Abstract
Chemotherapy with Temozolomide (TMZ), radiation and surgery are the primary methods to treat Glioblastoma Multiforme (GBM), the most common adult intracranial tumor with dismal outcome. GBM resistance to therapy is the main reason of poor patient outcomes. Thus, methods to overcome the resistance are an area of extensive research. This highlight focuses on three recently published articles on the mechanism of resistance and possible therapeutic intervention, including RNA treatment with stem cells. We showed a crucial role of the developmental Sonic Hedgehog (SHH) pathway in the acquisition and maintenance of TMZ resistance. SHH signaling caused TMZ resistance in GBM cells through an increase in the multiple drug resistance gene (MDR1). The SHH receptor, Patched-1 (PTCH1), negatively regulate SHH signaling. In GBM, miR-9 suppressed PTCH1 levels, resulting in the activation of SHH pathway. Thus, SHH signaling is independent of the ligand in resistant GBM cells. MiR-9 was also increased in chemoresistance CD133+ GBM cells. A potential method to reverse resistance was tested by delivering the anti-miR in bone marrow-derived Mesenchymal Stem Cells (MSCs). The anti-miR-9 was transferred into the resistant GBM cells through exosomes and gap junctional intercellular communication. We also review on-going clinical trials with inhibitor of SHH signaling, and also discuss drug delivery by cell therapy for GBM. While GBM treatment has proven to be a challenge, there are a number of novel approaches we are currently developing to manage this malignancy.
Keywords: miR-9, glioblastoma, temozolomide, sonic hedgehog, stem cell, exosome
Introduction
Glioblastoma multiforme (GBM) is the most common adult primary tumor of the central nervous system. Clinically, GBM presents a very difficult malignancy to treat, underscored by a 5-year survival of 3–5% [1]. GBM is typically treated with the alkylating agent Temozolomide (TMZ). Since 2010, the monoclonal antibody Bevacizumab (Avastin®) was approved to treat TMZ-resistant patients. GBMs uniformly exhibit resistance to chemotherapy and recur within a few weeks to months [2]. The mechanisms behind this resistance appear to be complex and could occur through synergistic mechanisms, such as cell cycle progression, upregulation of mismatch repair genes and increased activity of ATP-dependent drug efflux pumps [3–5].
Developmental and oncogenic pathways have often been shown to overlap, suggesting “reactivation” of developmental queues in malignancy. Examples of such pathways include those involving Notch, Bone morphogenic proteins, and Sonic Hedgehog (SHH) [6]. Here we review studies involving developmental pathways involving SHH and discuss the regulatory role of miR-9.
Neurodevelopmental of miR-9 regulation of TMZ resistance
MiRNA are small (18–22 bp) non-coding RNA molecules which regulate cellular processes including development, differentiation and oncogenesis [7]. MiRNA regulates protein expression by binding to the 3′ UTR of target mRNA to suppress translation. MiR-9 is a conserved miR known to regulate development, differentiation and migration of cells within the central nervous system [8]. MiR-9 has also been implicated in oncogenesis. In 2011, miR-9 it was shown to be upregulated in GBM cells, and then later shown to be upregulated within a “stem cell-like” subset of GBM cells [9,10]. Recently we reported on a novel target for miR-9 regulation, the SHH receptor, patched-1 (PTCH1) [11]. Interestingly, miR-9 has three genomic loci on chromosomes 1, 5, and 15. In neurodevelopment, miR-9-2 is known to be upregulated, resulting as the main source during development of the brain [12]. Consistent with cell development, our TMZ-resistant GBM model indicated that an upregulation of the miR-9-2 loci was the likely source of mature miR-9.
