Abstract
MIG-17, a secreted protease of the ADAMTS family, acts in the directed migration of gonadal distal tip cells (DTCs) through regulation of the gonadal basement membrane in Caenorhabditis elegans. Here, we show that MIG-17 is also required for the control of pharynx elongation during animal growth. We found that the pharynx was elongated in mig-17 mutants compared with wild type. MIG-17 localized to the pharyngeal basement membrane as well as to the gonadal basement membrane. The number of nuclei in the pharynx, and the pumping rate of the pharynx, were not affected in mig-17 mutants, suggesting that cells constituting the pharynx are elongated, although the pharynx functions normally in these mutants. In contrast to the control of DTC migration, MIG-18, a secreted cofactor of MIG-17, was not essential for pharynx length regulation. In addition, the downstream pathways of MIG-17 involving LET-2/type IV collagen, FBL-1/fibulin-1, and NID-1/nidogen, partly diverged from those in gonad development. These results indicate that basement membrane remodeling is important for organ length regulation, and suggest that MIG-17/ADAMTS functions in similar but distinct molecular machineries in pharyngeal and gonadal basement membranes.
Keywords: organ elongation, ADAMTS protease, basement membrane, pharynx
Organogenesis in animal development often involves elongation of tissues. For example, convergent extension tissue movements elongate the anterior-posterior axis of the vertebrate body plan (Keller 2002). Elongation of tubular epithelia occurs by cell shape change, cell rearrangement, and cell proliferation in both vertebrates and invertebrates (Andrew and Ewald 2010). While intracellular mechanisms have been the major focus of these studies, extracellular molecular mechanisms operating in organ elongation remain poorly understood. In the present study, we showed that a secreted metalloprotease of the ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) family acts in organ length regulation through modulating the basement membrane in C. elegans.
ADAMTS family proteins have received considerable attention because defects in many of these proteins have been linked to hereditary diseases related to disorders affecting the extracellular matrix (ECM) (Dubail and Apte 2015; Hubmacher and Apte 2015). We have been studying the function of the C. elegans mig-17 gene, which encodes an ADAMTS required for the regulated migration of the gonadal leader cells called distal tip cells (DTCs) (Kim and Nishiwaki 2015). MIG-17 is secreted from body wall muscle cells, and localizes to the basement membrane of the developing gonad, and acts in the directed migration of DTCs to generate the U-shaped gonad arms (Nishiwaki et al. 2000).
Genetic suppressor analyses of mig-17 mutants identified dominant gain-of-function (gf) mutations in two genes that encode basement membrane proteins, FBL-1/fibulin-1, and LET-2/α2 subunit of collagen IV (Kubota et al. 2004, 2008). The suppressor fbl-1(gf) mutations consist of substitutions of evolutionarily conserved amino acids within the second EGF-like motif of FBL-1C and D isoforms. Of these, the mutant FBL-1C protein acts in the suppression of mig-17 DTC migration defects. FBL-1C is secreted from the intestine, and recruited to the gonadal basement membrane by MIG-17 activity, where it is likely to be activated by MIG-17, and functions in directional control of DTC migration (Kubota et al. 2004). The suppression by fbl-1(gf) mutations depends on NID-1/nidogen, a basement membrane protein (Kubota et al. 2008). The two suppressor let-2(gf) mutations result in amino acid changes in the triple helical domain, and in the C-terminal noncollagenous domain. LET-2 protein that is localized to the gonadal basement membrane can be activated (cleaved or conformationally changed) by MIG-17 to control DTC migration. In contrast to fbl-1(gf) mutations, suppression by let-2(gf) mutations is NID-1 independent (Kubota et al. 2008). In addition to these basement membrane proteins, we recently identified a novel secreted protein, MIG-18, which acts as a cofactor of MIG-17 in the control of DTC migration (Kim et al. 2014). Like MIG-17, MIG-18 is secreted from the body wall muscle cells, and localizes to the gonadal basement membrane.
Here we report that mig-17 functions in the control of pharynx length. The pharynx is a complex organ made up of 62 cells, which includes epithelial cells, muscle cells, marginal cells, grand cells, and neurons (Altun and Hall 2009). The pharynx is an epithelial organ surrounded by a basement membrane. It is ∼60 μm long at hatching and elongates during four larval stages to ∼130 μm at the young adult stage. Although pharynx length did not differ between wild-type and mig-17 mutant animals during the first larval stage, the mig-17 pharynx became longer than that of wild type until the adult stage. We showed that the function of MIG-17 was as important for the control of pharynx length as for its function in DTC migration. Genetic analyses of genes that act in the MIG-17 pathway to regulate DTC migration, most of which are basement membrane proteins, revealed shared and organ-specific aspects of the molecular mechanisms regulating DTC migration and pharynx length. These results suggest that, although MIG-17/ADAMTS acts in the remodeling of multiple basement membranes, it can function in distinct molecular contexts depending on the tissues and organs.
Materials and Methods
Strains and genetic analysis
Culture and handling of C. elegans were conducted as described (Brenner 1974). Worms were cultured at 20°. The following mutations were used in this work: mig-17(k113, k135, k167, k169, k174) (Nishiwaki 1999; Nishiwaki et al. 2000), mig-18(k140, tm2007) (Kim et al. 2014), fbl-1(k201, k206, tk45) (Kubota et al. 2004), let-2(k193, k196) (Kubota et al. 2008), nid-1(cg118, cg119) (Kang and Kramer 2000), unc-42(e270), unc-119(e2498) (Brenner 1974; Maduro and Pilgrim 1995), emb-9(b117, b189, g34) (Gupta et al. 1997), cle-1(cg120) (Ackley et al. 2001), sdn-1(ok449) (Minniti et al. 2004), mig-6(k177) (Kawano et al. 2009), mig-23(k180) (Nishiwaki et al. 2004), mig-22(k141) and sqv-5(k172, k175) (Suzuki et al. 2006) and cogc-1(k179), cogc-3(k181) (Kubota et al. 2006). The mig-17(k160) was isolated by ethyl methanesulfonate mutagenesis and failed to complement mig-17(k174).
Microscopy
Pharynx length, body length and gonad migration phenotypes were determined using a Nomarski microscope (Axioplan 2; Zeiss). Pharynx and body length were analyzed using Image J software. Pharynx length was assessed by measuring the length of the pharyngeal region excepting the buccal cavity. Pharynx volume was assessed by measuring the area of the sagittal optical section of the pharynx. Analysis of gonadal phenotypes was performed at the young adult stage as described (Nishiwaki 1999). The patterns of expression of Venus fusion proteins were analyzed using a confocal laser scanning microscope (LSM5) equipped with a Plan-Neofluar 40× lens and controlled by PASCAL version 3.2 SP2 software (all from Zeiss).
Analysis of nuclear counts
Wild-type and mig-17(k174) young adult hermaphrodites with the transgenic markers qIs147[sur-5::gfp] (Kershner et al. 2014) and tkTSi1[emb-9::mCherry] were used. tkTSi1[emb-9::mCherry] was constructed as follows: the AvrII and SpeI restriction fragment from the emb-9::mCherry construct (Ihara et al. 2011) was inserted at the SpeI site of the miniMos vector pCFJ909, and the resulting plasmid was used to generate an animal having a single-copy insertion of emb-9::mCherry in linkage group I, tkTSi1[emb-9::mCherry] (Frokjaer-Jensen et al. 2014). The confocal images were obtained with a 0.5-μm z-series using a spinning-disk confocal scan head (CSU-X1; Yokogawa) that was mounted on a Zeiss Imager M2 microscope equipped with an EM-CCD camera (ImageEM; Hamamatsu Photonics). The nuclei surrounded by the pharyngeal basement membrane were counted.
