Abstract
Systems-scale profiling approaches have become widely used in translational research settings. The resulting accumulation of large-scale datasets in public repositories represents a critical opportunity to promote insight and foster knowledge discovery. However, resources that can serve as an interface between biomedical researchers and such vast and heterogeneous dataset collections are needed in order to fulfill this potential. Recently, we have developed an interactive data browsing and visualization web application, the Gene Expression Browser (GXB). This tool can be used to overlay deep molecular phenotyping data with rich contextual information about analytes, samples and studies along with ancillary clinical or immunological profiling data. In this note, we describe a curated compendium of 93 public datasets generated in the context of human monocyte immunological studies, representing a total of 4,516 transcriptome profiles. Datasets were uploaded to an instance of GXB along with study description and sample annotations. Study samples were arranged in different groups. Ranked gene lists were generated based on relevant group comparisons. This resource is publicly available online at http://monocyte.gxbsidra.org/dm3/landing.gsp.
Keywords: Monocyte, Transcriptomics, Gene Expression Browser, Immunology, Bioinformatics
Introduction
Platforms such as microarrays and, more recently, next generation sequencing have been leveraged to generate molecular profiles at the scale of entire systems. The global perspective gained using such approaches is potentially transformative. Transcriptome profiling enabled for instance the characterization of molecular perturbations that occur in the context of a wide range disease processes 1– 10. This in turn has provided opportunities for the discovery of biomarkers and for the development of novel therapeutic modalities 3, 11– 13. More recently such systems-scale profiling of the blood transcriptome has also been used to monitor response to vaccines or therapeutic drugs 14– 19. The democratization of these approaches has led to proliferation of data in public repositories: over 1.7 million individual transcriptome profiles from more than 65,000 studies have been deposited to date in the NCBI Gene Expression Omnibus (GEO), a public repository of transcriptome profiles.
Taken together this vast body of “collective data” holds the promise of accelerating the pace of biomedical discovery by creating countless opportunities for identifying and filling critical knowledge gaps. Building tools that provide biomedical researchers with the ability to seamlessly interact with collections of datasets along with rich contextual information is essential in promoting insight and enabling knowledge discovery. To address this need we have developed an interactive data browsing and visualization web application, the Gene Expression Browser (GXB).
GXB was described in a recent publication and is available as open source software on GitHub 20. This tool constitutes a simple interface for the browsing and interactive visualization of large volumes of heterogeneous data. Users can easily customize data plots by adding multiple layers of information, modifying the order of samples, and generating links that capture these settings which can be inserted in email communications or in publications. Accessing the tool via these links also provides access to rich contextual information that is essential for data interpretation. This includes access to gene information and relevant literature, study design information, detailed sample information as well as ancillary data 20.
In recent years, a large number of transcriptional studies have been conducted aiming at the characterization and functional classification of monocytes in health and disease. Monocytes are a population of immune cells found in the blood, bone marrow, and spleen. They constitute ~10% of the total circulating blood leukocytes in humans. They can remain in the blood circulation for up to 1–2 days, after which time, if they have not been recruited to a tissue, they die and are removed. They are considered the systemic reservoir of myeloid precursors for renewal of tissue macrophages and dendritic cells. Monocytes play a key role during immune response as professional phagocytes 21, 22, and producers of immune mediators 23, 24. Indeed, reports show that monocytes are recruited at the site of infections as innate effectors of the inflammatory response to microbes, killing pathogens via phagocytosis, production of reactive oxygen intermediate (ROIs) 25, reactive nitrogen intermediate (RNIs) 26, 27, myeloperoxidase (MPO) 28, 29, and producing inflammatory cytokines 30 that contribute to further amplifying the antimicrobial response 31.
Human monocytes are derived from hematopoietic stem cells in the bone marrow and are released into peripheral blood circulation upon maturation. They are divided into three major subsets based on the expression of cell surface markers CD14 and CD16. The most prevalent subset in the blood circulation, accounting for 90% of all monocytes, are the classical monocytes that express high levels of CD14 but low levels of CD16. The remaining 10% is divided into two subsets: intermediate monocyte with high expression of CD14 and CD16 (CD14+CD16+) and non-classical monocytes that express low levels of CD14 but high levels of CD16 (CD14dimCD16++ or CD14+CD16++) 32– 34.
