Abstract
Introduction
Bortezomib, the first proteasome inhibitor (PI) to be evaluated in humans, is approved in the USA and Europe for the treatment of patients with multiple myeloma, and in the USA for patients with relapsed mantle cell lymphoma (MCL).
Areas covered
This review examines the role of bortezomib in the therapy of non-Hodgkin’s lymphoma (NHL). Bortezomib may be particularly effective against the NF-κB-dependent activated B-cell subtype of diffuse large B-cell lymphoma. The combination of bortezomib with rituximab and dexamethasone represents a standard approach for the treatment of Waldenström’s macroglobulinemia, and that with bendamustine and rituximab has demonstrated excellent efficacy in follicular lymphoma. Combinations with other novel agents, such as inhibitors of cyclin-dependent kinases or histone deacetylases, also hold substantial promise in NHL. Unmet needs in NHL, competitor compounds, chemistry, pharmacokinetics, pharmacodynamics and safety and tolerability of bortezomib are also discussed.
Expert opinion
The success of bortezomib in MCL has validated the proteasome as a therapeutic target in NHL. Rational combinations, for example, with Bruton’s tyrosine kinase inhibitors or BH3-mimetics, may hold the key to optimizing the therapeutic potential of PIs in NHL. Future trials are likely to involve newer agents with improved pharmacodynamic (e.g., carfilzomib, marizomib) or pharmacokinetic (e.g., ixazomib, oprozomib) properties.
Keywords: bortezomib, cutaneous T-cell lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, marginal zone lymphoma, non-Hodgkin’s lymphoma, peripheral T-cell lymphoma, Waldenström’s macroglobulinemia
1. Introduction
The 26S proteasome is a large, ATP-dependent proteolytic machine that, as part of the ubiquitin proteasome system (UPS), represents the ultimate mechanism that eukaryotic cells use to ensure the quality of intracellular proteins through the selective destruction of misfolded or damaged polypeptides [1]. This orderly process of degradation of unwanted proteins tagged with ubiquitin is critical for normal cell cycling and function, protecting cells from heat shock or oxidative stress, and inhibition of the proteasome pathway results in cell-cycle arrest and apoptosis [1,2]. As dysregulation of the UPS may play a role in tumor progression, drug resistance and altered immune surveillance, the proteasome has emerged as an important and novel therapeutic target in cancer [2], and proteasome inhibitors (PIs) have become widely used drugs in the treatment of multiple myeloma (MM) and mantle cell lymphoma (MCL) over the past decade.
Although a proportion of cases of aggressive non-Hodgkin’s lymphoma (NHL) are curable by conventional chemotherapy, disease relapse portends a poor outcome, and new therapies are urgently needed [3,4]. Indolent subtypes of NHL are seldom cured by currently available therapies [5]. Among aggressive NHLs, the activated B-cell (ABC) subtype of diffuse large B-cell lymphoma (DLBCL) is characterized by constitutive NF-κB pathway activation and may be particularly sensitive to proteasome inhibition [3]. Proteasome inhibition can overwhelm the response of tumor cells to a variety of stressors such as lactic acidosis, chromosome instability, DNA damage, reactive oxygen species (ROS) and heat shock, and this can be exploited to sensitize and/or overload NHL cells to propel them beyond a point of no return [4]. Pharmacological proteasome inhibition affects a variety of pathways beyond NF-κB, and this strategy, as well as rational combination approaches, has been explored in multiple NHL subtypes. In this article, we summarize current knowledge regarding bortezomib (Box 1), the first PI to enter the clinic, in NHL and provide directions for the future.
Box 1. Drug summary.
| Drug name | Bortezomib |
| Phase | Launched (FDA approved 2006) |
| Indication | Mantle cell lymphoma (relapsed after one prior therapy) |
| Pharmacology description/mechanism of action | Reversible proteasome inhibitor |
| Route of administration | Subcutaneous, intravenous |
| Chemical structure |
|
| Pivotal trial(s) | PINNACLE [24] |
Pharmaprojects – copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
2. Overview of the market
The NHLs are a heterogeneous group of disorders, with DLBCL and follicular lymphoma (FL) comprising over half the cases, although many other subtypes exist [6]. Treatment of the B-cell NHLs (B-NHLs) has been transformed by the advent of anti-CD20 mAb therapy, the prototype of which is the chimeric molecule rituximab [7], whereas other agents in this class, for example, ofatumumab and obinutuzumab, are currently approved only for the treatment of chronic lymphocytic leukemia (CLL). In contrast, major therapeutic advances for T-cell lymphomas have largely been lacking [8]. However, significant challenges remain in the B-NHL space as well, with cures remaining elusive for the indolent B-NHLs [5] and a continuing poor prognosis for patients with aggressive B-NHLs that relapse after or are refractory to standard chemoimmunotherapy; novel therapies are, therefore, clearly needed for these patients [4].
In recent years, a number of new agents have been introduced, at least some of which promise to change the therapeutic landscape of NHL in the coming decades. The combination of bendamustine and rituximab represents a new standard of care for patients with indolent B-NHLs based on superior efficacy and safety compared to rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) demonstrated in the Phase III StiL NHL1 trial [9]. Whereas the use of radioimmunotherapy has declined, leading to the recent discontinuation of 131I-tositumomab, antibody–drug conjugates (ADCs) have emerged as one of the fastest developing classes of targeted therapy in hematological malignancies [10]. Although currently approved for use only in patients with relapsed Hodgkin’s or anaplastic large-cell lymphoma (ALCL), the CD30-targeted ADC brentuximab vedotin is being explored in multiple other NHL subtypes, both T- and B-lineage [11]. Similarly, inotuzumab ozogamycin, a CD22-targeted ADC, is under active investigation in B-NHLs in addition to B-cell acute lymphoblastic leukemia [11]. The folate analog pralatrexate and the histone deacetylase inhibitors (HDACIs) vorinostat, romidepsin and belinostat are approved for the treatment of T-cell NHLs (T-NHLs) [8]. The immunomodulatory agent lenalidomide recently received regulatory approval for the treatment of patients with MCL that has relapsed or progressed after at least two prior therapies, including bortezomib [12]. The B-cell receptor (BCR) signaling pathway represents a particularly attractive therapeutic target in lymphoid malignancies [13]. The first-in-class Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib is currently indicated for use in previously treated patients with MCL [14] or CLL [15], based on very high response rates (RRs) and demonstration of improved survival among the latter [16]. This agent is being studied in multiple B-cell malignancies and indications for its use are expected to expand rapidly [17]. Similarly, the phosphatidylinositol-3-kinase-δ inhibitor idelalisib appears highly promising in patients with relapsed indolent NHL [18] and CLL [19], and was recently approved by the FDA for patients with relapsed CLL, small lymphocytic lymphoma (SLL) or FL. Given the key pathogenetic role of cyclin D1 in MCL, it is not surprising that the selective cyclin-dependent kinase (CDK) 4/6 inhibitor palbociclib (PD0332991) has shown proof-of-principle in pharmacodynamic studies in patients with relapsed MCL [20]. Finally, the Bcl-2 antagonist ABT-199 (GDC-0199), with demonstration of marked efficacy in patients with relapsed or refractory CLL or SLL [21], is likely to play a key role in the future NHL therapeutic armamentarium.
3. Introduction to the compound
Bortezomib (Velcade®, formerly PS-341, Millennium Pharmaceuticals, Cambridge, MA, USA) was the first PI to enter the clinic. In a large Phase II trial in heavily pretreated subjects with MM, bortezomib yielded responses in 35% of patients that lasted a median of 12 months, leading to a median overall survival (OS) of 16 months [22]. These findings resulted in initial regulatory approval of bortezomib. Subsequently, a large Phase III trial in patients with relapsed MM demonstrated highly statistically significant improvements in overall response rate (ORR) and complete response (CR) rates, time to progression (TTP) and OS for bortezomib compared to high-dose dexamethasone [23]. A 33% RR to bortezomib (8% CRs) in patients with relapsed or refractory MCL led to US FDA approval for the drug in this setting [24]. Bortezomib is also approved for the frontline therapy of MM [25]. Most recently, the FDA approved bortezomib for subcutaneous (s.c.) administration based on an improved safety profile compared to that with intravenous (i.v.) administration in a randomized, Phase III, noninferiority study in patients with relapsed MM [26].
4. Chemistry
Bortezomib is a modified dipeptidyl boronic acid PI that was selected for further study after it demonstrated promising cytotoxic activity in an in vitro screen against a standard National Cancer Institute panel of 60 human tumor cell lines [27]. The naturally occurring compound lactalysin and synthetic peptide aldehydes were the first, albeit nonspecific PIs identified [28]. Bortezomib was developed with the concept that substitution of the aldehyde group with boronic acid would create compounds capable of forming reversible, covalent complexes with the proteasome, leading to enhanced potency and selectivity [29].
5. Pharmacodynamics
The intact 26S proteasome is the major site (~ 80%) of protein degradation in eukaryotic cells, responsible primarily for degrading intracellular proteins [28,30]. Present in both the nucleus and in the cytoplasm, it consists of a 20S cylindrical structure with a 19S regulatory ‘cap’ at each end [28]. The β-subunits (β1, β2 and β5) of the 20S proteasome are responsible for the proteolytic activities of the organelle, which have been classified as ‘chymotrypsin-like’, ‘trypsin-like’ and ‘caspase-like’ [28,30]. Bortezomib reversibly interacts with a threonine residue on the β-subunit that confers chymotrypsin proteolytic activity [30]. Proteins destined for proteasomal degradation become polyubiquitinated through the sequential action of ubiquitin-activating (E1), ubiquitin-conjugating (E2) and ubiquitin-ligating (E3) enzymes and are recognized by the proteasome by their polyubiquitin ‘tag’ [28]. The many proteasomal substrates include key cell-cycle regulatory proteins, such as cyclins, the endogenous CDK inhibitors p21 and p27, and the CDC25 family of phosphatases, the tumor suppressor p53 (the negative regulator of p53, MDM2, is itself an E3 ubiquitin ligase that targets p53 for proteasomal degradation), several proapoptotic and antiapoptotic proteins of the Bcl-2 family, oncoproteins such as c-fos, c-jun and N-myc, and I kappa B (IκB) and the inhibitor protein that maintains the transcription factor NF-κB in an inactivated state in the cytoplasm under normal conditions [28,30]. In addition, cell adhesion molecules, stress response enzymes, proinflammatory cytokines, pro-angiogenic factors and the unfolded protein response (UPR) are some of the many cellular processes affected by proteasomal activity [28,30].