Analysis of amphid sensory neurons
DiI staining of amphid neurons was performed as described (Hedgecock et al. 1985). Nomarski and fluorescence microscopy was performed using a Zeiss Axioplan 2 microscope equipped with both optical systems.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
mig-17 is required to achieve a pharynx of normal length
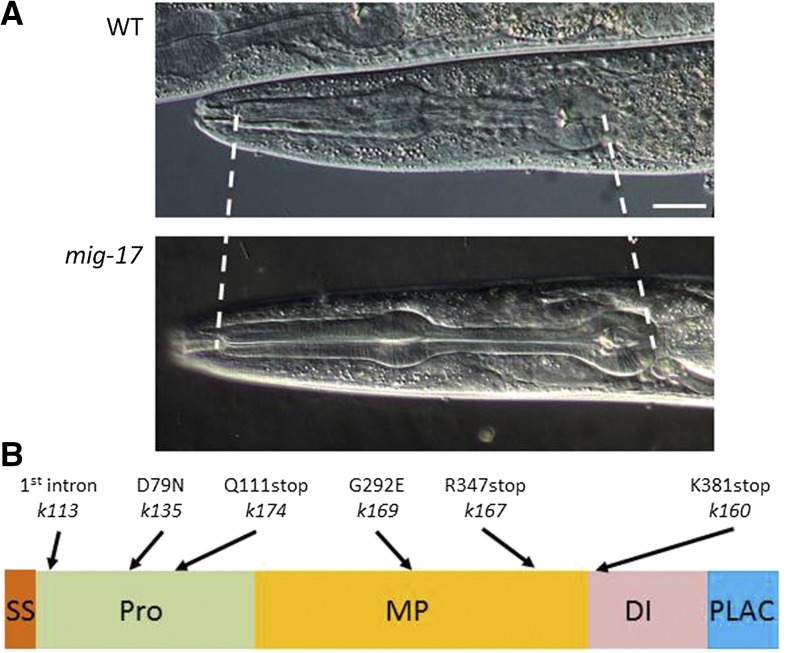
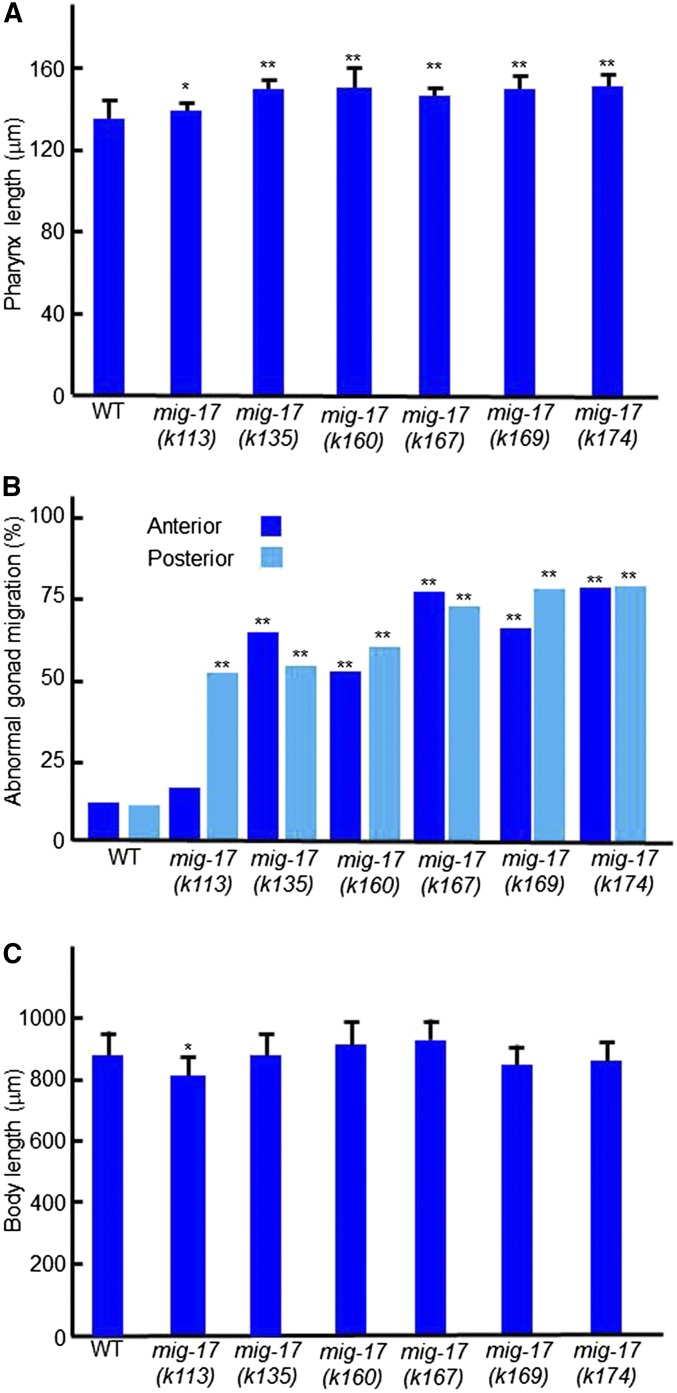
mig-17 was originally identified through mutations affecting directional migration of gonadal leader cells, the DTCs, in C. elegans (Nishiwaki et al. 2000). We found that the gene is also required to form a pharynx of the proper length: mig-17 mutants exhibited an elongated pharynx phenotype (Figure 1A). We analyzed six mutant alleles of mig-17, k113, k135, k160, k167, k169, and k174 (Figure 1B). We measured body length in addition to pharynx length to determine whether mutations affect pharyngeal length specifically or they affect overall size of the animal. All six mig-17 mutants showed pharynges that were significantly longer than those in the wild type. The putative null allele, k174, which had the strongest effect on DTC migration, also exhibited the strongest phenotype with respect to pharynx length. The k135 and k160 alleles, which had moderate effects on DTC migration, exhibited strong pharyngeal phenotypes comparable to that in k174 (Figure 2, A–C).
Figure 1.
Pharynx elongation phenotype and mutation sites of mig-17 mutants. (A) Nomarski micrographs of pharynges of wild-type and mig-17(k174) young adult hermaphrodites. Dashed lines depict anterior and posterior ends of the pharynx. Anterior is to the left, dorsal to the top. Bar: 20 μm. (B) mig-17 mutation sites. Positions of mutations are indicated along with the domain structure of the MIG-17 protein. SS, signal sequence; Pro, prodomain; MP, metalloprotease domain; DI, disintegrin domain; PLAC, protease and lacunin domain.
Figure 2.
Pharyngeal and gonadal phenotypes of mig-17 mutants. Young adult hermaphrodites were analyzed. (A–C) Pharynx lengths (n = 20) (A), % DTC migration defects (n = 60) (B), and body length (n = 20) (C) for each allele are shown. Data are shown as the mean ± SD for (A) and (C). P-values for Fisher’s exact test against wild type (WT) are indicated: ** P < 0.01, * P < 0.05.