SHH signaling is essential for the ventral-dorsal patterning of the CNS [13]. Our data revealed a novel method for activation of the SHH signaling pathway. Typical activation occurs by internalization of PTCH1 when bound to SHH. When PTCH1 is internalized, repression of intracellular signaling is released, resulting in the translocation of Gli1 transcription factor [14]. Yet, in our model PTCH1 expression was reduced, but SHH ligand synthesis was unchanged. MiR-9 overexpression in GBM cells resulted in SSH signaling and TMZ resistance. Chemoresistance in GBM cells was determine to be caused by increased expression of the Multiple Drug Resistance (MDR1) gene and ABCG2. Furthermore, patient-derived neurosphere cell lines from recurrent GBM also showed TMZ resistance. In these cell lines, miR-9-2, MDR1, and ABCG2 were also upregulated, while PTCH1 expression was decreased. The studies with the neurospheres from GBM recapitulated the data with cell lines.
Regulating the Sonic Hedgehog Pathway
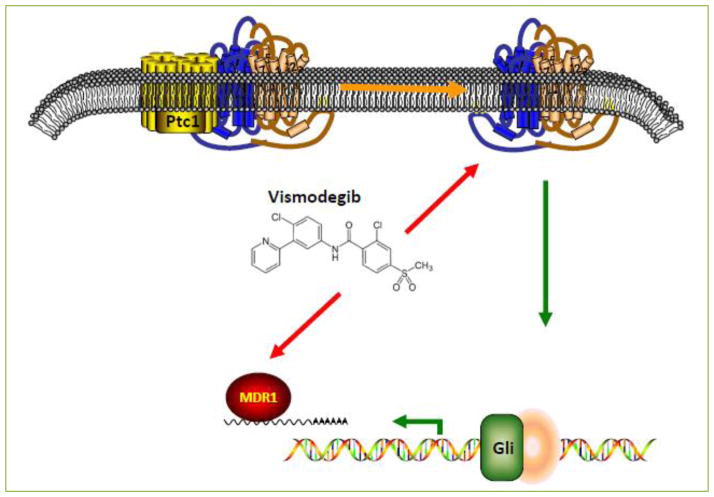
As part of our recent work we showed a role for Vismodegib (Erivedge®) in combination with TMZ to overcome the sonic hedgehog-dependent resistance. The advantage of Vismodegib therapy is inhibition of Smoothened, downstream of PTCH1, thus circumventing miR-9 activation in SHH signaling [15]. We selected Vismodegib since this drug has been FDA approved for basal cell carcinoma. It has also been shown to be a potent inhibitor of two downstream ABC transporters, ABCG2/BCRP1 and ABCB1/MDR1 [16]. We were able to show increased cell death and TMZ-induced caspase-3 activity, in combination with Vismodegib. The effect of Vismodegib was dose-dependent. A summary of the roles of Vismodegib is highlighted in Figure 1.
Figure 1. Diagrammatic representation of Vismodegib dual inhibition of SHH Signaling and MDR1 function.
Vismodegib inhibits SMO (the SHH activator, yellow) and MDR1 (red) at varying concentrations. This dual inhibition allows for reversal of TMZ resistance in GBM cells and increased TMZ-induced cell death.
Clinical trial NCT00980343 tested the role of Vismodegib in recurrent GBM (prior to or after surgery) for patients where were candidates for surgery. In the pediatric population, Vismodegib is currently being investigated as monotherapy for recurrent pontine glioma patients (NCT01774253). In addition, NCT01601184 is currently a phase I/II study to evaluate the role of Vismodegib along with TMZ for medulloblastoma patients. The validity of such a trial is due to the activation of SHH pathway and PTCH1 inactivating mutations. Together, these trials along with our data show great promise for targeting the SHH pathway with pharmacological inhibitors.
Cancer Stem Cells (CSCs) and Drug Resistance
The CSC hypothesis proposes the existence of self-renewal and tumor repopulating cells in the tumor [17]. The CSCs are believed to be chemoresistant and to have tumor regenerating properties. CD133 cell surface expression has been used as a marker to identify the CSC population within GBMs [18]. We published a number of experiments that showed chemoresistance of GBM CD133+ cells and showed that this was due to the upregulation of miR-9-2 and MDR1 [19].