Bortezomib induces tumor cell apoptosis in multiple lymphoid malignancies [31–37], primarily through NF-κB inhibition and, additionally, is capable of killing B-NHL cells via non-apoptotic (caspase-independent) mechanisms [36]. A gene expression signature of DLBCL cells sensitive (overexpression of activating transcription factors 3, 4 and 5, c-Jun, JunD and caspase-3) and resistant (overexpression of heat shock proteins 27, 70 and 90 and T-cell factor 4) to bortezomib has been proposed [38].
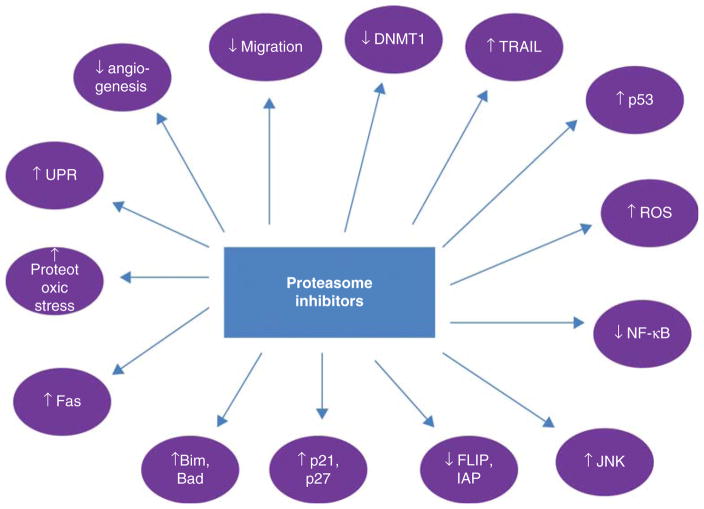
The mechanisms of PI lethality have been reviewed previously [39] and include (Figure 1):
Figure 1. Mechanisms of proteasome inhibitor lethality.
Modified with permission from [39].
Bad: Bcl-2-associated death promoter; Bim: Bcl-2 interacting mediator of cell death; DNMT1: DNA methyltransferase 1; ER: Endoplasmic reticulum; FLIP: FLICE-like inhibitory protein; IAP: Inhibitor of apoptosis; JNK: c-Jun N-terminal kinase; ROS: Reactive oxygen species; TRAIL: TNF-related apoptosis-inducing ligand; UPR: Unfolded protein response.
stabilization of p21, p27 and p53,
stabilization of c-Jun N-terminal kinase (JNK),
ROS generation,
inhibition of NF-κB activation,
inhibition of extracellular signal-regulated kinase (ERK) signaling,
disruption of the UPR, thereby leading to endoplasmic reticulum (ER) stress,
interference with tumor–microenvironment interactions,
inhibition of DNA repair,
upregulation/activation of pro-apoptotic Bcl-2 family proteins,
downregulation of several anti-apoptotic proteins, and
anti-angiogenic effects.
Bortezomib induces cell-cycle arrest and apoptosis in MCL cells [32]. Increased proteasomal degradation of p27 is associated with decreased OS in MCL [40]. MCL is characterized by constitutive activation of the NF-κB pathway [41,42]. The ability of IκB kinase inhibitors to induce apoptosis in MCL cells in vitro validated NF-κB as a therapeutic target in this disease [32,43]. However, although initially believed to be the major mechanism of bortezomib-induced apoptosis in MCL, inhibition of the NF-κB pathway may not represent the predominant mechanism of action of bortezomib in this disease [44,45]. It has been demonstrated that bortezomib induces apoptosis in MCL cells through ROS generation and upregulation of the BH3-only pro-apoptotic protein Noxa, thus displacing the apoptosis effector Bak from the anti-apoptotic protein myeloid cell leukemia 1 (Mcl-1) [46,47], potentially counteracting bortezomib-induced accumulation of the latter [48,49]. However, it is clear that in certain tumor types characterized by constitutive activation of NF-κB, such as the ABC subtype of DLBCL, bortezomib can significantly reverse resistance to chemotherapy [50]. The transcriptional repressor partial response (PR) domain zinc finger protein 1, Blimp1, appears to be a key mediator of bortezomib activity in MCL [51] as well as in T-cell lymphoma [52]. Finally, constitutive and BCR-induced activation of STAT3 are important signaling pathways targeted by bortezomib in leukemic MCL [53]. In peripheral T-cell lymphoma (PTCL), bortezomib inhibits cellular proliferation by downregulating microRNA 187, dephosphorylating ERK and Akt and degrading MYC [54].
Of note, PIs, and in particular bortezomib, have been shown to sensitize cells from a number of lymphoid malignancies to the lethal effects of chemotherapy [55,56], mAbs [31] and glucocorticoids [57], in large part by blocking the effects of NF-κB activation, a physiological response to cellular stress that leads to activation of transcription of genes for growth factors, stress response enzymes, cell adhesion molecules and apoptosis inhibitors [58–60].
6. Pharmacokinetics and metabolism
As noted above, bortezomib may be administered by the i.v. and s.c. routes with equivalent efficacy, at least in patients with MM [26]. Bortezomib is rapidly distributed into tissues after administration of a single dose, with an initial plasma distribution half-life of < 10 min, followed by a terminal elimination half-life of > 40 h [61]. Maximum proteasome inhibition occurs within 1 h and recovers close to baseline within 72 to 96 h after administration [61]. Plasma clearance decreases with repeat dosing, with associated increases in systemic exposure and terminal half-life [62]. The total systemic exposure after repeat dose administration is equivalent for the s.c. and i.v. routes, although the Cmax is lower after s.c. than after i.v. administration [63]. In animal studies, bortezomib distributes widely to peripheral tissues but does not accumulate [64]. The drug primarily undergoes oxidative metabolism via the CYP enzymes 3A4, 2C19 and 1A2, and to a much lesser extent, 2D6 and 2C9 [65]. The major metabolic pathway is deboronation of inactive metabolites [61]. Although the pathways of elimination of bortezomib in humans have not been characterized, dose adjustment is not necessary in the presence of renal impairment of any degree [66], whereas a lower starting dose is recommended in patients with moderate or severe hepatic impairment [67]. In general, bortezomib does not significantly induce or inhibit hepatic microsomal CYP450 enzymes except weak inhibition of 2C19, which is unlikely to be clinically significant [68]. However, coadministration of strong CYP3A4 inhibitors (e.g., ketoconazole) or inducers (e.g., rifampin) significantly impacts the systemic exposure of bortezomib [69,70], whereas the CYP2C19 inhibitor omeprazole has no effect [71]. St. John’s wort may decrease bortezomib exposure unpredictably and hence concomitant administration should be avoided.
7. Clinical efficacy
7.1 Mantle cell lymphoma
A very large number of clinical trials have evaluated bortezomib in different subtypes of NHL [72]; however, at present, MCL remains the only NHL subtype for the treatment of which bortezomib has received regulatory approval [73]. In a Phase II study of bortezomib in 26 patients with previously treated indolent B-NHL that included 11 patients with MCL, one patient with MCL achieved an unconfirmed complete response (CRu), four a PR and four achieved stable disease (SD) [74]. Another Phase II study reported a 41% ORR in 29 evaluable patients with relapsed or refractory MCL [75]. In the pivotal multicenter Phase II PINNACLE trial, bortezomib produced a 33% ORR in 141 assessable patients (out of 155 treated) with MCL and one to three prior therapies and exhibited a safety profile similar to that seen in patients with MM [24]. Median TTP was 6.7 months in the overall study population and 12.4 months in responding patients. Median OS was 23.5 months in the study population as a whole and 35.4 months in responders, with 1-year OS rates of 69 and 91%, respectively [76]. A smaller study conducted in Canada enrolled 29 patients with MCL, 13 of whom had not received prior chemotherapy [77]. There were 13 responders (46.4%) to bortezomib, including one CRu, and the median duration of response (DOR) was 10 months [77]. RRs were similar in previously untreated (46.2%) and treated (46.7%) patients [77]. In another multicenter, Phase II study in 40 heavily pretreated patients with MCL, the ORR to single-agent bortezomib was 47%, including 5 CRs and 14 PRs; importantly, the ORR (50 and 43%, respectively) and progression-free survival (PFS, 5.6 and 3.9 months, respectively) did not significantly differ between relapsed and refractory patients [78]. Key clinical trials of bortezomib monotherapy in MCL are summarized in Table 1.
Table 1.
Major Phase II studies of bortezomib monotherapy in mantle cell lymphoma.
| Ref. | Bortezomib dose and schedule | No. of patients | No. of prior therapies | Results |
|---|---|---|---|---|
| [24,76] | 1.3 mg/m2 on days 1, 4, 8, 11 (every 21 days) | 155 treated, 141 assessable for response | 1 (median) | 33% ORR, 8% CR/CRu, median TTP 6.7 months, median OS 23.5 months, median DOR 9.2 months |
| [77] | 1.3 mg/m2 on days 1, 4, 8, 11 (every 21 days) | 29 (13 previously untreated) | 0 in 13, 1 in 11, 2 in 5 | 46.4% ORR, 1 CRu, median DOR 10 months |
| [78] | 1.5 mg/m2 on days 1, 4, 8, 11 (every 21 days)* | 40 | 2 | 47% ORR (5 CRs) |
Dose reductions to 1.3 and 1.1 mg/m2 were allowed.
CR: Complete response; CRu: Unconfirmed complete response; DOR: Duration of response; ORR: Overall response rate; OS: Overall survival; TTP: Time to progression.
Two recent Phase II studies evaluated bortezomib in combination with modified rituximab, hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone followed by rituximab consolidation and maintenance in previously untreated MCL [79,80]. In the first 30-patient study, 77% achieved a CR/CRu, and the 3-year PFS and OS were 63 and 86%, respectively, after a median follow up of 42 months [79]. When these results were attempted to be confirmed in the cooperative group setting (E1405), consolidative autologous stem cell transplantation was allowed in place of rituximab maintenance, and rituximab consolidation was omitted [80]. The ORR was 95% and CR was achieved in 68% of the 75 patients enrolled [80]. After a median follow up of 4.5 years, 3-year PFS and OS were 72 and 88%, respectively, and there were no unexpected toxicities [80]. Bortezomib has been combined with R-CHOP in a dose-escalation study in 76 treatment-naïve patients with DLBCL (n = 40) or MCL (n = 36). In the MCL cohort, the evaluable and intention-to-treat (ITT) ORRs and CR/CRu rates were 91 and 81%, and 72 and 64%, respectively [81]. The 2-year PFS was 44%, and 2-year OS was 86% [81]. Based on marked synergism observed between bortezomib and HDACIs in multiple preclinical studies in MCL [82–84] and other NHL subtypes [85–87], clinical trials have explored bortezomib–H-DACI combinations in NHL. Synergism between PIs and HDACIs, best exploited clinically in MM [88,89], stems from inhibition by PIs of NF-κB, which is induced by HDACIs and limits their lethality, inhibition by HDACIs of aggresome formation, a physiologic response to proteasome inhibition, inhibition of chaperone protein function by HDACIs, which adds to the accumulation of misfolded proteins and ER stress caused by proteasome inhibition, among other actions common to both classes of agents, such as induction of ROS, inhibition of DNA repair, JNK activation, stabilization/induction of endogenous CDK inhibitors, upregulation of pro-apoptotic and downregulation of anti-apoptotic proteins. In early results from a Phase II trial of bortezomib and vorinostat in treatment-naïve or previously treated patients with MCL and patients with DLBCL who had received at least one prior systemic therapy, ORRs of 47% in the MCL cohort and 12% in the DLBCL cohort were reported [90]. A number of other bortezomib-containing combination regimens have been evaluated in patients with newly diagnosed or relapsed/refractory MCL (Table 2).
