Abstract
Hippocampal area CA2 has several features that distinguish it from CA1 and CA3, including a unique gene expression profile, failure to display long-term potentiation and relative resistance to cell death. A recent increase in interest in the CA2 region, combined with the development of new methods to define and manipulate its neurons, has led to some exciting new discoveries on the properties of CA2 neurons and their role in behaviour. Here, we review these findings and call attention to the idea that the definition of area CA2 ought to be revised in light of gene expression data.
The extended mammalian hippocampal formation is critical for learning and memory1, spatial navigation2,3, fear processing4 and social behaviour5, among other cognitive functions. The hippocampus proper consists of the Cornu Ammonis (CA) region, a strip of pyramidal neurons, and the dentate gyrus (DG), which consists of granule cells. The CA and DG are anatomically arranged in a curled structure that lends itself well to both in vivo and in vitro electrophysiological study (FIG. 1). Early neuroanatomists described two distinct areas of the rodent CA; the top portion, which consisted of small pyramidal neurons (regio superior of Cajal), and the lower portion, which consisted of larger pyramidal neurons (regio inferior of Cajal).
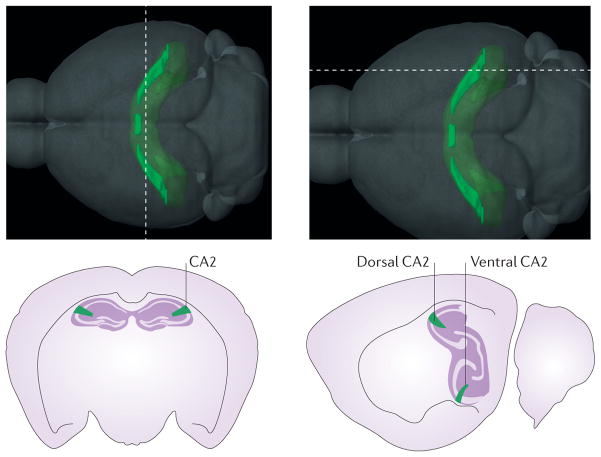
Figure 1. Hippocampal area CA2 in the mouse brain.
The top panel illustrates the location and gross structure of the mouse CA2 region, as defined by the Allen Institute for Brain Science on the basis of the differential expression of almost 50 genes. Thedashed lines indicate the positions of the slices that are represented in the bottom panels. Interestingly, most of these ‘CA2-enriched’ genes are not expressed in the ventral hippocampus of the mouse (lower right panel), even though other atlases have classically included CA2 in most of the ventral third of the hippocampus81. Images in the top panel are reproduced with permission from © 2015 Allen Institute for Brain Science. Allen Mouse Brain Atlas [Internet]. Available from: http://mouse.brain-map.org.
However, in 1934, Rafael Lorente de Nó noted that a small area of the regio inferior was sufficiently distinct in its cytoarchitecture and connectivity to warrant a separate nomenclature6. For this reason, he designated the three CA areas containing pyramidal neurons as CA1, CA2 and CA3; and the end portion within the blades of the DG, containing polymorphic cells, as CA4 (REF. 6). Lorente de Nó observed that the pyramidal cell bodies of the CA2, like those of the CA3, are larger than those found in the CA1 (REF. 6). However, he noted that CA2 pyramidal cell dendrites lack the specialized thorny excrescences associated with input from mossy fibres from the DG, which are characteristic of CA3 pyramidal neurons6. CA2 neurons also receive Schaffer collateral input from CA3 neurons, much like the cells of area CA1 (REF. 6).
Recent studies of the molecular attributes of CA2 neurons, however, support the use of an updated definition of this region that extends beyond the initial description by Lorente de Nó7–9. In addition, it has been shown that the presence of heavy axonal input from the supramammillary nucleus (SuM) and the paraventricular nucleus (PVN) of the hypothalamus may also be helpful when identifying the CA3–CA2 boundary, as the bulk of axonal projections from these regions terminate precisely at the site of molecular markers that delineate CA2 pyramidal neurons in rodents9–11. Thus, expression of genes enriched in the CA2 region, together with the distribution of SuM and PVN inputs to the hippocampus, may suffice to define CA2 in many species, rather than relying on Lorente de Nó’s definition that is dependent on the presence or absence of mossy fibre input12 (FIG. 2).