We first isolated CD133+ cells from U87 and T98G cell lines. Given the “stem cell” nature of CSCs, we asked if delayed cell cycle progression as a possible mechanism of TMZ resistance. Yet, CD133+ cells did not show any difference in cell cycle status when compared to CD133-cells. PTCH1 expression was decreased with concomitant increase in Gli1 expression. Thus we assayed for five possible PTCH1 targeting miRNAs, but only mature miR-9 was found to be increased in CD133+ cells from both cell lines. Specifically, miR-9-2 was upregulated in these cells and transfection with anti-miR-9 resulted in chemosensitivity.
CSC-targeted therapies offer a new opportunity to eradicate the tumor repopulating cells for GBM, which is undoubtedly a fatal malignancy. According to the CSC hypothesis, CSCs are slow cycling cells that provide the fuel for GBM growth and resistance [20]. By eliminating this subset of GBM cells, it would be expected that the tumor progression would be halted with chemotherapy-mediated death of the differentiated and rapidly cycling bulk tumor cells. On the downside for treatment to target CSCs, it would be important not to interfere with endogenous neural stem cells (NSCs).
MiRNA-targeted therapy for GBM
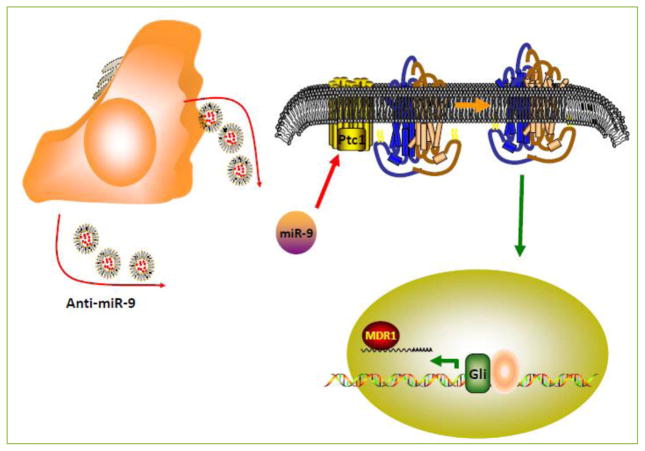
MiRNA-targeted therapy is a recent field with a growing interest in oncology. The current major hurdle in the development of this therapeutic approach is effective tissue targeting [21]. To overcome this difficulty, we recently published research showing the functional transfer of anti-miR-9 from Mesenchymal Stem Cells (MSCs) to GBM cells, through exosomes (Figure 2). MSCs exhibit an innate tropism to GBM cells in vivo. A comparison of the source of MSCs, adipose vs. bone marrow, showed no difference in tropism and migration to glioma capabilities [22]. The mechanisms of this tropism towards CNS lesions have been shown to be multifactorial [23]. Recent data has shown that neoadjuvant irradiation of gliomas enhanced MSC migration towards the glioma [24].
Figure 2. Anti-microRNA-9 targeted therapy by Mesenchymal Stem Cells (MSCs).
MSCs (orange) transfected with fluorescent anti-miR-9 showed intercellular-targeted therapy via MSC-derived Exosomes to GBM cells (yellow). Functional anti-miR-9 decreased endogenous miR-9 and reversed GBM TMZ chemoresistance.
We showed that MSCs can communicate with GBM cells through contact-dependent (gap junction formation) and contact-independent (exosome secretion) mechanisms [25]. GBM resistant cells also showed an increase in connexin-43 after treatment with TMZ [26]. Fluorescent-tagged anti-miR-9 was able to transfer most effectively through exosomes and decrease the expression of miR-9 in TMZ-resistant GBM cells. Along with this decrease in miR-9, MDR1 expression was reduced and the GBM cells exhibited greater sensitivity to TMZ.