Table 2.
Bortezomib-based combination therapies in mantle cell lymphoma.
| Ref. | Prior therapies | Bortezomib combination studied | Bortezomib dose and schedule | Phase | No. of patients | Results |
|---|---|---|---|---|---|---|
| [79] | None | VcR-CVAD × 6 with consolidation and MR in responders | 1.3 mg/m2 (days 1 and 4 every 21 days; first 7 patients received 1.5 mg/m2) | II | 30 | 90% ORR, 77% CR/CRu, 3-year PFS 63% and OS 86% after median f/u of 42 months |
| [80] | None | VcR-CVAD × 6 followed by ASCT or MR in responders | 1.3 mg/m2 (days 1 and 4 every 21 days) | II | 75 | 95% ORR, 68% CRs, 3-year PFS 72% and OS 88% after median f/u of 4.5 years |
| [81] | None | VcR-CHOP × 6 | 0.7, 1.0 or 1.3 mg/m2 (days 1 and 4 every 21 days) | I/II | 76 (40 DLBCL + 36 MCL) | MCL: evaluable ORR 91%, 72% CR/CRu; ITT ORR 81%, 64% CR/CRu; 2-year PFS 44% and OS 86% |
| [128] | 1 – 3 prior therapies | Bortezomib + gemcitabine (1000 mg/m2 on days 1, 8) | 1.0 mg/m2 (days 1, 4, 8, 11 every 21 days) | II | 26 | 60% ORR, 11.5% CRs, median PFS 11.4 months |
| [129] | Median 3 prior therapies | BDR × 6 with 4 doses of rituximab consolidation in responders* | 1.3 mg/m2 (days 1, 4, 8, 11 every 21 days) | II | 16 | 81.3% ORR, 43.8% CRs, median PFS 12.1 and OS 38.6 months |
| [130] | None | RiPAD + C × 6 (in responders; nonresponders received 4 cycles) | 1.3 mg/m2 (days 1, 4, 8, 11 every 35 days) | II | 39 | 79% ORR, 59% CRs, median PFS 26 months and OS not reached at 27 months |
| [90] | 2 | Bortezomib + vorinostat | 1.3 mg/m2 (days 1, 4, 8, 11 every 21 days) | II | 47 (26 DLBCL + 21 MCL) | MCL: 47% ORR, 12% CRs (all in bortezomib-naïve cohort) |
Rituximab given at a dose of 375 mg/m2 on day 1 and dexamethasone given at a dose of 40 mg orally on days 1 – 4.
ASCT: Autologous stem cell transplant; BDR: Bortezomib, dexamethasone, rituximab; CR: Complete response; CRu: Unconfirmed complete response; DLBCL: Diffuse large B-cell lymphoma; f/u: Follow up; ITT: Intention to treat; MCL: Mantle cell lymphoma; MR: Maintenance rituximab; ORR: Overall response rate; OS: Overall survival; PFS: Progression-free survival; RiPAD + C: Rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil; VcR-CHOP: Bortezomib, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; VcR-CVAD: Rituximab, bortezomib, modified hyperfractionated cyclophosphamide, doxorubicin, vincristine, dexamethasone.
7.2 Diffuse large B-cell lymphoma
In the bortezomib dose-escalation study in combination with R-CHOP, the ORR in the DLBCL cohort (n = 40) was 100% among evaluable patients, and the rate of CR/CRu was 86% [81]. In the ITT analysis, the ORR was 88%, and 75% achieved a CR/CRu [81]. The 2-year PFS and OS were 64 and 70%, respectively [81]. The authors concluded that bortezomib added to R-CHOP was safe and could improve outcomes, particularly in non-germinal center (non-GC) DLBCL [81]. In the relapsed/refractory setting, bortezomib has been combined with gemcitabine [91] and with ifosfamide, cisplatin, etoposide, rituximab and dexamethasone [92]; however, RRs did not meet predefined criteria in these studies.
7.3 Waldenström’s macroglobulinemia
The combination of bortezomib, dexamethasone and rituximab (BDR) has become a widely used standard in the management of Waldenström’s macroglobulinemia (WM). In the original report, bortezomib was administered on the standard dose and schedule (1.3 mg/m2 i.v. on days 1, 4, 8 and 11 every 21 days), dexamethasone (40 mg) was given orally on the same days as bortezomib, and rituximab was given on day 11 in order to minimize the risk of tumor flare [93]. Patients received four consecutive cycles for induction therapy and then four more cycles, each given 3 months apart, for maintenance therapy [93]. Among 23 symptomatic, previously untreated patients, the ORR was 96% and the major RR was 83% [93]. Responses occurred at a median of 1.4 months and 18 of 23 patients remained free of disease progression at a median follow up of 22.8 months [93]. Subsequent studies have explored the combination of weekly bortezomib (1.6 mg/m2 on days 1, 8 and 15 every 28 days) and rituximab (375 mg/m2 weekly in cycles 1 and 4 only of 6) in patients with relapsed/refractory [94] and newly diagnosed [95] WM, with demonstration of significant activity and minimal neurological toxicity (Table 3).
Table 3.
Major Phase II trials of bortezomib-based regimens in Waldenström’s macroglobulinemia.
| Ref. | Bortezomib combination studied | Bortezomib dose and schedule | No. of patients | Results |
|---|---|---|---|---|
| [93] | BDR | 1.3 mg/m2 on days 1, 4, 8, 11, every 21 days, dexamethasone 40 mg on same days, rituximab 375 mg/m2 on day 11 | 23 (previously untreated) | ORR 96%, 3 CRs, median time to response 1.4 months, 18 of 23 free of progression at median f/u of 22.8 months |
| [95] | Bortezomib and rituximab* | 1.6 mg/m2 on days 1, 8, 15, every 28 days | 26 (previously untreated) | 88% ORR, 1 CR and 1 near CR |
| [94] | Bortezomib and rituximab* | 1.6 mg/m2 on days 1, 8, 15, every 28 days | 37 (relapsed or refractory) | 81% ORR, 2 CR/near CRs, median TTP 16.4 months |
Rituximab given at a dose of 375 mg/m2 weekly in cycles 1 and 4.
BDR: Bortezomib, dexamethasone, rituximab; CR: Complete response; f/u: Follow up; ORR: Overall response rate; TTP: Time to progression.
7.4 Follicular, marginal zone and other indolent lymphomas
In a trial of bortezomib monotherapy (1.5 mg/m2 i.v. on days 1, 4, 8 and 11 of a 21-day cycle) in 77 patients with previously treated FL, MCL, CLL/SLL, WM or marginal zone lymphoma (MZL), 9 of 18 patients with FL responded, and 4 achieved CR, but the median time to treatment response was 12 weeks for FL, whereas it was only 4 weeks for the other histological subtypes [96]. A randomized Phase II study comparing this schedule to weekly administration (1.6 mg/m2 on days 1, 8, 15 and 22 of a 35-day cycle) in patients with recurrent or refractory FL found the latter schedule to be inferior in terms of RRs, although this did not translate to a difference in overall outcomes [97]. A total of 94 patients with advanced, previously untreated FL requiring therapy received bortezomib (1.3 mg/m2 on days 1 and 8) plus rituximab, cyclophosphamide, vincristine and prednisone on a Phase II trial [98]. The regimen was extremely well tolerated, and the ORR was 83%, with a 49% CR/CRu rate and 34% PRs [98]. In the Phase II VERTICAL study, 63 patients with relapsed or refractory FL received five 35-day cycles of bortezomib (1.6 mg/m2 i.v. on days 1, 8, 15 and 22), bendamustine (90 mg/m2 on days 1 and 2) and rituximab (375 mg/m2 on days 1, 8, 15, and 22 of cycle one and day 1 of subsequent cycles) [99]. The ORR was 88% (53% CRs), median DOR was 11.7 months and median PFS was 14.9 months [99]. A large (n = 676) Phase III trial recently compared bortezomib plus rituximab with rituximab alone in relapsed FL [100]. Patients could be rituximab-naïve or rituximab-sensitive [100]. After a median follow up of 33.9 months, median PFS was 11 months in the rituximab group and 12.8 months in the bortezomib plus rituximab group, a much lower magnitude of clinical benefit than expected [100]. Patients with PSMB1 P11A (G allele) and low CD68 expression seemed to have significantly longer PFS and greater clinical benefit with bortezomib and rituximab versus rituximab [101]. Table 4 summarizes the results of major Phase II and III clinical trials of bortezomib in FL.
Table 4.
Major clinical trials of bortezomib-based therapies in follicular lymphoma.
| Ref. | Prior therapies | Treatment regimen | Bortezomib dose and schedule | Phase | No. of patients | Results |
|---|---|---|---|---|---|---|
| [100] | At least 1 | Rituximab (375 mg/m2 on days 1, 8, 15, 22 of cycle 1 and day 1 of cycles 2 – 5) ± bortezomib | 1.6 mg/m2 on days 1, 8, 15, 22, every 35 days × 5 | III | 676 (340 in rituximab group + 336 in combination group) | Median PFS 11 months in rituximab group and 12.8 months in combination group (p = 0.039) |
| [99] | Median 2 | Bortezomib, rituximab (375 mg/m2 on days 1, 8, 15, 22 of cycle 1 and day 1 of cycles 2 – 5) and bendamustine (50, 70 or 90 mg/m2 on days 1 and 2) | 1.6 mg/m2 on days 1, 8, 15, 22, every 35 days × 5 | II | 73 (63 received 90 mg/m2 of bendamustine) | 88% ORR, 53% CRs, median DOR 11.7 months and PFS 14.9 months |
| [97] | Median 3 in biweekly arm, 3.8 in weekly arm | Monotherapy (comparison of two schedules) | 1.5 mg/m2 on days 1, 4, 8, 11, every 21 days up to 8 cycles versus 1.6 mg/m2 on days 1, 8, 15, 22, every 35 days up to 6 cycles. Two additional cycles allowed in both arms in responders | II | 87 (50 in biweekly arm + 37 in weekly arm | ITT ORR 30% in biweekly arm versus 22% in weekly arm (evaluable ORR 32 vs 23%); median DOR 16 and 15 months, and PFS 7 and 6 months, respectively, at median f/u of 36 and 38 months, respectively |
| [98] | None | BR-CVP for up to 8 cycles | 1.3 mg/m2 on days 1 and 8 of a 21-day cycle | II | 94 | 83% ORR, 49% CR/ CRu rate (ITT) |
BR-CVP: Bortezomib, rituximab, cyclophosphamide, vincristine, prednisone; CR: Complete response; CRu: Unconfirmed complete response; DOR: Duration of response; f/u: Follow up; ITT: Intention to treat; ORR: Overall response rate; PFS: Progression-free survival.