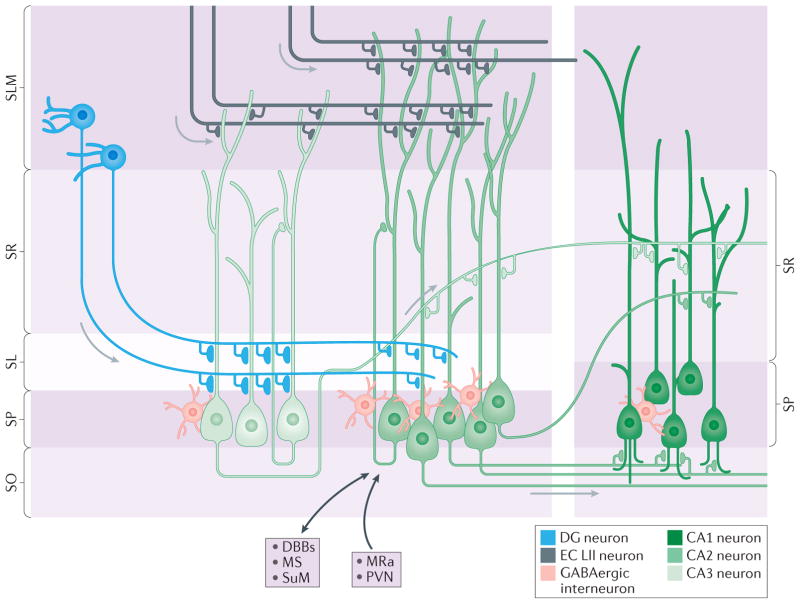
Figure 2. Connectivity of CA2 neurons within the rodent hippocampal circuit.
The CA2 region (medium green) is located between the CA3 region (light green) and the CA1 region (dark green) and is an integral part of the hippocampal circuitry. In rodents, the primary afferents contacting CA2 pyramidal neurons arise from three primary sources: dentate gyrus (DG) granule cells (shown in blue) that target the stratum lucidum (SL), CA3 neurons that target the stratum radiatum (SR), and medial and lateral entorhinal cortex layer II (EC LII) neurons (shown in dark grey) that target the stratum lacunosum-moleculare (SLM)9,10,53. This part of the perforant path was found to dip far into the area normally considered to be SR in monkeys and humans143. The main projection target of CA2 pyramidal neurons is ‘deep’ calbindin 1- immunonegative CA1 neurons with dendrites in stratum oriens (SO)9,29; CA2 neurons target the CA1 SR to a lesser extent29. Extrahippocampal inputs to CA2 (indicated by black arrows) include those from vasopressinergic neurons in the paraventricular nucleus (PVN) of the hypothalamus11,40 and from the median raphe (MRa), and reciprocal connections with the supramammillary nucleus (SuM)36,37,43, medial septum (MS) and diagonal bands of Broca (DBBs)10. Many different types of inhibitory interneurons are found in area CA2 and form synapses on CA2 pyramidal neurons. The density of reelin- and parvalbumin-immunopositive interneurons (shown in pink) is several-fold higher in area CA2 stratum pyramidale (SP) than in CA1 SP and CA3 SP60.
Area CA2 has attracted the interest of researchers because of its relatively high resistance to damage from injury13,14 and resistance to synaptic plasticity15 compared with other CA regions. CA2 pyramidal neurons also exhibit cellular signalling pathways and neuro-modulatory influences that are not present in other CA regions. In this Review, we first consider how hippocampal area CA2 was classically defined and distinguished from its neighbouring subfields. We also review more recent work on the connectivity, cellular morphology, electrophysiological properties and molecular signature of area CA2. We then highlight some of the exciting new findings regarding the unique role of area CA2 in behaviour. Finally, we discuss the remarkable resistance of area CA2 to both typical synaptic plasticity and certain neurobiological disease processes.
Identifying area CA2
A revised definition
When the CA regions were first described6, it was noted that the large pyramidal neurons of the regio inferior closest to the regio superior were different from the rest: they did not have large, complex spines on their proximal apical dendrites. Although the absence or presence of large specialized postsynaptic structures may distinguish CA2 neurons from those in CA3, another commonly used anatomical delineator, the absence of DG input, is not as reliable. Lorente de Nó’s illustrations of the mouse brain show DG axons ending at what he termed the CA3–CA2 border, even though many investigators have now observed that they extend almost to area CA1 in mice9. Indeed, the extent of the DG axon projection is species specific. For example, DG axons end in a bulb-like termination near CA2 in guinea pigs16, extend deep into area CA1 in cats17,18, and taper into area CA2 in rats and mice9,19 (BOX 1; FIG. 2). However, in the non-human primate hippocampus, it appears that CA3, defined as the area with pyramidal neurons having thorny excrescences, does correspond exactly to the area innervated by the DG axons6 (but for a more nuanced definition of human CA regions, see REF. 20). This classic designation of CA2 neurons in human and non-human primates, however, may simply be a consequence of the lack of extensive study with newer markers for area CA2. Alternatively, it may indeed be the case that area CA2 in primates is proportionately larger than that in rodents.
