Abstract
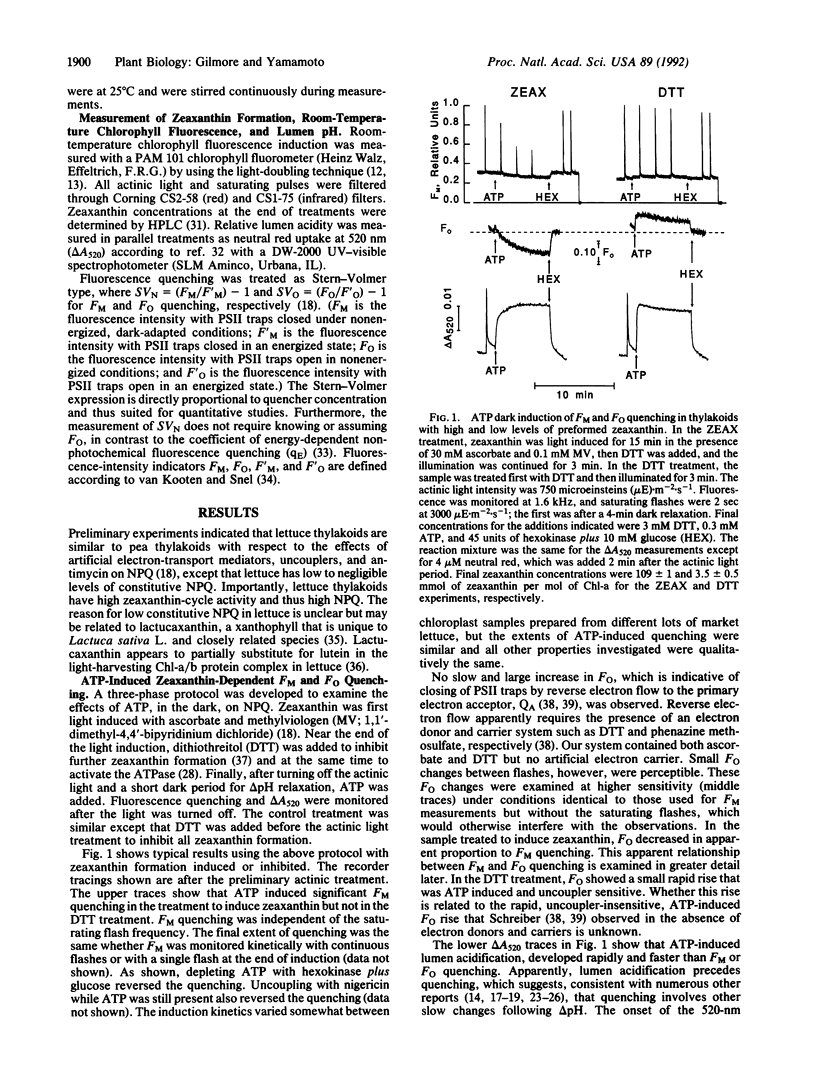
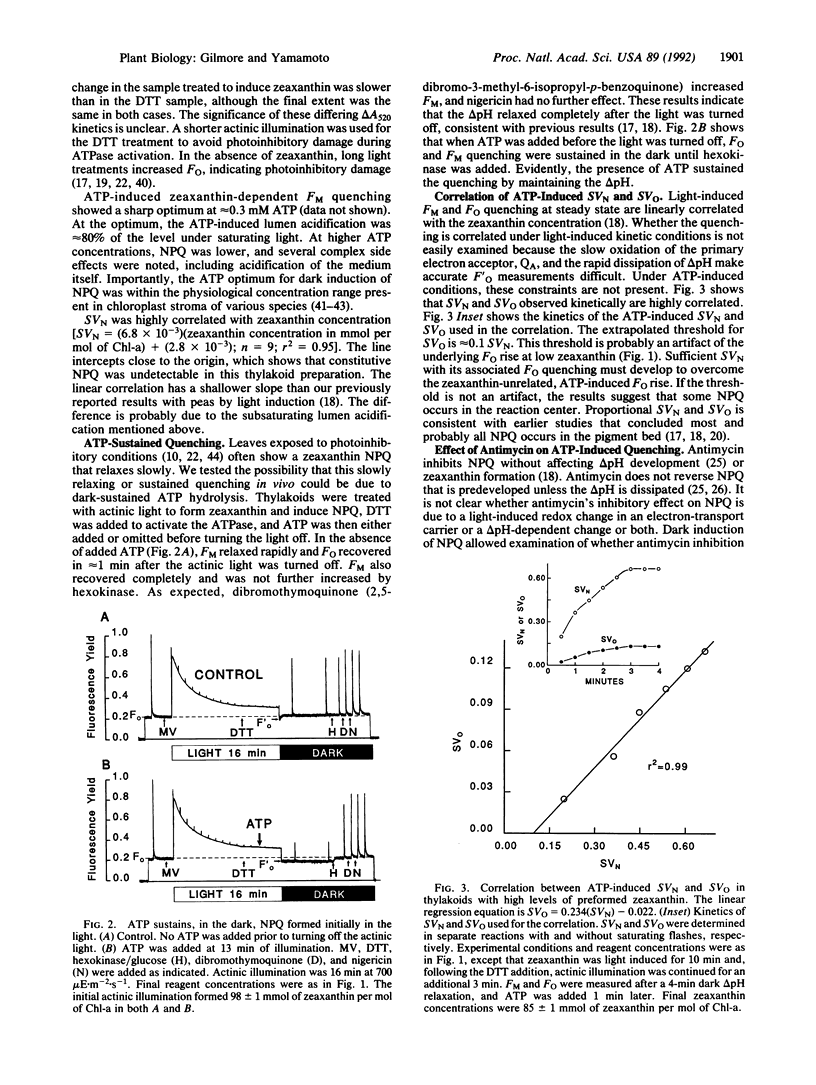
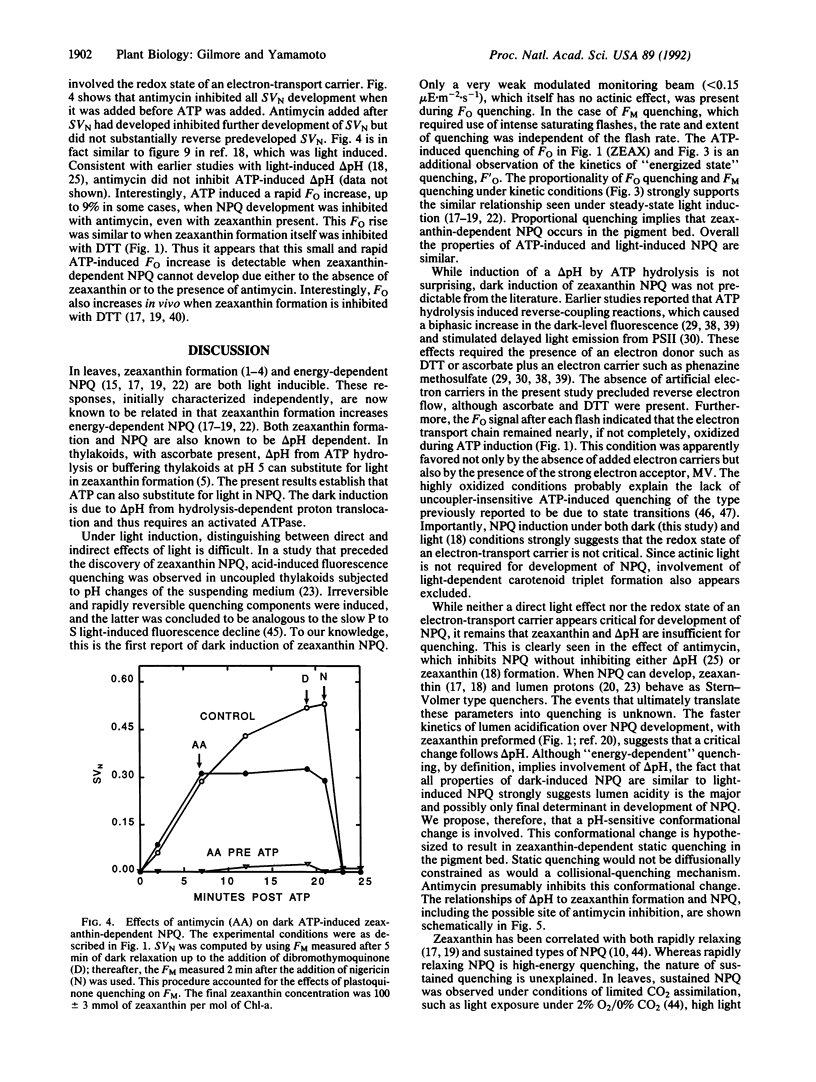
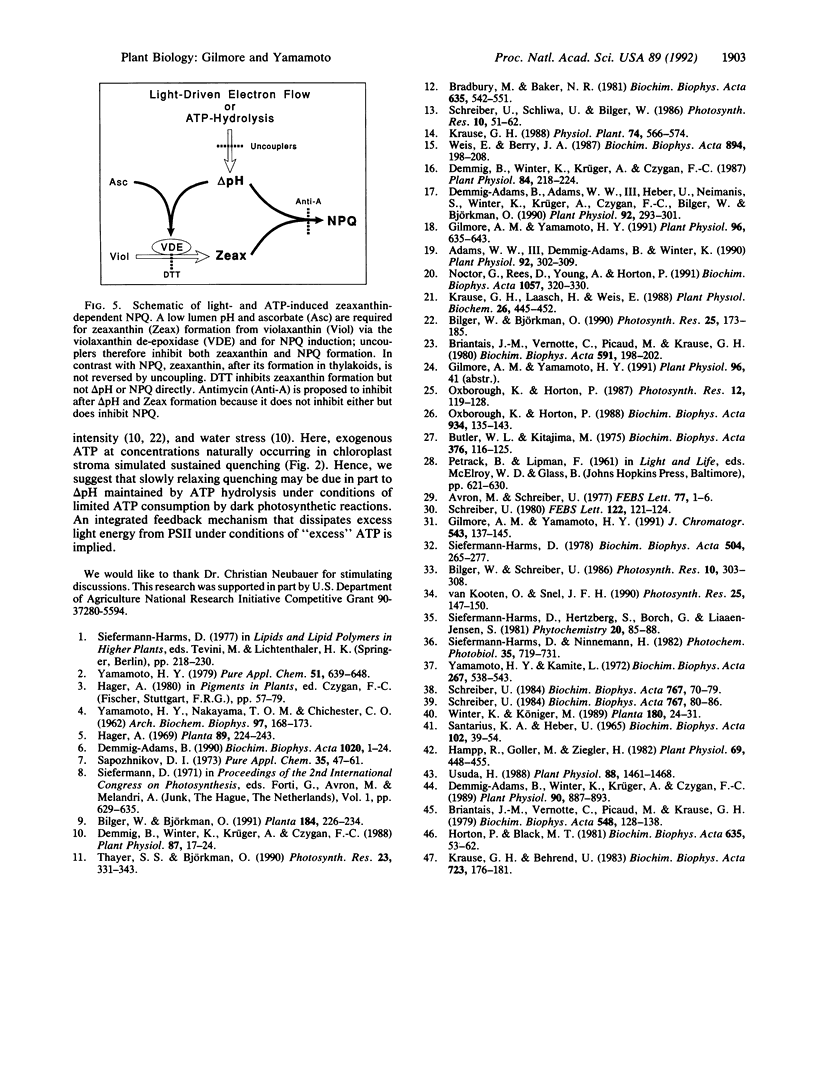
Zeaxanthin-dependent nonphotochemical fluorescence quenching is a light-induced activity in plants that apparently protects against the potentially damaging effects of excess light. We report a dark-induced nonphotochemical quenching in thylakoids of Lactuca sativa L. cv. Romaine mediated by ATP. This effect is due to low lumen pH from hydrolysis-dependent proton pumping and hence required an active ATPase. The induction was optimal at 0.3 mM ATP, a physiological concentration, and occurred under conditions of little or no reverse electron flow. The properties of ATP-induced quenching were in all respects examined similar to light-induced quenching, including antimycin inhibition of quenching induction but not delta pH. We conclude that zeaxanthin-dependent quenching depends directly on lumen pH and that the role of light is indirect. Although it is known that zeaxanthin and low lumen pH are insufficient for quenching to occur, the results apparently exclude the redox state of an electron-transport carrier or formation of light-induced carotenoid triplets as a further requirement. We propose that a slow pH-dependent conformational change together with zeaxanthin cause static quenching in the pigment bed; possibly antimycin inhibits this change. Furthermore, we suggest from