In this data note we are making available via GXB a curated compendium of 93 public datasets relevant to human monocyte immunobiology, representing a total of 4,516 transcriptome profiles.
Materials and methods
Identification of monocyte datasets
Potentially relevant datasets deposited in GEO were identified using an advanced query based on the Bioconductor package GEOmetadb and the SQLite database that captures detailed information on the GEO data structure; https://www.bioconductor.org/packages/release/bioc/html/GEOmetadb.html 35. The search query was designed to retrieve entries where the title and description contained the word Monocyte OR Monocytes, were generated from human samples, using Illumina or Affymetrix commercial platforms. The query result is appended with rich metadata from GEOmetadb that allows for manual filtering of the retrieved collection.
The relevance of each entry returned by this query was assessed individually. This process involved reading through the descriptions and examining the list of available samples and their annotations. Sometimes it was also necessary to review the original published report in which the design of the study and generation of the dataset is described in more detail. The datasets cover a broad range of human immunology studies investigating monocyte immunobiology in the context of diseases and through comparison with diverse cell populations and study types as illustrated by a graphical representation of relative occurrences of terms in the list of diseases loaded into our tool ( Figure 1). A wide range of cell types and diseases are represented. Ultimately, the collection was comprised of 93 curated datasets. It includes datasets generated from studies profiling primary human CD14+ cells isolated from patients with autoimmune diseases (7), bacterial, virus and parasite infections (7), cancer (4), cardiovascular diseases (4), kidney diseases (4), as well as monocytes isolated from healthy subjects (58) ( Figure 2). The 58 datasets in which monocytes were isolated from healthy subjects were classified based on whether profiling was conducted ex vivo or following in vitro experiments. In total 38 datasets were identified in which primary human CD14+ cells were stimulated or infected in in vitro experiments ( Figure 2). Among the many noteworthy datasets, there are 8 datasets investigating differences between monocytes subsets; classical (CD14++CD16-), intermediate (CD14+CD16+) and non-classical monocytes (CD14-CD16++) 32– 34 [GXB: GSE16836, GSE18565, GSE25913, GSE34515, GSE35457, GSE51997, GSE60601, GSE66936]. Another dataset from Banchereau and colleagues investigated responses of monocyte and dendritic cells to 13 different vaccines in vitro 36 [GXB: GSE44721]. The datasets that comprise our collection are listed in Table 1 and can be browsed interactively in GXB.
Figure 1. Thematic composition of the dataset collection.
Word frequencies extracted from text descriptions of the studies loaded into the GXB tool are depicted as a word cloud. The size of the words is proportional to their frequency.
Figure 2. Break down of the dataset collection by category.
The pie chart on the left panel indicates dataset frequencies by disease status. The chart on the right panel indicates the type of studies carried out for the 58 datasets consisting of monocyte obtained exclusively from healthy donors.
Table 1. List of datasets constituting the collection.
Dataset upload and annotation on GXB
Once a final selection had been made each dataset was downloaded from GEO in the SOFT file format. It was in turn uploaded on an instance of the Gene Expression Browser (GXB) hosted on the Amazon Web Services cloud. Available sample and study information were also uploaded. Samples were grouped according to possible interpretations of study results and ranking based on the different group comparisons that were computed (e.g. comparing monocyte isolated from case vs controls in studies where profiling was performed ex-vivo; or stimulated vs medium control in in vitro experiments).