Two Phase II studies have investigated bortezomib in mucosa-associated lymphoid tissue lymphomas [102,103]. In the first study in 16 patients (14 previously untreated), the ORR was 80% (43% CRs + 37% PRs), but toxicity was unexpectedly high at the dose used (1.5 mg/m2 on days 1, 4, 8 and 11 every 21 days) [103]. Four patients had relapsed after a median follow up of 23 months [103]. In the second larger study (n = 32) in relapsed or refractory patients, the drug was given at a dose of 1.3 mg/m2 on days 1, 4, 8 and 11 in 21-day cycles, and the ORR was 48% among 29 assessable patients [102]. The median DOR had not been reached yet after a median follow up of 24 months [102].
A number of trials have explored bortezomib, primarily in combination, in patients with indolent B-NHLs of varying histologies. In a community-based, single-agent Phase II study, 59 patients with relapsed or refractory indolent lymphoma received bortezomib according to the FDA-approved dose and schedule for up to eight cycles; responders could receive four additional cycles, while maintenance was optional [104]. A total of 1 patient obtained a CR, 3 a CRu, 3 a PR, 34 SD and 12 obtained progressive disease (PD) [104]. Median survival was 27.7 months, median PFS and TTP each 5.1 months, and median event-free survival was 1.8 months [104]. Modest activity was noted against FL and MZL [104]. A multicenter, randomized Phase II study evaluated weekly (1.6 mg/m2 on days 1, 8, 15 and 22 every 5 weeks for 3 cycles) or twice-weekly (standard schedule, 5 cycles) bortezomib in combination with rituximab (375 mg/m2 weekly for 4 weeks) in 81 relapsed or refractory patients with these two histological subtypes [105]. The weekly combination regimen seemed better tolerated, and efficacy was comparable between the two arms [105]. Several other Phase II studies of bortezomib plus rituximab have been conducted, both in the frontline [106] and relapsed/refractory [107,108] settings in patients with FL, MCL or WM. Unexpectedly high toxicity, particularly neurologic, was observed with twice-weekly administration of bortezomib in conjunction with rituximab in previously treated patients [107,108], whereas neurotoxicity was minimal in a frontline trial in predominantly FL patients that employed weekly dosing [106]. A multicenter, Phase II trial evaluated the combination of bendamustine (90 mg/m2 on days 1 and 4), bortezomib (1.3 mg/m2 on days 1, 4, 8 and 11) and rituximab (375 mg/m2 on day 1) given for six 28-day cycles in 30 patients with relapsed or refractory indolent lymphoma or MCL [109]. The ORR was 83% among 29 evaluable patients, with 15 CRs. With a median follow up of 24 months, 2-year PFS was 47% [109]. Bortezomib synergizes with the CDK inhibitor flavopiridol (alvocidib) at multiple levels (e.g., inhibition of NF-κB, JNK activation, downregulation by flavopiridol of anti-apoptotic Mcl-1, the proteasomal degradation of which is blocked by bortezomib, and counteraction of flavopiridol-induced downregulation of the endogenous CDK inhibitor p21 by bortezomib [110,111]), and a Phase I trial of this combination in recurrent or refractory B-cell neoplasms (MM, indolent lymphoma and MCL) reported an ORR of 44% (12% CR + 31% PR [112]). Clinical trial results with bortezomib in indolent lymphoma are listed in Table 5.
Table 5.
Clinical trials of bortezomib, alone and in combination, in indolent non-Hodgkin’s lymphomas.
| Ref. | Prior therapy | Bortezomib combination studied | Bortezomib dosing/frequency | Phase | No. of patients | Results |
|---|---|---|---|---|---|---|
| [103] | Variable (11 previously untreated) | Monotherapy | 1.5 mg/m2 on days 1, 4, 8, 11, every 21 days | II | 16 (all MALT lymphoma) | 80% ORR, 43% CRs; unexpectedly high toxicity |
| [102] | Median 2 prior therapies | Monotherapy | 1.3 mg/m2 on days 1, 4, 8, 11, every 21 days × up to 6 cycles | II | 32 enrolled, 31 treated, 29 assessable (all MALT lymphoma) | 48% ORR, 31% CRs, median DOR not reached after median f/u of 24 months |
| [105] | 0 to ≥ 3 (only 1 treatment-naïve patient) | Bortezomib + rituximab, 375 mg/m2 weekly × 4 weeks | 1.3 mg/m2 on days 1, 4, 8, 11 every 21 days × 5 cycles versus 1.6 mg/m2 on days 1, 8, 15, 22, every 35 days × 3 cycles | II | 41 in twice-weekly arm, 40 in weekly arm, FL or MZL | 49% ORR, 14% CR/CRu rate, median TTP 7 months and DOR not reached in twice-weekly arm; 43% ORR, 10% CR/CRu rate, median TTP 10 and DOR 9.3 months in weekly arm |
| [108] | Median 2 prior therapies | Bortezomib + rituximab, 375 mg/m2 on days 1 and 8 | 1.3 – 1.5 mg/m2 on days 1, 4, 8, 11, every 21 days × 1 – 5 cycles (median 3) | II | 25 (11 FL + 14 MCL) | ORR 40% (55% in FL patients, 29% in MCL patients); 2-year PFS 24% overall, 60% in responders |
| [104] | 1 to ≥ 3 (prior rituximab required) | Monotherapy | 1.3 mg/m2 on days 1, 4, 8, 11, every 21 days × up to 8 cycles | II | 60 enrolled (40 FL + 12 SLL + 7 MZL), 53 evaluable | ORR 13%, 1 CR, 3 CRu, median time to response 2.2, DOR 7.9, OS 27.7, PFS 5.1, TTP 5.1 and EFS 1.8 months |
| [109] | Median 4 prior therapies | Bortezomib + bendamustine 90 mg/m2 on days 1 and 4 + rituximab 375 mg/m2 on day 1 × 6 cycles | 1.3 mg/m2 on days 1, 4, 8, 11, every 28 days | II | 31 (16 FL + 3 MZL + 3 SLL + 7 MCL + 2 lymphoplasmacytic lymphoma), 29 evaluable | 83% ORR, 15 CRs, 2-year PFS 47% at a median f/u of 24 months, ORR 93% in FL patients and 71% in MCL patients |
| [74] | Median 3 prior therapies | Monotherapy | 1.5 mg/m2 on days 1,4,8,11, every 21 days | II | 26 registered (10 FL + 11 MCL + 3 SLL/CLL + 2 MZL), 24 assessable | 58% ORR, 1 CR (FL), 2 CRu (1 MCL, 1 FL) |
| [131] | Median 4 prior therapies in weekly group, 2 in twice weekly group | Rituximab, cyclophosphamide, bortezomib, prednisone every 21 days × 4; rituximab 375 mg/m2 on day 1 and prednisone 100 mg PO on days 2 – 6 | 1.3 – 1.8 mg/m2 on days 2 and 8 in weekly group; 1 – 1.5 mg/m2 on days 2, 5, 9, 12 in twice weekly group; bortezomib and cyclophosphamide (750 – 1000 mg/m2) alternately escalated | I | 57 (46 evaluable, 24 FL + 10 MCL + 6 MZL + 2 SLL + 4 transformed) | ORR 46% (23% CR/CRu) and 64% (36% CR/CRu) in weekly and twice weekly groups, respectively |
| [107] | Median 2 prior therapies | Bortezomib + rituximab 375 mg/m2 on day 1 every 21 days (twice-weekly bortezomib group) or on days 1, 8, 15 and 22 every 35 days (weekly bortezomib group) | 1.3 mg/m2 on days 1, 4, 8, 11, every 21 days versus 1.6 mg/m2 on days 1, 8, 15, 22, every 35 days | I/II | 49 (7 in Phase I and 42 in randomized Phase II portion; 21 MCL + 17 FL + 11 WM) | ORR 67% (MCL 11/19, FL 8/15, WM 9/10) |
| [106] | None | Bortezomib + rituximab 375 mg/m2 for 4 weekly doses in cycle 1 and on day 1 only in cycles 2 and 3 | 1.6 mg/m2 on days 1, 8, 15, 22, every 35 days × 3 | II | 42 (33 FL + 5 MZL + 3 SLL + 1 WM) | ITT ORR 70% (FL 76%), 40% CRs (FL 44%), 4-year PFS 44% (FL 44%) and OS 87% (FL 97%) |
| [112] | Median 2.5 prior therapies | Bortezomib + alvocidib (hybrid schedule of administration on days 1 and 8, MTD 30 mg/m2) | MTD 1.3 mg/m2 on days 1, 4, 8, 11, every 21 days | I | 16 (9 NHL + 7 MM) | 44% ORR, 12% CR |
| [119] | ≤ 3 prior therapies | Monotherapy | 1.8 mg/m2 weekly × 4 every 5 weeks × 3 cycles (median; range 1 – 10) | II | 26 (16 FL + 10 MCL), 22 assessable (14 FL + 8 MCL) | 18% ORR (14% in FL patients, 25% in MCL patients), 0% CRs |
| [96] | Median 3 prior therapies | Monotherapy | 1.5 mg/m2 on days 1, 4, 8, 11, every 21 days | II | 77 registered (22 FL + 40 MCL + 6 SLL/CLL + 8 MZL + 1 WM), 69 assessable | 45% ORR (40% on ITT analysis), 10 CRs; 9 of 18 (50%) evaluable FL patients responded with 4 CRs, 18 of 36 (50%) evaluable MCL patients responded with 6 CRs |
CLL: Chronic lymphocytic leukemia; CR: Complete response; CRu: Unconfirmed complete response; DOR: Duration of response; EFS: Event-free survival; FL: Follicular lymphoma; f/u: Follow up; ITT: Intention to treat; MALT: Mucosa-associated lymphoid tissue; MCL: Mantle cell lymphoma; MM: Multiple myeloma; MTD: Maximum tolerated dose; MZL: Marginal zone lymphoma; NHL: Non-Hodgkin’s lymphoma; ORR: Overall response rate; SLL: Small lymphocytic lymphoma; TTP: Time to progression; WM: Waldenström’s macroglobulinemia.