the ability of ATP to sustain quenching in the dark for extended periods that persistent or slowly reversible zeaxanthin quenching often observed in vivo may be due to sustained delta pH from ATP hydrolysis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. W., Demmig-Adams B., Winter K. Relative contributions of zeaxanthin-related and zeaxanthin-unrelated types of ;high-energy-state' quenching of chlorophyll fluorescence in spinach leaves exposed to various environmental conditions. Plant Physiol. 1990 Feb;92(2):302–309. doi: 10.1104/pp.92.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avron M., Schreiber U. Proton gradients as possible intermediary energy transducers during ATP-driven reverse electron flow in chloroplasts. FEBS Lett. 1977 May 1;77(1):1–6. doi: 10.1016/0014-5793(77)80180-4. [DOI] [PubMed] [Google Scholar]
- Bradbury M., Baker N. R. Analysis of the slow phases of the in vivo chlorophyll fluorescence induction curve. Changes in the redox state of photosystem II electron acceptors and fluorescence emission from photosystems I and II. Biochim Biophys Acta. 1981 May 13;635(3):542–551. doi: 10.1016/0005-2728(81)90113-4. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta. 1979 Oct 10;548(1):128–138. doi: 10.1016/0005-2728(79)90193-2. [DOI] [PubMed] [Google Scholar]
