Abstract
Animals must constantly assess their surroundings and integrate sensory cues to make appropriate behavioral and developmental decisions. Pheromones produced by conspecific individuals provide critical information regarding environmental conditions. Ascaroside pheromone concentration and composition are instructive in the decision of Caenorhabditis elegans to either develop into a reproductive adult or enter into the stress-resistant alternate dauer developmental stage. Pheromones are sensed by a small set of sensory neurons, and integrated with additional environmental cues, to regulate neuroendocrine signaling and dauer formation. To identify molecules required for pheromone-induced dauer formation, we performed an unbiased forward genetic screen and identified phd (pheromone response-defective dauer) mutants. Here, we describe new roles in dauer formation for previously identified neuronal molecules such as the WD40 domain protein QUI-1 and MACO-1 Macoilin, report new roles for nociceptive neurons in modulating pheromone-induced dauer formation, and identify tau tubulin kinases as new genes involved in dauer formation. Thus, phd mutants define loci required for the detection, transmission, or integration of pheromone signals in the regulation of dauer formation.
Keywords: C. elegans, dauer, pheromone, che-12, maco-1, qui-1, ttbk
Phenotypic plasticity in response to adverse environmental cues represents a bet-hedging strategy in an unpredictable environment (Avery 2014; Furness et al. 2015). A particularly well-studied form of phenotypic plasticity is facultative diapause, a hibernation-like state characterized by cessation of feeding and altered physiology (Denlinger 2002; Emerson et al. 2009; Guidetti et al. 2011; Schiesari and O’Connor 2013). The decision between entry into diapause or continued reproductive growth is mediated via the integration of environmental cues such as temperature, food, and light to regulate endocrine signaling (Golden and Riddle 1984b; Fielenbach and Antebi 2008; Emerson et al. 2009; Hahn and Denlinger 2011; Nylin 2013; Denlinger and Armbruster 2014; Furness et al. 2015). Although much is now known about the hormone signaling pathways that regulate diapause, particularly in invertebrates, the neuronal and molecular mechanisms that transduce and integrate sensory cues of multiple modalities to enable this adaptive developmental decision are poorly understood.
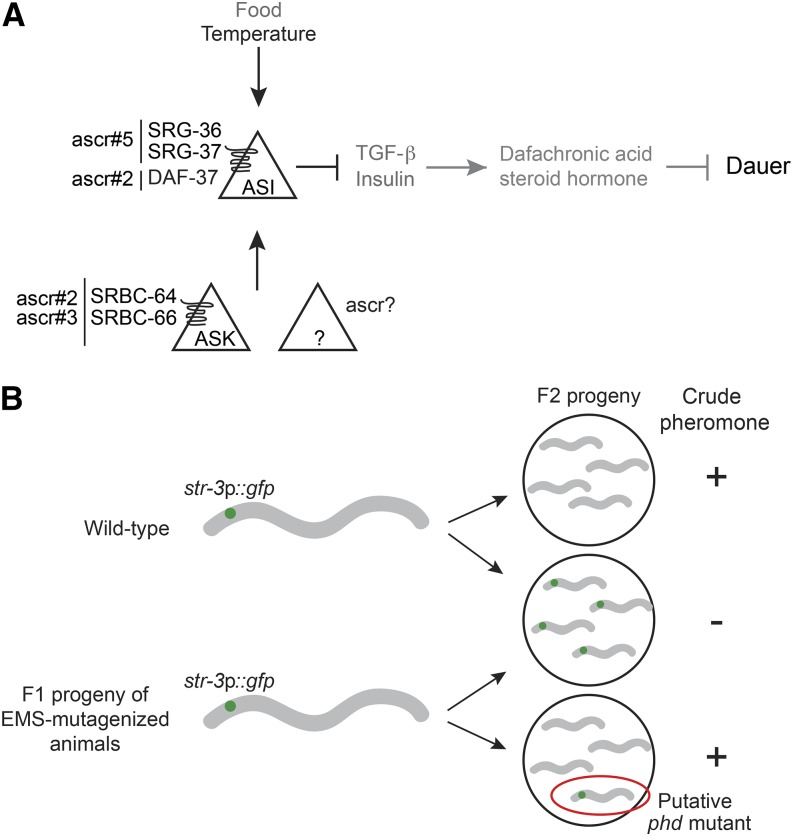
Caenorhabditis elegans provides an experimentally amenable system in which to dissect the genetic mechanisms that underlie entry into, and exit from, diapause. Shortly after hatching, C. elegans larvae assess environmental temperature, availability of food, and population density to choose between the alternate developmental trajectories of reproductive growth, or entry into the dauer diapause-like state (Cassada and Russell 1975; Swanson and Riddle 1981; Golden and Riddle 1982, 1984b). Genetic screens for mutants that enter constitutively into the dauer stage (dauer formation constitutive – Daf-c) or fail to form dauers (dauer formation defective – Daf-d) regardless of environmental conditions, have led to a detailed description of the neuroendocrine signaling pathways that underlie the dauer developmental decision (Albert et al. 1981; Riddle et al. 1981; Perkins et al. 1986; Albert and Riddle 1988; Vowels and Thomas 1992). In brief, environmental cues are integrated to modulate TGF-β and insulin signaling pathways, which act in parallel to regulate steroid hormone signaling (Thomas et al. 1993; Riddle and Albert 1997; Hu 2007; Fielenbach and Antebi 2008) (Figure 1A). Under adverse conditions, downregulation of expression of TGF-β and insulin-like peptide (ILP) genes in sensory neurons, such as ASI present in the head amphid organs, results in decreased steroid hormone signaling and dauer entry (Figure 1A). Conversely, under optimal conditions, increased TGF-β and ILP signaling promotes reproductive growth. Consequently, inappropriate modulation of TGF-β, ILP, or steroid hormone signaling results in Daf-d or Daf-c phenotypes.
Figure 1.
Identification of phd mutants. (A) Simplified model of the neuroendocrine signaling pathway regulating dauer formation in response to environmental cues. Ascarosides (ascr) are sensed by G protein-coupled receptors in ASI, in ASK, or possibly in other unidentified sensory neurons. High pheromone concentrations, low food availability, and high temperature cues are integrated to downregulate TGF-β (transforming growth factor-β) and insulin-like peptide gene expression primarily in the ASI sensory neurons. Downregulated steroid hormone signaling in turn promotes dauer formation. (B) Schematic of the forward genetic screen designed to identify putative phd mutants based on pheromone-mediated downregulation of str-3p::gfp expression in the ASI sensory neurons. See text for details. phd, pheromone response-defective dauer.
Although the neuroendocrine pathways underlying dauer formation are well described in C. elegans, the mechanisms by which environmental cues are sensed and integrated to regulate TGF-β and ILP signaling remain poorly characterized. Within the physiological temperature range, population density as assessed by pheromone concentrations is the instructive environmental trigger for dauer entry, whereas food availability and temperature play permissive roles (Golden and Riddle 1984a,b). C. elegans pheromones are now known to be comprised of an unexpectedly large number of small chemicals of diverse structures called ascarosides, derived from the sugar ascarylose (Jeong et al. 2005; Butcher et al. 2007; Edison 2009; Ludewig and Schroeder 2013). Pheromone signals detected directly by ASI and/or by other chemosensory neurons such as ASK regulate neuroendocrine gene expression in ASI as a function of environmental state (Figure 1A). The ascr#5 (C3, asc-ωC3, daumone 5) and ascr#2 (C6, asc-C6-MK, daumone 2) ascarosides have been shown to be detected by the SRG-36/SRG-37 and DAF-37 G protein-coupled receptors (GPCRs), respectively, acting in the ASI chemosensory neurons (McGrath et al. 2011; Park et al. 2012), whereas ascr#2 and ascr#3 (C9, asc-ΔC9, daumone 3) are also detected by the SRBC-64/SRBC-66 GPCRs in the ASK chemosensory neurons that regulate dauer formation (Kim et al. 2009) (Figure 1A). Sites of detection of other ascarosides in the dauer decision are largely uncharacterized. Moreover, additional signaling molecules required for the transduction of ascaroside signals within ASK or ASI, or for transmission of pheromone information from ASK or other pheromone-sensing neurons to ASI, are unidentified.
We performed an unbiased forward genetic screen to identify molecules required for both intra- and intercellular transduction of pheromone signals. A major goal of this screen was to identify mutants that may exhibit defects in dauer formation in response to subsets of ascarosides. Here, we report the isolation and characterization of pheromone response defective dauer (phd) mutants. We identified the causative lesions in a subset of these mutants via whole-genome sequencing and complementation. Our results describe previously uncharacterized roles for the WD40 domain-containing protein QUI-1, the HEAT repeat-containing protein CHE-12, the macoilin protein MACO-1, and tau tubulin kinases in pheromone-regulated dauer formation. Our analyses also define neuronal sites of action of these proteins in pheromone-induced dauer formation. We expect that loci identified in this screen will provide the foundation for additional studies focused on the complex mechanisms by which environmental cues are sensed, weighted, and integrated to direct a binary developmental decision.
Materials and Methods
C. elegans strains
C. elegans strains were maintained on nematode growth medium (NGM) agar plates at 20°, with Escherichia coli OP50 as a food source. The wild-type strain was N2 (Bristol). A list of all strains used in this work is provided in Supplemental Material, Table S1.