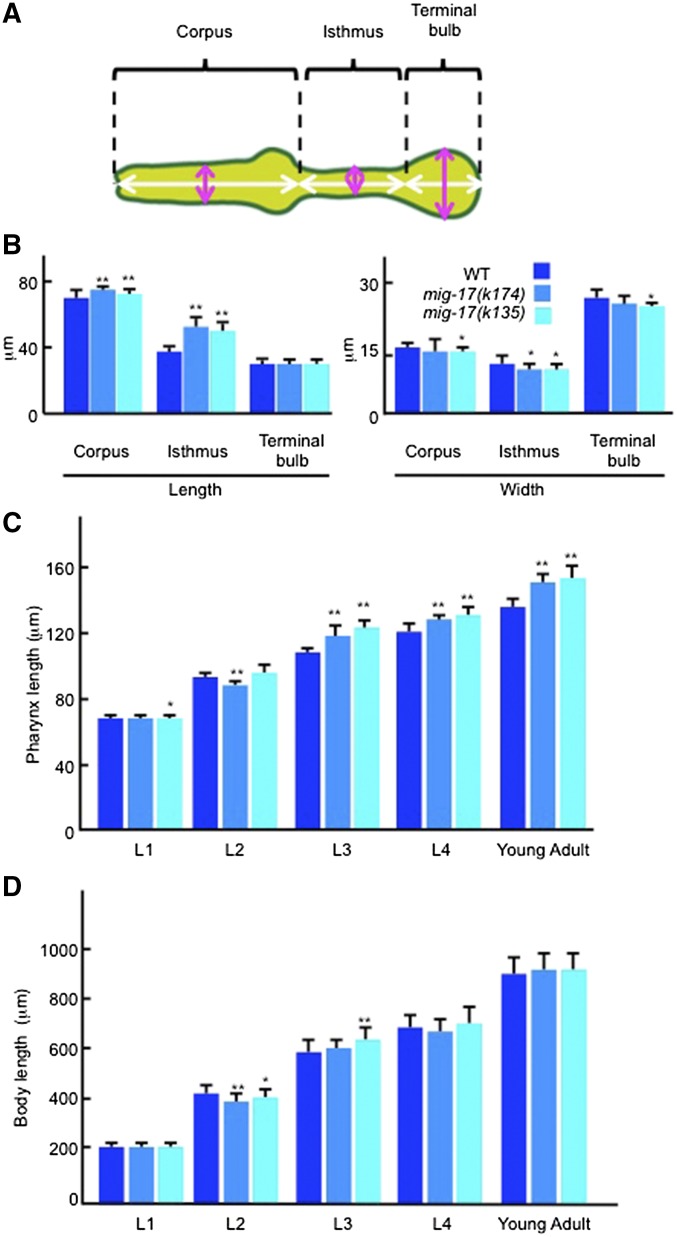
The pharynx can be divided into three parts, the corpus, isthmus and terminal bulb, from anterior to posterior (Figure 3A). We examined which part is elongated in the mig-17 mutants. In the strong k174 and k135 alleles, the corpus and isthmus were elongated, but the terminal bulb was not. In particular, lengthening of the isthmus was prominent in these alleles. As for the thickness of the pharyngeal tube, we detected slight thinning of the isthmus in both alleles relative to wild type (Figure 3B). We assessed pharynx volumes by examining the area of the sagittal optical section of wild type and mutant pharynges, and found no significant difference between them: 2354 ± 93 μm2 (n = 20) for wild type and 2377 ± 129 μm2 (n = 20) for mig-17(k174) (P = 0.528). These results suggest that the pharynx in mig-17 mutants extends along the anterior-posterior axis, and decreases in width without affecting its volume.
Figure 3.
Pharyngeal defects in mig-17 mutants. (A) The regions of the pharynx are indicated. Corpus, isthmus, and terminal bulb correspond to the muscular regions from pm1 to pm4, pm5, and from pm6 to pm8, respectively. Regions measured to determine length and width in (B) are shown by white and magenta bidirectional arrows, respectively. (B) Length and width of each pharyngeal region in young adult hermaphrodites (n = 20). The width was measured at the center of the corpus, isthmus, and terminal bulb. (C, D) Pharyngeal development in wild-type and mig-17 mutant animals (n = 20). Pharynx length (C) and body length (D). Worms having ∼10 germline nuclei, having gonad arms that recently completed the first turn and having Christmas tree-shaped vulval primordia were used as L2, L3, and L4 stage animals, respectively. Data are shown as the mean ± SD. P-values for Fisher’s exact test against WT are indicated: ** P < 0.01, * P < 0.05.
To understand developmental stages when MIG-17 is required for normal pharynx length, we examined when the pharyngeal abnormality becomes evident in k135 and k174 mutant animals. In both these alleles, pharynx elongation was observed later than the third larval (L3) stage but not during the L2 stage. Thus, it is possible that mig-17 function is required for pharynx length regulation after the L2 stage (Figure 3C).
Nuclear numbers, pumping rates, and length of the amphid sensory dendrites are not affected in mig-17 mutants
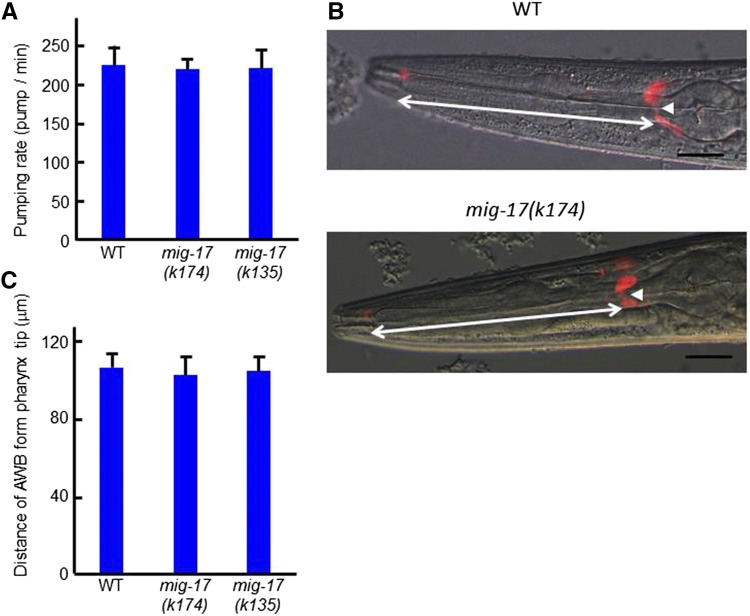
The difference in pharynx length between the wild type and mutants could reflect a difference either in cell number or cell length. We examined the number of nuclei in the pharynx in young adult animals expressing SUR-5-GFP and EMB-9-mCherry, markers for somatic nuclei and the basement membrane, respectively. The nuclear counts were at most 80; 79 ± 1.9 (n = 3) in the wild type, and 80 ± 0.4 (n = 5) in mig-17(k174). The numbers of nuclei in pm5 and mc2 cells constituting the isthmus were six and three, respectively, in all mig-17 and wild type animals examined, suggesting that nuclear number was not increased in the mig-17 mutants. Thus, it is likely that the length of cells constituting the pharynx is affected in the mutants.
Pumping frequencies did not differ between wild type and mig-17(k174) mutants (Figure 4A), suggesting that pharyngeal elongation does not affect pharyngeal function. We then examined whether mig-17 mutants affect the length of amphid sensory dendrites surrounding the pharynx. Labeling of the sensory neurons with DiI revealed that they were positioned and extended their sensory dendrites normally (Figure 4, B and C). Thus, mig-17 appears to control the length of the pharynx specifically.
Figure 4.
Pharyngeal pumping rates and length of AWB dendrites in mig-17 mutants. Young adult hermaphrodites were analyzed. (A) Pumping rates of WT and mig-17 animals. (B) Fluorescence and Nomarski merged images of WT and mig-17 animals stained with DiI. The cell body of the AWB neuron is indicated by arrowheads. (C) Distances of AWB neuronal cell bodies from the pharyngeal tip, shown by bidirectional arrows in (B). Data are shown as the mean ± SD (n = 20).
