Abstract
Metastasis is the spread of cancer cells around the body and the cause of the majority of cancer deaths. Metastasis is a very complex process in which cancer cells need to dramatically modify their cytoskeleton and cope with different environments to successfully colonize a secondary organ. In this review, we discuss recent findings pointing at Rho-ROCK or actomyosin force (or both) as major drivers of many of the steps required for metastatic success. We propose that these are important drug targets that need to be considered in the clinic to palliate metastatic disease.
Keywords: Rho-ROCK, actomyosin, metastasis, Rho
Introduction
Metastatic disease is still largely incurable because of its systemic distribution and resistance to current therapies, and it is the cause of more than 90% of cancer-related deaths 1, 2. In spite of its clinical importance, the underlying cellular and molecular mechanisms of cancer metastasis are only partially understood 3. Thus, improved knowledge of how cancer cells acquire metastatic traits is necessary to unravel novel drug targets and prognostic markers of distant relapse.
Metastasis is a complex multi-stage process by which cancer cells disseminate from primary tumors, survive in distant sites and eventually grow as secondary tumors 3. The main events of the metastatic cascade involve loss of cell-cell contacts, cancer cell migration, local invasion of the surrounding extracellular matrix (ECM), interactions with stroma, intravasation and transit into blood or lymphatic vessels, arrest at secondary sites, extravasation, survival and colonization of distant sites 4. Genetic alterations and deregulation of critical oncogenic signaling pathways affecting survival, proliferation, apoptosis, and cell motility, regulate many of these complex metastatic events 3, 5. In addition, the interaction with the tumor microenvironment such as ECM, growth-supportive stromal cells, inflammatory cells and endothelial cells strongly impacts the metastatic capabilities of cancer cells 6, 7.
Many signaling pathways have been reported to have an impact on metastasis and have been the focus of excellent reviews 8– 15. In the present review, we will focus on Rho-ROCK signaling and actomyosin contractility, key regulators of several main steps in metastasis. Rho-ROCK, through its actions on cytoskeletal dynamics and through regulation of critical signaling pathways, controls several cellular processes important for metastasis such as cell migration, local invasion, survival at the secondary site, and tumor outgrowth 16– 18.
Rho GTPases and metastasis
The Rho family of small GTPases plays crucial roles in the regulation of the actin cytoskeleton, cell polarity, cell migration, cell proliferation, invasion, and metastasis 19. Rho GTPases act as molecular switches cycling between a guanosine triphosphate (GTP)-bound active state and guanosine diphosphate (GDP)-bound inactive state to translate extracellular signals into different cellular responses 19. Their activity is controlled by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) 18. The best studied and most conserved Rho family members across eukaryotic species are Ras-related C3 botulinum toxin substrate 1 (Rac1), cell division control protein 42 homolog (Cdc42), and Ras homolog gene family member A (RhoA) 18. Rac1 stimulates lamellipodia formation 20, whereas RhoA regulates the formation of stress fibers or favors amoeboid migration depending on the cellular context and the properties of the matrix. RhoA bound to GTP leads to activation of its effectors Rho-associated protein kinases (ROCK1 and ROCK2) 21– 23. ROCK1/2 serine/threonine kinases promote actomyosin contractile force generation by decreasing myosin phosphatase activity and thereby increasing phosphorylation of myosin light chain 2 (MLC2) 24. On the other hand, Cdc42 induces filopodia formation 25, but Cdc42 signaling can also generate actomyosin contraction through p21 protein (Cdc42/Rac)-activated kinase 2 (PAK2) and myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK) kinases 26, 27. Deregulation of the Rho-ROCK signaling pathway has been found in a variety of cancer types and in several cases correlates with disease progression 28– 30 ( Table 1). Furthermore, inhibition of ROCK signaling could suppress migration and invasion in vitro and impair the metastatic process in vivo, suggesting that ROCK inhibitors might be potential anti-metastatic agents 30– 32.
Table 1. Rho, ROCK or actomyosin contractility are implicated in all stages of the metastatic cascade and in major cancer types.
Shown are examples in the literature of where different stages of the metastatic cascade have been shown to be influenced by Rho-ROCK and/or actomyosin contractility signalling. (SCC= Squamous cell carcinoma)
| Cancer type | Step of metastatic dissemination | Reference |
|---|---|---|
| Breast, colon | Local invasion and migration | 40, 46, 52, 68 |
| Breast | Intravasation | 40, 66, 71 |
| Breast | Survival in circulation, adhesion to
vessels, early lung colonization |
92 |
| Oesophageal | Invasion and survival in circulation | 104 |
| Lung | Transendothelial migration | 81 |
| Prostate | Transendothelial migration | 75 |
| SCC | Fibroblast mediated invasion and
migration |
42 |
| Melanoma | Local invasion and migration | 23, 32, 45, 46, 49, 105 |
| Melanoma | Intravasation, extravasation, survival in
circulation, adhesion to vessels, early lung colonization |
23, 32, 48, 54, 58, 92, 95– 97, 105 |
Rho/ROCK signaling and actomyosin contractility in early dissemination
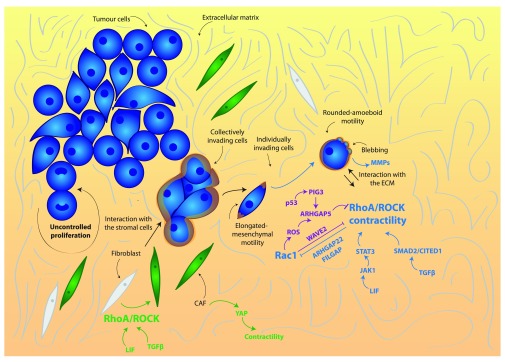
The ability of cancer cells to migrate into and invade surrounding tissue is a critical step in the metastatic cascade, which requires increased cell motility driven by altered cytoskeletal organization and contacts with the ECM and the stroma 33. Cancer cells can move either collectively or as individual cells 34, 35. The majority of tumors originate from epithelial tissues, and epithelial cancer cells that leave the primary tumor undergo a complex program called epithelial-mesenchymal transition (EMT). Incomplete or partial EMT allows collective migration in which cells can maintain cell-cell adhesions and migrate collectively in a coordinated manner as strands, sheets, or cell clusters. On the other hand, complete EMT is associated with the loss of cell-cell adhesions in favor of cell-ECM interactions and the concomitant acquisition of individual migratory characteristics 36, 37. After undergoing EMT, individual cancer cells can engage into elongated mesenchymal or rounded amoeboid modes of movement, distinguished by their different usage of signaling pathways. Mixed mesenchymal and amoeboid phenotypes have also been identified 38, 39. Individual cell migration seems to be required for blood-borne metastasis 40.