Short Gene Expression Brower tutorial
The GXB software has been described in detail in a recent publication 20. This custom software interface provides users with a means to easily navigate and filter the dataset collection available at http://monocyte.gxbsidra.org/dm3/landing.gsp. A web tutorial is also available online: http://monocyte.gxbsidra.org/dm3/tutorials.gsp#gxbtut. Briefly, datasets of interest can be quickly identified either by filtering using criteria from pre-defined lists on the left or by entering a query term in the search box at the top of the dataset navigation page. Clicking on one of the studies listed in the dataset navigation page opens a viewer designed to provide interactive browsing and graphic representations of large-scale data in an interpretable format. This interface is designed to present ranked gene lists and display expression results graphically in a context-rich environment. Selecting a gene from the rank ordered list on the left of the data-viewing interface will display its expression values graphically in the screen’s central panel. Directly above the graphical display drop down menus give users the ability: a) To change how the gene list is ranked; this allows the user to change the method used to rank the genes, or to include only genes that are selected for specific biological interest; b) To change sample grouping (Group Set button), in some datasets a user can switch between groups based on cell type to groups based on disease type, for example; c) To sort individual samples within a group based on associated categorical or continuous variables (e.g. gender or age); d) To toggle between the bar chart view and a box plot view, with expression values represented as a single point for each sample. Samples are split into the same groups whether displayed as a bar chart or box plot; e) To provide a color legend for the sample groups; f) To select categorical information that is to be overlaid at the bottom of the graph. For example, the user can display gender or smoking status in this manner; g) To provide a color legend for the categorical information overlaid at the bottom of the graph; and h) To download the graph as a png image or csv file for performing a separate analysis. Measurements have no intrinsic utility in absence of contextual information. It is this contextual information that makes the results of a study or experiment interpretable. It is therefore important to capture, integrate and display information that will give users the ability to interpret data and gain new insights from it. We have organized this information under different tabs directly above the graphical display. The tabs can be hidden to make more room for displaying the data plots, or revealed by clicking on the blue “show info panel” button on the top right corner of the display. Information about the gene selected from the list on the left side of the display is available under the “Gene” tab. Information about the study is available under the “Study” tab. Information available about individual samples is provided under the “Sample” tab. Rolling the mouse cursor over a bar chart's element while displaying the “Sample” tab lists any clinical, demographic, or laboratory information available for the selected sample. Finally, the “Downloads” tab allows advanced users to retrieve the original dataset for analysis outside this tool. It also provides all available sample annotation data for use alongside the expression data in third party analysis software. Other functionalities are provided under the “Tools” drop-down menu located in the top right corner of the user interface. Some of the notable functionalities available through this menu include: a) Annotations, which provides access to all the ancillary information about the study, samples and dataset organized across different tabs; b) Cross-project view, which provides the ability for a given gene to browse through all available studies; c) Copy link, which generates a mini-URL encapsulating information about the display settings in use and that can be saved and shared with others (clicking on the envelope icon on the toolbar inserts the url in an email message via the local email client); and d) Chart options, which gives user the option to customize chart labels.
Dataset validation
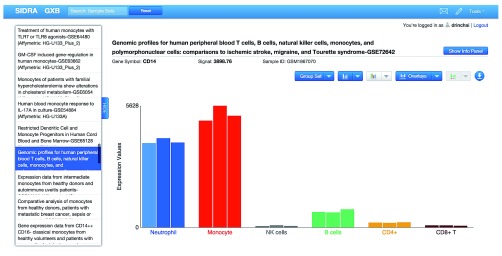
Quality control checks were performed with the examination of profiles of relevant biological indicators. Known leukocyte markers were used, such as CD14, which is expressed by monocytes and macrophages; as well as markers that would indicate significant contamination of the sample by other leukocyte populations: such as CD3, a T-cells marker; CD19, a B-cell marker; CD56, an NK cell marker ( Figure 3; The expression of the CD14 marker across all studies can be checked using the cross project functionality of GXB: http://monocyte.gxbsidra.org/dm3/geneBrowser/crossProject?probeID=201743_at&geneSymbol=CD14&geneID=929). In addition, expression of the XIST transcripts, in which expression is gender-specific, was also examined to determine its concordance with demographic information provided with the GEO submission.
Figure 3. Illustrative example showing the abundance levels of CD14 transcripts across samples in a given study.
The expression of this gene is indicative of the purity of primary human monocyte preparation; this marker is expected to be high in monocyte preparations and low in other leukocyte populations. In this view of the GXB expression of CD14 can be visualized across projects listed on the left.
Data availability
The data referenced by this article are under copyright with the following copyright statement: Copyright: © 2016 Rinchai D et al.
All datasets included in our curated collection are also available publically via the NCBI GEO website: http://www.ncbi.nlm.nih.gov/geo/; and are referenced throughout the manuscript by their GEO accession numbers (e.g. GSE25913). Signal files and sample description files can also be downloaded from the GXB tool under the “downloads” tab.
Acknowledgement
The authors would like to acknowledge all the investigators who decided to make their datasets publically available by sharing them in GEO.