EMS mutagenesis screen
L4 larvae of the strain CX3596 (kyIs128 [str-3p::gfp; lin-15+]) were mutagenized with ethylmethanesulfonate (EMS) using standard methods (Brenner 1974; Kutscher and Shaham 2014). F1 progeny of mutagenized animals were allowed to lay eggs on plates containing 3 units of crude pheromone (1 unit being defined as the amount necessary to induce 33% dauers on heat-killed OP50 bacteria at 25°). Young adult F2 animals were examined for bright GFP expression in the ASI neurons using a fluorescence dissection microscope. Putative mutants were singled out, and their progeny were retested on plates with and without crude pheromone.
Dye-filling and dauer formation assays
Animals were filled with DiI as described previously (Perkins et al. 1986; Herman and Hedgecock 1990) and examined under a compound microscope. Five pairs of sensory neurons in the head consistently and robustly dye-fill in wild-type animals; the ASI neurons dye-fill more variably and were excluded from this analysis. Mutants exhibiting weak or no dye-filling in at least one of these five head neuron pairs in ≥50% of animals were considered partially dye-fill defective (pDyf). Animals in which all five head sensory neuron pairs exhibited weak or no dye-filling in 50–80% of animals were considered strongly dye-fill defective (sDyf). Mutants that failed to dye-fill all five pairs of head neurons in 80–100% of animals were considered fully dye-fill defective (Dyf).
Dauer formation assays using ascarosides were performed as previously described (Neal et al. 2013). Animals were maintained under standard culture conditions at either 20° or 25° for at least three generations prior to being tested for dauer formation at 25°. For dauer formation experiments in the presence of quinine, 3 µL of a 100 mM stock solution of quinine hydrochloride dihydrate (Sigma) was added to the molten agar during the preparation of assay plates.
Quantification of str-3p::gfp expression
Five well-fed and growth-synchronized adult animals were grown on NGM assay plates containing either ethanol or a pheromone mixture (1 µM each of ascr#2, ascr#3 and ascr#5 or 1 unit crude pheromone) for 4–5 hr until they laid 60–80 eggs. Adult animals were removed, and assay plates were placed at 25° for 60–80 hr until eggs developed into young adults. For GFP quantification, animals were anesthetized using 50 mM sodium azide on an agar pad and were visualized using a Zeiss Axio Imager. All strains were assayed in parallel in two independent experiments.
Genetic complementation and linkage mapping
To perform complementation testing among isolated alleles, phd mutant strains were first injected with a fluorescent marker (unc-122p::dsRed) to enable identification of cross-progeny. Males from strains carrying the marker as an extrachromosomal array were mated with hermaphrodites from an unmarked strain, and the F2 progeny of marked F1 hermaphrodites from the cross were examined for dauer formation and expression of str-3p::gfp on pheromone-containing plates. Complementation with wild-type was used to determine whether alleles were dominant or recessive.
To map alleles to linkage groups, we first examined expression of str-3p::gfp in the F2 progeny of phd mutants mated to strains containing recessive mutations on each linkage group leading to visible phenotypes (Fay 2013). We also crossed putative phd mutants to strains containing dominant markers on each linkage group, and examined dauer formation in their progeny. We were unable to map using single nucleotide polymorphisms between the N2 Bristol and the Hawaiian CB4856 C. elegans isolates (Jakubowski and Kornfeld 1999; Wicks et al. 2001; Davis et al. 2005), since CB4856 animals were Daf-d under all tested conditions.
Whole-genome resequencing
Mutants were not outcrossed prior to sequencing. Sequencing libraries from genomic DNA isolated from each phd mutant, and sequencing on the Illumina Genome Analyzer IIx platform, were prepared and performed as previously described (Sarin et al. 2008). Demultiplexing, alignment and preliminary sequence analysis were performed using the MAQGene analysis pipeline (Bigelow et al. 2009). Sequence data were reanalyzed using the CloudMap pipeline and default parameters in the published workflow (Minevich et al. 2012). Shared polymorphisms among strains were excluded from analysis (Sarin et al. 2010). Polymorphisms unique to each strain that were identified by at least five consensus sequence reads were further followed. Gene models were derived from information in WormBase (www.wormbase.org) and were plotted using WormWeb Tools generated by Nikhil Bhatla (www.wormweb.org).
Molecular biology
Genomic rescue fragments were amplified from wild-type genomic DNA. Upstream (5′) and downstream (3′) untranslated sequences included in the genomic fragments were the following (relative to the longest predicted isoform in genome release WS220): che-12: 837 bp 5′, 100 bp 3′; maco-1: 1886 bp 5′, 92 bp 3′; ttbk-3: 1109 bp 5′, 242 bp 3′. The fosmid clone encompassing the qui-1 locus WRM0616aH02 was obtained from Source Bioscience.
cDNA sequences were reverse transcribed from a library generated from the RNA of mixed stage wild-type animals. cDNAs were cloned into the pGEM-T Easy vector (Promega) and were confirmed by sequencing. The qui-1 cDNA was the generous gift of Paolo Bazzicalupo and Elia di Schiavi, the che-12 cDNA was the generous gift of Shai Shaham, and the maco-1 cDNA was the generous gift of Mario de Bono. The following promoters were used for cell-specific expression: ADL (sre-1Δp, 1.8 kb), ASH (sra-6p, 3.0 kb; also drives weaker expression in ASI), ASI (srg-47p, 1.0 kb), ASK (sra-9p, 2.9 kb), AWC+ASE (ceh-36p, 1.9 kb), and ASJ (trx-1p, 1.0 kb). Expression of the che-12 cDNA was driven by 0.9 kb of che-12 upstream regulatory sequences.
Transcriptional reporters of ttbk gene expression were generated by amplifying upstream regulatory sequences from wild-type genomic DNA (ttbk-3, 0.7 kb; ttbk-4, 0.4 kb; ttbk-5, 0.8 kb; ttbk-6, 3.2 kb; ttbk-7, 3.0 kb) and fusing them with a gfp expression cassette by either overlap extension PCR or by cloning into an expression vector. GFP-tagged ttbk-3 and ttbk-4 cDNA sequences were generated by overlap extension PCR to mutate the stop codons and fuse GFP in frame. The qui-1 cDNA was fused in frame with a four amino acid linker and an artificial intron-containing mCherry reporter gene. Constructs were confirmed by sequencing.
Microscopy
L1 larvae were dye-filled with DiI in M9 buffer and were imaged on a Zeiss Axio Imager.M2 microscope using a 63 × (NA 1.4) oil objective. Larvae were mounted on 2% agarose pads on a microscope slide and were immobilized in 10 mM levamisole (Sigma). Z-stacks (0.25–0.5 µm per slice) were acquired using a Hamamatsu Orca camera. Image analysis and cell identification was performed using Zen Pro (Zeiss) and FIJI (NIH) imaging software.
For cilia length measurements, 1-day-old adult worms were transferred to a 2% agarose pad on a microscope slide and immobilized using 10 mM levamisole. Animals were visualized and images were captured as above. Cilia were measured using the segmented line tool in FIJI imaging software. Expression of daf-7p::gfp(ksIs2) was examined using a 63 × oil objective. All measurements were performed blind to the genotype.
Statement on data and reagent availability
All reagents including strains and DNA constructs are freely available upon request. Whole-genome sequencing data have been deposited at the NCBI Sequence Read Archive (accession PRJNA314001; http://www.ncbi.nlm.nih.gov/bioproject/314001).
Results and Discussion
Rationale for screen design
Quantitative dauer formation assays under controlled conditions are laborious, and are not readily amenable to high throughput screening (Neal et al. 2013). We and others previously showed that ASI-specific expression of a GFP reporter, driven under the regulatory sequences of the str-3 GPCR gene, is strongly downregulated by pheromones (Peckol et al. 2001; Nolan et al. 2002; Kim et al. 2009) (Figure 1B), and that this downregulation is decreased in animals that are mutant for the ASK-expressed srbc-64/srbc-66 pheromone receptor genes (Kim et al. 2009). Therefore, we reasoned that screening for mutants unable to downregulate str-3p::gfp expression upon pheromone exposure may allow us to identify mutants defective in pheromone sensation, and/or signal transmission, in the context of dauer formation.
Following mutagenesis of str-3p::gfp-expressing parent animals by ethyl methanesulfonate (EMS), we screened F2 progeny representing approximately 38,500 haploid genomes for their ability to repress str-3p::gfp expression when grown on plates containing crude pheromone and plentiful food (see Materials and Methods) (Figure 1B). This screen allowed us to identify 129 phd mutants that continued to express str-3p::gfp upon pheromone exposure. Preliminary data from this screen have been reported previously (Kim et al. 2009).
Initial characterization of phd mutants
We first determined whether the isolated mutants were Daf-c or Daf-d. Four of the 129 mutants were Daf-c and were not examined further. Many Daf-d mutants exhibit ciliary or dendritic structural defects in the chemosensory neurons of the head amphid or tail phasmid organs (Thomas et al. 1993; Vowels and Thomas 1994); these defects are readily detected due to the inability of a subset of these sensory neurons, including ASK and ASI, to fill with lipophilic dyes such as DiI (Perkins et al. 1986; Herman and Hedgecock 1990; Starich et al. 1995). Thus, dye-filling serves as a convenient initial screen for animals with such morphological abnormalities. Of the mutants, 58% (75/129) exhibited either complete or nearly complete defects in dye-filling and were not considered further. Of the remaining mutants, we analyzed 26 strains that were sufficiently healthy and fertile to permit quantification of dauer formation.
