemental Material, Figure S1). In this study, we further analyzed the expression of MMP13 mRNA in the ovary of Hyline-brown hens during development. A stably elevated mRNA expression of chicken MMP13 from 60 d to 159 d was observed, and, at 159 d, it was significantly higher than at the other three developmental stages (P < 0.05) (Figure 1A). Similarly, the protein level of chicken MMP13 in the ovary was also increased during chicken development, reaching the highest level at 159 d (P < 0.05) (Figure 1B).
Figure 1.
Expression of MMP13 in ovaries from 60-, 90-, 123-, and 159-d-old hens. (A) Messenger RNA expression of MMP13 was analyzed by real-time PCR. (B) Expression of MMP13 protein was analyzed by Western blot analysis. A band of approximately 48 kDa corresponding to the molecular mass of MMP13 was detected. β-actin (41 kDa) was used as the loading control. Data are presented as mean ± SEM from at least four independent experiments. Bars with different superscript letters are significantly different (P < 0.05).
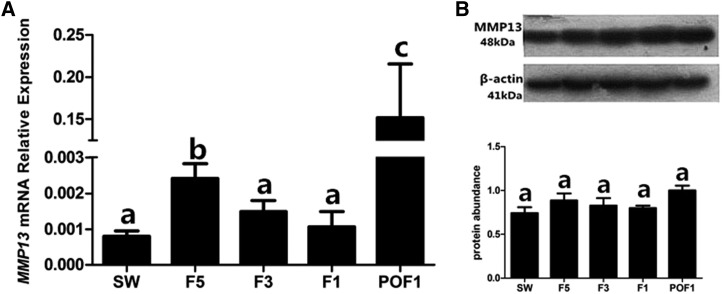
Expression of MMP13 mRNA and protein during chicken follicular development
The expression of MMP13 mRNA increased from SW to F5, then decreased to F1 and dramatically increased again in POF1 follicles (P < 0.05) (Figure 2A). The expression of MMP13 protein among SW, F5, F3, F1 to POF1 follicles was not significant (P > 0.05) (Figure 2B).
Figure 2.
Expression of MMP13 in different follicles from 159-d-old hen ovaries. (A) Expression of MMP13 mRNA in small white follicles (SW), fifth largest follicles (F5), third largest follicles (F3), first largest follicles (F1), and newly postovulatory follicles (POF1). (B) Expression of chicken MMP13 protein was analyzed by Western blot analysis. β-actin (41 kDa) was used as the loading control. Data are presented as mean ± SEM from at least four independent experiments. Bars with different superscript letters are significantly different (P < 0.05).