Funding Statement
DR, SB and DC received support from the Qatar Foundation.
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 1 approved
References
- 1. Bennett L, Palucka AK, Arce E, et al. : Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197(6):711–23. 10.1084/jem.20021553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffiths MJ, Shafi MJ, Popper SJ, et al. : Genomewide analysis of the host response to malaria in Kenyan children. J Infect Dis. 2005;191(10):1599–611. 10.1086/429297 [DOI] [PubMed] [Google Scholar]
- 3. Mejias A, Dimo B, Suarez NM, et al. : Whole blood gene expression profiles to assess pathogenesis and disease severity in infants with respiratory syncytial virus infection. PLoS Med. 2013;10(11):e1001549. 10.1371/journal.pmed.1001549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moal V, Textoris J, Ben Amara A, et al. : Chronic hepatitis E virus infection is specifically associated with an interferon-related transcriptional program. J Infect Dis. 2013;207(1):125–32. 10.1093/infdis/jis632 [DOI] [PubMed] [Google Scholar]
- 5. Mostafavi S, Battle A, Zhu X, et al. : Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Mol Psychiatry. 2014;19(12):1267–74. 10.1038/mp.2013.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Novershtern N, Subramanian A, Lawton LN, et al. : Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell. 2011;144(2):296–309. 10.1016/j.cell.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Panelli MC, Wang E, Phan G, et al. : Gene-expression profiling of the response of peripheral blood mononuclear cells and melanoma metastases to systemic IL-2 administration. Genome Biol. 2002;3(7): RESEARCH0035. 10.1186/gb-2002-3-7-research0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pascual V, Allantaz F, Patel P, et al. : How the study of children with rheumatic diseases identified interferon-alpha and interleukin-1 as novel therapeutic targets. Immunol Rev. 2008;223(1):39–59. 10.1111/j.1600-065X.2008.00643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smih F, Desmoulin F, Berry M, et al. : Blood signature of pre-heart failure: a microarrays study. PLoS One. 2011;6(6):e20414. 10.1371/journal.pone.0020414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stamova B, Xu H, Jickling G, et al. : Gene expression profiling of blood for the prediction of ischemic stroke. Stroke. 2010;41(10):2171–7. 10.1161/STROKEAHA.110.588335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berry MP, Graham CM, McNab FW, et al. : An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466(7309):973–7. 10.1038/nature09247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martínez-Llordella M, Lozano JJ, Puig-Pey I, et al. : Using transcriptional profiling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest. 2008;118(8):2845–57. 10.1172/JCI35342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Newell KA, Asare A, Kirk AD, et al. : Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120(6):1836–47. 10.1172/JCI39933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gaucher D, Therrien R, Kettaf N, et al. : Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205(13):3119–31. 10.1084/jem.20082292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hecker M, Hartmann C, Kandulski O, et al. : Interferon-beta therapy in multiple sclerosis: the short-term and long-term effects on the patients' individual gene expression in peripheral blood. Mol Neurobiol. 2013;48(3):737–56. 10.1007/s12035-013-8463-1 [DOI] [PubMed] [Google Scholar]
- 16. Li S, Rouphael N, Duraisingham S, et al. : Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15(2):195–204. 10.1038/ni.2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Obermoser G, Presnell S, Domico K, et al. : Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38(4):831–44. 10.1016/j.immuni.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oswald M, Curran ME, Lamberth SL, et al. : Modular analysis of peripheral blood gene expression in rheumatoid arthritis captures reproducible gene expression changes in tumor necrosis factor responders. Arthritis Rheumatol. 2015;67(2):344–51. 10.1002/art.38947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Querec TD, Akondy RS, Lee EK, et al. : Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10(1):116–25. 10.1038/ni.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Speake C, Presnell S, Domico K, et al. : An interactive web application for the dissemination of human systems immunology data. J Transl Med. 2015;13:196. 10.1186/s12967-015-0541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Auffray C, Sieweke MH, Geissmann F: Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–92. 10.1146/annurev.immunol.021908.132557 [DOI] [PubMed] [Google Scholar]