Catalytic activity, disintegrin (DI) domain, and N-glycosylation are essential for pharynx length regulation
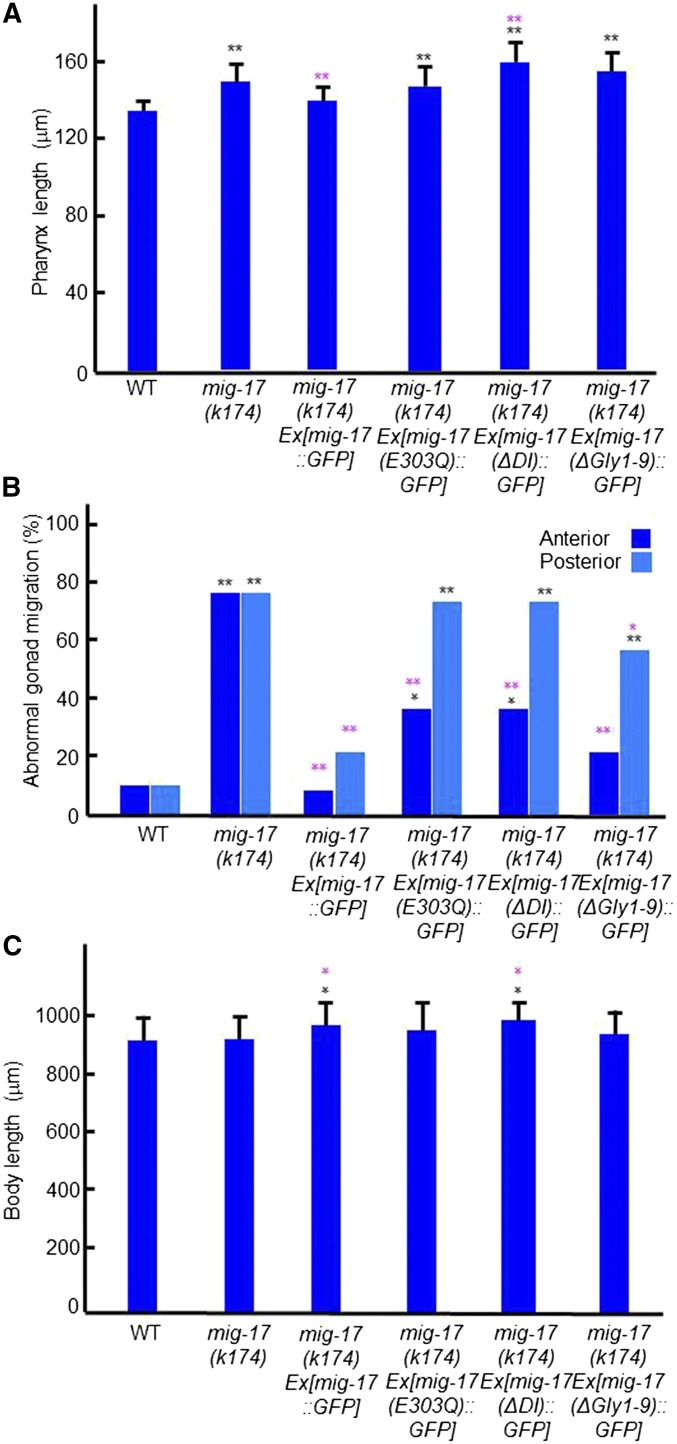
To explore the function of individual domains of MIG-17, as well as its glycosylation, in the regulation of pharynx length and DTC migration, we conducted transgenic rescue experiments of mig-17(k174) using extrachromosomal transgenic arrays (Ihara and Nishiwaki 2007). The transgenic array containing the wild-type mig-17::GFP fusion construct Ex[mig-17::GFP] fully rescued both pharyngeal and gonadal phenotypes (Figure 5, A–C). The transgenic arrays Ex[mig-17(E303Q)::GFP], which lacks catalytic activity, Ex[mig-17(ΔDI)::GFP], which lacks the DI domain, and Ex[mig-17(ΔGly1-9)::GFP], which lacks all nine potential N-glycosylation sites, failed to rescue the pharyngeal phenotype. Similar results were obtained for the posterior gonadal defects, although these mutant constructs significantly rescued the anterior gonadal defects (Figure 5B). These results indicate that the catalytic activity, the DI domain, and N-glycan modifications are essential for regulating pharynx length.
Figure 5.
Transgenic rescue experiments of mig-17 pharynx and gonadal defects. mig-17(k174) young adult hermaphrodites having extrachromosomal arrays Ex[mig-17::GFP], Ex[mig-17(E303Q)::GFP], Ex[mig-17(ΔDI)::GFP], and Ex[mig-17(ΔGly1-9)::GFP] (Ihara and Nishiwaki 2007) were analyzed. (A) Pharynx length. Data are shown as the mean ± SD (n = 30). (B) Gonad abnormality (n = 30 for WT, n = 60 for others). (C) Body length. Data are shown as the mean ± SD (n = 30). P-values for Fisher’s exact test against WT (black) and mig-17(k174) (magenta) are indicated: ** P < 0.01, *P < 0.05.
MIG-17-Venus localizes to the pharyngeal basement membrane
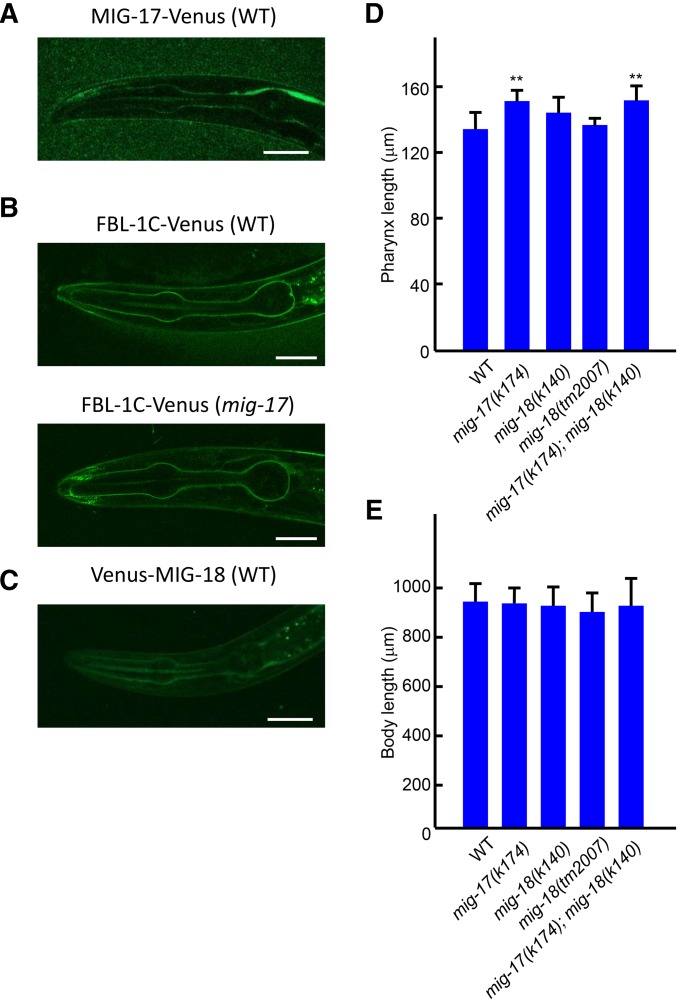
We examined expression of the MIG-17-Venus functional fusion. MIG-17-Venus was observed to localize to the pharyngeal basement membrane (Figure 6A) as well as to the gonadal basement membrane (Kim et al. 2014). The pharyngeal localization of MIG-17-Venus was first detected in the twofold stage embryo and continued to the adult stage. The localization was strongest in L3 larvae, and became weaker in late larval and adult animals (data not shown).
Figure 6.
Localization of MIG-17, FBL-1C and MIG-18, and effects of mig-18 mutations on pharynx length. (A–C) Confocal images of L3 larvae expressing MIG-17-Venus in WT (A), FBL-1C-Venus in WT (upper), and mig-17(k174) mutant (lower) (B), and Venus-MIG-18 in WT (C) backgrounds. Bar: 20 μm. (D), (E) Effects of mig-18 mutations on pharynx length. Pharynx length (D) and body length (E) of young adult hermaphrodites were analyzed. Data are shown as the mean ± SD (n = 20). P-values for Fisher’s exact test against WT are indicated: ** P < 0.01.