Rho/ROCK signaling and actomyosin contractility in cancer cells
Actomyosin contractility driven by Rho or ROCK signaling is key in controlling tumor dissemination, as all forms of cell migration require a certain degree of actomyosin force 34, 41. During collective cell migration, actomyosin contractility is high around the edges of groups of invading cancer cells, which generates pulling forces between the substrate and the follower cells, together with a prominent actomyosin ring at lateral regions of the groups to maintain coupling between cells and collective forward movement 42, 43. On the other hand, in individual migration, the contractile cortex is crucially important for amoeboid to intermediate forms of movement, and some degree of contractility is also required to retract protrusions in mesenchymal migration 39, 44– 46. The mesenchymal mode of movement is characterized by an elongated, spindle-like shape, high levels of adhesion, and Rac-dependent adhesive actin-rich protrusions 23, 46, 47. On the other side of the spectrum, in amoeboid migration, cancer cells adopt a rounded or irregular morphology with blebs as functional protrusions. Amoeboid motility is promoted by high levels of RhoA/Ras homolog gene family member C (RhoC) or ROCK-driven actomyosin contractility and requires lower levels of adhesion that allow higher speeds of movement 46– 50.
Cancer cell migration is a dynamic process, and individual cancer cells can switch between modes of movement to adapt to the changing microenvironment and facilitate tumor dissemination. Different cues will favor either a mesenchymal-amoeboid transition (MAT) or an amoeboid-mesenchymal transition (AMT) 23, 45, 49, 51, 52. Their core regulatory network is the mutually inhibitory circuit between Rac1 and Rho GTPase signaling in migrating cells ( Figure 1). Higher Rac1 activity promotes cell elongation and permits long actin-rich protrusions characteristic of mesenchymal migration. Moreover, active Rac1 negatively regulates Rho or ROCK signaling and suppresses amoeboid movement. On the other hand, active Rho or ROCK supports bleb-based amoeboid migration 23, 45, 49, 51, 52 and limits excessive Rac1-dependent adhesion via regulation of the Rac GAPs ARHGAP22 and filamin-A-associated Rho GTPase activation protein (FilGAP) 23, 53. Furthermore, cancer cells control amoeboid migration at the transcriptional level under circumstances in which matrix compliance allows sustained actomyosin contractility ( Figure 1). Different chemical cues have been shown to control this process. For instance, amoeboid melanoma cells support contractility, establishing a positive feedback loop with the cytokines leukemia inhibitory factor (LIF)/IL6 and the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway to maintain Rho-ROCK activity 49. As a result of high STAT3 activity, very contractile cells secrete different factors, including matrix metalloprotease 9 (MMP-9). MMP-9 promotes the generation of actomyosin contractile force and bleb-driven migration through a positive feedback loop via CD44 binding and increased MLC2 phosphorylation to sustain amoeboid invasion 48. Moreover, amoeboid contractile cells secrete high levels of transforming growth factor beta (TGFβ), and downstream of it a Sma- and Mad-related protein 2 (SMAD2)-Cbp/P300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain (CITED1) transcriptional network sustains actomyosin contractility 54. In addition, the physical properties of the matrix play an important role in establishing a balance between actomyosin levels and adhesion to regulate optimal migration efficiency 34, 39, 47, 55, 56. Increased ECM density results in increased matrix stiffness, in which cells sense and respond by increasing Rho-mediated actomyosin contractility 57. Furthermore, slow mesenchymal cells can switch to fast amoeboid migrating modes under conditions of low adhesiveness and high physical confinement 47, 56.
Figure 1. Rho/ROCK and actomyosin contractility in early dissemination.
ROCK-driven actomyosin contractility is stimulated by extracellular signals such as leukemia inhibitory factor (LIF) and transforming growth factor beta (TGFβ) to promote rounded amoeboid cancer cell motility. Rounded amoeboid cells display blebbing as well as high levels of actomyosin contractility and a rounded morphology. They interact with the extracellular matrix (ECM) by physically deforming it and by secreting metalloproteases (MMPs). In the stroma, ROCK-driven actomyosin contractility promotes the transformation of fibroblasts into cancer-associated fibroblasts (CAFs), driven by Yes-associated protein (YAP) as well as by extracellular factors. Blue indicates positive regulators of contractility, purple indicates negative regulators of contractility and orange lines indicate actomyosin contractility. Abbreviations: CAF, carcinoma-associated fibroblasts; CITED1, Cbp/P300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain, 1; FilGAP, filamin-A-associated Rho GTPase activation protein; JAK, Janus kinase; RhoA, Ras homolog gene family member A; ROCK, Rho-associated protein kinase; SMAD2, Sma- and Mad-related protein 2; STAT3, signal transducer and activator of transcription 3.
The ability to switch between different modes of migration is an important factor for metastatic dissemination, as cancer cells have to migrate through a range of ECMs to escape the primary tumor and spread to distant organs. Therefore, anti-metastatic treatments should target the ability of tumor cells to cope with such variability. Recently, it has been described that potent ROCK inhibitors are able to strongly inhibit actomyosin contractility and collapse the actomyosin cytoskeleton, blocking both mesenchymal and amoeboid modes of movement 32.
Intra-vital imaging studies have shown that bleb-driven highly contractile amoeboid migration is favored in the invasive fronts of melanomas and breast cancers 23, 29, 45, 48, 49, 58. Furthermore, in these studies, it has been shown that treatment with ROCK inhibitors or actomyosin perturbations (or both) is able to decrease tumor cell motility in vivo 23, 29, 32, 45, 49, 58. Hence, ROCK inhibition could effectively impair local invasion and dissemination of cancer cells ( Figure 1).