- 22. van Furth R, Cohn ZA, Hirsch JG, et al. : The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–52. [PMC free article] [PubMed] [Google Scholar]
- 23. Narni-Mancinelli E, Soudja SM, Crozat K, et al. : Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo. PLoS Pathog. 2011;7(12):e1002457. 10.1371/journal.ppat.1002457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grimm MJ, Vethanayagam RR, Almyroudis NG, et al. : Monocyte- and macrophage-targeted NADPH oxidase mediates antifungal host defense and regulation of acute inflammation in mice. J Immunol. 2013;190(8):4175–84. 10.4049/jimmunol.1202800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fang FC: Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2(10):820–32. 10.1038/nrmicro1004 [DOI] [PubMed] [Google Scholar]
- 26. Dinauer MC, Deck MB, Unanue ER: Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J Immunol. 1997;158(12):5581–3. [PubMed] [Google Scholar]
- 27. Endres R, Luz A, Schulze H, et al. : Listeriosis in p47 phox-/- and TRp55 -/- mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 1997;7(3):419–32. 10.1016/S1074-7613(00)80363-5 [DOI] [PubMed] [Google Scholar]
- 28. Aratani Y, Koyama H, Nyui S, et al. : Severe impairment in early host defense against Candida albicans in mice deficient in myeloperoxidase. Infect Immun. 1999;67(4):1828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Albrecht D, Jungi TW: Luminol-enhanced chemiluminescence induced in peripheral blood-derived human phagocytes: obligatory requirement of myeloperoxidase exocytosis by monocytes. J Leukoc Biol. 1993;54(4):300–6. [DOI] [PubMed] [Google Scholar]
- 30. Gavrilin MA, Bouakl IJ, Knatz NL, et al. : Internalization and phagosome escape required for Francisella to induce human monocyte IL-1beta processing and release. Proc Natl Acad Sci U S A. 2006;103(1):141–6. 10.1073/pnas.0504271103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serbina NV, Jia T, Hohl TM, et al. : Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–52. 10.1146/annurev.immunol.26.021607.090326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ancuta P, Liu KY, Misra V, et al. : Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16- monocyte subsets. BMC Genomics. 2009;10:403. 10.1186/1471-2164-10-403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frankenberger M, Hofer TP, Marei A, et al. : Transcript profiling of CD16-positive monocytes reveals a unique molecular fingerprint. Eur J Immunol. 2012;42(4):957–74. 10.1002/eji.201141907 [DOI] [PubMed] [Google Scholar]
- 34. Wong KL, Tai JJ, Wong WC, et al. : Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–31. 10.1182/blood-2010-12-326355 [DOI] [PubMed] [Google Scholar]
- 35. Zhu Y, Davis S, Stephens R, et al. : GEOmetadb: powerful alternative search engine for the Gene Expression Omnibus. Bioinformatics. 2008;24(23):2798–800. 10.1093/bioinformatics/btn520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Banchereau R, Baldwin N, Cepika AM, et al. : Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun. 2014;5: 5283. 10.1038/ncomms6283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iwata M, Sandstrom RS, Delrow JJ, et al. : Functionally and phenotypically distinct subpopulations of marrow stromal cells are fibroblast in origin and induce different fates in peripheral blood monocytes. Stem Cells Dev. 2014;23(7):729–40. 10.1089/scd.2013.0300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Llaverias G, Pou J, Ros E, et al. : Monocyte gene-expression profile in men with familial combined hyperlipidemia and its modification by atorvastatin treatment. Pharmacogenomics. 2008;9(8):1035–54. 10.2217/14622416.9.8.1035 [DOI] [PubMed] [Google Scholar]