Currently, the Phase I Clinical Trial NCT02015819 at the City of Hope Medical Center is in the process of assessing the feasibility of Stem Cell-based therapies for recurrent GBM. Although NCT02015819 uses another stem cell, NSCs, the goal is the same as our studies with MSCs; namely to deliver cytosine deaminase in ectopic expression in NSCs [27]. More importantly, this trial will assess the T-cell response to stem cell based therapy.
Conclusions
Overcoming and treating GBM resistance and recurrence has been a difficult clinical challenge. Although medical management of resistance is limited at this time to TMZ and Bevacizumab, we have taken great strides to understand the mechanisms of resistance and thus, attempt personalized and targeted therapy [28]. Our recent data has highlighted the dichotomy known to exist between malignancy and development. The SHH pathway is a well-established pillar of neural development and thus it is no surprise that cancer cells utilize this mechanism to resist therapy and recur. We have shown the potential in using SHH inhibition to enhance TMZ efficacy. In addition, the usage of Vismodegib allowed for simultaneous targeting of SHH and MDR1 [29].
MiRNA, once considered “junk” DNA products, have now become a major area of research, highlighting the role of MiRs in many biological processes. Among the regulatory roles of miRs is the coordination of development and the parallel process, oncogenesis. MiR-9 is known to have critical roles in neuron development and migration [30], yet has been associated with malignancies of the CNS such as GBM and Medulloblastoma [31,32]. MiR-9 also has important roles in the development of cancers outside of the CNS such as breast, colon, cervical, ovarian, and gastric cancer [33–36]. Here we highlighted the oncogenic role of miR-9 in GBM and provided a mechanism to reverse miR-9 upregulation using exosomal targeted anti-miR-9.
The CSC hypothesis provides an explanation for the heterogeneity and recurrence of tumors such as GBMs. Recent pharmaceutical research aims to target and eradicate CSC populations in GBMs. Here we also highlight our work showing that CSCs exhibit innate resistance to TMZ because of miR-9 upregulation and subsequent MDR1 expression. This opens the door to two novel targets for CSC-based therapy, miR-9 and MDR1.
Current clinical trials indicate the great promise that exists in a future to overcome this uniformly fatal malignancy. GBMs have been shown to activate a number of pathways and utilize various methods of TMZ resistance. Yet, SHH inhibition may prove to be key to overcome the resistance and subsequent recurrence of TMZ-treated GBMs.
Abbreviations
- GBM
glioblastoma multiforme
- SHH
sonic hedgehog
- MSC
mesenchymal stem cell
- CSC
cancer stem cell
- MDR1
multiple drug resistance gene 1
- miR
microRNA
- PTCH1
patched-1
- NSC
neural stem cell
Footnotes
Conflicting interests
The author(s) declare that they have no Conflicting interests.