Rho/ROCK signaling and actomyosin contractility in the stroma
Within the tumor, a variety of non-cancer stromal cells interact with the cancer cells promoting tumorigenesis 7. Actomyosin contractility not only is fundamental for cancer cell migration and invasion but also is crucial for maintenance of the carcinoma-associated fibroblasts (CAFs) phenotype, an important stromal component in the tumor microenvironment 7. Actomyosin contractility activated by ROCK signaling and the LIF/JAK/STAT pathway is crucial for CAF-dependent pro-invasive physical remodeling of the ECM favoring tumor aggressiveness and dissemination 42, 49, 59, 60. Additionally, actomyosin contractility, Src function, and matrix stiffening induced by TGFβ, are required for Yes-associated protein (YAP) activation in CAFs to promote ECM remodeling and cancer cell invasion, and to generate a positive feedback loop that helps to maintain the CAF phenotype 61 ( Figure 1). Moreover, contractility in CAFs has been shown to modulate EMT and metastasis-initiating cell properties in breast cancer models 62.
Therefore, some degree of actomyosin contractility is essential for both cancer cells and stroma for efficient cell movement in the initial steps of the metastatic cascade 34, 41, 49, 59, 61, and some factors such as TGFβ and LIF can stimulate contractility both in cancer cells and in fibroblasts.
Rho/ROCK signaling and actomyosin contractility in transendothelial migration
After local invasion within the primary tumor microenvironment, cancer cells need to spread throughout the body and colonize new organs to form metastases. They do so by exploiting the vascular and lymphatic systems. The process through which cancer cells enter and exit vessels crossing the endothelial layer is known as transendothelial migration, which is extremely complex and involves the interaction with several different cell types, such as platelets, immune cells and endothelial cells, and the activation of a variety of signaling pathways 63. These events are in some cases similar to those occurring during inflammation or infection, when immune cells need to enter and exit vessels. In fact, parallels between cancer cell and immune cell migration allow for interesting speculation in areas of cancer cell dissemination that are still not fully understood.
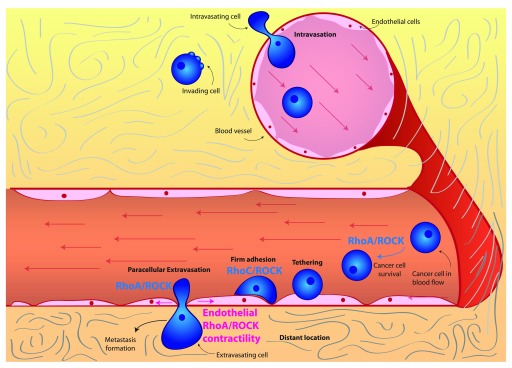
Intravasation. The first step in this metastatic cascade is intravasation, the entry of tumor cells into blood vessels. Intravasation depends on the weakening of cell-cell junctions between endothelial cells, which allows cancer cells to squeeze in between adjacent endothelial cells and enter the vessel lumen 63. From a molecular perspective, not as much is known about intravasation compared with other steps in the metastatic cascade as this is an experimentally challenging step to study 64, 65. In fact, intravasation is dependent on the ability of cancer cells to invade towards blood vessels, so it is difficult to distinguish between genes involved in invasion and intravasation 63. RhoA signaling has been linked to the process of intravasation 66 ( Figure 2). Specifically, RhoA activity in cancer cells is thought to be stimulated by macrophage contact and leads to the formation of invadopodia. Invadopodia are instrumental in the degradation and eventual breakdown of the matrix barrier, which allows for tumor cell intravasation. Furthermore, highly contractile, rounded amoeboid melanoma cells have been shown to intravasate more efficiently than low-contractility elongated cells in vivo 67, 68. Once in the bloodstream, cells are transported throughout the body by the blood flow ( Figure 2).
Figure 2. Rho/ROCK and actomyosin contractility in intravasation and extravasation.
RhoC/ROCK signaling promotes survival of cancer cells in the blood flow as well as adhesion to the endothelium and extravasation. ROCK-driven actomyosin contractility within endothelial cells can be stimulated by secreted factors and is essential for cancer cell extravasation. Abbreviations: RhoC, Ras homolog gene family member C; ROCK, Rho-associated protein kinase.
Extravasation. Eventually, cancer cells flowing through the bloodstream need to exit blood vessels to form secondary tumors. This process is known as extravasation and entails several sequential steps. First of all, cancer cells form loose adhesions to the vascular endothelium, which is known as tethering. These loose adhesions then are tightened to form firm adhesions: firmly adhering cells then can cross the endothelial barrier and extravasate 63.
The best-studied mechanism for extravasation is known as paracellular extravasation, during which cancer cells exit the vessel by squeezing in between endothelial cells. An alternative mechanism for cancer cell extravasation is transcellular extravasation, where tumor cells exit the vessel by going through endothelial cells 63, 69. Transcellular extravasation has been observed in immune cells 70 and has also been identified in cancer cells, where it probably plays a role in some cases 71.
Rho/ROCK signaling and actomyosin contractility in cancer cells
Rho or ROCK-driven actomyosin contractility within extravasating cells has been shown to play an important role. For instance, in monocytes, RhoA activity has been shown to be necessary for tail retraction during extravasation 72. In the context of transcellular extravasation, monocytes can rely on RhoA and ROCK signaling 73, 74.
On the other hand, in prostate cancer cells, it is RhoC and ROCK signaling that is essential for interaction with endothelial cells, promoting adhesion and paracellular extravasation 75. As a result of its role in promoting extravasation, RhoC signaling is a key driver of tumor dissemination and metastasis 75, in part explaining how RhoC was one of the first genes identified as a metastasis driver 76. Furthermore, RhoA and RhoC have been shown to drive adhesion to the endothelium and transendothelial migration in breast and prostate cancer cells 77, 78. Consequently, rounded-amoeboid cancer cells with high levels of RhoA or ROCK-driven actomyosin contractility are more efficient during transendothelial migration than elongated cells both in vitro and in vivo 67, 68, 79. Additional evidence supporting the importance of RhoA-driven contractility in transendothelial migration comes from studies examining the role of RhoA regulators. For instance, FilGAP, a Rac GAP, promotes RhoA signaling and rounded-amoeboid motility by suppressing Rac, and as a consequence it enhances in vivo extravasation of breast cancer cells 53. Conversely, the RhoA GAP ARHGAP7 has been shown to be a negative regulator of transendothelial migration in thymic lymphoma 80.