- 39. Maouche S, Poirier O, Godefroy T, et al. : Performance comparison of two microarray platforms to assess differential gene expression in human monocyte and macrophage cells. BMC Genomics. 2008;9:302. 10.1186/1471-2164-9-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu X, Chung AY, Wu I, et al. : Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008;29(5):691–703. 10.1016/j.immuni.2008.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wrzesinski SH, Fisher JL, Ernstoff MS: Genetic profiles of plasmacytoid (BDCA-4 expressing) DC subtypes-clues to DC subtype function in vivo. Exp Hematol Oncol. 2013;2(1):8. 10.1186/2162-3619-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Butchar JP, Cremer TJ, Clay CD, et al. : Microarray analysis of human monocytes infected with Francisella tularensis identifies new targets of host response subversion. PLoS One. 2008;3(8):e2924. 10.1371/journal.pone.0002924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Boomgaarden I, Egert S, Rimbach G, et al. : Quercetin supplementation and its effect on human monocyte gene expression profiles in vivo. Br J Nutr. 2010;104(3):336–45. 10.1017/S0007114510000711 [DOI] [PubMed] [Google Scholar]
- 44. Karlsson KR, Cowley S, Martinez FO, et al. : Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp Hematol. 2008;36(9):1167–75. 10.1016/j.exphem.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Szanto A, Balint BL, Nagy ZS, et al. : STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33(5):699–712. 10.1016/j.immuni.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poliska S, Csanky E, Szanto A, et al. : Chronic obstructive pulmonary disease-specific gene expression signatures of alveolar macrophages as well as peripheral blood monocytes overlap and correlate with lung function. Respiration. 2011;81(6):499–510. 10.1159/000324297 [DOI] [PubMed] [Google Scholar]
- 47. Strunnikova NV, Barb J, Sergeev YV, et al. : Loss-of-function mutations in Rab escort protein 1 (REP-1) affect intracellular transport in fibroblasts and monocytes of choroideremia patients. PLoS One. 2009;4(12):e8402. 10.1371/journal.pone.0008402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ingersoll MA, Spanbroek R, Lottaz C, et al. : Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115(3):e10–9. 10.1182/blood-2009-07-235028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fuentes-Duculan J, Suárez-Fariñas M, Zaba LC, et al. : A subpopulation of CD163-positive macrophages is classically activated in psoriasis. J Invest Dermatol. 2010;130(10):2412–22. 10.1038/jid.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Semnani RT, Keiser PB, Coulibaly YI, et al. : Filaria-induced monocyte dysfunction and its reversal following treatment. Infect Immun. 2006;74(8):4409–17. 10.1128/IAI.01106-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmelzer C, Niklowitz P, Okun JG, et al. : Ubiquinol-induced gene expression signatures are translated into altered parameters of erythropoiesis and reduced low density lipoprotein cholesterol levels in humans. IUBMB Life. 2011;63(1):42–8. 10.1002/iub.413 [DOI] [PubMed] [Google Scholar]
- 52. Smythies LE, Shen R, Bimczok D, et al. : Inflammation anergy in human intestinal macrophages is due to Smad-induced IkappaBalpha expression and NF-kappaB inactivation. J Biol Chem. 2010;285(25):19593–604. 10.1074/jbc.M109.069955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grigoryev YA, Kurian SM, Avnur Z, et al. : Deconvoluting post-transplant immunity: cell subset-specific mapping reveals pathways for activation and expansion of memory T, monocytes and B cells. PLoS One. 2010;5(10):e13358. 10.1371/journal.pone.0013358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen LY, Eberlein M, Alsaaty S, et al. : Cooperative and redundant signaling of leukotriene B 4 and leukotriene D 4 in human monocytes. Allergy. 2011;66(10):1304–11. 10.1111/j.1398-9995.2011.02647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu F, Lei W, O'Rourke JP, et al. : Oncogenic mutations cause dramatic, qualitative changes in the transcriptional activity of c-Myb. Oncogene. 2006;25(5):795–805. 10.1038/sj.onc.1209105 [DOI] [PubMed] [Google Scholar]
- 56. Allantaz F, Cheng DT, Bergauer T, et al. : Expression profiling of human immune cell subsets identifies miRNA-mRNA regulatory relationships correlated with cell type specific expression. PLoS One. 2012;7(1):e29979. 10.1371/journal.pone.0029979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Santer DM, Wiedeman AE, Teal TH, et al. : Plasmacytoid dendritic cells and C1q differentially regulate inflammatory gene induction by lupus immune complexes. J Immunol. 2012;188(2):902–15. 10.4049/jimmunol.1102797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hulsmans M, Geeraert B, De Keyzer D, et al. : Interleukin-1 receptor-associated kinase-3 is a key inhibitor of inflammation in obesity and metabolic syndrome. PLoS One. 2012;7(1):e30414. 