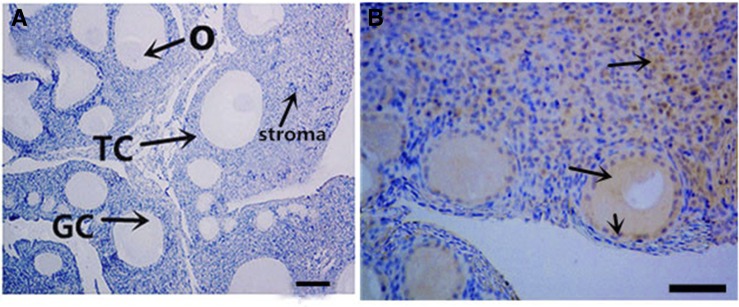
Spatiotemporal changes in the localization of chicken MMP13 protein
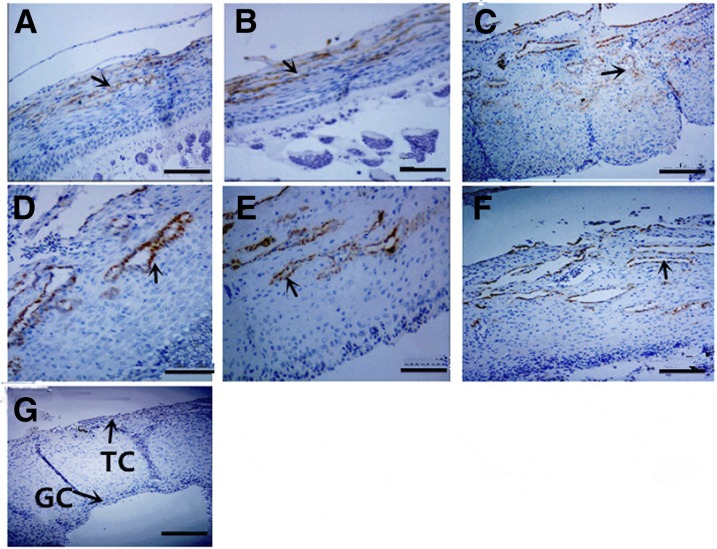
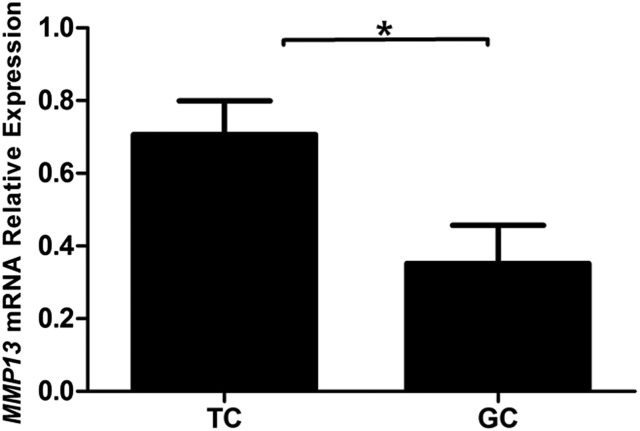
Cellular localization of MMP13 protein was examined in chicken sexually immature (45-d-old) and mature (159-d-old) ovaries. In sexually immature chicken ovaries, MMP13 protein staining was observed in stroma cells and primordial follicles (Figure 3). In sexually mature chicken ovaries, MMP13 protein is localized mainly in theca cell layers of all sized follicles, and in follicles of F5, F3, F1, and POF1, its expression is restricted mainly to blood vessels of theca cell layers (Figure 4). In chicken preovulatory follicles, MMP13 mRNA level was significantly higher in theca cells than in granulosa cells (Figure 5).
Figure 3.
Immunohistochemistry of chicken MMP13 expression in 45-d-old hen ovaries. (A) Negative control with PBS used in place of the primary antibody. (B) Localization of MMP13 protein in the ovaries of 45-d-old chickens; arrowheads indicate the position of strongly stained protein. GC, granulosa cells; TC, theca cells; O, oocyte. Bar for (A): 100 μm; (B): 200 μm.
Figure 4.
Immunohistochemistry detection of chicken MMP13 protein in different follicles from the 159-d-old hen ovaries. (A) SW, (B) SY, (C) F5, (D) F3, (E) F1, (F) POF1, and (G) negative control with PBS used in place of the primary antibody. Arrowheads indicate the position of strongly stained protein. GC, granulosa cells; TC, theca cells. Bar for (A–G): 200 μm
Figure 5.
mRNA expression of chicken MMP13 in theca cells (TC) and granulosa cells (GC). * P < 0.05.