- Briantais J. M., Vernotte C., Picaud M., Krause G. H. Chlorophyll fluorescence as a probe for the determination of the photo-induced proton gradient in isolated chloroplasts. Biochim Biophys Acta. 1980 Jun 10;591(1):198–202. doi: 10.1016/0005-2728(80)90233-9. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B., Adams W. W., Heber U., Neimanis S., Winter K., Krüger A., Czygan F. C., Bilger W., Björkman O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1990 Feb;92(2):293–301. doi: 10.1104/pp.92.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig-Adams B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Induction and Relaxation Kinetics of the Dissipation of Excess Excitation Energy in Leaves in 2% O(2), 0% CO(2). Plant Physiol. 1989 Jul;90(3):887–893. doi: 10.1104/pp.90.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Zeaxanthin and the Heat Dissipation of Excess Light Energy in Nerium oleander Exposed to a Combination of High Light and Water Stress. Plant Physiol. 1988 May;87(1):17–24. doi: 10.1104/pp.87.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A. M., Yamamoto H. Y. Zeaxanthin Formation and Energy-Dependent Fluorescence Quenching in Pea Chloroplasts under Artificially Mediated Linear and Cyclic Electron Transport. Plant Physiol. 1991 Jun;96(2):635–643. doi: 10.1104/pp.96.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampp R., Goller M., Ziegler H. Adenylate Levels, Energy Charge, and Phosphorylation Potential during Dark-Light and Light-Dark Transition in Chloroplasts, Mitochondria, and Cytosol of Mesophyll Protoplasts from Avena sativa L. Plant Physiol. 