References
- 1.Naydenov E, Tzekov C, Minkin K, Nachev S, Romansky K, Bussarsky V. Long-term survival with primary glioblastoma multiforme: a clinical study in bulgarian patients. Case Reports in Oncol. 2011;4:1–11. doi: 10.1159/000323432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verhoeff JJ, van Tellingen O, Claes A, Stalpers LJ, van Linde ME, Richel DJ, et al. Concerns about anti-angiogenic treatment in patients with glioblastoma multiforme. BMC Cancer. 2009;9:e444. doi: 10.1186/1471-2407-9-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smalley S, Chalmers AJ, Morley SJ. mTOR inhibition and levels of the DNA repair protein MGMT in T98G glioblastoma cells. Mol Cancer. 2014;13:e144. doi: 10.1186/1476-4598-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veringa SJ, Biesmans D, van Vuurden DG, Jansen MH, Wedekind LE, Horsman I, et al. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS One. 2013;8:e61512. doi: 10.1371/journal.pone.0061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q, Qian J, Wang J, Luo C, Chen J, Hu G, et al. Knockdown of RLIP76 expression by RNA interference inhibits invasion, induces cell cycle arrest, and increases chemosensitivity to the anticancer drug temozolomide in glioma cells. J Neuro-Oncol. 2013;112:73–82. doi: 10.1007/s11060-013-1045-2. [DOI] [PubMed] [Google Scholar]
- 6.Cruceru ML, Neagu M, Demoulin JB, Constantinescu SN. Therapy targets in glioblastoma and cancer stem cells: lessons from haematopoietic neoplasms. J Cell Mol Med. 2013;17:1218–1235. doi: 10.1111/jcmm.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha M, Kim VN. Regulation of microRNA biogenesis. Nature Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 8.Yuva-Aydemir Y, Simkin A, Gascon E, Gao FB. MicroRNA-9: functional evolution of a conserved small regulatory RNA. RNA Biol. 2011;8:557–564. doi: 10.4161/rna.8.4.16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TM, Huang W, Park R, Park PJ, Johnson MD. A developmental taxonomy of glioblastoma defined and maintained by MicroRNAs. Cancer Res. 2011;71:3387–3399. doi: 10.1158/0008-5472.CAN-10-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schraivogel D, Weinmann L, Beier D, Tabatabai G, Eichner A, Zhu JY, et al. CAMTA1 is a novel tumour suppressor regulated by miR-9/9* in glioblastoma stem cells. EMBO J. 2011;30:4309–4322. doi: 10.1038/emboj.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munoz JL, Rodriguez-Cruz V, Ramkissoon SH, Ligon KL, Greco SJ, Rameshwar P. Temozolomide resistance in glioblastoma occurs by miRNA-9-targeted PTCH1, independent of sonic hedgehog level. Oncotarget. 2015;6:1190–1201. doi: 10.18632/oncotarget.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun E, Shi Y. MicroRNAs: Small molecules with big roles in neurodevelopment and diseases. Exp Neurol. doi: 10.1016/j.expneurol.2014.08.005. In press. [DOI] [PubMed] [Google Scholar]
- 13.Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–4063. doi: 10.1242/dev.036624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhry Z, Rikani AA, Choudhry AM, Tariq S, Zakaria F, Asghar MW, et al. Sonic hedgehog signalling pathway: a complex network. Annals Neurosci. 2014;21:28–31. doi: 10.5214/ans.0972.7531.210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dijkgraaf GJ, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 16.Chen YJ, Kuo CD, Chen SH, Chen WJ, Huang WC, Chao KS, et al. Small-molecule synthetic compound norcantharidin reverses multi-drug resistance by regulating Sonic hedgehog signaling in human breast cancer cells. PLoS One. 2012;7:e37006. doi: 10.1371/journal.pone.0037006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam F, Qiao B, Smith RA, Gopalan V, Lam AK. Cancer stem cell: Fundamental experimental pathological concepts and updates. Exp Mol Pathol. 2015;98:184–191. doi: 10.1016/j.yexmp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Choy W, Nagasawa DT, Trang A, Thill K, Spasic M, Yang I. CD133 as a marker for regulation and potential for targeted therapies in glioblastoma multiforme. Neurosurg Clinics North Am. 2012;23:391–405. doi: 10.1016/j.nec.