We further analyzed the dye-filling, pheromone-mediated repression of str-3p::gfp expression in ASI, and dauer formation phenotypes of the selected mutants. We also included a mutant (oy141) that failed to dye-fill for comparison purposes in these assays. Nineteen of these strains exhibited wild-type dye-filling in young adult animals (Table 1), suggesting that the ASK and ASI pheromone-sensing neurons are likely to be generated and correctly specified in these mutants. Four and three strains exhibited partial and more severe defects in dye-filling, respectively (Table 1). As expected, the ability of a mixture of ascr#2, ascr#3 and ascr#5 (1 μm each) to repress str-3p::gfp expression in ASI was strongly affected in the srbc-64; srbc-66, and srg-36srg-37 pheromone receptor mutants, and was compromised in the majority of the examined mutant strains to varying extents (Table 1). The extent of the gene expression defect was not fully correlated with the dye-fill phenotypes of these mutants (Table 1). Thus, a subset of mutants with strong defects in pheromone-mediated str-3p::gfp repression exhibited wild-type dye-filling (oy109, oy134; Table 1) and conversely, mutants with dye-fill defects downregulated str-3p::gfp expression in the presence of pheromone (oy106, oy127; Table 1). All mutants, with the exception of oy143, also exhibited strong defects in dauer formation in response to two concentrations of ascr#2, regardless of their dye-fill or str-3p::gfp expression phenotype (Table 1). Although the different phenotypes of these phd mutants could arise from mutations in distinct loci in these nonoutcrossed strains, these results nevertheless indicate that pheromone-dependent repression of str-3p::gfp is a useful screening tool for the identification of mutants exhibiting defects in pheromone-regulated dauer formation.
Table 1. Initial phenotypic characterization of phd mutants.
| Straina | Visible Phenotypesb | Dye-Fillingc | str-3p::gfp Expression Indexd | Proportion of Dauers Formed on:e | ||
|---|---|---|---|---|---|---|
| ascr#2 (nM) | ||||||
| 0f | 60 | 600 | ||||
| WT | WT | n/a | 0 ± 0 | 0.4 ± 0.1 | 0.7 ± 0.1 | |
| str-3p::gfp | WT | 0.35 | 0 ± 0 | 0.5 ± 0.1 | 0.6 ± 0.1 | |
| srbc-64; srbc-66 | Adult egg production delayed | WT | 0.87 | 0 ± 0 | 0.1 ± 0.0 | 0.1 ± 0.1 |
| srg-36 srg-37 | n/d | 0.82 | n/d | n/d | n/d | |
| oy103 | Inviable eggs (25°, crude pheromone) | WT | 0.57 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.0 |
| oy104 | WT | 0.59 | 0 ± 0 | 0.1 ± 0.1 | 0.4 ± 0.1 | |
| oy105 | Social, eggs laid at edge of plate | WT | 0.53 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| oy106 | pDyf | 0.44 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.0 | |
| oy107 | egl, slow development | WT | 0.52 | 0 ± 0 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| oy108 | WT | 0.62 | 0 ± 0 | 0.1 ± 0.1 | 0.2 ± 0.1 | |
| oy109 | egl, slow development | WT | 0.93 | 0.1 ± 0.0 | 0 ± 0 | 0 ± 0 |
| oy113 | pDyf | 0.62 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| oy117 | WT | 0.65 | 0 ± 0 | 0 ± 0 | 0.2 ± 0.1 | |
| oy118 | WT | 0.56 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.0 | |
| oy119 | WT | 0.42 | 0 ± 0 | 0 ± 0.1 | 0.3 ± 0.1 | |
| oy120 | WT | 0.66 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| oy125 | unc | WT | 0.60 | 0 ± 0 | 0.1 ± 0.0 | 0.1 ± 0.1 |
| oy126 | WT | 0.46 | 0 ± 0 | 0.1 ± 0.0 | 0.1 ± 0.1 | |
| oy127 | egl, slow development | sDyf | 0.44 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.0 |
| oy129 | unc | pDyf | 0.68 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| oy131 | egl, slow development | pDyf | 0.55 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| oy134 | Asynchronous growth | WT | 0.93 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| oy135 | Adult egg production delayed | WT | 0.71 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.0 |
| oy137 | Slow development | sDyf | 0.74 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| oy138 | unc | WT | 0.47 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.1 |
| oy139 | lon, rol, thin body size | WT | 0.54 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| oy140 | WT | 0.56 | 0 ± 0 | 0 ± 0 | 0.1 ± 0.0 | |
| oy141 | Dyf | 0.95 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| oy142 | sDyf | 0.85 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| oy143 | WT | 0.57 | 0 ± 0 | 0.3 ± 0.1 | 0.6 ± 0.1 | |
| oy144 | Inviable eggs (25°, crude pheromone) | WT | n/d | 0 ± 0 | 0 ± 0 | 0.1 ± 0.0 |
ascr, ascaroside; WT, wild-type; n/a, not applicable; n/d, not done; egl, delayed egg-laying; pDyf, partially dye-fill defective; unc, uncoordinated; sDyf, strongly dye-fill defective; lon, long; rol rollers; Dyf, fully dye-fill defective.
All strains, except WT, contain stably integrated copies of the str-3p::gfp fusion gene (kyIs128).
Phenotypes are reported only if they are observed in > 50% of animals. Social indicates that animals aggregate.
Animals were filled with DiI and amphid neurons were visualized. Criteria for classification as WT, pDyf, sDyf, and Dyf are described in Materials and Methods.
str-3p::gfp expression was observed in animals grown in the presence or absence of 1 µM each ascr#2, ascr#3, and ascr#5 at 25°, and a subjective expression value with arbitrary values of 0–10 was assigned to each animal. The index presented is the ratio of expression in animals grown on pheromone, divided by the expression in control animals. n = 30 animals/condition/trial; at least two independent trials.
Numbers shown are the proportion of dauers formed in the given condition from two (0 nM) or three (60 nM, 600 nM) independent experiments at 25° with 40–110 animals per assay. Two technical replicates were performed in each experiment. Errors are SEM (standard errors of the mean).
Plates contained 6 µL of ethanol which was used as the diluent for ascr#2.
phd mutants exhibit defects in dauer formation in response to specific ascarosides
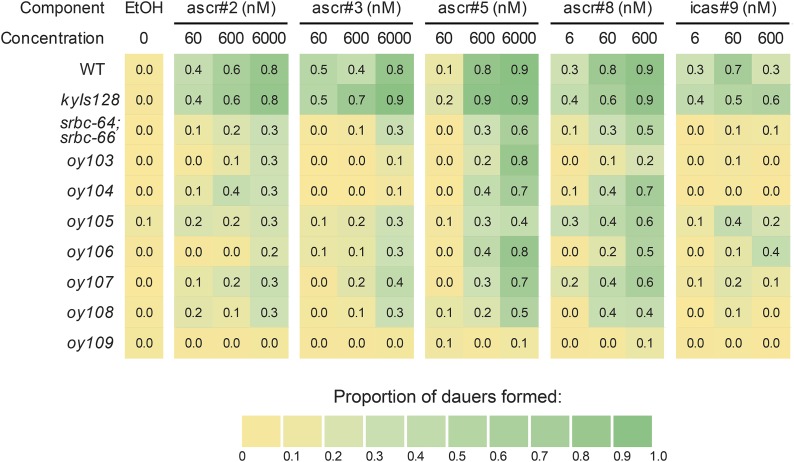
Given the strong defects in ascr#2-induced dauer formation in the majority of examined phd mutants, we next asked whether these mutants exhibit similarly strong defects in dauer formation in response to additional ascarosides. We selected seven phd mutants (oy103–oy109), which exhibited a range of defects in pheromone-mediated repression of str-3p::gfp expression, and examined dauer formation in response to multiple concentrations of ascr#3, ascr#5, ascr#8 (asc-ΔC7-PABA), and icas#9 (C5, IC-asc-C5), in addition to ascr#2 (Figure 2 and Table S2).
Figure 2.
Dauer formation defects exhibited by phd mutants in response to different ascarosides. Shown are the proportions of dauers formed by strains of the indicated genotypes in response to different concentrations of ascr#2, ascr#3, ascr#5, ascr#8, and icas#9 at 25°. Numbers shown are the average from three biologically independent assays of two technical replicates each. Averages with SEM (standard error of the mean) are shown in Table S2. All strains with the exception of wild-type (WT) and srbc-64; srbc-66 contain stably integrated copies of the str-3p::gfp transgene (kyIs128). The srbc-64(tm1946) and srbc-66(tm2943) alleles were used. ascr, ascarisode; EtOH, ethanol; phd, pheromone response-defective dauer.
oy103:
oy103 mutants exhibited strong defects in dauer formation in response to all concentrations of ascr#2, ascr#3, ascr#8, and icas#9. However, oy103 mutants retained the ability to form dauers in response to high concentrations of ascr#5 (Figure 2 and Table S2).
oy104:
oy104 mutants were strongly defective in dauer formation in response to all examined concentrations of ascr#3 and icas#9, and less defective to ascr#2. Dauer formation in response to ascr#5 and ascr#8 was only weakly affected (Figure 2 and Table S2). Since responses to ascr#2, ascr#3, and icas#9 require ASK (Kim et al. 2009) (S. J. Neal and P. Sengupta, unpublished results), oy104 may be a mutation in a molecule required for ascaroside-specific signal transduction within ASK, between ASK and ASI, or within ASI.
oy105:
oy105 mutants exhibited an overall decrease in dauer formation in response to all concentrations of examined ascarosides (Figure 2 and Table S2), suggesting a generalized defect, or dampening, of pheromone-induced dauer formation in this mutant background.
oy106:
oy106 mutants exhibited defects in dauer formation in response to all examined concentrations of ascr#2 and ascr#3, and strong defects in response to lower concentrations of ascr#8 and icas#9 (Figure 2 and Table S2). Weak defects were also observed in response to low ascr#5 concentrations (Figure 2 and Table S2).
oy107:
oy107 animals exhibited strong defects in dauer formation in response to all concentrations of icas#9, and weaker defects in response to all other examined ascarosides, including low concentrations of ascr#5 (Figure 2 and Table S2).
oy108:
Dauer formation defects in oy108 mutants resembled those of oy105, with the exception that these mutants failed to form dauers in response to any concentration of icas#9 (Figure 2 and Table S2).
oy109:
Unlike the other examined phd mutants, oy109 mutants exhibited strong defects in dauer formation in response to all concentrations of all examined ascarosides (Figure 2 and Table S2). This mutant also exhibited strong defects in pheromone-mediated repression of str-3p::gfp expression, similar to oy141 and oy142 mutants (Table 1). However, in contrast to the strong dye-fill defects exhibited by oy141 and oy142 mutants, dye-filling was unaffected in oy109 animals (Table 1). Thus, oy109 may represent a mutation in a gene required for the specific transduction of ascaroside signals.