7.5 T-cell lymphomas
Bortezomib was studied in patients with relapsed or refractory cutaneous T-cell lymphoma (CTCL) or PTCL-not otherwise specified (PTCL-NOS) with isolated skin involvement in a small study (n = 15) [113]. The ORR was 67% among 12 assessable patients, with 2 (17%) CRs and 6 (50%) PRs, whereas the remaining 4 patients had PD [113]. Bortezomib was also combined with CHOP in a Phase II study in 46 patients with PTCLs of various histological subtypes [114]. The ORR was 76% and the CR rate was 65%; 3-year OS and PFS were 47 and 35%, respectively [114]. Patients with PTCL-NOS, angioimmunoblastic T-cell lymphoma and ALCL accounted for the vast majority of responses, whereas those with extranodal natural killer/T-cell lymphoma, nasal type responded poorly [114].
8. Safety and tolerability
Peripheral neuropathy, often painful, is the most important toxicity of bortezomib encountered in clinical practice; bortezomib-induced peripheral neuropathy (BIPN) occurred in 37 – 44% of patients on MM clinical trials [115]. BIPN is usually, but not always, reversible on treatment cessation, and the cumulative bortezomib dose is its single most important predictor [115]. BIPN is usually sensory, distal, symmetric and affects the feet more than the hands, although orthostatic hypotension from dysautonomia can occur in about 10% of patients [116,117]. In the noninferiority trial that led to the approval of bortezomib administration by the s.c. route, peripheral neuropathy of any grade, grade ≥ 2 and grade ≥ 3, was all significantly less common with s.c. than with i.v. administration [26]. Rates of BIPN may also be significantly reduced by once-weekly administration of bortezomib while preserving efficacy, at least in patients with MM [118]. In the setting of B-NHL, RRs were inferior in some monotherapy studies with weekly compared with twice-weekly administration, although overall outcomes were similar [97,119], while efficacy was similar between the two schedules in other studies that also used rituximab [105,107], with better tolerability for weekly dosing observed in a large, multicenter, randomized Phase II study [105] that prompted weekly administration of bortezomib in combination with rituximab in the Phase III trial versus rituximab alone in FL [100]. Other major adverse effects of bortezomib include gastrointestinal toxicity such as diarrhea or constipation, fatigue and neutropenia and thrombocytopenia [116], the latter due to a transient, reversible, inhibition of platelet release from megakaryocytes [120]. Herpes zoster reactivation is a well-recognized complication of bortezomib treatment, and concomitant antiviral prophylaxis is highly recommended [116,121]. Intriguingly, a bortezomib-induced non-necrotizing cutaneous vasculitis may portend a better clinical response in some patients with B-NHL and should not necessarily prompt cessation of drug [122].
9. Regulatory affairs
The initial US (fast track) approval of bortezomib occurred in 2003 based on data from the Phase II SUMMIT [22] and CREST [123] trials in patients with relapsed/refractory MM. Approval in Europe followed in 2004. Subsequently, the drug obtained regular approval in 2005 based on the findings of the Phase III APEX trial, in which it significantly improved RRs, TTP and survival in 669 patients with relapsed MM compared to high-dose dexamethasone [23]. As discussed above, FDA approval for use in patients with relapsed MCL [73] followed in 2006 when the Phase II PINNACLE trial showed a RR of 33% in 155 patients with previously treated MCL [24]. The drug is not approved in Europe for this indication. The Phase III VISTA trial in 682 previously untreated transplant-ineligible patients with MM led to the approval of bortezomib in combination with melphalan and prednisone (VMP) for the upfront treatment of MM in 2008 [25]. The drug was approved for s.c. administration in 2011 after an open-label, randomized (2:1), Phase III study in 222 bortezomib-naïve patients with relapsed MM demonstrated noninferiority (compared to i.v. administration) with significantly less peripheral neuropathy [26].
In 2012, the FDA granted accelerated approval to carfilzomib (Kyprolis™, Onyx, formerly PR-171), a cell-permeable tetrapeptide epoxyketone that irreversibly and selectively inhibits the chymotrypsin-like site of the proteasome, for the treatment of patients with relapsed/refractory MM [124]. A number of other PIs, some reversible and others irreversible, administered via oral or i.v. routes, are currently in various phases of development in an effort to overcome mechanisms of resistance to bortezomib inherent to the proteasome itself [125]. Millennium Pharmaceuticals is actively pursuing the development of ixazomib (MLN9708), an oral, reversible PI, and Phase III trials in both relapsed/refractory (NCT01564537) and newly diagnosed (NCT01850524) patients with MM are underway.
10. Conclusion
Although currently registered in the USA only for MCL, bortezomib has clear activity in multiple NHL subtypes, including FL, WM, MZL, DLBCL and CTCL. Much attention has been focused on bortezomib in non-GC DLBCL. In WM, the BDR regimen represents a standard of care. The combination of bortezomib, bendamustine and rituximab is highly efficacious in indolent lymphoma, particularly FL. Rational combinations likely hold the key to further optimizing the therapeutic potential of bortezomib in NHL.
11. Expert opinion
Bortezomib, the prototypical first-in-class PI, has clearly established an important role in the treatment of multiple B- and T-cell malignancies, including NHL. It offers several practical and theoretical advantages over other classes of chemotherapeutic agents, both conventional and more novel, that is, its unique target (i.e., the proteasome), the potential for therapeutic selectivity, the ability to inhibit the NF-κB pathway and its relatively non-myelosuppressive toxicity profile. Until and unless other PIs are definitively shown to be superior to bortezomib in respect to improved tolerability or increased activity, it is very likely to continue to be prescribed well into the future in diseases for which it has earned approval, that is, MM and MCL. However, it is also very likely to be used in other forms of NHL for which approval has not yet been granted, but where it appears to have significant activity, such as WM, indolent NHL, non-GC-DLBCL and PTCL, primarily in combination with other cytotoxic agents. The major impact that this agent is likely to have stems from its ability to disrupt a variety of cytoprotective signaling pathways and, in so doing, it lowers the threshold for apoptosis induction in NHL cells by other agents or regimens, for example, CHOP or dose-adjusted infusional etoposide, prednisone, vincristine, cyclophosphamide and doxorubicin. Consequently, in the future, physicians are most likely to prescribe bortezomib in combination with established NHL regimens, possibly in genetically defined NHL subtypes found to be most susceptible to its actions (e.g., ABC-DLBCL). Despite the success of bortezomib in NHL to date, many questions remain to be addressed, and which could have tangible effects on its future use and development. For example, will next-generation PIs, for example, carfilzomib, shown to be active in bortezomib-resistant myeloma, demonstrate similar efficacy or advantages in the setting of NHL? Will orally active and putatively less neurotoxic PIs such as ixazomib prove equally or more effective than bortezomib in NHL, and eventually supplant it? The major questions to be addressed include defining the mechanism of action of bortezomib in NHL, which will be critical for its optimal use in the future. Answers to these questions will undoubtedly have a critical impact on the extent of bortezomib use in NHL over the next 5 – 10 years. Finally, the ultimate use of bortezomib or analogous agents in NHL in the future will also depend on the development of new therapeutic strategies, particularly those involving other, more specifically targeted agents rather than standard cytotoxic regimens. For example, rational strategies combining bortezomib with BTK inhibitors, for example, ibrutinib (BTK signals downstream to NF-κB and this may be a particularly effective strategy against DLBCL as well as MCL [126]), CDK inhibitors (e.g., pan-CDK inhibitors such as alvocidib, or CDK4/6 inhibitors, e.g., palbociclib), HDACIs or BH3-mimetics, for example, ABT-199 (via JNK activation and ER stress induction [127]), are likely to emerge in the coming years, and could have a significant impact on the therapeutic landscape for bortezomib in NHL in the foreseeable future.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents, received or pending, or royalties.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1•.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426(6968):895–9. doi: 10.1038/nature02263. Excellent review of protein homeostasis within cells. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23(3):630–9. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 3•.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362(15):1417–29. doi: 10.1056/NEJMra0807082. Excellent review of the differences between biologic subtypes of diffuse large B-cell lymphoma (DLBCL) defined by gene expression profiling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahadevan D, Fisher RI. Novel therapeutics for aggressive non-hodgkin’s lymphoma. J Clin Oncol. 2011;29(14):1876–84. doi: 10.1200/JCO.2010.32.7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lunning MA, Vose JM. Management of indolent lymphoma: where are we now and where are we going. Blood Rev. 2012;26(6):279–88. doi: 10.1016/j.blre.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Campo E, Swerdlow SH, Harris NL, et al. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011;117(19):5019–32. doi: 10.1182/blood-2011-01-293050. Insightful review of key changes to the WHO classification of lymphoid neoplasms made in the 2008 version. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366(21):2008–16. doi: 10.1056/NEJMct1114348. [DOI] [PubMed] [Google Scholar]
- 8.Foss FM, Zinzani PL, Vose JM, et al. Peripheral T-cell lymphoma. Blood. 2011;117(25):6756–67. doi: 10.1182/blood-2010-05-231548. [DOI] [PubMed] [Google Scholar]
- 9••.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203–10. doi: 10.1016/S0140-6736(12)61763-2. Report of the StiL non-Hodgkin’s lymphoma (NHL)1 trial that established bortezomib and rituximab as a frontline regimen of choice for the treatment of indolent B-cell NHLs (B-NHLs) [DOI] [PubMed] [Google Scholar]