Polymorphisms in the critical promoter region of the chicken MMP13 gene
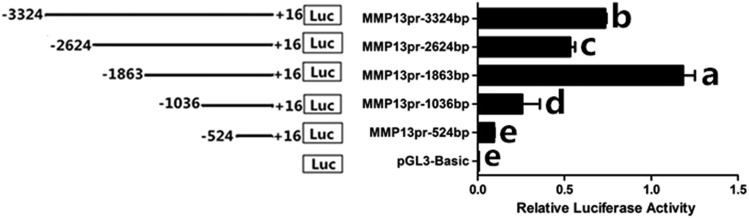
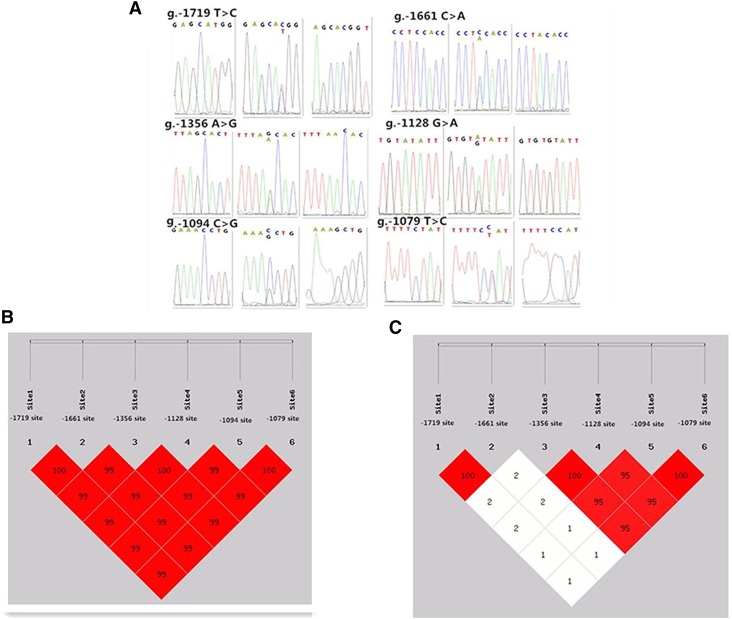
We further identified a critical region controlling the transcription of chicken MMP13 gene, and found that deletion from −1863 bp to −1036 bp significantly decreased the promoter activity as assayed by luciferase activity (P < 0.05) (Figure 6). In this region, six single nucleotide polymorphisms (SNPs), i.e., g. –1719 T > C (rs731299043), g.–1661 C > A (rs314003171), g.–1356 G > A(ss1971458461) identified by this study, g.–1128 A > G (rs316193109), g.–1094 C > G (rs315077856), and g.–1079 T > C (rs312778897) were found, among which complete linkage disequilibrium exist between g.–1719 and g.–1661, g.–1356 and g.–1128, and g.–1094 and g.–1079 loci, respectively (Figure 7). At SNPs g.–1719 T > C, g.–1661 C > A, g.–1356 G > A, g.–1128 A > G, g.–1094 C > G, g.–1079 T > C, alleles T, C, G, A, C, and T were predominant in White Recessive Rock, Hy-line brown, Wenchang, Jining Bairi, and Wenshang Barred chicken populations, respectively (Table 2).
Figure 6.
Luciferase assay of chicken MMP13 gene promoter transfections. Schematic structure of various progressive deletion in the 5′ flanking region of the chicken MMP13 gene, and the activity of the corresponding truncated chicken MMP13 promoters. Luciferase vectors with progressive 5′ deletions were constructed and transiently transfected into theca cells as described. The pGL3-basic vector was used as a control. A renilla luciferase reporter plasmid was used as the internal control to correct for transfection efficiency. Data are presented as mean ± SEM from at least four replicates for each construct. Means with the different lowercase letters within the same column are significantly different (P < 0.05).
Figure 7.
Six single nucleotide polymorphisms identified in the promoter region from −1863 bp to −1036 bp of chicken MMP13 gene (A) and the linkage disequilibrium (LD) result by the online software SHEsis analysis (B and C). Normalized LD was shown as D’ (B) and R2 (C), the values of D’ and R2 are in the range between 0 and 100%. D’ can be seen as a frequency independent metric, and R2 is a measure of frequency. D’ and R2 value are 100% when the chain is in complete LD.