10.1371/journal.pone.0030414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhen A, Krutzik SR, Levin BR, et al. : CD4 ligation on human blood monocytes triggers macrophage differentiation and enhances HIV infection. J Virol. 2014;88(17):9934–46. 10.1128/JVI.00616-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Irvine KM, Gallego P, An X, et al. : Peripheral blood monocyte gene expression profile clinically stratifies patients with recent-onset type 1 diabetes. Diabetes. 2012;61(5):1281–90. 10.2337/db11-1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schenk M, Krutzik SR, Sieling PA, et al. : NOD2 triggers an interleukin-32-dependent human dendritic cell program in leprosy. Nat Med. 2012;18(4):555–63. 10.1038/nm.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chauncey KM, Lopez MC, Sidhu G, et al. : Bacillus anthracis' lethal toxin induces broad transcriptional responses in human peripheral monocytes. BMC Immunol. 2012;13:33. 10.1186/1471-2172-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henig N, Avidan N, Mandel I, et al. : Interferon-beta induces distinct gene expression response patterns in human monocytes versus T cells. PLoS One. 2013;8(4):e62366. 10.1371/journal.pone.0062366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Haniffa M, Shin A, Bigley V, et al. : Human tissues contain CD141 hi cross-presenting dendritic cells with functional homology to mouse CD103 + nonlymphoid dendritic cells. Immunity. 2012;37(1):60–73. 10.1016/j.immuni.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hou W, Gibbs JS, Lu X, et al. : Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119(13):3128–31. 10.1182/blood-2011-09-379479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ganesh K, Das A, Dickerson R, et al. : Prostaglandin E 2 induces oncostatin M expression in human chronic wound macrophages through Axl receptor tyrosine kinase pathway. J Immunol. 2012;189(5):2563–73. 10.4049/jimmunol.1102762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chittezhath M, Dhillon MK, Lim JY, et al. : Molecular profiling reveals a tumor-promoting phenotype of monocytes and macrophages in human cancer progression. Immunity. 2014;41(5):815–29. 10.1016/j.immuni.2014.09.014 [DOI] [PubMed] [Google Scholar]
- 68. Ismail N, Wang Y, Dakhlallah D, et al. : Macrophage microvesicles induce macrophage differentiation and miR-223 transfer. Blood. 2013;121(6):984–95. 10.1182/blood-2011-08-374793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hyder LA, Gonzalez J, Harden JL, et al. : TREM-1 as a potential therapeutic target in psoriasis. J Invest Dermatol. 2013;133(7):1742–51. 10.1038/jid.2013.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teles RM, Graeber TG, Krutzik SR, et al. : Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339(6126):1448–53. 10.1126/science.1233665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wheelwright M, Kim EW, Inkeles MS, et al. : All- trans retinoic acid-triggered antimicrobial activity against Mycobacterium tuberculosis is dependent on NPC2. J Immunol. 2014;192(5):2280–90. 10.4049/jimmunol.1301686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shalova IN, Lim JY, Chittezhath M, et al. : Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1α. Immunity. 2015;42(3):484–98. 10.1016/j.immuni.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 73. Hamm A, Prenen H, Van Delm W, et al. : Tumour-educated circulating monocytes are powerful candidate biomarkers for diagnosis and disease follow-up of colorectal cancer. Gut. 2015; pii: gutjnl-2014-308988. 10.1136/gutjnl-2014-308988 [DOI] [PubMed] [Google Scholar]
- 74. Martinez FO, Helming L, Milde R, et al. : Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 2013;121(9):e57–69. 10.1182/blood-2012-06-436212 [DOI] [PubMed] [Google Scholar]
- 75. Levine AJ, Horvath S, Miller EN, et al. : Transcriptome analysis of HIV-infected peripheral blood monocytes: gene transcripts and networks associated with neurocognitive functioning. J Neuroimmunol. 2013;265(1–2):96–105. 10.1016/j.jneuroim.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Radom-Aizik S, Zaldivar FP , Jr, Haddad F, et al. : Impact of brief exercise on circulating monocyte gene and microRNA expression: implications for atherosclerotic vascular disease. Brain Behav Immun. 2014;39:121–9. 10.1016/j.bbi.2014.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kyogoku C, Smiljanovic B, Grün JR, et al. : Cell-specific type I IFN signatures in autoimmunity and viral infection: what makes the difference? PLoS One. 2013;8(12):e83776. 