1982 Feb;69(2):448–455. doi: 10.1104/pp.69.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P., Black M. T. Light-dependent quenching of chlorophyll fluorescence in pea chloroplasts induced by adenosine 5'-triphosphate. Biochim Biophys Acta. 1981 Mar 12;635(1):53–62. doi: 10.1016/0005-2728(81)90006-2. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Sapozhnikov D. I. Investigation on the violaxanthin cycle. Pure Appl Chem. 1973;35(1):47–61. doi: 10.1351/pac197335010047. [DOI] [PubMed] [Google Scholar]
- Siefermann-Harms D. The accumulation of neutral red in illuminated thylakoids. Biochim Biophys Acta. 1978 Nov 9;504(2):265–277. doi: 10.1016/0005-2728(78)90175-5. [DOI] [PubMed] [Google Scholar]
- Usuda H. Adenine Nucleotide Levels, the Redox State of the NADP System, and Assimilatory Force in Nonaqueously Purified Mesophyll Chloroplasts from Maize Leaves under Different Light Intensities. Plant Physiol. 1988 Dec;88(4):1461–1468. doi: 10.1104/pp.88.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO H. Y., NAKAYAMA T. O., CHICHESTER C. O. Studies on the light and dark interconversions of leaf xanthophylls. Arch Biochem Biophys. 1962 Apr;97:168–173. doi: 10.1016/0003-9861(62)90060-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto H. Y., Kamite L. The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500-nm region. Biochim Biophys Acta. 1972 Jun 23;267(3):538–543. doi: 10.1016/0005-2728(72)90182-x. [DOI] [PubMed] [Google Scholar]