In summary, we identified mutants that exhibit dauer formation defects in response to specific ascarosides, particularly at low concentrations (oy104, oy106, and oy107), a mutant (oy103) which is defective in dauer formation in response to all ascarosides with the exception of high concentrations of ascr#5, a mutant (oy105) that exhibits generally decreased dauer formation regardless of ascaroside identity, and a mutant (oy109) that fails to form dauers at any concentrations of all examined ascarosides.
Genetic and molecular analyses of phd genes
We attempted to map each allele to candidate linkage groups based on both their pheromone-induced dauer and str-3p::gfp expression phenotypes (see Materials and Methods). Genetic mapping and complementation analyses suggested that the oy103–oy109 alleles described above represent recessive mutations in complementing genes (data not shown). Alleles were tentatively mapped to the following linkage groups: oy103 II, oy104 I, oy105 IV, oy106 V, oy107 IV, oy108 I, and oy109 II. To identify the causal mutations, we sequenced the genomes of these phd mutants and identified unique variants in each strain (Table S3). We focused on nonsense mutations in genes located on candidate linkage groups associated with each allele, and performed rescue experiments with wild-type sequences of each candidate gene. Although we were unable to rescue the mutant phenotypes of oy103, oy104 and oy109, as described below, we identified genes affected by the oy105, oy106, oy107, and oy108 mutations.
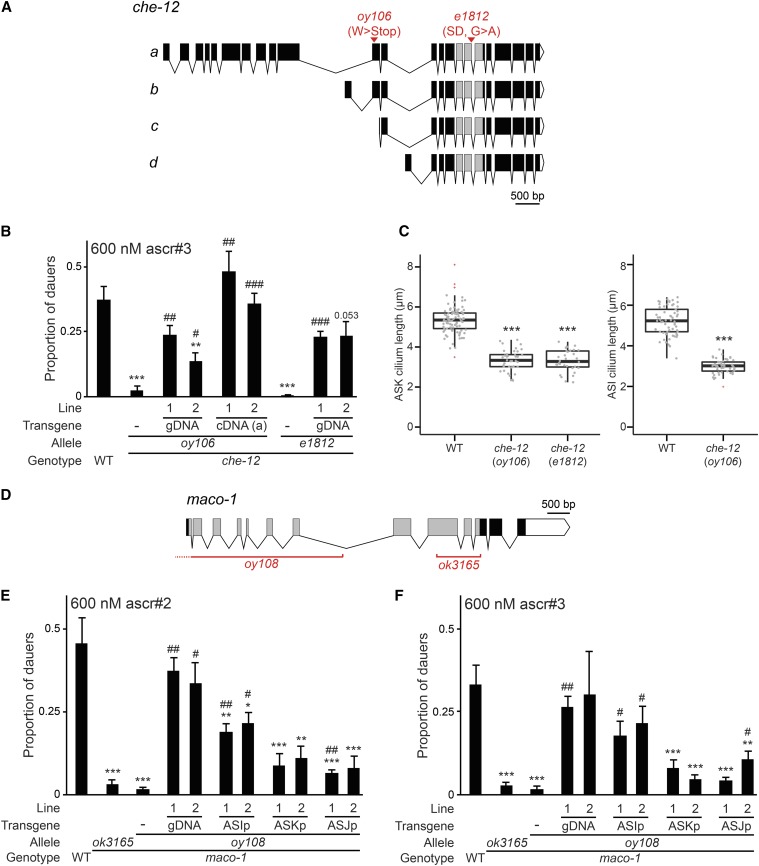
oy106 is a mutation in the che-12 ciliary gene
The oy106 strain contains a premature STOP codon in the che-12 gene (Figure 3A) (Perkins et al. 1986; Starich et al. 1995; Bacaj et al. 2008) that is predicted to affect the two longer che-12 isoforms (Figure 3A). The independently isolated che-12(e1812) mutant also exhibited strong defects in ascaroside-induced dauer formation (Figure 3B). ascr#3-induced dauer formation defects in oy106 and che-12(e1812) mutants were significantly rescued by wild-type genomic che-12 sequences, as well as a che-12 cDNA expressed under che-12 upstream regulatory sequences (Figure 3B). We conclude that oy106 is an allele of che-12.
Figure 3.
The CHE-12 HEAT domain and MACO-1 macoilin proteins regulate pheromone-induced dauer formation. (A) Predicted exon/intron structures of che-12 isoforms (a–d). Gray boxes indicate exons predicted to encode HEAT repeats, white triangles indicate 3′ UTRs. The locations of mutations in the oy106 and e1812 alleles are indicated. (B) Dauers formed by animals of the indicated genotypes in response to 600 nM ascr#3 at 25°. Lines refer to independent transgenic strains carrying the indicated transgenes on extrachromosomal arrays. The che-12, a cDNA isoform was expressed under 0.9 kb che-12 upstream regulatory sequences (Bacaj et al. 2008). Each data point is the average of at least three biologically independent assays of 40–110 animals each. Errors are SEM. ** and *** indicate different from wild-type at P < 0.01 and 0.001, respectively; #, ##, and ### indicate different from che-12(oy106) or che-12(e1812) at P < 0.05, 0.01, and 0.001, respectively (ANOVA and Games-Howell posthoc test). (C) Lengths of ASK (left) and ASI (right) cilia in animals of the indicated genotypes. Cilia were visualized via expression of srbc-66p::mCherry (ASK) and str-3p::gfp (ASI). Top and bottom bounds of boxes indicate 25th and 75th percentiles, respectively. Medians are indicated by thick horizontal bars. *** indicates different at P < 0.001 from wild-type (t-test). Outliers are indicated by red + symbols. (D) Predicted exon/intron structure of the maco-1 genomic locus. Gray boxes indicate exons encoding the macoilin domain, white triangle indicates 3′ UTR. The extent of the deletions in maco-1 alleles is indicated. (E and F) Dauers formed by animals of the indicated genotypes in response to 600 nM ascr#2 (E) or 600 nM ascr#3 (F) at 25°. Lines refer to independent transgenic strains carrying the indicated transgenes on extrachromosomal arrays. The maco-1 cDNA was expressed under srg-47 (ASI), sra-9 (ASK), or trx-1 (ASJ) promoters. Each data point is the average of at least three biologically independent assays of 40–110 animals each. Errors are SEM.*, **, and *** indicate different from wild-type at P < 0.05, 0.01, and 0.001, respectively; #, ##, and ### indicate different from maco-1(oy108) at P < 0.05, 0.01, and 0.001, respectively (ANOVA and Games-Howell posthoc test). ascr, ascaroside; cDNA, complementary DNA; gDNA, genomic DNA; SD, splice donor; SEM, standard error of the mean; UTR, untranslated region; WT, wild-type.
che-12 encodes a HEAT repeat-containing protein belonging to the tubulin-binding Crescerin1 family (Bacaj et al. 2008; Das et al. 2015). che-12 is expressed in a subset of amphid sensory neurons, including ASK and ASI, that exhibit simple, rod-like ciliated sensory endings (Perkins et al. 1986; Bacaj et al. 2008; Doroquez et al. 2014; Das et al. 2015). CHE-12 is localized to cilia, and ciliary ultrastructure is disrupted in che-12 mutants (Bacaj et al. 2008; Das et al. 2015). Cilia house sensory signal transduction molecules and are formed by the process of intraflagellar transport (IFT), which is required for the movement of proteins within cilia (Rosenbaum and Witman 2002; Pedersen and Rosenbaum 2008). Previous studies have indicated that CHE-12 is unlikely to be a component of the IFT complex but requires IFT for ciliary localization (Perkins et al. 1986; Bacaj et al. 2008). Based on these observations and the roles of other CHE-12 family members, CHE-12 has recently been proposed to be a microtubule-binding and polymerizing protein that is required for correct ciliogenesis and cilia structure maintenance (Bacaj et al. 2008; Das et al. 2015).
We confirmed that ASK cilia length is significantly shorter in both che-12 alleles (Figure 3C). In addition, ASI cilia length was also similarly decreased in che-12(oy106) mutants (Figure 3C). We conclude that the described role of CHE-12 in regulating the formation and maintenance of cilia in neurons such as ASK and ASI is consistent with the observed pheromone-induced dauer formation defects upon loss of che-12 function.
oy108 is a mutation in the maco-1 Macoilin gene
We noted a large deletion in the oy108 carrying strain that affected two genes including the maco-1 macoilin locus (Figure 3D). The expression of wildtype genomic maco-1 sequences was sufficient to rescue dauer formation in oy108 in response to both ascr#2 and ascr#3 (Figure 3, E and F). Moreover, animals carrying the independently isolated maco-1(ok3165) putative null allele also exhibited dauer formation defects similar to those exhibited by oy108 animals (Figure 3, E and F). Thus, oy108 affects maco-1 function.