MIG-17 activity is required for efficient accumulation of FBL-1C/fibulin-1C in the gonadal basement membrane, which is important for directional migration of gonadal DTCs (Kubota et al. 2004; Kim et al. 2014). We examined whether FBL-1C also localizes to the pharyngeal basement membrane, and whether the localization is affected by mig-17 mutations. We found that FBL-1C-Venus, a functional fusion (Kubota et al. 2004), localized to the pharyngeal basement membrane, but its accumulation was not reduced in the mig-17(k174) mutant background compared with the wild type (Figure 6B, and Supplemental Material, Figure S1), suggesting that, in contrast to the case in the gonadal basement membrane, MIG-17 is not required for the recruitment of FBL-1C to the pharyngeal basement membrane.
MIG-18, a cofactor of MIG-17, is not required for the control of pharynx length
MIG-18 is a novel secreted protein whose loss-of-function mutations result in DTC migration defects similar to those in the mig-17 mutations. Based on the genetic evidence, we proposed that it acts as a cofactor of MIG-17 to regulate DTC migration (Kim et al. 2014). We examined MIG-18 expression using Venus-MIG-18, a functional fusion protein (Kim et al. 2014). We observed that Venus-MIG-18 localizes to the pharyngeal basement membrane from the twofold embryonic stage through the adult stage, and that the basement membrane accumulation was strongest during the L3 stage (Figure 6C, and data not shown). Thus, MIG-18 localized to the pharynx similar to MIG-17. Mutants with the putative null alleles, mig-18(k140) and mig-18(tm2007), had pharynges of a similar length to those in wild-type animals. The length of the pharynx in mig-17(k174); mig-18(k140) double mutants was elongated and comparable to that of the mig-17(k174) single mutants (Figure 6, D and E). These results suggest that MIG-18 is not required for the regulation of pharynx length, although it has a substantial function in DTC migration (Kim et al. 2014).
Mutations in type IV collagen and fibulin-1 suppress the pharyngeal phenotype of mig-17
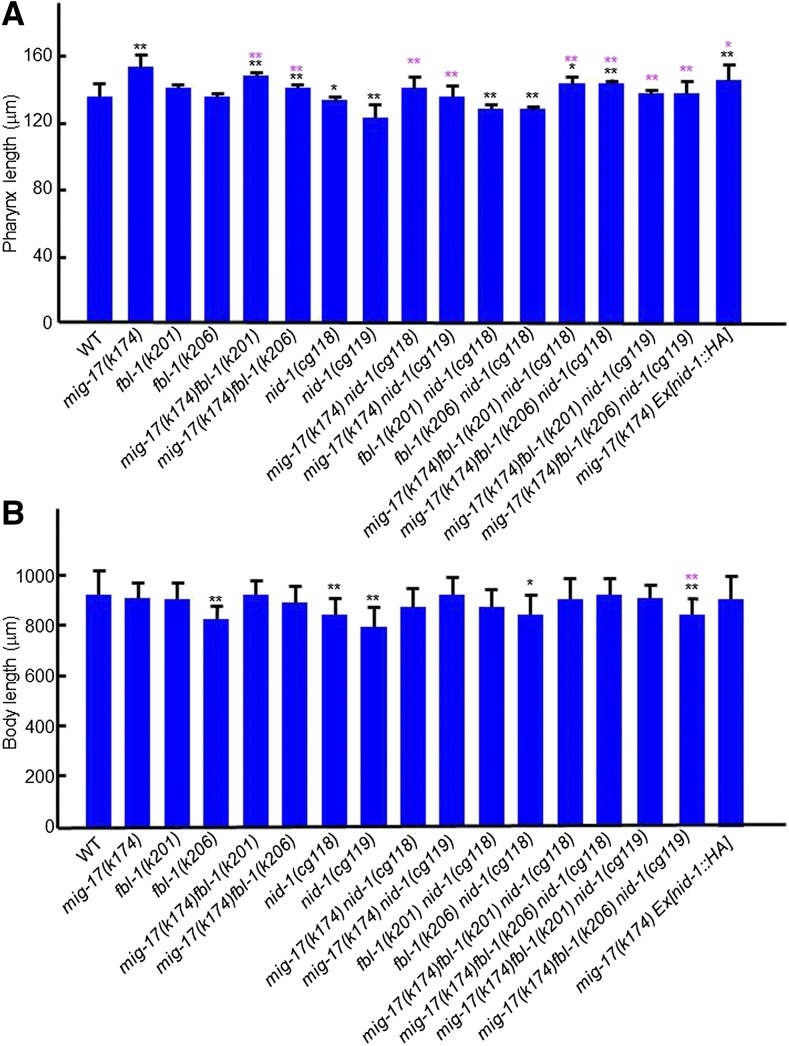
The gonadal DTC migration defects of mig-17 mutants can be suppressed by the gain-of-function mutations caused by amino acid substitutions in let-2 (encoding the α2 subunit of type IV collagen) and fbl-1 (encoding fibulin-1) (Kubota et al. 2004, 2008). When mig-17(k174) was combined with suppressor let-2 mutations k193 or k196, k193 suppressed the pharyngeal phenotype, whereas k196 did not (Figure 7, A and B). When mig-17(k174) was combined with suppressor fbl-1 mutations k201 or k206, they both suppressed the pharyngeal defects (Figure 8, A and B). Because the suppression of gonadal defects of mig-17 by fbl-1(gf) mutations depends on nid-1, which encodes nidogen in C. elegans (Kubota et al. 2008), we asked whether this is also the case in pharyngeal defects. First, we combined the nid-1(cg118) (partial loss of function), or nid-1(cg119) (complete loss of function), allele with mig-17(k174), and observed that they each suppressed the pharyngeal phenotype of mig-17 (Figure 8, A and B). This was unexpected because nid-1(cg119) enhances mig-17(k174) with respect to gonadal defects (Kubota et al. 2008). When nid-1 mutants were then combined with mig-17(k174); fbl-1(k201) or mig-17; fbl-1(k206), the mig-17 pharynx length defect was still suppressed. Overexpression of NID-1-hemagglutinin (HA) partially suppresses the DTC migration defects of mig-17 mutants (Kubota et al. 2008). We examined pharynx length in a mig-17(k174) mutant that overexpresses NID-1-HA (Figure 8, A and B) and observed partial suppression in the transgenic line. Therefore, either a reduction or increase in the NID-1 dosage appears to suppress the mig-17 pharynx phenotype. Thus, it is likely that, although let-2, fbl-1 and nid-1 act to control the length of the pharynx in the MIG-17 pathway, their roles are not always the same as their roles in the control of gonadal DTC migration.
Figure 7.