Table 2. Allele frequency of six SNPs in the promoter region of the chicken MMP13 gene.
| Locus or Haplotype | Allele | White Recessive Rock (n = 510) | Hy-Line Brown (n = 45) | Wenchang (n = 53) | Jining Bairi (n = 37) | Wenshang Barred (n = 46) |
|---|---|---|---|---|---|---|
| g.–1719T > C | T | 0.917 | 1.000 | 0.774 | 0.838 | 0.935 |
| g.–1661C > A | C | 0.917 | 1.000 | 0.774 | 0.838 | 0.772 |
| g.–1356G > A | G | 0.819 | 0.600 | 0.953 | 0.622 | 0.989 |
| g.–1128A > G | A | 0.819 | 0.600 | 0.679 | 0.595 | 0.804 |
| g.–1094C > G | C | 0.825 | 0.600 | 0.755 | 0.676 | 0.989 |
| g.–1079T > C | T | 0.825 | 0.600 | 0.755 | 0.676 | 0.837 |
Associations of diplotypes of chicken MMP13 gene with egg production traits
To analyze the associations of SNPs in the chicken MMP13 promoter region with egg production traits, haplotypes were constructed using the two SNPs of g.–1356 G > A and g.–1079 T > C. Three haplotypes of A (G-1356/T-1079), C (A-1356/T-1079), and D (A-1356/C-1079) were detected in the White Recessive Rock population. Due to the fact that the frequency of haplotype C was less than 0.01, association with egg production traits was analyzed only with diplotypes AA, AD, and DD. The results indicated that hens with diplotype DD had earlier age at first laying than those with diplotype AA and AD (P < 0.05), but its effect on egg number at 32 wk was not significant (P > 0.05) (Table 3).
Table 3. Effect of the diplotypes of the SNPs g.–1356 and g.–1079 on laying traits in the White Recessive Rock chicken population.
| Diplotype | Number | Age at First Laying | Egg Number at 32 wk |
|---|---|---|---|
| AA | 335 | 176.27 ± 0.48 a | 31.65 ± 0.39 |
| AD | 162 | 176.73 ± 0.69 a | 31.35 ± 0.55 |
| DD | 8 | 168.86 ± 3.33 b | 35.57 ± 2.62 |
Means with the different lowercase letters within the same column are significantly different (P < 0.05).
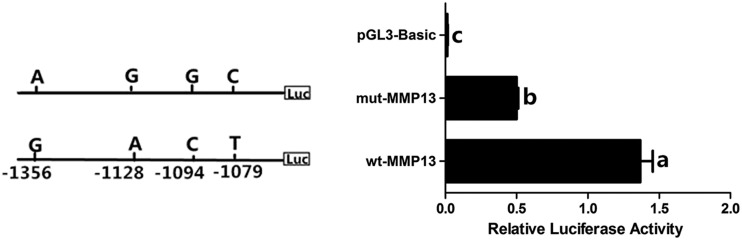
Genetic effect of the SNPs on chicken MMP13 expression
To further investigate the genetic effect of promoter SNPs on chicken MMP13 expression, the transcriptional activity of the 5′-flanking region of the MMP13 gene, which contains the G-1356/A-1128/C-1094/T-1079 and A-1356/G-1128/G-1094/C-1079 (designated as wt-MMP13 and mut-MMP13), respectively, was compared. As shown in Figure 8, the wt-MMP13 promoter showed significantly higher transcriptional activity than mut-MMP13 (P < 0.05).
Figure 8.
Effect of the six single nucleotide polymorphisms on chicken MMP13 transcriptional activity in theca cells. Data are presented as mean ± SEM from at least four replicates for each construct.
Discussion
In chicken, during follicle growth and ovulation, MMPs and their associated endogenous inhibitors, TIMPs, play critical roles in ECM remodeling. Our previous study has characterized the expression and regulation mechanism of chicken MMP1, MMP3, and MMP9 in the ovary and ovarian follicles (Zhu et al. 2014), and identified an indel polymorphism in the promoter region of the chicken MMP9 that is associated with egg number at 28 wk (Zhu and Jiang 2014). From a transcriptome study, we also found that the MMP13 mRNA is elevated in sexually mature chicken ovary (Zhu et al. 2014); therefore, in this study, we further analyzed the expression dynamics and regulation of chicken MMP13 transcription, and identified a haplotype that is associated with the trait “age at first laying”.