MACO-1 is expressed broadly in the C. elegans nervous system, and is localized to the rough endoplasmic reticulum (Arellano-Carbajal et al. 2011; Miyara et al. 2011). maco-1 mutants exhibit pleiotropic neuronal defects, and this protein has been suggested to play a role in the trafficking of transmembrane proteins that regulate neuronal excitability (Arellano-Carbajal et al. 2011; Miyara et al. 2011). We examined whether MACO-1 acts in specific sensory neuron types to regulate dauer formation. We found that expression of wild-type maco-1 sequences in ASI rescued both ascr#2- and ascr#3-regulated dauer formation defects of maco-1(oy108) mutants more strongly than expression in either the ASK or ASJ sensory neurons (Figure 3, E and F). Expression of the daf-7 TGF-β ligand in ASI (Ren et al. 1996; Schackwitz et al. 1996) was unaffected upon loss of maco-1 function [Figure S1; 100% of wild-type and maco-1(ok3165) animals expressed daf-7p::gfp in ASI; n = 20 each]. Since maco-1 mutants retained the ability to form dauers in response to ascr#5 (Figure 2 and Table S1), we propose that MACO-1 may play a role in the trafficking of as yet unidentified proteins required for transducing ascaroside-specific signals in ASI to regulate dauer formation.
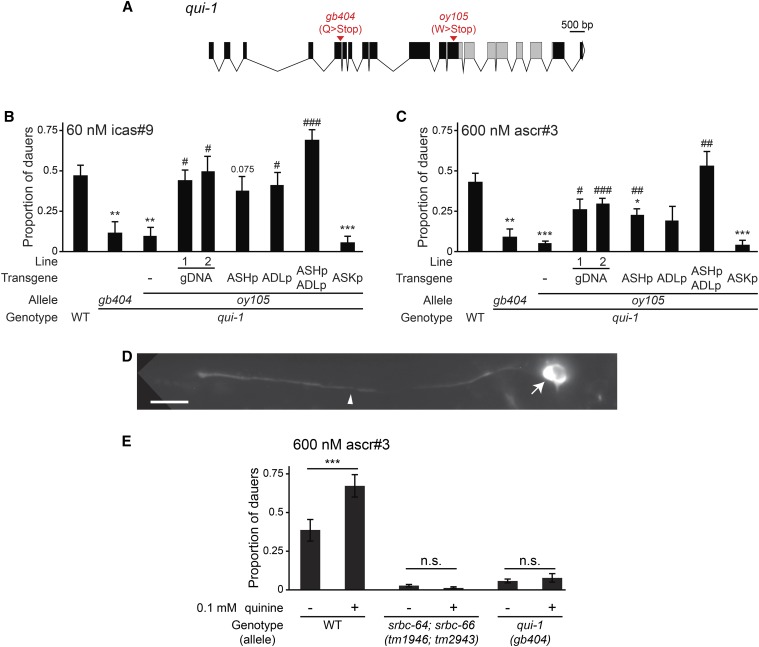
The QUI-1 WD40 repeat-containing protein acts in nociceptive neurons to facilitate pheromone-induced dauer formation in the presence of noxious chemicals
The oy105 strain contains a premature termination codon in the predicted coding region of the qui-1 gene (Figure 4A) (Hilliard et al. 2004). We obtained significant rescue of both icas#9- and ascr#3-induced dauer formation defects of oy105 mutants with a fosmid containing qui-1 genomic sequences (Figure 4, B and C). The independently isolated qui-1(gb404) mutant (Hilliard et al. 2004) (Figure 4A) also exhibited dauer formation defects that were qualitatively similar to those of oy105 mutants (Figure 4, B and C). Together with additional observations described below, these results indicate that oy105 is an allele of qui-1.
Figure 4.
QUI-1 acts in the ASH and ADL nociceptive neurons to increase pheromone-induced dauer formation by noxious chemicals. (A) Predicted exon/intron structure of the qui-1 genomic locus. Gray boxes indicate exons predicted to encode WD40 repeats, white triangle indicates 3′ UTR. The location and nature of the mutations in qui-1 alleles are indicated. (B and C) Dauers formed by animals of the indicated genotypes in response to 60 nM icas#9 (B) or 600 nM ascr#3 (C) at 25°. Lines refer to independent transgenic strains carrying the indicated transgenes on extrachromosomal arrays. The qui-1 cDNA was expressed under the sra-9 (ASK), sra-6 (ASH, also drives expression weakly and variably in ASI), sre-1Δp (ADL), or both (ASH/ASI/ADL) promoters. Each data point is the average of at least three biologically independent assays of 40–110 animals each. Errors are SEM.*, **, and *** indicate different from wild-type at P < 0.05, 0.01, and 0.001, respectively; #, ##, and ### indicate different from qui-1(oy105) at P < 0.05, 0.01, and 0.001, respectively (ANOVA and Games-Howell posthoc test). (D) Representative image of the localization of QUI-1::mCherry in ASH in an adult hermaphrodite. Arrow and arrowhead indicate cell soma and dendrite, respectively. The bright fluorophore signal in the soma likely represents aggregation due to overexpression. Note exclusion from the nucleus. The fusion protein was also detected weakly in the cilia. Anterior is at left. Scale bar: 10 μm. (E) Dauers formed by animals of the indicated genotypes in response to 600 nM ascr#3 and 0.1 mM quinine at 25°. Each data point is the average of three biologically independent assays of 40–110 animals each. Errors are SEM. For the indicated pairwise comparisons, *** indicates P < 0.001 and n.s. indicates not statistically significant (two-tailed t-test). ascr, ascaroside; cDNA, complementary DNA; gDNA, genomic DNA; SEM, standard error of the mean; UTR, untranslated region; WT, wild-type.
qui-1 alleles were originally identified in a genetic screen for mutants defective in their ability to avoid a subset of aqueous repellents including quinine, as well as low pH (Hilliard et al. 2004). qui-1 encodes a large protein of undefined function, containing multiple WD40 repeats implicated in protein-protein interactions (Figure 4A) (Hilliard et al. 2004; Xu and Min 2011; Zhang and Zhang 2015). qui-1 was shown to be expressed in multiple sensory and nonsensory neurons including in the ASH and ADL nociceptive chemosensory neurons (Hilliard et al. 2004); the ASH neurons have previously been shown to respond to bitter compounds such as quinine (Hilliard et al. 2005). A GFP-tagged QUI-1 protein localized to the cytoplasm and was excluded from the nuclei of many, but not all, expressing cells (Hilliard et al. 2004). A role for QUI-1 in dauer formation has not previously been described.
To first determine where QUI-1 acts to regulate dauer formation, we performed cell-specific rescue experiments. Intriguingly, we found that expression of wild-type qui-1 sequences in either the ASH or ADL nociceptive neurons partially rescued the dauer formation defects of qui-1(oy105) mutants, whereas expression in both neuron types fully rescued dauer formation in this mutant background (Figure 4B). qui-1 was reported to not be expressed in ASK (Hilliard et al. 2004), and consistent with this observation, expression of qui-1 in ASK failed to rescue (Figure 4B). We reexamined the subcellular localization of QUI-1 in ASH by expressing a mCherry-tagged QUI-1 fusion protein under a cell-specific promoter. QUI-1::mCherry was localized to the ASH cytosol but excluded from nuclei (Figure 4D).
The general decrease in dauer formation in qui-1 mutants in response to multiple ascarosides implies that QUI-1 may regulate a shared aspect of dauer formation such as modulation of neuroendocrine signaling, possibly via regulation of general pheromone responsiveness. Neither ASH nor ADL have previously been implicated in dauer formation, although ADL mediates aversion to ascr#3 in adult hermaphrodites (Jang et al. 2012). Since ascr#3-induced dauer formation is largely mediated by ASK, we considered the hypothesis that QUI-1 acts in ASH and ADL to modulate ASK-mediated pheromone signaling. ASH and ADL have been previously suggested to respond to aversive food stimuli to promote adult aggregation behavior, which is in part mediated by pheromone signaling (White et al. 1986; De Bono et al. 2002; Macosko et al. 2009; Jang et al. 2012). Along with ASK, ASH and ADL are present in a hub-and-spoke network motif in which spoke sensory neurons are connected to the RMG hub motor/interneuron via gap junctions (Macosko et al. 2009; Jang et al. 2012). Moreover, ADL, ASH, and ASK are connected via chemical synapses in a feedforward circuit with ADL being the most upstream, and ASK being the most downstream, neurons in the circuit (White et al. 1986). Thus, it is plausible that ASH/ADL activity modulates ASK pheromone signaling.