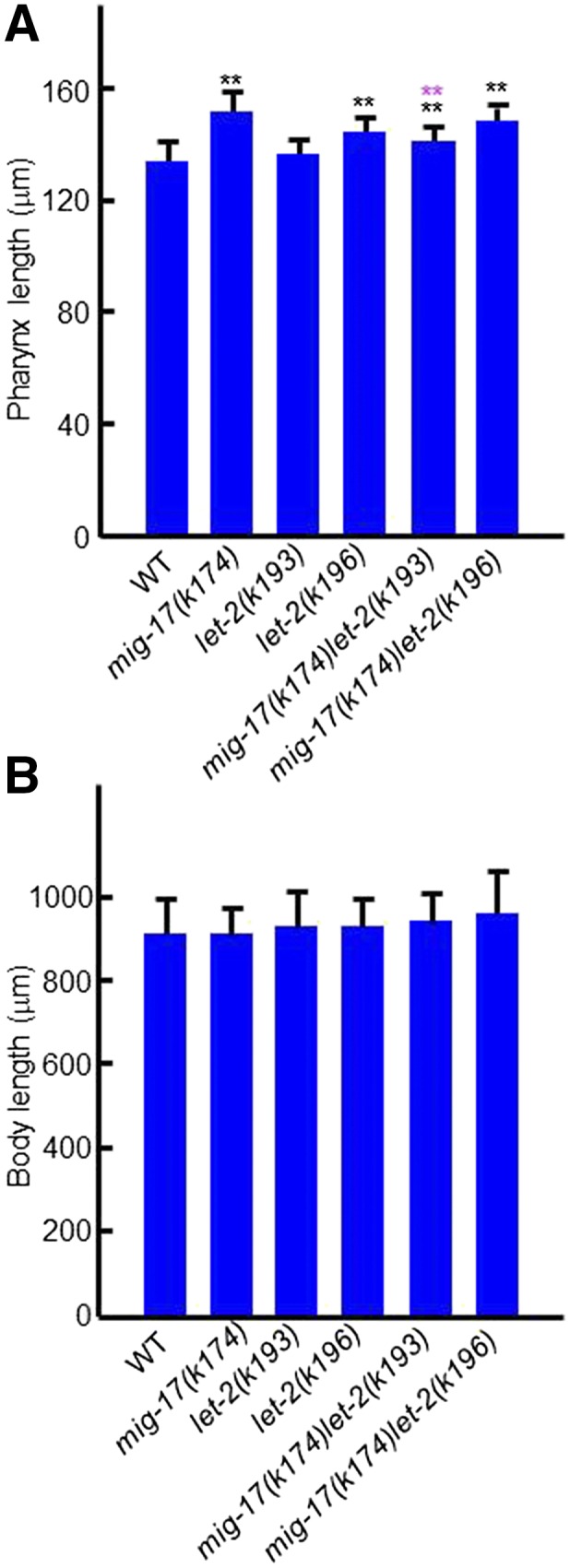
Effects of let-2 mutations on pharynx length. (A), (B) Pharynx length (A) and body length (B) of young adult hermaphrodites were analyzed. Data are shown as the mean ± SD (n = 20). P-values for Fisher’s exact test against WT (black) and mig-17(k174) (magenta) are indicated: ** P < 0.01.
Figure 8.
Effects of fbl-1 and nid-1 mutations on pharynx length. (A), (B) Pharynx length (A) and body length (B) of young adult hermaphrodites were analyzed. Data are shown as the mean ± SD (n = 20). P-values for Fisher’s exact test against WT (black) and mig-17(k174) (magenta) are indicated: ** P < 0.01, * P < 0.05.
Glycosylation mutants having mig-17-like gonadal defects exhibit short body length phenotypes
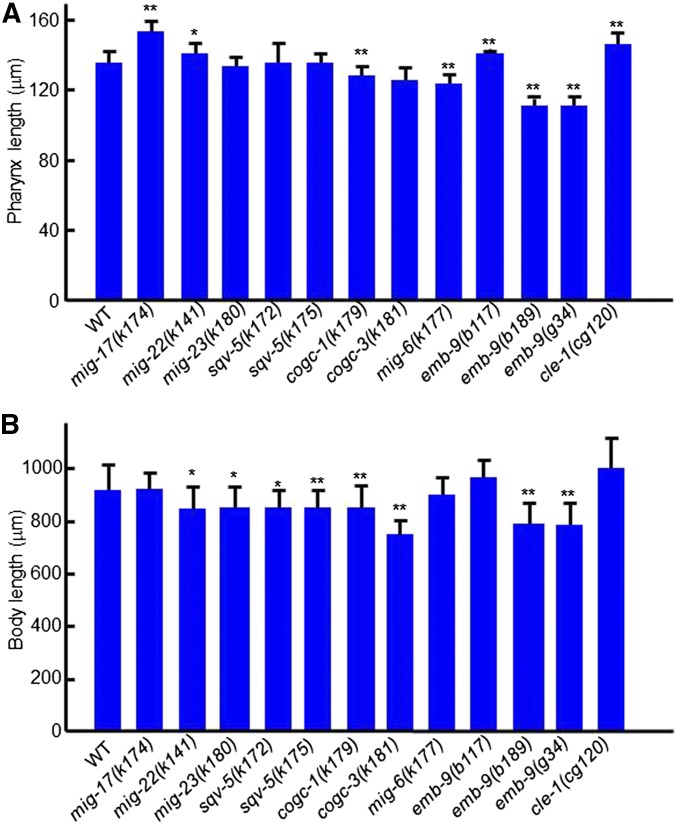
Mutations in genes that act in protein glycosylation in the Golgi apparatus cause gonadal DTC migration-defective phenotypes similar to those observed in the mig-17 mutants. These include sqv-5 (chondroitin synthase), mig-22 (chondroitin polymerizing factor), mig-23 (Golgi NDPase), and cogc-1 and cogc-3 (components of conserved oligomeric Golgi complex) (Nishiwaki et al. 2004; Kubota et al. 2006; Suzuki et al. 2006). We examined mutant alleles of these genes, and found that each of these mutations resulted in a shorter body length relative to the wild type, and that a slight elongation of the pharynx was present only in mig-22(k141) animals (Figure 9, A and B). Because of the shorter body length, it was not clear whether the other mutants affected pharynx length. Although mig-23, cogc-1, and cogc-3 mutants partially compromise the function of MIG-17 in DTC migration through their misglycosylation (Nishiwaki et al. 2004; Kubota et al. 2006), it may still function adequately to control pharynx length.
Figure 9.
Effects of mutations affecting glycosylation and basement membrane components on pharynx length. (A), (B) Pharynx length (A) and body length (B) of young adult hermaphrodites were analyzed. Data are shown as the mean ± SD (n = 20). P-values for Fisher’s exact test against WT are indicated: ** P < 0.01, * P < 0.05.
Mutations in basement membrane proteins affect the length of the pharynx and body
mig-6 encodes the basement membrane protein papilin. The class-s alleles of mig-6, which affect its short splicing isoform, exhibit intergenic noncomplementation with mig-17 mutants with respect to the DTC migration phenotype, and therefore mig-6 is proposed to act in the same genetic pathway as mig-17 to control DTC migration (Kawano et al. 2009). The mig-6(k177) class-s mutants, however, showed a rather shortened pharynx (Figure 9, A and B), suggesting that mig-6 may not function with mig-17 to control the length of the pharynx. emb-9 encodes the α1 subunit of type IV collagen, which forms a collagen trimer with the α2 subunit/LET-2. Animals carrying one of three emb-9 mutations, which are embryonic or larval lethal at 25°, were reared at 20°, at which temperature they remain viable. The b117 allele produced a slightly elongated pharynx, whereas the b189 and g34 alleles had shorter pharynges and bodies compared with the wild type. We also examined an allele of cle-1, which encodes basement membrane type XVIII collagen (Ackley et al. 2001) and observed a significant increase in the length of the pharynx, as was noted in the emb-9(b177) mutants (Figure 9, A and B). These results indicate that mutations in basement membrane collagens affect the control of the pharynx and body length.