Cancer cells that successfully extravasate need to cross the vascular basement membrane that surrounds the vessel 63. Since actomyosin contractility has been shown to promote the secretion of proteases in rounded amoeboid cells 48, it is tempting to speculate that highly contractile extravasating cells could have an advantage when crossing the vascular basement membrane.
Rho/ROCK signaling and actomyosin contractility in endothelial cells
In order for paracellular extravasation to occur, cancer cells need to weaken cell-cell junctions within the endothelium. This can be mediated by regulating Rho or ROCK signaling and actomyosin contractility within the endothelial cells themselves ( Figure 2). Lung cancer cells have been shown to induce adherens junction disassembly by stimulating actomyosin contractility through Rho/ROCK in endothelial cells 81. Furthermore, thrombin stimulation of endothelial cells has been shown to induce ROCK activity and subsequently lead to cytoskeletal remodeling, junction disruption, and endothelial permeability 82, 83. Tumor-derived thrombin induces endothelial gap formation and transendothelial migration 84. Furthermore, cancer cells have been shown to use thrombin within blood vessels in order to promote metastasis 85. This prompts the speculation that actomyosin contraction in endothelial cells could be controlled by thrombin produced by cancer cells.
As well as leading to junction disassembly, actomyosin contractility in endothelial cells allows for endothelial cell retraction 86, 87, which increases endothelial permeability. Moreover, ROCK-driven actomyosin contractility in endothelial cells has been shown to prevent endothelial cell re-spreading downstream of ephrin-B signaling, which maintains increased endothelial permeability 88. Conversely, ROCK inhibition has been shown to decrease endothelial permeability after hemorrhage 89, 90. Although these studies have not been conducted in cancer models, ROCK activity in endothelial cells could be similarly regulated while in contact with disseminating cancer cells.
In brief, we speculate that the ability of cancer cells to form secondary tumors is to a certain extent dependent on their ability to manipulate the cytoskeleton of endothelial cells; thus, increasing endothelial permeability could be a crucial step to promote extravasation. More work is needed to validate the roles of Rho/ROCK or actomyosin contractility (or both) in tumor cells during both cancer intravasation and extravasation.
Rho/ROCK signaling and actomyosin contractility in metastatic colonization
Following extravasation at secondary sites, cancer cells that survive can form micro-metastasis and colonize new sites. In order for this colonization to take place, cancer cells must be able to adhere to endothelial cells, extravasate, survive and proliferate at the secondary site. The first few hours of colonization are crucial in determining the success of this process, as cells will undergo apoptosis if they do not adhere to their new niche. Furthermore, once established, cells must be able to evade the immune response in order to survive 91. Although we have discussed that Rho/ROCK signaling is important for early dissemination, there is also evidence to suggest that Rho/ROCK signaling, actomyosin contractility or its regulators, or a combination of these are important for efficient colonization at secondary sites.
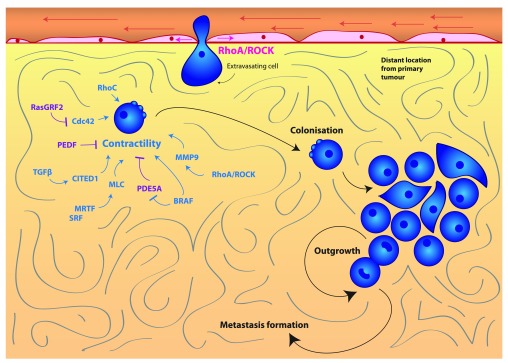
In vivo studies where cancer cells are injected intravenously (i.e., experimental metastasis assays) show that high levels of actomyosin contractility play a role in seeding of and colonizing the lung. For instance, cells selected for efficient colonization in the lung such as the highly metastatic A375M2 melanoma cell line have higher levels of RhoC 76, RhoA 23 and phosphorylated MLC2 48 when compared with low metastatic A375P melanoma cells.
Several studies have confirmed the importance of the initial hours in seeding during colonization. For example, serum response factor (SRF) co-activators myocardin-related transcription factors (MRTFs) are able to control the expression of MLC2 92 ( Figure 3). MRTF and SRF are both important for early stages of lung colonization in breast cancer and melanoma 92. Furthermore, depletion of MLC2 itself has also been shown to reduce lung colonization 92. Conversely, enhanced actomyosin contractility favors colonization: for example, depletion of the actomyosin contractility suppressors Rac1 and its GEF dedicator of cytokinesis 3 (DOCK3) favors early lung colonization 23. In melanoma, pigment epithelium-derived factor (PEDF) reduces lung colonization and suppresses lung tumor outgrowth 93, 94. PEDF is a negative regulator of Rho-ROCK signaling through supporting DOCK3-Rac1 activity 95 ( Figure 3). Furthermore, oncogenic BRAF suppresses phosphodiesterase 5A (PDE5A), which in turn inhibits actomyosin contractility 96 ( Figure 3). Therefore, re-expression of PDE5A reduces the ability of melanoma cells to colonize the lung and prevents short-term survival and long-term cancer growth in the lung 96.
Figure 3. Rho/ROCK and actomyosin contractility in colonization and metastasis.
Actomyosin contractility promotes cancer cell colonization and outgrowth at a secondary site to form metastases. Contractility is under the control of a wide variety of pathways, including SRF/MRTF, TGFβ-SMAD-CITED1, MMP-9, BRAF-V600E and Cdc42 signaling. Blue indicates positive regulators of contractility, and purple indicates negative regulators of contractility. Abbreviations: CITED1, Cbp/P300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain, 1; Cdc42, cell division control protein 42 homolog; MLC2, myosin light chain 2; MMP, matrix metallopeptidase; MRTF, myocardin-related transcription factors; PDE5A, phosphodiesterase 5A; PEDF, pigment epithelium-derived factor; RasGRF2, Ras protein-specific guanine nucleotide-releasing factor 2; ROCK, Rho-associated protein kinase; SMAD, Sma- and Mad-related protein; SRF, serum response factor; TGFβ, transforming growth factor beta.