Since both ASH and ADL respond to noxious stimuli (Kaplan and Horvitz 1993; Hart et al. 1995; Maricq et al. 1995; Troemel et al. 1997; Sambongi et al. 1999; Hilliard et al. 2002, 2004, 2005), we asked whether activation of one or both of these neurons by noxious chemicals promotes dauer formation, and whether this facilitation is decreased in qui-1 mutants. To address this issue, we tested whether quinine sensed by ASH (Hilliard et al. 2005) enhances pheromone-induced dauer formation. We found that the addition of 0.1 mM quinine enhanced dauer formation in response to ascr#3 in wild-type animals (Figure 4E). Importantly, this enhancement was abolished not only in qui-1(gb404) mutants, but also in animals mutant for the srbc-64 and srbc-66 ascr#3 receptor genes that are expressed specifically in ASK (Figure 4E). A simple interpretation of these results is that QUI-1-dependent activation of ASH or ADL by noxious chemicals, possibly from food, facilitates pheromone signaling from ASK to result in increased dauer formation. This facilitation could occur at the level of pheromone responsiveness or pheromone signal output from ASK. ASH and ASI have recently been shown to reciprocally inhibit each other in the context of adult nociceptive behavior (Guo et al. 2015). Thus, QUI-1 activity in ASH/ADL may also modulate pheromone responsiveness in ASI to regulate dauer formation. While the precise cellular functions of QUI-1 in sensory signaling remain to be determined, analysis of the cellular loci of function of this protein reveals a role for network activity in modulating pheromone responses in dauer formation.
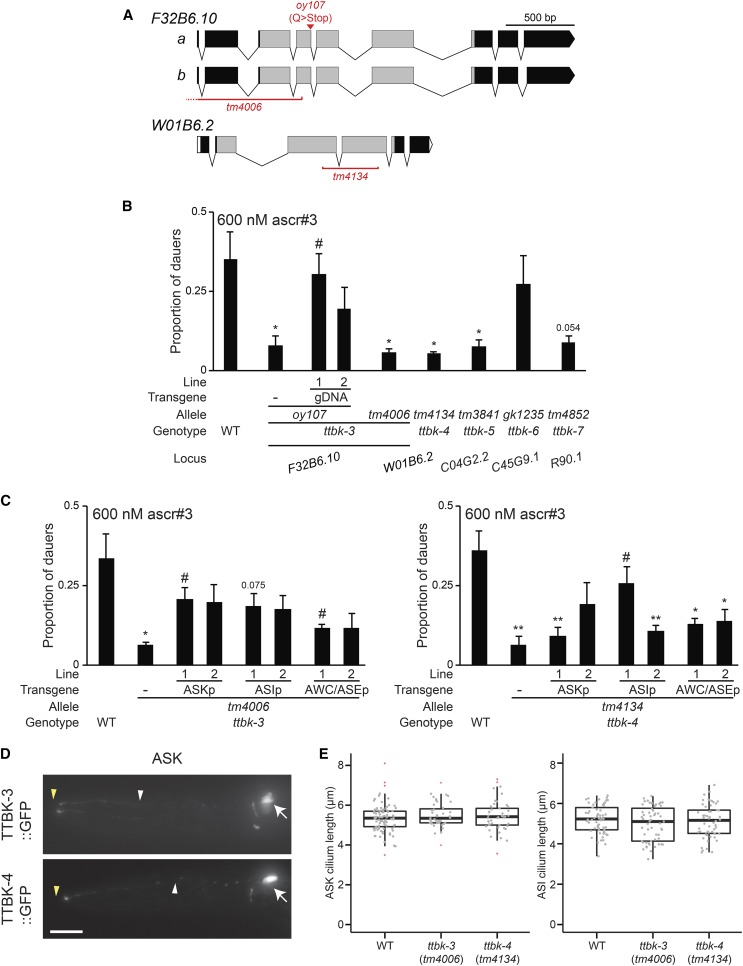
Tau tubulin kinases regulate pheromone-induced dauer formation
The oy107 mutation is predicted to result in a truncated protein encoded by the F32B6.10 gene (Figure 5A). The ascr#3-induced dauer formation defects of oy107 mutants were partly rescued upon expression of wild-type F32B6.10 genomic sequences (Figure 5B). In addition, animals carrying the F32B6.10(tm4006) deletion allele (Figure 5A) exhibited dauer formation defects similar to those exhibited by oy107 mutants (Figure 5B), indicating that oy107 is an allele of F32B6.10.
Figure 5.
Multiple members of the TTBK (tau tubulin kinase-like) protein family regulate pheromone-induced dauer formation. (A) Predicted exon/intron structures of F32B6.10 (ttbk-3) and W01B6.2 (ttbk-4). Gray boxes indicate exons predicted to encode the kinase domains. The location and nature of the mutations in F32B6.10 and W01B6.2 alleles are indicated. (B and C) Dauers formed by animals of the indicated genotypes in response to 600 nM ascr#3 at 25°. Lines refer to independent transgenic strains carrying the indicated transgenes on extrachromosomal arrays. ttbk cDNAs were expressed in ASK under the sra-9 promoter, in ASI under the srg-47 promoter, and in AWC/ASE under the ceh-36 promoter. Each data point is the average of at least three biologically independent assays of 40–110 animals each. Errors are SEM.* and ** indicate different from wild-type at P < 0.05 and 0.01, respectively; # indicates different from the relevant ttbk mutant at P < 0.05, 0.01, and 0.001, respectively (ANOVA and Games-Howell posthoc test). (D) Representative images of the localization patterns of TTBK-3 and TTBK-4 fusion proteins in ASK. Arrows, white and yellow arrowheads indicate cell soma, dendrites and cilia, respectively. Anterior is at left. Scale: 10 μm. (E) Lengths of ASK (left) and ASI (right) cilia in animals of the indicated genotypes. Wild-type data are repeated from Figure 3C. Cilia were visualized via expression of srbc-66p::mCherry (ASK) and str-3p::gfp (ASI). Top and bottom bounds of boxes indicate 25th and 75th percentiles, respectively. Medians are indicated by thick horizontal bars. Outliers are indicated by red + symbols. ascr, ascaroside; gDNA, genomic DNA; GFP, green fluorescent protein; SEM, standard errors of the mean; WT, wild-type.
F32B6.10 encodes a tau tubulin kinase (TTBK)-like enzyme belonging to the casein kinase I (CK1) superfamily of kinases (Manning 2005; Ikezu and Ikezu 2014). TTBK proteins were originally identified by their ability to phosphorylate the microtubule-associated protein tau, as well as tubulin (Takahashi et al. 1995; Tomizawa et al. 2001; Sato et al. 2006). The presence of hyper-phosphorylated tau in brain neurofibrillary tangles is a characteristic of tauopathies, including Alzheimer’s disease (Grundke-Iqbal et al. 1986; Wolozin et al. 1986; Iqbal et al. 1989), and genetic variants in TTBK1 have been associated with the late-onset form of this disease (Vazquez-Higuera et al. 2011; Yu et al. 2011). In contrast to TTBK1, whose expression is restricted to the central nervous system (Sato et al. 2006; Lund et al. 2013), TTBK2 is expressed more broadly in multiple tissues in mammals (Houlden et al. 2007). TTBK2 has recently been shown to play a role in ciliogenesis and cellular processes such as regulation of transporter activity (Alesutan et al. 2012; Almilaji et al. 2013; Liao et al. 2015). Variants in TTBK2 have been linked to spinocerebellar ataxia (Houlden et al. 2007), although the causal relationship between TTBK2 function and this neurodegenerative disease is unclear. Interestingly, the TTBK family appears to be greatly expanded in C. elegans, with the C. elegans genome predicted to encode 32 TTBK-like proteins (Manning 2005). A subset of these kinases belonging to the larger superfamily has been analyzed in C. elegans models of neurodegenerative diseases (Kraemer et al. 2006; Liachko et al. 2014), but the endogenous functions of these proteins are largely unknown.
We noted that genes encoding two additional TTBK-like enzymes, W01B6.2 and C04G2.2, are located within 200 kb of F32B6.10 on linkage group IV (www.wormbase.org). Given the sequence homology, we asked whether mutations in one or both of these linked ttbk genes also affect dauer formation. As shown in Figure 5B, loss of the linked W01B6.2 and C04G2.2, but not of the unlinked C45G9.1 TTBK-like genes, resulted in dauer formation defects similar to those exhibited by F32B6.10 mutants. Mutations in the unlinked R90.1 ttbk gene also resulted in pheromone-induced dauer formation defects (Figure 5B). These observations suggest that a subset of these kinases contributes to pheromone-induced dauer formation. We henceforth refer to F32B6.10 as ttbk-3, W01B6.2 as ttbk-4, C04G2.2 as ttbk-5, C45G9.1 as ttbk-6, and R90.1 as ttbk-7.
We characterized the expression patterns of ttbk-3-7 by examining the expression of gfp driven by their upstream regulatory sequences. Expression of transcriptional ttbkp::gfp fusion genes was weak and variable, but was observed primarily in neurons, including subsets of sensory neurons in the amphid sense organs of the head (Figure S2). In particular, ttbk-3, ttbk-4, and ttbk-5 transcriptional fusion genes were expressed in either or both of the pheromone-sensing ASK and ASI sensory neurons (Figure S2). Additional expressing neurons could not be reliably identified due to variable and weak expression of these reporter genes. We next performed cell-specific rescue experiments to identify the site(s) of action of ttbk-3 and ttbk-4 in the regulation of dauer formation. Expression of ttbk-3 and ttbk-4 in either ASI or ASK rescued ascr#3-induced dauer formation defects of ttbk-3(tm4006) and ttbk-4(tm4134) mutants more strongly than expression in the AWC/ASE sensory neurons (Figure 5, C and D). Functional GFP-tagged TTBK-3 and TTBK-4 fusion proteins were localized throughout the cell in ASK, but appeared to be excluded from sensory cilia (Figure 5E). These results indicate that TTBK-3 and TTBK-4 function in ASI and ASK can regulate dauer formation.