Discussion
MIG-17 activity is involved in pharynx length regulation
In this study, we showed that an ADAMTS family metalloprotease, MIG-17, which is required for directional migration of gonadal DTCs, also acts in the regulation of pharynx length. We found that the pharynx of mig-17 mutants becomes longer compared with the wild type after the L2 stage. Consistent with this observation, MIG-17-Venus strongly accumulated in the pharyngeal basement membrane during the L3 stage. These results suggest that MIG-17 negatively regulates pharynx elongation from the basal side of the pharynx. By expressing various mutant constructs in the mig-17(k174) null mutant, we found that the catalytic activity, the C-terminal DI domain and N-glycosylation of MIG-17 are essential for the regulation of pharynx length as in the case of the control of DTC migration. We previously showed that the MIG-17 pro-enzyme is converted into the mature enzyme by autoproteolytic removal of its prodomain (Ihara and Nishiwaki 2007). The catalytic activity of mature MIG-17 is important for controlling DTC migration, especially for migration of the posterior DTCs. Also, the DI domain is essential for prodomain processing (Ihara and Nishiwaki 2007). Thus it is likely that MIG-17 can be regulated in a similar manner in the pharyngeal basement membrane.
MIG-17 acts through different molecular mechanisms in pharynx and gonad development
An analysis of molecules involved in the MIG-17 pathway that regulates DTC migration revealed distinct aspects of these molecules in the regulation of pharynx length. MIG-18, a cofactor of MIG-17 in gonadal DTC migration, was not required for pharynx length regulation. FBL-1C, which accumulates in the gonadal basement membrane in a strongly MIG-17-dependent manner, accumulated normally in the pharyngeal basement membrane in the absence of MIG-17.
Although both let-2 alleles, k193 and k196, strongly suppress DTC migration defects (Kubota et al. 2008), only k193 suppressed the pharynx phenotype of mig-17. k193 and k196 are amino acid substitutions in the C-terminal noncollagenous (NC1) domain and the triple helical domain of type IV collagen, respectively. The NC1 domain is important for the linkage of NC1 domains of two type IV collagen trimers (Yurchenco and Schittny 1990; Sundaramoorthy et al. 2002). Thus, the type IV collagen meshwork that is formed in k193 mutants could be partially disorganized because of the aberrant formation of intermolecular bonds between NC1 domains. In contrast, k196 could affect folding or stability of part of the triple-helical structure, which may also result in disorganization of the meshwork. We speculate that the suppressor let-2 mutations could mimic the type IV collagen remodeling in the basement membrane that is normally elicited by MIG-17. Therefore, the different suppression outcomes by k196 in the formation of the pharynx and the gonad could reflect the different actions of MIG-17 in these basement membrane regions.
The nid-1 mutations suppressed the mig-17 pharyngeal phenotype, in contrast to its enhancement of mig-17 gonadal defects. nid-1 appears to act antagonistically to mig-17 in the regulation of pharynx length. The observation that nid-1 mutations themselves lead to shorter pharynges compared with wild type supports this idea (Figure 8A). NID-1 strongly localizes to the basement membrane of migrating DTCs but weakly to the pharyngeal basement membrane (Kang and Kramer 2000). In addition, nid-1 produces three different splicing isoforms, NID-1A, B, and C (Kang and Kramer 2000). Thus, it might be possible that qualitative and/or quantitative differences in NID-1 expression may be the cause for distinct activities of NID-1 in the MIG-17–dependent mechanisms in the pharynx and the gonad.
Basement membrane collagens are involved in pharynx length regulation
Basement membrane type IV collagen plays important roles in tissue morphogenesis (Morrissey and Sherwood 2015). We also showed in this study that mutations in type IV collagen affect pharynx length. Four of the five type IV collagen mutants examined—let-2(k196) and emb-9(b117, b189, and g34)—consist of substitutions of the glycines in the tripeptide repeats (Gly-X-Y) in the triple helical domain. Interestingly, let-2(k196) and emb-9(b117) showed an elongated pharynx, whereas emb-9(b189) and emb-9(g34) had a shortened pharynx. The type IV collagen molecule is a triple-helical trimer consisting of two α1/EMB-9 subunits and one α2/LET-2 subunit. The triple helix is formed by successive Gly-X-Y repeats, where X is often proline and Y is often lysine (Kramer 2005). Both EMB-9 and LET-2 molecules have intrinsic interruptions in the Gly-X-Y sequence, which are more often found in the N-terminal half of the triple-helical domain, and therefore the thermal stability of these molecules is higher in the C-terminal than the N-terminal region (Sibley et al. 1994). The lethal phenotypes of glycine substitution mutations in emb-9 are stronger when they occur in the N-terminal half (b189 and g34) than in the C-terminal half (b117) (Gupta et al. 1997). The let-2(k196) mutation is in the C-terminal half (Kubota et al. 2008). Therefore it might be possible that glycine substitutions in the C-terminal high-stability region result in pharynx elongation, whereas those in the N-terminal low-stability region result in pharyngeal shortening. We speculate that MIG-17 may modulate the structure or conformation of the C-terminal half of the triple-helical domain to achieve a pharynx of the proper length.
We found that cle-1(cg120) results in pharynx elongation similar to that in the emb-9(b117) and let-2(k196) mutants. CLE-1/type XVIII collagen is widely distributed in the basement membrane, and cle-1(cg120) is a deletion mutant lacking the NC1 domain, which contains the endostatin (an angiogenesis inhibitor in mammals) domain (Ackley et al. 2001). Because cle-1(cg120) mutants show DTC migration defects (that phenotypically differ from those of mig-17) (Ackley et al. 2001), endostatin is suggested to have a role in this process. Thus, it might be possible that endostatin also acts in pharynx length control.
Pharynx elongation in mig-17 mutants
Our finding indicates that the pharynx of mig-17 mutants is elongated because of the elongation of cells rather than an increase in the cell number. The pharynx is ∼60 μm long when worms hatch, and it elongates to ∼130 μm long by the adult stage (about a twofold increase). During the same period, the body size grows from ∼200 to 900 μm (an ∼4.5-fold increase). Thus, the rate of pharyngeal elongation is much lower than that of body elongation. Recently, it was shown that the embryonic pharynx behaves like a spring in which the anterior end is attached to the mouth, and the posterior end attached to the surrounding epidermis (Kelley et al. 2015). During embryonic 1.5- and 3-fold stages, the pharyngeal spring is stretched with increasing tensile force along the anterior-posterior axis because of the elongation of the epidermis. If this is also the case in the larval pharynx, the active elongation of the larval body, or the elongation of the epidermis, should stretch the pharynx during its elongation. Because epidermal elongation is much greater than pharyngeal elongation, we should postulate some slippage between the pharynx end and the epidermis. Because the pharyngeal end interacts with the surrounding epidermis through their basement membranes, MIG-17 may normally act to weaken the friction or adhesiveness between these basement membranes to produce a pharynx of proper length. Loss of MIG-17 activity may enhance the adhesion and therefore confer a greater stretching force, resulting in pharynx elongation. However, it is also possible that the larval pharynx expands actively rather than passively by stretching. In this case, MIG-17 may act to stiffen the pharyngeal basement membrane to inhibit its overgrowth. Interestingly, type IV collagen functions in organ morphogenesis through providing a constricting force in Drosophila (Haigo and Bilder 2011; Pastor-Pareja and Xu 2011). MIG-17 may affect the stiffness of the pharyngeal basement membrane similarly by modulating the type IV collagen meshwork. Further studies are needed to distinguish between these possibilities.
ADAMTS function controlling organ shape can be evolutionarily conserved
Our finding demonstrates that MIG-17/ADAMTS functions in organ shape control through its remodeling activity in the basement membrane in C. elegans. Another ADAMTS in C. elegans, ADT-2, regulates body size by modulating TGFβ signaling and cuticle collagen organization (Fernando et al. 2011). This function in shape control appears to be conserved in mammalian ADAMTS proteins, which also act in ECM remodeling. Mutations in human ADAMTS10 and ADAMTS17 cause Weill-Marchesani syndrome, which is characterized by short stature and eye abnormalities including microspherophakia (the lens of the eye is smaller than normal and spherically shaped) (Dagoneau et al. 2004; Morales et al. 2009; Shah et al. 2014). It is worth noting that MIG-17, which acts in pharynx length regulation in C. elegans, has a structural similarity to ADAMTS10 and 17 in that they all share C-terminal protease and lacunin (PLAC) domains (Ihara and Nishiwaki 2007). Further analysis of C. elegans ADAMTS proteinases would shed light on the detailed molecular mechanisms and the consequent physical interactions between the ECM and the cells that finally determine organ shape.