As mentioned earlier, Cdc42 can also promote actomyosin contractility in cancer cells 26. Further evidence of the importance of actomyosin contractility in early colonization has been shown by experiments in which loss of Ras protein-specific guanine nucleotide-releasing factor 2 (RasGRF2), an inhibitor of Cdc42 97, enhanced colonization of the lungs in a Rac-independent manner. This was associated with higher actomyosin contractility levels 97 ( Figure 3).
TGFβ signaling plays an important role in promoting cancer cell colonization 40, 54, 98 ( Figure 3). We recently found that TGFβ increases actomyosin contractility in melanoma cells 54. While TGFβ is known to promote EMT 99 in epithelial cancers, in melanoma TGFβ signals through SMAD2 and the adaptor CITED1 to support contractile amoeboid migration 54. TGFβ no longer sustains lung colonization in melanoma cells if the SMAD2-CITED1 axis is not functional 54, which serves to highlight the multiple levels in which actomyosin contractility promotes colonization.
Furthermore, ROCK regulates expression of several MMPs, including MMP-9, which promote early stages of lung colonization 48 ( Figure 3). While MMPs exert their catalytic function in degradation of the ECM during local invasion, the non-catalytic roles of MMP-9 could promote the survival of cancer cells at the metastatic secondary sites. For example, it has been shown that non-catalytic functions of MMP-9 regulate STAT3 functions to drive survival in B-cell chronic lymphocytic leukemia (B-CLL) cells 100.
From these results, it is clear that positive and negative regulators of Rho/ROCK signaling or actomyosin contractility (or both) are critical for cancer cells to efficiently colonize the metastatic sites in experimental metastasis models.
We have highlighted the crucial role that Rho/ROCK signaling or actomyosin contractility play in dissemination and metastatic colonization using a range of experimental cancer models. A highly contractile phenotype is clearly critical for effective cancer colonization, ultimately supporting the idea of developing drugs to inhibit actomyosin contractility. In vivo validation of the role of Rho/ROCK signaling or actomyosin contractility (or both) in metastasis is important to qualify these signaling modules as potential drug targets. Experimental metastasis models are insightful for understanding the processes of extravasation and colonization to the lungs, but recapitulation of the entire metastatic cascade, including local invasion, dissemination and intravasation, requires the use of spontaneous metastasis models 101. Indeed, it has recently been shown that a new class of ROCK inhibitors has the ability to prevent both experimental and spontaneous metastases formation 32. It will be of great importance to combine these mouse models with non-invasive cell-tracking techniques 102, 103 to understand the entire process and how early Rho/ROCK signaling should be targeted in order to effectively block the metastatic cascade.
Abbreviations
CAF, carcinoma-associated fibroblasts; CITED1, Cbp/P300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain, 1; Cdc42, cell division control protein 42 homolog; DOCK3, dedicator of cytokinesis 3; ECM, extracellular matrix; EMT, epithelial-mesenchymal transition; FilGAP, filamin-A-associated Rho GTPase activation protein; GEF, guanine nucleotide exchange factor; GAP, GTPase activation protein; GTP, guanosine triphosphate; JAK, Janus kinase; LIF, leukemia inhibitory factor; MLC2, myosin light chain 2; MMP, matrix metallopeptidase; MRTF, myocardin-related transcription factors; PDE5A, phosphodiesterase 5A; PEDF, pigment epithelium-derived factor; Rac1, Ras-related C3 botulinum toxin substrate 1; RasGRF2, Ras protein-specific guanine nucleotide-releasing factor 2; RhoA, Ras homolog gene family member A; RhoC, Ras homolog gene family member C; ROCK, Rho-associated protein kinase; SMAD2, Sma- and Mad-related protein 2; SRF, serum response factor; STAT, signal transducer and activator of transcription; TGFβ, transforming growth factor beta.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Michael Olson, Cancer Research UK Beatson Institute, Glasgow, UK
Martin A Schwartz, Department of Internal Medicine, Cardiovascular Research Center, Yale University School of Medicine, New Haven, CT, USA
Funding Statement
This work was supported by Cancer Research UK C33043/A12065 (Victoria Sanz-Moreno and Irene Rodriguez-Hernandez) and Royal Society RG110591 (Victoria Sanz-Moreno). Irene Rodriguez-Hernandez is supported by Fundacion Alfonso Martin Escudero, Gaia Cantelli by the Medical Research Council (C97993H), and Bruce Fanshawe by the King’s Bioscience Institute and the Guy’s and St Thomas’ Charity Prize PhD Program in Biomedical and Translational Science.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Valastyan S, Weinberg RA: Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–92. 10.1016/j.cell.2011.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gupta GP, Massagué J: Cancer metastasis: building a framework. Cell. 2006;127(4):679–95. 10.1016/j.cell.2006.11.001 [DOI] [PubMed] [Google Scholar]
- 3. Vanharanta S, Massagué J: Origins of metastatic traits. Cancer Cell. 2013;24(4):410–21. 10.1016/j.ccr.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Talmadge JE, Fidler IJ: AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70(14):5649–69. 10.1158/0008-5472.CAN-10-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim S: New and emerging factors in tumorigenesis: an overview. Cancer Manag Res. 2015;7:225–39. 10.2147/CMAR.S47797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Psaila B, Lyden D: The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–93. 10.1038/nrc2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quail DF, Joyce JA: Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anastas JN, Moon RT: WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. 10.1038/nrc3419 [DOI] [PubMed] [Google Scholar]
- 9. Padua D, Massagué J: Roles of TGFbeta in metastasis. Cell Res. 2009;19(1):89–102. 10.1038/cr.2008.316 [DOI] [PubMed] [Google Scholar]
- 10. Wakefield LM, Hill CS: Beyond TGFβ: roles of other TGFβ superfamily members in cancer. Nat Rev Cancer. 2013;13(5):328–41. 10.1038/nrc3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen DX, Massagué J: Genetic determinants of cancer metastasis. Nat Rev Genet. 2007;8(5):341–52. 10.1038/nrg2101 [DOI] [PubMed] [Google Scholar]