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Munoz JL, Rodriguez-Cruz V, Rameshwar P. High expression of miR-9 in CD133+ glioblastoma cells in chemoresistance to temozolomide. J Cancer Stem Cell Res. 2015;3:e1003. doi: 10.14343/JCSCR.2015.3e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang TW, Choi SW, Yang SR, Shin TH, Kim HS, Yu KR, et al. Growth arrest and forced differentiation of human primary glioblastoma multiforme by a novel small molecule. Sci Reports. 2014;4:e5546. doi: 10.1038/srep05546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rooij E, Purcell AL, Levin AA. Developing microRNA therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 22.Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A. Mesenchymal stem cells derived from adipose tissue vs bone marrow: in vitro comparison of their tropism towards gliomas. PLoS One. 2013;8:e58198. doi: 10.1371/journal.pone.0058198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Z, Jiang Z, Huang J, Huang S, Li Y, Sheng F, et al. Mesenchymal stem cells show little tropism for the resting and differentiated cancer stem cell-like glioma cells. Intl J Oncol. 2014;44:1223–1232. doi: 10.3892/ijo.2014.2284. [DOI] [PubMed] [Google Scholar]
- 24.Kim SM, Oh JH, Park SA, Ryu CH, Lim JY, Kim DS, et al. Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells. 2010;28:2217–2228. doi: 10.1002/stem.543. [DOI] [PubMed] [Google Scholar]
- 25.Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol Ther Nucleic acids. 2013;2:e126. doi: 10.1038/mtna.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munoz JL, Rodriguez-Cruz V, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Temozolomide resistance in glioblastoma cells occurs partly through epidermal growth factor receptor-mediated induction of connexin 43. Cell Death & Dis. 2014;5:e1145. doi: 10.1038/cddis.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5:ra59. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wick W, Weller M, Weiler M, Batchelor T, Yung AW, Platten M. Pathway inhibition: emerging molecular targets for treating glioblastoma. Neuro-Oncol. 2011;13:566–579. doi: 10.1093/neuonc/nor039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz JL, Rodriguez-Cruz V, Greco SJ, Nagula V, Scotto KW, Rameshwar P. Temozolomide induces the production of epidermal growth factor to regulate MDR1 expression in glioblastoma cells. Mol Cancer Ther. 2014;13:2399–2411. doi: 10.1158/1535-7163.MCT-14-0011. [DOI] [PubMed] [Google Scholar]
- 30.Rotkrua P, Akiyama Y, Hashimoto Y, Otsubo T, Yuasa Y. MiR-9 downregulates CDX2 expression in gastric cancer cells. J Intl Cancer. 2011;129:2611–2620. doi: 10.1002/ijc.25923. [DOI] [PubMed] [Google Scholar]
- 31.Fiaschetti G, Abela L, Nonoguchi N, Dubuc AM, Remke M, Boro A, et al. Epigenetic silencing of miRNA-9 is associated with HES1 oncogenic activity and poor prognosis of medulloblastoma. Br J Cancer. 2014;110:636–647. doi: 10.1038/bjc.2013.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Z, Wang L, Li G, Liu H, Fan F, Li Z, et al. Increased expression of microRNA-9 predicts an unfavorable prognosis in human glioma. Mol Cell Biochem. 2013;384:263–268. doi: 10.1007/s11010-013-1805-5. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Gao G, Hu X, Wang Y, Schwarz JK, Chen JJ, et al. Activation of miR-9 by human papillomavirus in cervical cancer. Oncotarget. 2014;5:11620–11630. doi: 10.18632/oncotarget.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Selcuklu SD, Donoghue MT, Rehmet K, de Souza Gomes M, Fort A, Kovvuru P, et al. MicroRNA-9 inhibition of cell proliferation and identification of novel miR-9 targets by transcriptome profiling in breast cancer cells. J Biol Chem. 2012;287:29516–29528. doi: 10.1074/jbc.M111.335943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su YH, Zhou Z, Yang KP, Wang XG, Zhu Y, Fa XE. MIR-142-5p and miR-9 may be involved in squamous lung cancer by regulating cell cycle related genes. Eur Rev Med Pharm Sci. 2013;17:3213–3220. [PubMed] [Google Scholar]
- 36.Zhu L, Chen H, Zhou D, Li D, Bai R, Zheng S, et al. MicroRNA-9 up-regulation is involved in colorectal cancer metastasis via promoting cell motility. Med Oncol. 2012;29:1037–1043. doi: 10.1007/s12032-011-9975-z. [DOI] [PubMed] [Google Scholar]