MMP13, also called collagenase-3, was first cloned from a cDNA library derived from a breast tumor (Freije et al. 1994). In this study, we first validated the expression dynamics of MMP13 mRNA in Jining Bairi and Hy-line hens by qRT-PCR (Figure S1), and found that, in both breeds, its mRNA expression level was significantly increased in sexually matured ovaries (140 d for Jining Bairi hens, 300 d for Hy-line hens) compared with sexually immature ovaries (90 d for Jining Bairi hens, 70 d for Hy-line hens), which is consistent with transcriptome results (Zhu et al. 2014). Second, we analyzed the expression dynamics of chicken MMP13 during chicken development from 60-d-old to 159-d-old hens, and found that MMP13 expression in the ovary of 159-d-old hens was significantly higher than other developmental stages, suggesting that MMP13 plays a role in the proper function of the ovary in laying hens. When expression of MMP13 was further analyzed in different sized follicles of sexually mature Hy-line hens, two expression peaks in F5 and POF1 follicles at mRNA level, and one in POF1 follicles at the protein level were revealed, suggesting MMP13 is an important enzyme in follicle selection and follicle regression. In rat ovary, the expression of collagenase-3 mRNA was upregulated by 32-fold at 48 h after eCG injection; MMP13 is likely responsible for the extracellular matrix remodeling associated with follicular growth (Cooke et al. 1999), and, during formation and regression of the rat CL, collagenase-3 had a separate expression pattern, being expressed only in the regressing CL, suggesting a specific role of MMP13 in luteal regression (Liu et al. 1999). In chicken, although formation of a CL is not observed, follicle regression is a similar process; consistently, a higher expression of MMP13 was also observed in POF1. This suggests that chicken MMP13 is also important in follicle regression.
The distribution of chicken MMP13 protein in the stroma cells of sexually immature chicken ovary, and in the theca cells of sexually mature chicken ovary, suggest that MMP13 is involved in the action of a cascade of proteolytic events for folliculogenesis, and proper follicle function. In ovarian follicles, the expression of MMP13 is significantly higher in theca cells than in granulosa cells. These results suggest that the MMP13 is secreted mainly by theca cells to regulate follicle growth and ovulations. In accordance with the results of this study, MMP13 was also shown to be localized in rat ovary (Balbin et al. 1996), and in the granulosal and thecal layers of bovine preovulatory follicles (Bakke et al. 2004). In addition, the expression of MMP13 is stimulated by LH in rat (Komar et al. 2001), PGF2α in sheep (Ricke et al. 2002), and GnRH in cattle (Bakke et al. 2004). In broiler hens consuming feed ad libitum compared to feed-restricted hens, granulosa cells from F1 follicles have less collagenase-3-like gelatinolytic activity (Liu et al. 2014), consistent with the fact that less ovulation of mature follicle occurs in the former, emphasizing the importance of MMP13 in chicken follicle maturation. Whether the expression of MMP13 in laying hens is regulated by the aforementioned hormones requires further investigation. Studies have indicated that MMP13 plays a role in the processes of vascularization and ossification (Inada et al. 2004; Negev et al. 2008), and induction and expression of MMP13 coincided with the onset of angiogenesis and blood vessel formation in the chorioallantoic membrane (Zijlstra et al. 2004). We also found that chicken MMP13 was expressed in the veins of the theca cell layer; consistently, the expression of VEGFA mRNA exhibits a similar pattern (Figure S2), which suggests that MMP13 is likely involved in angiogenesis. Further study is required to clarify the relationship between MMP13 and VEGFA in chicken follicle growth.