10.1371/journal.pone.0083776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tilton JC, Johnson AJ, Luskin MR, et al. : Diminished production of monocyte proinflammatory cytokines during human immunodeficiency virus viremia is mediated by type I interferons. J Virol. 2006;80(23):11486–97. 10.1128/JVI.00324-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wu JQ, Sassé TR, Saksena MM, et al. : Transcriptome analysis of primary monocytes from HIV-positive patients with differential responses to antiretroviral therapy. Virol J. 2013;10:361. 10.1186/1743-422X-10-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sharma S, Jin Z, Rosenzweig E, et al. : Widely divergent transcriptional patterns between SLE patients of different ancestral backgrounds in sorted immune cell populations. J Autoimmun. 2015;60:51–8. 10.1016/j.jaut.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Laudanski K, Miller-Graziano C, Xiao W, et al. : Cell-specific expression and pathway analyses reveal alterations in trauma-related human T cell and monocyte pathways. Proc Natl Acad Sci U S A. 2006;103(42):15564–9. 10.1073/pnas.0607028103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reynolds LM, Taylor JR, Ding J, et al. : Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat Commun. 2014;5: 5366. 10.1038/ncomms6366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mosig S, Rennert K, Büttner P, et al. : Monocytes of patients with familial hypercholesterolemia show alterations in cholesterol metabolism. BMC Med Genomics. 2008;1:60. 10.1186/1755-8794-1-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Däbritz J, Weinhage T, Varga G, et al. : Reprogramming of monocytes by GM-CSF contributes to regulatory immune functions during intestinal inflammation. J Immunol. 2015;194(5):2424–38. 10.4049/jimmunol.1401482 [DOI] [PubMed] [Google Scholar]
- 85. Elavazhagan S, Fatehchand K, Santhanam V, et al. : Granzyme B expression is enhanced in human monocytes by TLR8 agonists and contributes to antibody-dependent cellular cytotoxicity. J Immunol. 2015;194(6):2786–95. 10.4049/jimmunol.1402316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee J, Breton G, Oliveira TY, et al. : Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med. 2015;212(3):385–99. 10.1084/jem.20141442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bergenfelz C, Larsson AM, von Stedingk K, et al. : Systemic Monocytic-MDSCs Are Generated from Monocytes and Correlate with Disease Progression in Breast Cancer Patients. PLoS One. 2015;10(5):e0127028. 10.1371/journal.pone.0127028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Liu B, Dhanda A, Hirani S, et al. : CD14 ++CD16 + Monocytes Are Enriched by Glucocorticoid Treatment and Are Functionally Attenuated in Driving Effector T Cell Responses. J Immunol. 2015;194(11):5150–60. 10.4049/jimmunol.1402409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cho HJ, Shashkin P, Gleissner CA, et al. : Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics. 2007;29(2):149–60. 10.1152/physiolgenomics.00051.2006 [DOI] [PubMed] [Google Scholar]
- 90. Du X, Tang Y, Xu H, et al. : Genomic profiles for human peripheral blood T cells, B cells, natural killer cells, monocytes, and polymorphonuclear cells: comparisons to ischemic stroke, migraine, and Tourette syndrome. Genomics. 2006;87(6):693–703. 10.1016/j.ygeno.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 91. Meier P, Antonov J, Zbinden R, et al. : Non-invasive gene-expression-based detection of well-developed collateral function in individuals with and without coronary artery disease. Heart. 2009;95(11):900–8. 10.1136/hrt.2008.145383 [DOI] [PubMed] [Google Scholar]
- 92. Woszczek G, Chen LY, Nagineni S, et al. : Leukotriene D 4 induces gene expression in human monocytes through cysteinyl leukotriene type I receptor. J Allergy Clin Immunol. 2008;121(1):215–21.e1. 10.1016/j.jaci.2007.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu PT, Stenger S, Li H, et al. : Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 94. Stegmaier K, Ross KN, Colavito SA, et al. : Gene expression-based high-throughput screening(GE-HTS) and application to leukemia differentiation. Nat Genet. 2004;36(3):257–63. 10.1038/ng1305 [DOI] [PubMed] [Google Scholar]
- 95. Dower K, Ellis DK, Saraf K, et al. : Innate immune responses to TREM-1 activation: overlap, divergence, and positive and negative cross-talk with bacterial lipopolysaccharide. J Immunol. 2008;180(5):3520–34. 10.4049/jimmunol.180.5.3520 [DOI] [PubMed] [Google Scholar]