Since TTBK proteins have been implicated in the regulation of neuronal morphology and ciliogenesis, we asked whether neuronal morphology, including ciliary morphology, is altered in these mutants. The pattern and extent of dye uptake by a subset of sensory neurons, including the ASK and ASI neurons, was unaffected in ttbk-3(oy107) mutants (Table 1), indicating that these neurons are generated and specified, and that their ciliary sensory endings are grossly unaffected in this mutant background. Dye-filling was also unaffected in ttbk-4(tm4134) mutants (100% adults filled with dye; n = 40). We also directly visualized ASI and ASK neuronal morphology via the expression of soluble gfp driven under cell-specific reporters. The positions of ASI and ASK cell soma and cellular and ciliary morphologies did not appear to be grossly altered in examined neurons in ttbk-3 or ttbk-4 mutants (Figure 5E and Figure S3). We conclude that TTBK-3 and TTBK-4 do not regulate sensory neuron cellular architecture to regulate pheromone-induced dauer formation.
Conclusions
In summary, we have shown that we successfully identified mutations in genes required for dauer formation using pheromone-mediated str-3p::gfp repression as a screening tool. This screening strategy can now be readily scaled up and saturated via the use of automated sorting devices (Doitsidou et al. 2008; Crane et al. 2012; Entchev et al. 2015). Our pilot screen allowed us to identify genes such as che-12, mutations in which affect sensory neuron development and/or morphology. We also described new roles for previously identified genes such as maco-1 and qui-1 in the regulation of dauer formation. Moreover, analysis of the role of qui-1 in dauer formation allowed us to demonstrate that pheromone-induced dauer formation is modulated as a function of environmental noxious chemicals, and that this modulation is mediated by QUI-1-dependent activity of the ASH and ADL nociceptive neurons. We also identified a subfamily of TTBK-like enzymes that plays a role in dauer formation, suggesting that further analyses of TTBK protein function in this process may provide new insights into the roles of these conserved molecules in the regulation of sensory neuron function. Continued investigation of mutants and genes isolated in this and related screens in the future will allow us to better understand how animals sense and integrate environmental information to drive critical binary developmental decisions.
Supplementary Material
Acknowledgments
We are grateful to Alex Boyanov, Gregory Minevich, and Oliver Hobert for performing whole-genome resequencing and for assistance with sequence analyses, Munzareen Khan for assistance with experiments, Elia di Schiavi for sharing unpublished reagents and information on qui-1, and the Caenorhabditis Genetics Center and the National BioResource Project (Japan) for strains. We thank Michael O’Donnell for critical comments on the manuscript. This work was funded in part by the National Institutes of Health (R37 GM56223, P.S.), the National Science Foundation (IOS 1256488, P.S.), the Human Frontiers Science Program (RGY0042/2010, P.S. and R.A.B.), the Ellison Medical Foundation (AG-NS-0963-12, R.A.B.), in addition to the Daegu Gyeongbuk Institute of Science and Technology Research and Development Program of the Ministry of Science, an Information and Communication Technology (ICT) and Future Planning grant (15-BD-06), and the National Research Foundation of Korea (NRF-2015R1D1A1A09061430) (all awarded to K.K.).
Footnotes
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.115.026450/-/DC1
Communicating editor: S. Lee
Literature Cited
- Albert P. S., Riddle D. L., 1988. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev. Biol. 126: 270–293. [DOI] [PubMed] [Google Scholar]
- Albert P. S., Brown S. J., Riddle D. L., 1981. Sensory control of dauer larva formation in Caenorhabditis elegans. J. Comp. Neurol. 198: 435–451. [DOI] [PubMed] [Google Scholar]
- Alesutan I., Sopjani M., Dermaku-Sopjani M., Munoz C., Voelkl J., et al. , 2012. Upregulation of Na-coupled glucose transporter SGLT1 by Tau tubulin kinase 2. Cell. Physiol. Biochem. 30: 458–465. [DOI] [PubMed] [Google Scholar]
- Almilaji A., Munoz C., Hosseinzadeh Z., Lang F., 2013. Upregulation of Na+,Cl(-)-coupled betaine/gamma-amino-butyric acid transporter BGT1 by Tau tubulin kinase 2. Cell. Physiol. Biochem. 32: 334–343. [DOI] [PubMed] [Google Scholar]
- Arellano-Carbajal F., Briseno-Roa L., Couto A., Cheung B. H., Labouesse M., et al. , 2011. Macoilin, a conserved nervous system-specific ER membrane protein that regulates neuronal excitability. PLoS Genet. 7: e1001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L., 2014. A model of the effect of uncertainty on the C. elegans L2/L2d decision. PLoS One 9: e100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaj T., Lu Y., Shaham S., 2008. The conserved proteins CHE-12 and DYF-11 are required for sensory cilium function in Caenorhabditis elegans. Genetics 178: 989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow H., Doitsidou M., Sarin S., Hobert O., 2009. MAQGene: software to facilitate C. elegans mutant genome sequence analysis. Nat. Methods 6: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher R. A., Fujita M., Schroeder F. C., Clardy J., 2007. Small molecule signaling of dauer formation in C. elegans. Nat. Chem. Biol. 3: 420–422. [DOI] [PubMed] [Google Scholar]
- Cassada R. C., Russell R. L., 1975. The dauer larva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46: 326–342. [DOI] [PubMed] [Google Scholar]
- Crane M. M., Stirman J. N., Ou C. Y., Kurshan P. T., Rehg J. M., et al. , 2012. Autonomous screening of C. elegans identifies genes implicated in synaptogenesis. Nat. Methods 9: 977–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Dickinson D. J., Wood C. C., Goldstein B., Slep K. C., 2015. Crescerin uses a TOG domain array to regulate microtubules in the primary cilium. Mol. Biol. Cell 26: 4248–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. W., Hammarlund M., Harrach T., Hullett P., Olsen S., et al. , 2005. Rapid single nucleotide polymorphism mapping in C. elegans. BMC Genomics 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bono M., Tobin D. M., Davis M. W., Avery L., Bargmann C. I., 2002. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419: 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger D. L., 2002. Regulation of diapause. Annu. Rev. Entomol. 47: 93–122. [DOI] [PubMed] [Google Scholar]
- Denlinger D. L., Armbruster P. A., 2014. Mosquito diapause. Annu. Rev. Entomol. 59: 73–93. [DOI] [PubMed] [Google Scholar]
- Doitsidou M., Flames N., Lee A. C., Boyanov A., Hobert O., 2008. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nat. Methods 5: 869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroquez D. B., Berciu C., Anderson J. R., Sengupta P., Nicastro D., 2014. A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in C. elegans. eLife 3: 01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison A. S., 2009. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Curr. Opin. Neurobiol. 19: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson K. J., Bradshaw W. E., Holzapfel C. M., 2009. Complications of complexity: integrating environmental, genetic and hormonal control of insect diapause. Trends Genet. 25: 217–225. [DOI] [PubMed] [Google Scholar]
- Entchev E. V., Patel D. S., Zhan M., Steele A. J., Lu H., et al. , 2015. A gene-expression-based neural code for food abundance that modulates lifespan. eLife 4: e06259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay D. S., 2013. Classical genetic methods (December 30, 2013), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.165.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielenbach N., Antebi A., 2008. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 22: 2149–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness A. I., Lee K., Reznick D. N., 2015. Adaptation in a variable environment: Phenotypic plasticity and bet-hedging during egg diapause and hatching in an annual killifish. Evolution 69: 1461–1475. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L., 1982. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 218: 578–580. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L., 1984a A Caenorhabditis elegans dauer-inducing pheromone and an antagonistic component of the food supply. J. Chem. Ecol. 10: 1265–1280. [DOI] [PubMed] [Google Scholar]
- Golden J. W., Riddle D. L., 1984b The Caenorhabditis elegans dauer larva: developmental effects of pheromone, food, and temperature. Dev. Biol. 102: 368–378. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., et al. , 1986. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 83: 4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti R., Altiero T., Rebecchi L., 2011. On dormancy strategies in tardigrades. J. Insect Physiol. 57: 567–576. [DOI] [PubMed] [Google Scholar]
- Guo M., Wu T. H., Song Y. X., Ge M. H., Su C. M., et al. , 2015. Reciprocal inhibition between sensory ASH and ASI neurons modulates nociception and avoidance in Caenorhabditis elegans. Nat. Commun. 6: 5655. [DOI] [PubMed] [Google Scholar]
- Hahn D. A., Denlinger D. L., 2011. Energetics of insect diapause. Annu. Rev. Entomol. 56: 103–121. [DOI] [PubMed] [Google Scholar]
- Hart A., Sims S., Kaplan J., 1995. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature 378: 82–85. [DOI] [PubMed] [Google Scholar]
- Herman R. K., Hedgecock E. M., 1990. Limitation of the size of the vulval primordium of Caenorhabditis elegans by lin-15 expression in surrounding hypodermis. Nature 348: 169–171. [DOI] [PubMed] [Google Scholar]
- Hilliard M. A., Bargmann C. I., Bazzicalupo P., 2002. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr. Biol. 12: 730–734. [DOI] [PubMed] [Google Scholar]
- Hilliard M. A., Bergamasco C., Arbucci S., Plasterk R. H., Bazzicalupo P., 2004. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 23: 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M. A., Apicella A. J., Kerr R., Suzuki H., Bazzicalupo P., et al. , 2005. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 24: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden H., Johnson J., Gardner-Thorpe C., Lashley T., Hernandez D., et al. , 2007. Mutations in TTBK2, encoding a kinase implicated in tau phosphorylation, segregate with spinocerebellar ataxia type 11. Nat. Genet. 39: 1434–1436. [DOI] [PubMed] [Google Scholar]