As the expression dynamics of chicken MMP13 accompany follicle growth and ovulation, we set out to analyze the regulatory mechanism of MMP13 transcription and identified a critical region stimulating its transcription. In this region, six SNPs were identified, among which haplotype D caused by SNPs at –1356 and –1079 sites has a positive effect on the age at first laying trait in the White Recessive Rock population, and hens of diplotype DD are expected to lay the first egg about 8 d earlier (Table 3). Moreover, luciferase assay indicated that the promoter of a chicken MMP13 gene harboring G-1356/A-1128/C-1094/T-1079 has a higher transcriptional activity than one harboring A-1356/G-1128/G-1094/C-1079. Using Genomatix Online Software (http://www.genomatix.de/index.html), transcription factors that can bind to these cis-elements were predicted. The two polymorphic alleles may have different binding affinity for MEL1 DNA-binding domains 2 (MELS), Kruppel-like factor 7 (KLF7), and Grainyhead-like2 (Grhl2) at g.−1356 (G > A), g.−1128 (A > G), and g.−1094(C > G), respectively. MEL1 encodes a zinc finger protein, and overexpression of a MEL1S lacking PR domain blocked granulocytic differentiation, acting as one of the causative factors in the pathogenesis of myeloid leukemia (Nishikata et al. 2003). Chicken KLF7 contains three C2H2-type zinc fingers domain at the C-terminus (Zhang et al. 2013) that are important regulators of cell proliferation and differentiation in several different organ systems (Laub et al. 2005; Lei et al. 2005; Caiazzo et al. 2010). Grhl2 belongs to the grainyhead-like transcription factor family, plays an important role in growth and development, neural tube closure, and epithelial cell differentiation (Werth et al. 2010; Pyrgaki et al. 2011; Senga et al. 2012). Whether these SNPs affect transcription of chicken MMP13 by interfering with the binding of MELS, KLF7, and Grhl2 remains to be determined.
In conclusion, in laying hens, the expression of both chicken MMP13 mRNA and protein was increased significantly in the ovary of 159-d-old hens and POF1 follicles, and chicken MMP13 protein was predominantly expressed in theca cells of sexually mature ovaries. Positive cis-acting element controlling chicken MMP13 transcription was identified, and, in this region, six SNPs were found and genotyped in chicken populations. In the White Recessive Rock population, hens with A–1356-C–1079 haplotype had earlier age at first laying than those with the G–1356-T–1079 haplotype, and exhibited lower transcriptional activity by luciferase assay. These results collectively suggest that MMP13 plays an important role in chicken follicle growth and POF regression, and that polymorphisms in its promoter region could be used as molecular markers for improving the trait age at first laying in chicken breeding.
Supplementary Material
Acknowledgments
We are grateful to Jihua Zhao and Xibo Qiao for sample collection. This work was funded by the National Science Foundation of China (NSFC 31272435), and Agricultural Elite Breeds Project of Shandong Province (2015).
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.027755/-/DC1
Communicating editor: D. J. de Koning
Literature Cited
- Bakke L. J., Li Q., Cassar C. A., Dow M. P., Pursley J. R., et al. , 2004. Gonadotropin surge-induced differential upregulation of collagenase-1 (MMP-1) and collagenase-3 (MMP-13) mRNA and protein in bovine preovulatory follicles. Biol. Reprod. 71: 605–612. [DOI] [PubMed] [Google Scholar]
- Balbin M., Fueyo A., Lopez J. M., Díez-Itza I., Velasco G., et al. , 1996. Expression of collagenase-3 in the rat ovary during the ovulatory process. J. Endocrinol. Invest. 149: 405–415. [DOI] [PubMed] [Google Scholar]
- Caiazzo M., Colucci-D’Amato L., Esposito M. T., Parisi S., Stifani S., et al. , 2010. Transcription factor KLF7 regulates differentiation of neuroectodermal and mesodermal cell lineages. Exp. Cell Res. 316: 2365–2376. [DOI] [PubMed] [Google Scholar]