- 12. Alizadeh AM, Shiri S, Farsinejad S: Metastasis review: from bench to bedside. Tumour Biol. 2014;35(9):8483–523. 10.1007/s13277-014-2421-z [DOI] [PubMed] [Google Scholar]
- 13. Powell E, Piwnica-Worms D, Piwnica-Worms H: Contribution of p53 to metastasis. Cancer Discov. 2014;4(4):405–14. 10.1158/2159-8290.CD-13-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Polivka J, Jr, Janku F: Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142(2):164–75. 10.1016/j.pharmthera.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 15. Jiang WG, Sanders AJ, Katoh M, et al. : Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35(Suppl):S244–75. 10.1016/j.semcancer.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 16. Orgaz JL, Herraiz C, Sanz-Moreno V: Rho GTPases modulate malignant transformation of tumor cells. Small GTPases. 2014;5(4):e29019. 10.4161/sgtp.29019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rath N, Olson MF: Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012;13(10):900–8. 10.1038/embor.2012.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ridley AJ: Rho GTPase signalling in cell migration. Curr Opin Cell Biol. 2015;36:103–12. 10.1016/j.ceb.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaffe AB, Hall A: Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. 10.1146/annurev.cellbio.21.020604.150721 [DOI] [PubMed] [Google Scholar]
- 20. Ridley AJ, Paterson HF, Johnston CL, et al. : The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70(3):401–10. 10.1016/0092-8674(92)90164-8 [DOI] [PubMed] [Google Scholar]
- 21. Amano M, Chihara K, Kimura K, et al. : Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275(5304):1308–11. 10.1126/science.275.5304.1308 [DOI] [PubMed] [Google Scholar]
- 22. Kimura K, Ito M, Amano M, et al. : Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science. 1996;273(5272):245–8. 10.1126/science.273.5272.245 [DOI] [PubMed] [Google Scholar]
- 23. Sanz-Moreno V, Gadea G, Ahn J, et al. : Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135(3):510–23. 10.1016/j.cell.2008.09.043 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Ito M, Nakano T, Erdodi F, et al. : Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259(1–2):197–209. 10.1023/B:MCBI.0000021373.14288.00 [DOI] [PubMed] [Google Scholar]
- 25. Nobes CD, Hall A: Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62. 10.1016/0092-8674(95)90370-4 [DOI] [PubMed] [Google Scholar]
- 26. Gadea G, Sanz-Moreno V, Self A, et al. : DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells. Curr Biol. 2008;18(19):1456–65. 10.1016/j.cub.2008.08.053 [DOI] [PubMed] [Google Scholar]
- 27. Wilkinson S, Paterson HF, Marshall CJ: Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7(3):255–61. 10.1038/ncb1230 [DOI] [PubMed] [Google Scholar]
- 28. Sahai E, Marshall CJ: RHO-GTPases and cancer. Nat Rev Cancer. 2002;2(2):133–42. 10.1038/nrc725 [DOI] [PubMed] [Google Scholar]
- 29. Herraiz C, Calvo F, Pandya P, et al. : Reactivation of p53 by a Cytoskeletal Sensor to Control the Balance Between DNA Damage and Tumor Dissemination. J Natl Cancer Inst. 2016;108(1): pii: djv289. 10.1093/jnci/djv289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu S, Goldstein RH, Scepansky EM, et al. : Inhibition of rho-associated kinase signaling prevents breast cancer metastasis to human bone. Cancer Res. 2009;69(22):8742–51. 10.1158/0008-5472.CAN-09-1541 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Patel RA, Liu Y, Wang B, et al. : Identification of novel ROCK inhibitors with anti-migratory and anti-invasive activities. Oncogene. 2014;33(5):550–5. 10.1038/onc.2012.634 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Sadok A, McCarthy A, Caldwell J, et al. : Rho kinase inhibitors block melanoma cell migration and inhibit metastasis. Cancer Res. 2015;75(11):2272–84. 10.1158/0008-5472.CAN-14-2156 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Hanahan D, Weinberg RA: Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Friedl P, Wolf K: Plasticity of cell migration: a multiscale tuning model. J Cell Biol. 2010;188(1):11–9. 10.1083/jcb.200909003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolf K, Wu YI, Liu Y, et al. : Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9(8):893–904. 10.1038/ncb1616 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Thiery JP, Acloque H, Huang RY, et al. : Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 37. Tsai JH, Yang J: Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–206. 10.1101/gad.225334.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedl P, Alexander S: Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009. 10.1016/j.cell.2011.11.016 [DOI] [PubMed] [Google Scholar]
- 39. Lämmermann T, Sixt M: Mechanical modes of 'amoeboid' cell migration. Curr Opin Cell Biol. 2009;21(5):636–44. 10.1016/j.ceb.2009.05.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Giampieri S, Manning C, Hooper S, et al. : Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11(11):1287–96. 10.1038/ncb1973 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Sanz-Moreno V, Marshall CJ: The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol. 2010;22(5):690–6. 10.1016/j.ceb.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 42. Gaggioli C, Hooper S, Hidalgo-Carcedo C, et al. : Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–400. 10.1038/ncb1658 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Friedl P, Wolf K, Zegers MM: Rho-directed forces in collective migration. Nat Cell Biol. 2014;16(3):208–10. 10.1038/ncb2923 [DOI] [PubMed] [Google Scholar]
- 44. Charras GT, Hu CK, Coughlin M, et al. : Reassembly of contractile actin cortex in cell blebs. J Cell Biol. 2006;175(3):477–90. 10.1083/jcb.200602085 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Tozluoğlu M, Tournier AL, Jenkins RP, et al. : Matrix geometry determines optimal cancer cell migration strategy and modulates response to interventions. Nat Cell Biol. 2013;15(7):751–62. 10.1038/ncb2775 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Sahai E, Marshall CJ: Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5(8):711–9. 10.1038/ncb1019 [DOI] [PubMed] [Google Scholar]