- 10.Palanca-Wessels MC, Press OW. Advances in the treatment of hematologic malignancies using immunoconjugates. Blood. 2014;123(15):2293–301. doi: 10.1182/blood-2013-10-492223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robak T, Robak E. Current phase II antibody-drug conjugates for the treatment of lymphoid malignancies. Expert Opin Investig Drugs. 2014;23(7):911–24. doi: 10.1517/13543784.2014.908184. [DOI] [PubMed] [Google Scholar]
- 12••.Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688–95. doi: 10.1200/JCO.2013.49.2835. The trial that led to FDA approval of lenalidomide for the treatment of relapsed/refractory mantle cell lymphoma (MCL) following two prior therapies, including bortezomib. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12(3):229–43. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–16. doi: 10.1056/NEJMoa1306220. The trial that led to FDA approval of ibrutinib for the treatment of patients with MCL following one prior therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. The trial that led to FDA approval of ibrutinib for the treatment of patients with chronic lymphocytic leukemia (CLL) following one prior therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–23. doi: 10.1056/NEJMoa1400376. The RESONATE trial that demonstrated the superiority of ibrutinib over ofatumumab in patients with relapsed CLL in terms of both response rates and survival. This study also led to the approval of ibrutinib for the treatment of patients with CLL with a 17p deletion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendriks RW, Yuvaraj S, Kil LP. Targeting bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14(4):219–32. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 18••.Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–18. doi: 10.1056/NEJMoa1314583. The trial that led to FDA approval of idelalisib for patients with relapsed small lymphocytic lymphoma (SLL) and follicular lymphoma (FL) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. The trial that led to FDA approval of idelalisib, in combination with rituximab, for patients with relapsed CLL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Leonard JP, LaCasce AS, Smith MR, et al. Selective CDK4/6 inhibition with tumor responses by PD0332991 in patients with mantle cell lymphoma. Blood. 2012;119(20):4597–607. doi: 10.1182/blood-2011-10-388298. Small study demonstrating proof-of-principle for selective cyclin-dependent kinase (CDK)4/6 inhibition in MCL. [DOI] [PubMed] [Google Scholar]
- 21•.BCL-2 inhibitor yields high response in CLL and SLL. Cancer Discov. 2014;4(2):OF5,8290. doi: 10.1158/2159-8290.CD-NB2013-178. CD-NB2013-178 Phase I study of the Bcl-2-selective BH3-mimetic ABT-199 showing very promising efficacy in CLL/SLL. [DOI] [PubMed] [Google Scholar]
- 22••.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–17. doi: 10.1056/NEJMoa030288. The SUMMIT trial that led to accelerated approval of bortezomib for the treatment of relapsed multiple myeloma (MM) [DOI] [PubMed] [Google Scholar]
- 23••.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–98. doi: 10.1056/NEJMoa043445. The APEX trial that led to full FDA approval of bortezomib for the treatment of relapsed MM. [DOI] [PubMed] [Google Scholar]
- 24••.Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24(30):4867–74. doi: 10.1200/JCO.2006.07.9665. First report of the PINNACLE trial that led to FDA approval for bortezomib for the treatment of relapsed MCL. [DOI] [PubMed] [Google Scholar]
- 25.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–17. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 26••.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12(5):431–40. doi: 10.1016/S1470-2045(11)70081-X. The trial that led to FDA approval for subcutaneous administration of bortezomib. [DOI] [PubMed] [Google Scholar]
- 27.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–22. [PubMed] [Google Scholar]
- 28•.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4(5):349–60. doi: 10.1038/nrc1361. Excellent review of the discovery of proteasome inhibitors (PIs) and early clinical development of bortezomib. [DOI] [PubMed] [Google Scholar]
- 29••.Adams J, Behnke M, Chen S, et al. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorg Med Chem Lett. 1998;8(4):333–8. doi: 10.1016/s0960-894x(98)00029-8. Original report of potent and selective proteasome inhibition by boronic acid derivatives. [DOI] [PubMed] [Google Scholar]
- 30•.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5(5):417–21. doi: 10.1016/s1535-6108(04)00120-5. Excellent review of the discovery of PIs and early clinical development of bortezomib. [DOI] [PubMed] [Google Scholar]
- 31.Tan C, Waldmann TA. Proteasome inhibitor PS-341, a potential therapeutic agent for adult T-cell leukemia. Cancer Res. 2002;62(4):1083–6. [PubMed] [Google Scholar]
- 32.Pham LV, Tamayo AT, Yoshimura LC, et al. Inhibition of constitutive NF-kappa B activation in mantle cell lymphoma B cells leads to induction of cell cycle arrest and apoptosis. J Immunol. 2003;171(1):88–95. doi: 10.4049/jimmunol.171.1.88. [DOI] [PubMed] [Google Scholar]
- 33.Bonvini P, Zorzi E, Basso G, Rosolen A. Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma. Leukemia. 2007;21(4):838–42. doi: 10.1038/sj.leu.2404528. [DOI] [PubMed] [Google Scholar]
- 34.Juvekar A, Manna S, Ramaswami S, et al. Bortezomib induces nuclear translocation of IkappaBalpha resulting in gene-specific suppression of NF-kappaB–dependent transcription and induction of apoptosis in CTCL. Mol Cancer Res. 2011;9(2):183–94. doi: 10.1158/1541-7786.MCR-10-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasr R, El-Sabban ME, Karam JA, et al. Efficacy and mechanism of action of the proteasome inhibitor PS-341 in T-cell lymphomas and HTLV-I associated adult T-cell leukemia/lymphoma. Oncogene. 2005;24(3):419–30. doi: 10.1038/sj.onc.1208212. [DOI] [PubMed] [Google Scholar]
- 36.Olejniczak SH, Blickwedehl J, Belicha-Villanueva A, et al. Distinct molecular mechanisms responsible for bortezomib-induced death of therapy-resistant versus -sensitive B-NHL cells. Blood. 2010;116(25):5605–14. doi: 10.1182/blood-2009-12-259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sors A, Jean-Louis F, Pellet C, et al. Down-regulating constitutive activation of the NF-kappaB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood. 2006;107(6):2354–63. doi: 10.1182/blood-2005-06-2536. [DOI] [PubMed] [Google Scholar]
- 38.Shringarpure R, Catley L, Bhole D, et al. Gene expression analysis of B-lymphoma cells resistant and sensitive to bortezomib. Br J Haematol. 2006;134(2):145–56. doi: 10.1111/j.1365-2141.2006.06132.x. [DOI] [PubMed] [Google Scholar]
- 39.Holkova B, Grant S. Proteasome inhibitors in mantle cell lymphoma. Best Pract Res Clin Haematol. 2012;25(2):133–41. doi: 10.1016/j.beha.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiarle R, Budel LM, Skolnik J, et al. Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood. 2000;95(2):619–26. [PubMed] [Google Scholar]
- 41.Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122(10):3416–23. doi: 10.1172/JCI61272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perez-Galan P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117(1):26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roue G, Perez-Galan P, Lopez-Guerra M, et al. Selective inhibition of IkappaB kinase sensitizes mantle cell lymphoma B cells to TRAIL by decreasing cellular FLIP level. J Immunol. 2007;178(3):1923–30. doi: 10.4049/jimmunol.178.3.1923. [DOI] [PubMed] [Google Scholar]
- 44.Rizzatti EG, Mora-Jensen H, Weniger MA, et al. Noxa mediates bortezomib induced apoptosis in both sensitive and intrinsically resistant mantle cell lymphoma cells and this effect is independent of constitutive activity of the AKT and NF-kappaB pathways. Leuk Lymphoma. 2008;49(4):798–808. doi: 10.1080/10428190801910912. [DOI] [PubMed] [Google Scholar]
- 45.Yang DT, Young KH, Kahl BS, et al. Prevalence of bortezomib-resistant constitutive NF-kappaB activity in mantle cell lymphoma. Mol Cancer. 2008;7:40. doi: 10.1186/1476-4598-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perez-Galan P, Roue G, Villamor N, et al. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and noxa activation independent of p53 status. Blood. 2006;107(1):257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 47.Weniger MA, Rizzatti EG, Perez-Galan P, et al. Treatment-induced oxidative stress and cellular antioxidant capacity determine response to bortezomib in mantle cell lymphoma. Clin Cancer Res. 2011;17(15):5101–12. doi: 10.1158/1078-0432.CCR-10-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derouet M, Thomas L, Cross A, et al. Granulocyte macrophage colony-stimulating factor signaling and proteasome inhibition delay neutrophil apoptosis by increasing the stability of mcl-1. J Biol Chem. 2004;279(26):26915–21. doi: 10.1074/jbc.M313875200. [DOI] [PubMed] [Google Scholar]
- 49•.Nencioni A, Hua F, Dillon CP, et al. Evidence for a protective role of mcl-1 in proteasome inhibitor-induced apoptosis. Blood. 2005;105(8):3255–62. doi: 10.1182/blood-2004-10-3984. Demonstration that proteasome inhibition upregulates myeloid cell leukemia-1 (Mcl-1), a possible mechanism of resistance to PIs and a basis for some synergistic strategies with agents that downregulate Mcl-1, such as CDK9 inhibitors. [DOI] [PubMed] [Google Scholar]
- 50••.Dunleavy K, Pittaluga S, Czuczman MS, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113(24):6069–76. doi: 10.1182/blood-2009-01-199679. Demonstration that bortezomib may be particularly effective against the NF-κB-dependent activated B-cell (ABC) subtype of DLBCL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desai S, Maurin M, Smith MA, et al. PRDM1 is required for mantle cell lymphoma response to bortezomib. Mol Cancer Res. 2010;8(6):907–18. doi: 10.1158/1541-7786.MCR-10-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao WL, Liu YY, Zhang QL, et al. PRDM1 is involved in chemoresistance of T-cell lymphoma and down-regulated by the proteasome inhibitor. Blood. 2008;111(7):3867–71. doi: 10.1182/blood-2007-08-108654. [DOI] [PubMed] [Google Scholar]
- 53.Baran-Marszak F, Boukhiar M, Harel S, et al. Constitutive and B-cell receptor-induced activation of STAT3 are important signaling pathways targeted by bortezomib in leukemic mantle cell lymphoma. Haematologica. 2010;95(11):1865–72. doi: 10.3324/haematol.2009.019745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan ZX, Wu LL, Xue K, et al. MicroRNA187 overexpression is related to tumor progression and determines sensitivity to bortezomib in peripheral T-cell lymphoma. Leukemia. 2014;28(4):880–7. doi: 10.1038/leu.2013.291. [DOI] [PubMed] [Google Scholar]