- Hu P. J., 2007. Dauer (August 08, 2007), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.144.1, http://www.wormbook.org. [Google Scholar]
- Ikezu S., Ikezu T., 2014. Tau-tubulin kinase. Front. Mol. Neurosci. 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Grundke-Iqbal I., Smith A. J., George L., Tung Y. C., et al. , 1989. Identification and localization of a tau peptide to paired helical filaments of Alzheimer disease. Proc. Natl. Acad. Sci. USA 86: 5646–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski J., Kornfeld K., 1999. A local, high-density, single-nucleotide polymorphism map used to clone Caenorhabditis elegans cdf-1. Genetics 153: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H., Kim K., Neal S. J., Macosko E. Z., Kim D., et al. , 2012. Neuromodulatory state and sex specify alternative behaviors through antagonistic synaptic pathways in C. elegans. Neuron 75: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong P. Y., Jung M., Yim Y. H., Kim H., Park M., et al. , 2005. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 433: 541–545. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Horvitz H., 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90: 2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Sato K., Shibuya M., Zeiger D. M., Butcher R. A., et al. , 2009. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 326: 994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer B. C., Burgess J. K., Chen J. H., Thomas J. H., Schellenberg G. D., 2006. Molecular pathways that influence human tau-induced pathology in Caenorhabditis elegans. Hum. Mol. Genet. 15: 1483–1496. [DOI] [PubMed] [Google Scholar]
- Kutscher L. M., Shaham S., 2014. Forward and reverse mutagenesis in C. elegans (January 17, 2014), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.167.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liachko N. F., McMillan P. J., Strovas T. J., Loomis E., Greenup L., et al. , 2014. The tau tubulin kinases TTBK1/2 promote accumulation of pathological TDP-43. PLoS Genet. 10: e1004803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J. C., Yang T. T., Weng R. R., Kuo C. T., Chang C. W., 2015. TTBK2: A tau protein kinase beyond tau phosphorylation. BioMed Res. Int. 2015: 575170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig A. H., Schroeder F. C., 2013. Ascaroside signaling in C. elegans (January 18, 2013), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.155.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund H., Cowburn R. F., Gustafsson E., Stromberg K., Svensson A., et al. , 2013. Tau-tubulin kinase 1 expression, phosphorylation and co-localization with phospho-Ser422 tau in the Alzheimer’s disease brain. Brain Pathol. 23: 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko E. Z., Pokala N., Feinberg E. H., Chalasani S. H., Butcher R. A., et al. , 2009. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 458: 1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning G., 2005. Genomic overview of protein kinases (December 13, 2005), WormBook, ed. The C. elegans Research Community WormBook, /10.1895/wormbook.1.60.1, http://www.wormbook.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq A. V., Peckol E., Bargmann C. I., 1995. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature 378: 78–81. [DOI] [PubMed] [Google Scholar]
- McGrath P. T., Xu Y., Ailion M., Garrison J. L., Butcher R. A., et al. , 2011. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minevich G., Park D. S., Blankenberg D., Poole R. J., Hobert O., 2012. CloudMap: a cloud-based pipeline for analysis of mutant genome sequences. Genetics 192: 1249–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyara A., Ohta A., Okochi Y., Tsukada Y., Kuhara A., et al. , 2011. Novel and conserved protein macoilin is required for diverse neuronal functions in Caenorhabditis elegans. PLoS Genet. 7: e1001384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal S. J., Kim K., Sengupta P., 2013. Quantitative assessment of pheromone-induced dauer formation in Caenorhabditis elegans. Methods Mol. Biol. 1068: 273–283. [DOI] [PubMed] [Google Scholar]
- Nolan K. M., Sarafi-Reinach T. R., Horne J. G., Saffer A. M., Sengupta P., 2002. The DAF-7 TGF-beta signaling pathway regulates chemosensory receptor gene expression in C. elegans. Genes Dev. 16: 3061–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylin S., 2013. Induction of diapause and seasonal morphs in butterflies and other insects: knowns, unknowns and the challenge of integration. Physiol. Entomol. 38: 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D., O’Doherty I., Somvanshi R. K., Bethke A., Schroeder F. C., et al. , 2012. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 109: 9917–9922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckol E. L., Troemel E. R., Bargmann C. I., 2001. Sensory experience and sensory activity regulate chemosensory receptor gene expression in C. elegans. Proc. Natl. Acad. Sci. USA 98: 11032–11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L. B., Rosenbaum J. L., 2008. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85: 23–61. [DOI] [PubMed] [Google Scholar]
- Perkins L. A., Hedgecock E. M., Thomson J. N., Culotti J. G., 1986. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev. Biol. 117: 456–487. [DOI] [PubMed] [Google Scholar]
- Ren P., Lim C. S., Johnsen R., Albert P. S., Pilgrim D., et al. , 1996. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science 274: 1389–1391. [DOI] [PubMed] [Google Scholar]
- Riddle D. L., Albert P. S., 1997. Genetic and environmental regulation of dauer larva development, pp. 739–768 in C. elegans II, edited by Riddle D. S., Blumenthal T., Meyer B. J., Priess J. R. Cold Spring Harbor Press, Cold Spring Harbor. [PubMed] [Google Scholar]
- Riddle D. L., Swanson M. M., Albert P. S., 1981. Interacting genes in nematode dauer larva formation. Nature 290: 668–671. [DOI] [PubMed] [Google Scholar]
- Rosenbaum J. L., Witman G. B., 2002. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 3: 813–825. [DOI] [PubMed] [Google Scholar]
- Sambongi Y., Nagae T., Liu Y., Yoshimizu T., Takeda K., et al. , 1999. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport 10: 753–757. [DOI] [PubMed] [Google Scholar]
- Sarin S., Prabhu S., O’Meara M. M., Pe’er I., Hobert O., 2008. Caenorhabditis elegans mutant allele identification by whole-genome sequencing. Nat. Methods 5: 865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin S., Bertrand V., Bigelow H., Boyanov A., Doitsidou M., et al. , 2010. Analysis of multiple ethyl methanesulfonate-mutagenized Caenorhabditis elegans strains by whole-genome sequencing. Genetics 185: 417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Cerny R. L., Buescher J. L., Ikezu T., 2006. Tau-tubulin kinase 1 (TTBK1), a neuron-specific tau kinase candidate, is involved in tau phosphorylation and aggregation. J. Neurochem. 98: 1573–1584. [DOI] [PubMed] [Google Scholar]
- Schackwitz W. S., Inoue T., Thomas J. H., 1996. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron 17: 719–728. [DOI] [PubMed] [Google Scholar]
- Schiesari L., O’Connor M. B., 2013. Diapause: delaying the developmental clock in response to a changing environment. Curr. Top. Dev. Biol. 105: 213–246. [DOI] [PubMed] [Google Scholar]
- Starich T. A., Herman R. K., Kari C. K., Yeh W.-H., Schackwitz W. S., et al. , 1995. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics 139: 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. M., Riddle D. L., 1981. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev. Biol. 84: 27–40. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tomizawa K., Sato K., Ohtake A., Omori A., 1995. A novel tau-tubulin kinase from bovine brain. FEBS Lett. 372: 59–64. [DOI] [PubMed] [Google Scholar]
- Thomas J. H., Birnby D. A., Vowels J. J., 1993. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics 134: 1105–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa K., Omori A., Ohtake A., Sato K., Takahashi M., 2001. Tau-tubulin kinase phosphorylates tau at Ser-208 and Ser-210, sites found in paired helical filament-tau. FEBS Lett. 492: 221–227. [DOI] [PubMed] [Google Scholar]
- Troemel E. R., Kimmel B. E., Bargmann C. I., 1997. Reprogramming chemotaxis responses: sensory neurons define olfactory preferences in C. elegans. Cell 91: 161–169. [DOI] [PubMed] [Google Scholar]
- Vazquez-Higuera J. L., Martinez-Garcia A., Sanchez-Juan P., Rodriguez-Rodriguez E., Mateo I., et al. , 2011. Genetic variations in tau-tubulin kinase-1 are linked to Alzheimer’s disease in a Spanish case-control cohort. Neurobiol. Aging 32: 550.e5–550e9. [DOI] [PubMed] [Google Scholar]
- Vowels J. J., Thomas J. H., 1992. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 130: 105–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels J. J., Thomas J. H., 1994. Multiple chemosensory defects in daf-11 and daf-21 mutants of Caenorhabditis elegans. Genetics 138: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S., 1986. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond., B 314: 1–340. [DOI] [PubMed] [Google Scholar]
- Wicks S. R., Yeh R. T., Gish W. R., Waterston R. H., Plasterk R. H., 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
- Wolozin B. L., Pruchnicki A., Dickson D. W., Davies P., 1986. A neuronal antigen in the brains of Alzheimer patients. Science 232: 648–650. [DOI] [PubMed] [Google Scholar]
- Xu C., Min J., 2011. Structure and function of WD40 domain proteins. Protein Cell 2: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N. N., Yu J. T., Xiao J. T., Zhang H. W., Lu R. C., et al. , 2011. Tau-tubulin kinase-1 gene variants are associated with Alzheimer’s disease in Han Chinese. Neurosci. Lett. 491: 83–86. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang F., 2015. The multifunctions of WD40 proteins in genome integrity and cell cycle progression. J Genomics 3: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.