- 47. Liu YJ, Le Berre M, Lautenschlaeger F, et al. : Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell. 2015;160(4):659–72. 10.1016/j.cell.2015.01.007 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Orgaz JL, Pandya P, Dalmeida R, et al. : Diverse matrix metalloproteinase functions regulate cancer amoeboid migration. Nat Commun. 2014;5: 4255. 10.1038/ncomms5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sanz-Moreno V, Gaggioli C, Yeo M, et al. : ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20(2):229–45. 10.1016/j.ccr.2011.06.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Lorentzen A, Bamber J, Sadok A, et al. : An ezrin-rich, rigid uropod-like structure directs movement of amoeboid blebbing cells. J Cell Sci. 2011;124(Pt 8):1256–67. 10.1242/jcs.074849 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Pinner S, Sahai E: Imaging amoeboid cancer cell motility in vivo. J Microsc. 2008;231(3):441–5. 10.1111/j.1365-2818.2008.02056.x [DOI] [PubMed] [Google Scholar]
- 52. Wyckoff JB, Pinner SE, Gschmeissner S, et al. : ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16(15):1515–23. 10.1016/j.cub.2006.05.065 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Saito K, Ozawa Y, Hibino K, et al. : FilGAP, a Rho/Rho-associated protein kinase-regulated GTPase-activating protein for Rac, controls tumor cell migration. Mol Biol Cell. 2012;23(24):4739–50. 10.1091/mbc.E12-04-0310 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Cantelli G, Orgaz JL, Rodriguez-Hernandez I, et al. : TGF-β-Induced Transcription Sustains Amoeboid Melanoma Migration and Dissemination. Curr Biol. 2015;25(22):2899–914. 10.1016/j.cub.2015.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doyle AD, Petrie RJ, Kutys ML, et al. : Dimensions in cell migration. Curr Opin Cell Biol. 2013;25(5):642–9. 10.1016/j.ceb.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ruprecht V, Wieser S, Callan-Jones A, et al. : Cortical contractility triggers a stochastic switch to fast amoeboid cell motility. Cell. 2015;160(4):673–85. 10.1016/j.cell.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Provenzano PP, Keely PJ: Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. J Cell Sci. 2011;124(Pt 8):1195–205. 10.1242/jcs.067009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pinner S, Sahai E: PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10(2):127–37. 10.1038/ncb1675 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Albrengues J, Bourget I, Pons C, et al. : LIF mediates proinvasive activation of stromal fibroblasts in cancer. Cell Rep. 2014;7(5):1664–78. 10.1016/j.celrep.2014.04.036 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Albrengues J, Bertero T, Grasset E, et al. : Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat Commun. 2015;6: 10204. 10.1038/ncomms10204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Calvo F, Ege N, Grande-Garcia A, et al. : Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15(6):637–46. 10.1038/ncb2756 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Del Pozo Martin Y, Park D, Ramachandran A, et al. : Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell Rep. 2015;13(11):2456–69. 10.1016/j.celrep.2015.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Reymond N, d'Água BB, Ridley AJ: Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–70. 10.1038/nrc3628 [DOI] [PubMed] [Google Scholar]
- 64. Harney AS, Arwert EN, Entenberg D, et al. : Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2 hi Macrophage-Derived VEGFA. Cancer Discov. 2015;5(9):932–43. 10.1158/2159-8290.CD-15-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patsialou A, Bravo-Cordero JJ, Wang Y, et al. : Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. Intravital. 2013;2(2):e25294. 10.4161/intv.25294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, et al. : Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2014;33(33):4203–12. 10.1038/onc.2013.377 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Sahai E: Illuminating the metastatic process. Nat Rev Cancer. 2007;7(10):737–49. 10.1038/nrc2229 [DOI] [PubMed] [Google Scholar]
- 68. Sahai E, Garcia-Medina R, Pouysségur J, et al. : Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J Cell Biol. 2007;176(1):35–42. 10.1083/jcb.200605135 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Tremblay PL, Huot J, Auger FA: Mechanisms by which E-selectin regulates diapedesis of colon cancer cells under flow conditions. Cancer Res. 2008;68(13):5167–76. 10.1158/0008-5472.CAN-08-1229 [DOI] [PubMed] [Google Scholar]
- 70. Engelhardt B, Wolburg H: Mini-review: Transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34(11):2955–63. 10.1002/eji.200425327 [DOI] [PubMed] [Google Scholar]
- 71. Khuon S, Liang L, Dettman RW, et al. : Myosin light chain kinase mediates transcellular intravasation of breast cancer cells through the underlying endothelial cells: a three-dimensional FRET study. J Cell Sci. 2010;123(Pt 3):431–40. 10.1242/jcs.053793 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Worthylake RA, Lemoine S, Watson JM, et al. : RhoA is required for monocyte tail retraction during transendothelial migration. J Cell Biol. 2001;154(1):147–60. 10.1083/jcb.200103048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Honing H, van den Berg TK, van der Pol SM, et al. : RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukoc Biol. 2004;75(3):523–8. 10.1189/jlb.0203054 [DOI] [PubMed] [Google Scholar]
- 74. Heasman SJ, Carlin LM, Cox S, et al. : Coordinated RhoA signaling at the leading edge and uropod is required for T cell transendothelial migration. J Cell Biol. 2010;190(4):553–63. 10.1083/jcb.201002067 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Reymond N, Im JH, Garg R, et al. : RhoC and ROCKs regulate cancer cell interactions with endothelial cells. Mol Oncol. 2015;9(6):1043–55. 10.1016/j.molonc.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Clark EA, Golub TR, Lander ES, et al. : Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406(6795):532–5. 10.1038/35020106 [DOI] [PubMed] [Google Scholar]