- 55.Mozos A, Roue G, Lopez-Guillermo A, et al. The expression of the endoplasmic reticulum stress sensor BiP/GRP78 predicts response to chemotherapy and determines the efficacy of proteasome inhibitors in diffuse large b-cell lymphoma. Am J Pathol. 2011;179(5):2601–10. doi: 10.1016/j.ajpath.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vega MI, Martinez-Paniagua M, Jazirehi AR, et al. The NF-kappaB inhibitors (bortezomib and DHMEQ) sensitise rituximab-resistant AIDS-B-non-hodgkin lymphoma to apoptosis by various chemotherapeutic drugs. Leuk Lymphoma. 2008;49(10):1982–94. doi: 10.1080/10428190802357071. [DOI] [PubMed] [Google Scholar]
- 57.Chandra J, Niemer I, Gilbreath J, et al. Proteasome inhibitors induce apoptosis in glucocorticoid-resistant chronic lymphocytic leukemic lymphocytes. Blood. 1998;92(11):4220–9. [PubMed] [Google Scholar]
- 58••.Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274(5288):784–7. doi: 10.1126/science.274.5288.784. Early demonstration of the role of NF-κB in resistance to apoptosis induced by cancer chemotherapy. [DOI] [PubMed] [Google Scholar]
- 59•.Wang CY, Mayo MW, Korneluk RGS, Jr, et al. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281(5383):1680–3. doi: 10.1126/science.281.5383.1680. Demonstration of the mechanisms through which NF-κB suppresses apoptosis. [DOI] [PubMed] [Google Scholar]
- 60•.Wang CY, Cusack JC, Jr, Liu R, Baldwin AS., Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med. 1999;5(4):412–17. doi: 10.1038/7410. Demonstration that NF-κB inhibition can be a valuable strategy to overcome resistance to cancer chemotherapy. [DOI] [PubMed] [Google Scholar]
- 61.Schwartz R, Davidson T. Pharmacology, pharmacokinetics, and practical applications of bortezomib. Oncology (Williston Park) 2004;18(14 Suppl 11):14–21. [PubMed] [Google Scholar]
- 62.Reece DE, Sullivan D, Lonial S, et al. Pharmacokinetic and pharmacodynamic study of two doses of bortezomib in patients with relapsed multiple myeloma. Cancer Chemother Pharmacol. 2011;67(1):57–67. doi: 10.1007/s00280-010-1283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moreau P, Karamanesht II, Domnikova N, et al. Pharmacokinetic, pharmacodynamic and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin Pharmacokinet. 2012;51(12):823–9. doi: 10.1007/s40262-012-0010-0. [DOI] [PubMed] [Google Scholar]
- 64.Hemeryck A, Geerts R, Monbaliu J, et al. Tissue distribution and depletion kinetics of bortezomib and bortezomib-related radioactivity in male rats after single and repeated intravenous injection of 14 C-bortezomib. Cancer Chemother Pharmacol. 2007;60(6):777–87. doi: 10.1007/s00280-007-0424-9. [DOI] [PubMed] [Google Scholar]
- 65.Uttamsingh V, Lu C, Miwa G, Gan LS. Relative contributions of the five major human cytochromes P450, 1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the proteasome inhibitor bortezomib. Drug Metab Dispos. 2005;33(11):1723–8. doi: 10.1124/dmd.105.005710. [DOI] [PubMed] [Google Scholar]
- 66.Leal TB, Remick SC, Takimoto CH, et al. Dose-escalating and pharmacological study of bortezomib in adult cancer patients with impaired renal function: a national cancer institute organ dysfunction working group study. Cancer Chemother Pharmacol. 2011;68(6):1439–47. doi: 10.1007/s00280-011-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LoRusso PM, Venkatakrishnan K, Ramanathan RK, et al. Pharmacokinetics and safety of bortezomib in patients with advanced malignancies and varying degrees of liver dysfunction: phase I NCI organ dysfunction working group study NCI-6432. Clin Cancer Res. 2012;18(10):2954–63. doi: 10.1158/1078-0432.CCR-11-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu C, Gallegos R, Li P, et al. Investigation of drug-drug interaction potential of bortezomib in vivo in female sprague-dawley rats and in vitro in human liver microsomes. Drug Metab Dispos. 2006;34(4):702–8. doi: 10.1124/dmd.105.008060. [DOI] [PubMed] [Google Scholar]
- 69.Hellmann A, Rule S, Walewski J, et al. Effect of cytochrome P450 3A4 inducers on the pharmacokinetic, pharmacodynamic and safety profiles of bortezomib in patients with multiple myeloma or non-hodgkin’s lymphoma. Clin Pharmacokinet. 2011;50(12):781–91. doi: 10.2165/11594410-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 70.Venkatakrishnan K, Rader M, Ramanathan RK, et al. Effect of the CYP3A inhibitor ketoconazole on the pharmacokinetics and pharmacodynamics of bortezomib in patients with advanced solid tumors: a prospective, multicenter, open-label, randomized, two-way crossover drug-drug interaction study. Clin Ther. 2009;31(Pt 2):2444–58. doi: 10.1016/j.clinthera.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 71.Quinn DI, Nemunaitis J, Fuloria J, et al. Effect of the cytochrome P450 2C19 inhibitor omeprazole on the pharmacokinetics and safety profile of bortezomib in patients with advanced solid tumours, non-hodgkin’s lymphoma or multiple myeloma. Clin Pharmacokinet. 2009;48(3):199–209. doi: 10.2165/00003088-200948030-00006. [DOI] [PubMed] [Google Scholar]
- 72•.Mato AR, Feldman T, Goy A. Proteasome inhibition and combination therapy for non-hodgkin’s lymphoma: from bench to bedside. Oncologist. 2012;17(5):694–707. doi: 10.1634/theoncologist.2011-0341. Comprehensive review of bortezomib for the treatment of NHL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kane RC, Dagher R, Farrell A, et al. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13(18 Pt 1):5291–4. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- 74.O’Connor OA, Wright J, Moskowitz C, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2005;23(4):676–84. doi: 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 75.Goy A, Younes A, McLaughlin P, et al. Phase II study of proteasome inhibitor bortezomib in relapsed or refractory B-cell non-hodgkin’s lymphoma. J Clin Oncol. 2005;23(4):667–75. doi: 10.1200/JCO.2005.03.108. [DOI] [PubMed] [Google Scholar]
- 76•.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20(3):520–5. doi: 10.1093/annonc/mdn656. Updated results of the PINNACLE trial that led to bortezomib approval for relapsed MCL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the national cancer institute of canada clinical trials group trial IND. 150. Ann Oncol. 2007;18(1):116–21. doi: 10.1093/annonc/mdl316. [DOI] [PubMed] [Google Scholar]
- 78.O’Connor OA, Moskowitz C, Portlock C, et al. Patients with chemotherapy-refractory mantle cell lymphoma experience high response rates and identical progression-free survivals compared with patients with relapsed disease following treatment with single agent bortezomib: Results of a multicentre phase 2 clinical trial. Br J Haematol. 2009;145(1):34–9. doi: 10.1111/j.1365-2141.2008.07466.x. [DOI] [PubMed] [Google Scholar]
- 79.Chang JE, Peterson C, Choi S, et al. VcR-CVAD induction chemotherapy followed by maintenance rituximab in mantle cell lymphoma: a wisconsin oncology network study. Br J Haematol. 2011;155(2):190–7. doi: 10.1111/j.1365-2141.2011.08820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang JE, Li H, Smith MR, et al. Phase 2 study of VcR-CVAD with maintenance rituximab for untreated mantle cell lymphoma: an eastern cooperative oncology group study (E1405) Blood. 2014;123(11):1665–73. doi: 10.1182/blood-2013-08-523845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruan J, Martin P, Furman RR, et al. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29(6):690–7. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 82•.Heider U, von Metzler I, Kaiser M, et al. Synergistic interaction of the histone deacetylase inhibitor SAHA with the proteasome inhibitor bortezomib in mantle cell lymphoma. Eur J Haematol. 2008;80(2):133–42. doi: 10.1111/j.1600-0609.2007.00995.x. Early demonstration of synergism between bortezomib and the histone deacetylase inhibitor (HDACI) vorinostat in MCL cells. [DOI] [PubMed] [Google Scholar]
- 83.Paoluzzi L, Scotto L, Marchi E, et al. Romidepsin and belinostat synergize the antineoplastic effect of bortezomib in mantle cell lymphoma. Clin Cancer Res. 2010;16(2):554–65. doi: 10.1158/1078-0432.CCR-09-1937. [DOI] [PubMed] [Google Scholar]
- 84.Rao R, Nalluri S, Fiskus W, et al. Role of CAAT/enhancer binding protein homologous protein in panobinostat-mediated potentiation of bortezomib-induced lethal endoplasmic reticulum stress in mantle cell lymphoma cells. Clin Cancer Res. 2010;16(19):4742–54. doi: 10.1158/1078-0432.CCR-10-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85•.Dai Y, Chen S, Kramer LB, et al. Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res. 2008;14(2):549–58. doi: 10.1158/1078-0432.CCR-07-1934. Early demonstration of synergism between bortezomib and HDACIs in CLL cells. [DOI] [PubMed] [Google Scholar]
- 86.Bhalla S, Balasubramanian S, David K, et al. PCI-24781 induces caspase and reactive oxygen species-dependent apoptosis through NF-kappaB mechanisms and is synergistic with bortezomib in lymphoma cells. Clin Cancer Res. 2009;15(10):3354–65. doi: 10.1158/1078-0432.CCR-08-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87•.Zhang QL, Wang L, Zhang YW, et al. The proteasome inhibitor bortezomib interacts synergistically with the histone deacetylase inhibitor suberoylanilide hydroxamic acid to induce T-leukemia/lymphoma cells apoptosis. Leukemia. 2009;23(8):1507–14. doi: 10.1038/leu.2009.41. Early demonstration of synergism between bortezomib and the HDACI vorinostat in T-cell NHL (T-NHL) cells. [DOI] [PubMed] [Google Scholar]
- 88.Jagannath S, Dimopoulos MA, Lonial S. Combined proteasome and histone deacetylase inhibition: a promising synergy for patients with relapsed/refractory multiple myeloma. Leuk Res. 2010;34(9):1111–18. doi: 10.1016/j.leukres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 89.Hideshima T, Richardson PG, Anderson KC. Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma. Mol Cancer Ther. 2011;10(11):2034–42. doi: 10.1158/1535-7163.MCT-11-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Holkova B, Perkins EB, Sokol L, et al. A phase II trial of bortezomib and vorinostat in mantle cell lymphoma and diffuse large B-cell lymphoma. ASH Annual Meeting Abstracts. 2011;118(21):779. doi: 10.1016/j.clml.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 91.Evens AM, Rosen ST, Helenowski I, et al. A phase I/II trial of bortezomib combined concurrently with gemcitabine for relapsed or refractory DLBCL and peripheral T-cell lymphomas. Br J Haematol. 2013;163(1):55–61. doi: 10.1111/bjh.12488. [DOI] [PubMed] [Google Scholar]
- 92.Elstrom RL, Andemariam B, Martin P, et al. Bortezomib in combination with rituximab, dexamethasone, ifosfamide, cisplatin and etoposide chemoimmunotherapy in patients with relapsed and primary refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53(8):1469–73. doi: 10.3109/10428194.2012.656629. [DOI] [PubMed] [Google Scholar]