Supplementary Material
Acknowledgments
We thank Noriko Nakagawa for technical assistance. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources and from Shohei Mitani through the National Bioresource Project for the nematode. This work was supported by a Grant-in-Aid for Scientific Research (B) by the Ministry of Education, Culture, Sports, Science and Technology to K.N. (22111005).
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.028019/-/DC1
Communicating editor: D. S. Fay
Literature Cited
- Ackley B. D., Crew J. R., Elamaa H., Pihlajaniemi T., Kuo C. J., et al. , 2001. The NC1/endostatin domain of Caenorhabditis elegans type XVIII collagen affects cell migration and axon guidance. J. Cell Biol. 152: 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun, Z.F. and D.H. Hall, 2009 Alimentary system, overview. In WormAtlas. 10.3908/wormatlas.1.2. Edited for the web by Laura A. Herndon. Last revision June 15, 2010. [Google Scholar]
- Andrew D. J., Ewald A. J., 2010. Morphogenesis of epithelial tubes: insights into tube formation, elongation, and elaboration. Dev. Biol. 341: 34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagoneau N., Benoist-Lasselin C., Huber C., Faivre L., Megarbane A., et al. , 2004. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 75: 801–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubail J., Apte S. S., 2015. Insights on ADAMTS proteases and ADAMTS-like proteins from mammalian genetics. Matrix Biol. 44–46: 24–37. [DOI] [PubMed] [Google Scholar]
- Fernando T., Flibotte S., Xiong S., Yin J., Yzeiraj E., et al. , 2011. C. elegans ADAMTS ADT-2 regulates body size by modulating TGFbeta signaling and cuticle collagen organization. Dev. Biol. 352: 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer-Jensen C., Davis M. W., Sarov M., Taylor J., Flibotte S., et al. , 2014. Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nat. Methods 11: 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M. C., Graham P. L., Kramer J. M., 1997. Characterization of alpha1(IV) collagen mutations in Caenorhabditis elegans and the effects of alpha1 and alpha2(IV) mutations on type IV collagen distribution. J. Cell Biol. 137: 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo S. L., Bilder D., 2011. Global tissue revolutions in a morphogenetic movement controlling elongation. Science 331: 1071–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Thomson J. N., Perkins L. A., 1985. Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev. Biol. 111: 158–170. [DOI] [PubMed] [Google Scholar]
- Hubmacher D., Apte S. S., 2015. ADAMTS proteins as modulators of microfibril formation and function. Matrix Biol. 47: 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara S., Nishiwaki K., 2007. Prodomain-dependent tissue targeting of an ADAMTS protease controls cell migration in Caenorhabditis elegans. EMBO J. 26: 2607–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara S., Hagedorn E. J., Morrissey M. A., Chi Q., Motegi F., et al. , 2011. Basement membrane sliding and targeted adhesion remodels tissue boundaries during uterine-vulval attachment in Caenorhabditis elegans. Nat. Cell Biol. 13: 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. H., Kramer J. M., 2000. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol. Biol. Cell 11: 3911–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T., Zheng H., Merz D. C., Kohara Y., Tamai K. K., et al. , 2009. C. elegans mig-6 encodes papilin isoforms that affect distinct aspects of DTC migration, and interacts genetically with mig-17 and collagen IV. Development 136: 1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., 2002. Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298: 1950–1954. [DOI] [PubMed] [Google Scholar]
- Kelley M., Yochem J., Krieg M., Calixto A., Heiman M. G., et al. , 2015. FBN-1, a fibrillin-related protein, is required for resistance of the epidermis to mechanical deformation during C. elegans embryogenesis. eLife 4: e06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershner A. M., Shin H., Hansen T. J., Kimble J., 2014. Discovery of two GLP-1/Notch target genes that account for the role of GLP-1/Notch signaling in stem cell maintenance. Proc. Natl. Acad. Sci. USA 111: 3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Nishiwaki K., 2015. Control of the basement membrane and cell migration by ADAMTS proteinases: lessons from C. elegans genetics. Matrix Biol. 44–46: 64–69. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Kitano Y., Mori M., Takano T., Harbaugh T. E., et al. , 2014. The novel secreted factor MIG-18 acts with MIG-17/ADAMTS to control cell migration in Caenorhabditis elegans. Genetics 196: 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., 2005. Basement membranes (September 1, 2005), WormBook, ed. The C. elegans Research Community, WormBook, /10.1895/wormbook.1.16.1, http://www.wormbook.org.. [Google Scholar]
- Kubota Y., Kuroki R., Nishiwaki K., 2004. A fibulin-1 homolog interacts with an ADAM protease that controls cell migration in C. elegans. Curr. Biol. 14: 2011–2018. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Sano M., Goda S., Suzuki N., Nishiwaki K., 2006. The conserved oligomeric Golgi complex acts in organ morphogenesis via glycosylation of an ADAM protease in C. elegans. Development 133: 263–273. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Ohkura K., Tamai K. K., Nagata K., Nishiwaki K., 2008. MIG-17/ADAMTS controls cell migration by recruiting nidogen to the basement membrane in C. elegans. Proc. Natl. Acad. Sci. USA 105: 20804–20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro M., Pilgrim D., 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minniti A. N., Labarca M., Hurtado C., Brandan E., 2004. Caenorhabditis elegans syndecan (SDN-1) is required for normal egg laying and associates with the nervous system and the vulva. J. Cell Sci. 117: 5179–5190. [DOI] [PubMed] [Google Scholar]
- Morales J., Al-Sharif L., Khalil D. S., Shinwari J. M., Bavi P., et al. , 2009. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am. J. Hum. Genet. 85: 558–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey M. A., Sherwood D. R., 2015. An active role for basement membrane assembly and modification in tissue sculpting. J. Cell Sci. 128: 1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K., 1999. Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics 152: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K., Hisamoto N., Matsumoto K., 2000. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science 288: 2205–2208. [DOI] [PubMed] [Google Scholar]
- Nishiwaki K., Kubota Y., Chigira Y., Roy S. K., Suzuki M., et al. , 2004. An NDPase links ADAM protease glycosylation with organ morphogenesis in C. elegans. Nat. Cell Biol. 6: 31–37. [DOI] [PubMed] [Google Scholar]
- Pastor-Pareja J. C., Xu T., 2011. Shaping cells and organs in Drosophila by opposing roles of fat body-secreted collagen IV and perlecan. Dev. Cell 21: 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M. H., Bhat V., Shetty J. S., Kumar A., 2014. Whole exome sequencing identifies a novel splice-site mutation in ADAMTS17 in an Indian family with Weill-Marchesani syndrome. Mol. Vis. 20: 790–796. [PMC free article] [PubMed] [Google Scholar]
- Sibley M. H., Graham P. L., von Mende N., Kramer J. M., 1994. Mutations in the alpha 2(IV) basement membrane collagen gene of Caenorhabditis elegans produce phenotypes of differing severities. EMBO J. 13: 3278–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy M., Meiyappan M., Todd P., Hudson B. G., 2002. Crystal structure of NC1 domains. Structural basis for type IV collagen assembly in basement membranes. J. Biol. Chem. 277: 31142–31153. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Toyoda H., Sano M., Nishiwaki K., 2006. Chondroitin acts in the guidance of gonadal distal tip cells in C. elegans. Dev. Biol. 300: 635–646. [DOI] [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C., 1990. Molecular architecture of basement membranes. FASEB J. 4: 1577–1590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.