- Cooke R. G., 3rd, Nothnick W. B., Komar C., Burns P., Curry Jr. T. E., 1999. Collagenase and gelatinase messenger ribonucleic acid expression and activity during follicular development in the rat ovary. Biol. Reprod. 61: 1309–1316. [DOI] [PubMed] [Google Scholar]
- Curry T. E., Jr, Osteen K. G., 2001. Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol. Reprod. 64: 1285–1296. [DOI] [PubMed] [Google Scholar]
- Curry T. E., Jr, Osteen K. G., 2003. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr. Rev. 24: 428–465. [DOI] [PubMed] [Google Scholar]
- Curry T. E., Jr, Smith M. F., 2006. Impact of extracellular matrix remodeling on ovulation and the folliculo-luteal transition. Semin. Reprod. Med. 24: 228–241. [DOI] [PubMed] [Google Scholar]
- Fata J. E., Ho A. T., Leco K. J., Moorehead R. A., Khokha R., 2000. Cellular turnover and extracellular matrix remodeling in female reproductive tissues: functions of metalloproteinases and their inhibitors. Cell. Mol. Life Sci. 57: 77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freije J. M., Díez-Itza I., Balbín M., Sánchez L. M., Blasco R., et al. , 1994. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J. Biol. Chem. 269: 16766–16773. [PubMed] [Google Scholar]
- Gilbert A. B., Evans A. J., Perry M. M., Davidson M. H., 1977. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J. Reprod. Fertil. 50: 179–181. [DOI] [PubMed] [Google Scholar]
- Gilbert A. B., Perry M. M., Waddington D., Hardie M. A., 1983. Role of atresia in establishing the follicular hierarchy in the ovary of the domestic hen (Gallus domesticus). J. Reprod. Fertil. 69: 221–227. [DOI] [PubMed] [Google Scholar]
- Huh M. I., Lee Y. M., Seo S. K., Kang B. S., Chang Y., et al. , 2007. Roles of MMP/TIMP in regulating matrix swelling and cell migration during chick corneal development. J. Cell. Biochem. 101: 1222–1237. [DOI] [PubMed] [Google Scholar]
- Inada M., Wang Y., Byrne M. H., Rahman M. U., Miyaura C., et al. , 2004. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc. Natl. Acad. Sci. USA 101: 17192–17197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar C. M., Matousek M., Mitsube K., Mikuni M., Brännström M., et al. , 2001. Effects of genistein on the periovulatory expression of messenger ribonucleic acid for matrix metalloproteinases and tissue inhibitors of metalloproteinases in the ratovary. Reproduction 121: 259–265. [DOI] [PubMed] [Google Scholar]
- Laub F., Lei L., Sumiyoshi H., Kajimura D., Dragomir C., et al. , 2005. Transcription factor KLF7 is important for neuronal morphogenesis in selected regions of the nervous system. Mol. Cell. Biol. 25: 5699–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H., Furth E. E., Kalluri R., Wakenell P., Kallen C. B., et al. , 1999. Induction of matrix metalloproteinases and collagenolysis in chick embryonic membranes before hatching. Biol. Reprod. 60: 183–189. [DOI] [PubMed] [Google Scholar]
- Lei L., Laub F., Lush M., Romero M., Zhou J., et al. , 2005. The zinc finger transcription factor Klf7 is required for TrkA gene expression and development of nociceptive sensory neurons. Genes Dev. 19: 1354–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K., Olofsson J. I., Wahlberg P., Ny T., 1999. Distinct expression of gelatinase A [matrix metalloproteinase (MMP)-2], collagenase-3 (MMP-13), membrane type MMP 1 (MMP-14), and tissue inhibitor of MMPs type 1 mediated by physiological signals during formation and regression of the rat corpus luteum. Endocrinology 140: 5330–5338. [DOI] [PubMed] [Google Scholar]