- 93•.Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009;27(23):3830–5. doi: 10.1200/JCO.2008.20.4677. First report of high efficacy of the bortezomib, dexamethasone and rituximab regimen for the treatment of Waldenström’s macroglobulinemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghobrial IM, Hong F, Padmanabhan S, et al. Phase II trial of weekly bortezomib in combination with rituximab in relapsed or relapsed and refractory waldenstrom macroglobulinemia. J Clin Oncol. 2010;28(8):1422–8. doi: 10.1200/JCO.2009.25.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghobrial IM, Xie W, Padmanabhan S, et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with waldenstrom macroglobulinemia. Am J Hematol. 2010;85(9):670–4. doi: 10.1002/ajh.21788. [DOI] [PubMed] [Google Scholar]
- 96•.O’Connor OA, Portlock C, Moskowitz C, et al. Time to treatment response in patients with follicular lymphoma treated with bortezomib is longer compared with other histologic subtypes. Clin Cancer Res. 2010;16(2):719–26. doi: 10.1158/1078-0432.CCR-08-2647. Demonstration that FL takes longer to respond to bortezomib than other NHL subtypes. [DOI] [PubMed] [Google Scholar]
- 97.Ribrag V, Tilly H, Casasnovas O, et al. Efficacy and toxicity of two schedules of bortezomib in patients with recurrent or refractory follicular lymphoma: a randomised phase II trial from the groupe d’etude des lymphomes de l’adulte (GELA) Eur J Cancer. 2013;49(4):904–10. doi: 10.1016/j.ejca.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 98.Sehn LH, MacDonald D, Rubin S, et al. Bortezomib ADDED to R-CVP is safe and effective for previously untreated advanced-stage follicular lymphoma: a phase II study by the national cancer institute of canada clinical trials group. J Clin Oncol. 2011;29(25):3396–401. doi: 10.1200/JCO.2010.33.6594. [DOI] [PubMed] [Google Scholar]
- 99•.Fowler N, Kahl BS, Lee P, et al. Bortezomib, bendamustine, and rituximab in patients with relapsed or refractory follicular lymphoma: the phase II VERTICAL study. J Clin Oncol. 2011;29(25):3389–95. doi: 10.1200/JCO.2010.32.1844. Marked efficacy of the bortezomib, bendamustine, and rituximab regimen against FL observed in a Phase II trial. [DOI] [PubMed] [Google Scholar]
- 100•.Coiffier B, Osmanov EA, Hong X, et al. Bortezomib plus rituximab versus rituximab alone in patients with relapsed, rituximab-naive or rituximab-sensitive, follicular lymphoma: a randomised phase 3 trial. Lancet Oncol. 2011;12(8):773–84. doi: 10.1016/S1470-2045(11)70150-4. Large, Phase III trial comparing bortezomib + rituximab with rituximab alone in FL that found considerably lower benefit for adding bortezomib to rituximab than anticipated. [DOI] [PubMed] [Google Scholar]
- 101.Coiffier B, Li W, Henitz ED, et al. Prespecified candidate biomarkers identify follicular lymphoma patients who achieved longer progression-free survival with bortezomib-rituximab versus rituximab. Clin Cancer Res. 2013;19(9):2551–61. doi: 10.1158/1078-0432.CCR-12-3069. [DOI] [PubMed] [Google Scholar]
- 102.Conconi A, Martinelli G, Lopez-Guillermo A, et al. Clinical activity of bortezomib in relapsed/refractory MALT lymphomas: results of a phase II study of the international extranodal lymphoma study group (IELSG) Ann Oncol. 2011;22(3):689–95. doi: 10.1093/annonc/mdq416. [DOI] [PubMed] [Google Scholar]
- 103.Troch M, Jonak C, Mullauer L, et al. A phase II study of bortezomib in patients with MALT lymphoma. Haematologica. 2009;94(5):738–42. doi: 10.3324/haematol.2008.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Di Bella N, Taetle R, Kolibaba K, et al. Results of a phase 2 study of bortezomib in patients with relapsed or refractory indolent lymphoma. Blood. 2010;115(3):475–80. doi: 10.1182/blood-2009-08-233155. [DOI] [PubMed] [Google Scholar]
- 105.de Vos S, Goy A, Dakhil SR, et al. Multicenter randomized phase II study of weekly or twice-weekly bortezomib plus rituximab in patients with relapsed or refractory follicular or marginal-zone B-cell lymphoma. J Clin Oncol. 2009;27(30):5023–30. doi: 10.1200/JCO.2008.17.7980. [DOI] [PubMed] [Google Scholar]
- 106.Evens AM, Smith MR, Lossos IS, et al. Frontline bortezomib and rituximab for the treatment of newly diagnosed high tumour burden indolent non-hodgkin lymphoma: a multicentre phase II study. Br J Haematol. 2014;166(4):514–20. doi: 10.1111/bjh.12915. [DOI] [PubMed] [Google Scholar]
- 107.Agathocleous A, Rohatiner A, Rule S, et al. Weekly versus twice weekly bortezomib given in conjunction with rituximab, in patients with recurrent follicular lymphoma, mantle cell lymphoma and waldenstrom macroglobulinaemia. Br J Haematol. 2010;151(4):346–53. doi: 10.1111/j.1365-2141.2010.08340.x. [DOI] [PubMed] [Google Scholar]
- 108.Baiocchi RA, Alinari L, Lustberg ME, et al. Phase 2 trial of rituximab and bortezomib in patients with relapsed or refractory mantle cell and follicular lymphoma. Cancer. 2011;117(11):2442–51. doi: 10.1002/cncr.25792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Friedberg JW, Vose JM, Kelly JL, et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-hodgkin lymphoma. Blood. 2011;117(10):2807–12. doi: 10.1182/blood-2010-11-314708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110•.Dai Y, Rahmani M, Grant S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK- and NF-kappaB-dependent process. Oncogene. 2003;22(46):7108–22. doi: 10.1038/sj.onc.1206863. Early demonstration of synergism at the preclinical level between bortezomib and the pan-CDKI flavopiridol (alvocidib) [DOI] [PubMed] [Google Scholar]
- 111.Dai Y, Rahmani M, Pei XY, et al. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104(2):509–18. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- 112•.Holkova B, Perkins EB, Ramakrishnan V, et al. Phase I trial of bortezomib (PS-341; NSC 681239) and alvocidib (flavopiridol; NSC 649890) in patients with recurrent or refractory B-cell neoplasms. Clin Cancer Res. 2011;17(10):3388–97. doi: 10.1158/1078-0432.CCR-10-2876. Promising efficacy of the combination of bortezomib and flavopiridol (alvocidib) against MM and indolent B-NHL seen in a Phase I study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zinzani PL, Musuraca G, Tani M, et al. Phase II trial of proteasome inhibitor bortezomib in patients with relapsed or refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(27):4293–7. doi: 10.1200/JCO.2007.11.4207. [DOI] [PubMed] [Google Scholar]
- 114.Kim SJ, Yoon DH, Kang HJ, et al. Bortezomib in combination with CHOP as first-line treatment for patients with stage III/IV peripheral T-cell lymphomas: a multicentre, single-arm, phase 2 trial. Eur J Cancer. 2012;48(17):3223–31. doi: 10.1016/j.ejca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Cavaletti G, Jakubowiak AJ. Peripheral neuropathy during bortezomib treatment of multiple myeloma: a review of recent studies. Leuk Lymphoma. 2010;51(7):1178–87. doi: 10.3109/10428194.2010.483303. [DOI] [PubMed] [Google Scholar]
- 116.Delforge M. Bortezomib for previously untreated multiple myeloma. Expert Opin Pharmacother. 2011;12(16):2553–64. doi: 10.1517/14656566.2011.622266. [DOI] [PubMed] [Google Scholar]
- 117.Mohty B, El-Cheikh J, Yakoub-Agha I, et al. Peripheral neuropathy and new treatments for multiple myeloma: background and practical recommendations. Haematologica. 2010;95(2):311–19. doi: 10.3324/haematol.2009.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23):4745–53. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 119.Gerecitano J, Portlock C, Moskowitz C, et al. Phase 2 study of weekly bortezomib in mantle cell and follicular lymphoma. Br J Haematol. 2009;146(6):652–5. doi: 10.1111/j.1365-2141.2009.07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lonial S, Waller EK, Richardson PG, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. 2005;106(12):3777–84. doi: 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008;26(29):4784–90. doi: 10.1200/JCO.2007.14.9641. [DOI] [PubMed] [Google Scholar]
- 122.Gerecitano J, Goy A, Wright J, et al. Drug-induced cutaneous vasculitis in patients with non-hodgkin lymphoma treated with the novel proteasome inhibitor bortezomib: a possible surrogate marker of response? Br J Haematol. 2006;134(4):391–8. doi: 10.1111/j.1365-2141.2006.06201.x. [DOI] [PubMed] [Google Scholar]
- 123.Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–72. doi: 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 124.Kortuem KM, Stewart AK. Carfilzomib. Blood. 2013;121(6):893–7. doi: 10.1182/blood-2012-10-459883. [DOI] [PubMed] [Google Scholar]
- 125•.Ruschak AM, Slassi M, Kay LE, Schimmer AD. Novel proteasome inhibitors to overcome bortezomib resistance. J Natl Cancer Inst. 2011;103(13):1007–17. doi: 10.1093/jnci/djr160. Thorough review of novel PIs developed to circumvent resistance to bortezomib. [DOI] [PubMed] [Google Scholar]
- 126•.Dasmahapatra G, Patel H, Dent P, et al. The bruton tyrosine kinase (BTK) inhibitor PCI-32765 synergistically increases proteasome inhibitor activity in diffuse large-B cell lymphoma (DLBCL) and mantle cell lymphoma (MCL) cells sensitive or resistant to bortezomib. Br J Haematol. 2013;161(1):43–56. doi: 10.1111/bjh.12206. First report of synergism at the preclinical level between ibrutinib and bortezomib against DLBCL (both ABC- and GCB-subtypes) and MCL. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 127.Dasmahapatra G, Lembersky D, Rahmani M, et al. Bcl-2 antagonists interact synergistically with bortezomib in DLBCL cells in association with JNK activation and induction of ER stress. Cancer Biol Ther. 2009;8(9):808–19. doi: 10.4161/cbt.8.9.8131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 128.Kouroukis CT, Fernandez LA, Crump M, et al. A phase II study of bortezomib and gemcitabine in relapsed mantle cell lymphoma from the national cancer institute of canada clinical trials group (IND 172) Leuk Lymphoma. 2011;52(3):394–9. doi: 10.3109/10428194.2010.546015. [DOI] [PubMed] [Google Scholar]
- 129.Lamm W, Kaufmann H, Raderer M, et al. Bortezomib combined with rituximab and dexamethasone is an active regimen for patients with relapsed and chemotherapy-refractory mantle cell lymphoma. Haematologica. 2011;96(7):1008–14. doi: 10.3324/haematol.2011.041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Houot R, Le Gouill S, Ojeda Uribe M, et al. Combination of rituximab, bortezomib, doxorubicin, dexamethasone and chlorambucil (RiPAD+C) as first-line therapy for elderly mantle cell lymphoma patients: results of a phase II trial from the GOELAMS. Ann Oncol. 2012;23(6):1555–61. doi: 10.1093/annonc/mdr450. [DOI] [PubMed] [Google Scholar]
- 131.Gerecitano J, Portlock C, Hamlin P, et al. Phase I trial of weekly and twice-weekly bortezomib with rituximab, cyclophosphamide, and prednisone in relapsed or refractory non-hodgkin lymphoma. Clin Cancer Res. 2011;17(8):2493–501. doi: 10.1158/1078-0432.CCR-10-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]