- 77. Reymond N, Im JH, Garg R, et al. : Cdc42 promotes transendothelial migration of cancer cells through β1 integrin. J Cell Biol. 2012;199(4):653–68. 10.1083/jcb.201205169 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Yagi H, Tan W, Dillenburg-Pilla P, et al. : A synthetic biology approach reveals a CXCR4-G 13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal. 2011;4(191):ra60. 10.1126/scisignal.2002221 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Borrull A, Ghislin S, Deshayes F, et al. : Nanog and Oct4 overexpression increases motility and transmigration of melanoma cells. J Cancer Res Clin Oncol. 2012;138(7):1145–54. 10.1007/s00432-012-1186-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Sabbir MG, Prieditis H, Ravinsky E, et al. : The role of Dlc1 isoform 2 in K-Ras2 G12D induced thymic cancer. PLoS One. 2012;7(7):e40302. 10.1371/journal.pone.0040302 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Li B, Zhao WD, Tan ZM, et al. : Involvement of Rho/ROCK signalling in small cell lung cancer migration through human brain microvascular endothelial cells. FEBS Lett. 2006;580(17):4252–60. 10.1016/j.febslet.2006.06.056 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Gavard J, Gutkind JS: Protein kinase C-related kinase and ROCK are required for thrombin-induced endothelial cell permeability downstream from Galpha12/13 and Galpha 11/q. J Biol Chem. 2008;283(44):29888–96. 10.1074/jbc.M803880200 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. van Nieuw Amerongen GP, van Delft S, Vermeer MA, et al. : Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87(4):335–40. 10.1161/01.RES.87.4.335 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 84. Zhang P, Feng S, Liu G, et al. : Mutant B-Raf(V600E) Promotes Melanoma Paracellular Transmigration by Inducing Thrombin-mediated Endothelial Junction Breakdown. J Biol Chem. 2016;291(5):2087–106. 10.1074/jbc.M115.696419 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Nierodzik ML, Karpatkin S: Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10(5):355–62. 10.1016/j.ccr.2006.10.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Riento K, Ridley AJ: Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–56. 10.1038/nrm1128 [DOI] [PubMed] [Google Scholar]
- 87. Wysolmerski RB, Lagunoff D: Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci U S A. 1990;87(1):16–20. 10.1073/pnas.87.1.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Groeger G, Nobes CD: Co-operative Cdc42 and Rho signalling mediates ephrinB-triggered endothelial cell retraction. Biochem J. 2007;404(1):23–9. 10.1042/BJ20070146 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Huang B, Krafft PR, Ma Q, et al. : Fibroblast growth factors preserve blood-brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol Dis. 2012;46(1):204–14. 10.1016/j.nbd.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Fujii M, Duris K, Altay O, et al. : Inhibition of Rho kinase by hydroxyfasudil attenuates brain edema after subarachnoid hemorrhage in rats. Neurochem Int. 2012;60(3):327–33. 10.1016/j.neuint.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Gajewski TF, Schreiber H, Fu YX: Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–22. 10.1038/ni.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Medjkane S, Perez-Sanchez C, Gaggioli C, et al. : Myocardin-related transcription factors and SRF are required for cytoskeletal dynamics and experimental metastasis. Nat Cell Biol. 2009;11(3):257–68. 10.1038/ncb1833 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Orgaz JL, Ladhani O, Hoek KS, et al. : 'Loss of pigment epithelium-derived factor enables migration, invasion and metastatic spread of human melanoma'. Oncogene. 2009;28(47):4147–61. 10.1038/onc.2009.284 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Garcia M, Fernandez-Garcia NI, Rivas V, et al. : Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor. Cancer Res. 2004;64(16):5632–42. 10.1158/0008-5472.CAN-04-0230 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 95. Ladhani O, Sánchez-Martinez C, Orgaz JL, et al. : Pigment epithelium-derived factor blocks tumor extravasation by suppressing amoeboid morphology and mesenchymal proteolysis. Neoplasia. 2011;13(7):633–42. 10.1593/neo.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Arozarena I, Sanchez-Laorden B, Packer L, et al. : Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19(1):45–57. 10.1016/j.ccr.2010.10.029 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Calvo F, Sanz-Moreno V, Agudo-Ibáñez L, et al. : RasGRF suppresses Cdc42-mediated tumour cell movement, cytoskeletal dynamics and transformation. Nat Cell Biol. 2011;13(7):819–26. 10.1038/ncb2271 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Padua D, Zhang XH, Wang Q, et al. : TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77. 10.1016/j.cell.2008.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 99. Thiery JP: Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2(6):442–54. 10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- 100. Redondo-Muñoz J, Ugarte-Berzal E, Terol MJ, et al. : Matrix metalloproteinase-9 promotes chronic lymphocytic leukemia b cell survival through its hemopexin domain. Cancer Cell. 2010;17(2):160–72. 10.1016/j.ccr.2009.12.044 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 101. Gould SE, Junttila MR, de Sauvage FJ: Translational value of mouse models in oncology drug development. Nat Med. 2015;21(5):431–9. 10.1038/nm.3853 [DOI] [PubMed] [Google Scholar]
- 102. Kircher MF, Gambhir SS, Grimm J: Noninvasive cell-tracking methods. Nat Rev Clin Oncol. 2011;8(11):677–88. 10.1038/nrclinonc.2011.141 [DOI] [PubMed] [Google Scholar]
- 103. Brader P, Serganova I, Blasberg RG: Noninvasive molecular imaging using reporter genes. J Nucl Med. 2013;54(2):167–72. 10.2967/jnumed.111.099788 [DOI] [PubMed] [Google Scholar]
- 104. Lawler K, Foran E, O'Sullivan G, et al. : Mobility and invasiveness of metastatic esophageal cancer are potentiated by shear stress in a ROCK- and Ras-dependent manner. Am J Physiol Cell Physiol. 2006;291(4):C668–77. 10.1152/ajpcell.00626.2005 [DOI] [PubMed] [Google Scholar]
- 105. Reymond N, Riou P, Ridley AJ: Rho GTPases and cancer cell transendothelial migration. Methods Mol Biol. 2012;827:123–42. 10.1007/978-1-61779-442-1_9 [DOI] [PubMed] [Google Scholar]
- 106. Parri M, Taddei ML, Bianchini F, et al. : EphA2 reexpression prompts invasion of melanoma cells shifting from mesenchymal to amoeboid-like motility style. Cancer Res. 2009;69(5):2072–81. 10.1158/0008-5472.CAN-08-1845 [DOI] [PubMed] [Google Scholar]