- Liu Z. C., Xie Y. L., Chang C. J., Su C. M., Chen Y. H., et al. , 2014. Feed intake alters immune cell functions and ovarian infiltration in broiler hens: implications for reproductive performance. Biol. Reprod. 90: 134. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta C (T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Negev M. H., Simsa S., Tong A., Genina O., Ornan E. M., 2008. Expression of matrix metalloproteinases during vascularization and ossification of normal and impaired avian growth plate. J. Anim. Sci. 86: 1306–1315. [DOI] [PubMed] [Google Scholar]
- Nishikata I., Sasaki H., Iga M., Tateno Y., Imayoshi S., et al. , 2003. A novel EVI1 gene family, MEL1, lacking a PR domain (MEL1S) is expressed mainly in t (1; 3) (p36; q21)-positive AML and blocks G-CSF-induced myeloid differentiation. Blood 102: 3323–3332. [DOI] [PubMed] [Google Scholar]
- Ny T., Wahlberg P., Brandstrom I. J., 2002. Matrix remodeling in the ovary: regulation and functional role of the plasminogen activator and matrix metalloproteinase systems. Mol. Cell. Endocrinol. 187: 29–38. [DOI] [PubMed] [Google Scholar]
- Ohnishi J., Ohnishi E., Shibuya H., Takahashi T., 2005. Functions for proteinases in the ovulatory process. Biochim. Biophys. Acta 1751: 95–109. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A., Ewald A. J., Werb Z., 2007. Matrix metalloproteinases and the regulation of tissue remodeling. Nat. Rev. Mol. Cell Biol. 8: 221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrgaki C., Liu A., Niswander L., 2011. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. J. Dev. Biol. 353: 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke W. A., Smith G. W., Smith M. F., 2002. Matrix metalloproteinase expression and activity following prostaglandin F (2alpha)-induced luteolysis. Biol. Reprod. 66: 685–691. [DOI] [PubMed] [Google Scholar]
- Rodgers R. J., Irving-Rodgers H. F., Russell D. L., 2003. Extracellular matrix of the developing ovarian follicle. Reproduction 126: 415–424. [DOI] [PubMed] [Google Scholar]
- Senga K., Mostov K. E., Mitaka T., Miyajima A., Tanimizu N., 2012. Grainyhead-like 2 regulates epithelial morphogenesis by establishing functional tight junctions through the organization of a molecular network among claudin3, claudin4, and Rab25. J. Mol. Cell Biol. 23: 2845–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. F., Ricke W. A., Bakke L. J., Dow M. P., Smith G. W., 2002. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol. Cell. Endocrinol. 191: 45–56. [DOI] [PubMed] [Google Scholar]
- Sudaresan N. R., Saxena V. K., Sastry K. V., Anish D., Marcus Leo M. D., et al. , 2008. Caspase-mediated apoptosis in chicken postovulatory follicle regression. Vet Res. Commun. 32: 13–19. [DOI] [PubMed] [Google Scholar]
- Werth M., Walentin K., Aue A., Schönheit J., Wuebken A., et al. , 2010. The transcription factor grainyhead-like2 regulates the molecular composition of the epithelial apical junctional complex. J. Dev. 137: 3835–3845. [DOI] [PubMed] [Google Scholar]
- Zhang Z. W., Wang Z. P., Zhang K., Wang N., Li H., 2013. Cloning, tissue expression and polymorphisms of chicken Krüppel-like factor 7 gene. J. Anim. Sci. 84: 535–542. [DOI] [PubMed] [Google Scholar]
- Zhu G., Jiang Y., 2014. Polymorphism, genetic effect and association with egg production traits of chicken matrix metalloproteinases 9 promoter. J. Anim. Sci. 27: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Kang L., Wei Q., Cui X., Wang S., et al. , 2014. Expression and regulation of MMP1, MMP3 and MMP9 in the chicken ovary in response to gonadotropin, sex hormones and TGFβ1. Biol. Reprod. 90: 1–11. [DOI] [PubMed] [Google Scholar]
- Zijlstra A., Aimes R. T., Zhu D., Regazzoni K., Kupriyanova T., et al. , 2004. Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3). J. Biol. Chem. 279: 27633–27645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplementary file File S1 contains genotype and phenotype data of chicken individuals used for diplotype and egg production trait association analysis. Sequence data are available at GenBank and the accession number is 1971458461 for SNP g. -1356G>A.