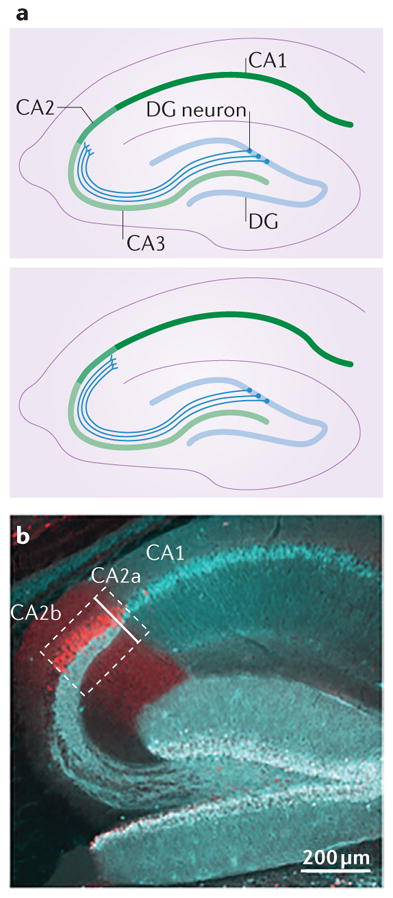
Box 1. A case for subdividing area CA2.
Rafael Lorente de Nó defined the CA subfields of the hippocampus in both the mouse and the macaque, and designated CA2 as the area of the CA region with large pyramidal neurons lacking the thorny excrescences that are characteristic of the mossy fibre synapses onto CA3 neurons6 (see the figure, part a, top panel). Although his drawings clearly indicate that he designated the CA2 CA3 border as the point at which the dentate gyrus (DG) axons end, the expression pattern of molecules that are known to be enriched in area CA2 (for example, striatum-enriched protein-tyrosine phosphatase (STEP), Purkinje cell protein 4 (PCP4) and regulator of G protein signalling 14 (RGS14)) differs from this interpretation of the CA2 CA3 border. Indeed, in mice, it appears that only a very small proportion of the CA2 pyramidal neurons defined by the expression of such molecules also fall under the classic definition of area CA2 (REF. 19) (that is, neurons lacking DG input; see the figure, part a, bottom panel, and part b).
In mice, most of the CA2 neurons defined by the expression of such molecules receive functional DG input, as depicted in the figure (part a, bottom panel), but with smaller synapses than those made onto neurons of the CA3 (REF. 9). Therefore, it is not surprising that some of the CA3 neurons that are closest to area CA2 have firing properties more similar to those in area CA2 than to the rest of the CA3(REFS 87,88). A case can even be made that, in the human hippocampus, area CA2 should be subdivided into subregions CA2a, CA2b and CA2c, based on the packing density of cell bodies expressing high levels of chromogranin A (CgA)20; CA2a can be identified by the presence of CgA-expressing pyramidal neurons intermingled with CgA-negative (CA1-like) neurons, CA2b can be identified by the presence of CgA-expressing neurons across the full depth of the stratum pyramidale, and CA2c can be identified as having a thicker layer and less intense CgA expression than the other regions20. Note that, in this particular study, area CA2 was identified by the termination of DG axons and the presence of neurons lacking CgA expression. In the non-human primate hippocampus, as in the rodent hippocampus, the DG axons seem to extend into CA2, suggesting that a CA2a and CA2b designation could also be valid128.
In rodents, at least, the absence or presence of DG input onto CA2 neurons can be easily identified, and so could be used to designate CA2a and CA2b, respectively (see the figure, part b). The image in part b shows mouse CA2 pyramidal neurons labelled with an antibody against PCP4 and DG neurons and their axons labelled with an antibody against calbindin 1. Note the nearly full coverage of the CA2 with fibres originating from the DG. Part b is from REF. 9, Nature Publishing Group.
Given these species differences, it is not surprising that there has been much debate over whether CA2 is truly a distinct region from CA3 (REFS 20–22). This debate has led to some confusion in the literature, but we note that several investigators have observed that neurons in CA3 immediately bordering CA2 (here, called the distal portion of CA3a) have much in common with those in CA2, despite the fact that CA2 neurons appear to lack the postsynaptic specializations that are characteristic of CA3 pyramidal neurons (for examples, see REFS 19,23).
Many of the clearest indications that area CA2 was different from its neighbouring CA subfields initially came from the study of expression of specific molecules such as growth factors24,25. A systematic approach to the search for genes highly enriched in the hippocampal subfields has greatly expanded our understanding of the anatomical divisions7,26. Newly identified markers for CA2 pyramidal neurons in rodents, such as the proteins α-actinin 2 (ACTN2), Purkinje cell protein 4 (PCP4; also known as PEP19), striatum-enriched protein-tyrosine phosphatase (STEP; also known as PTPN5), regulator of G protein signalling 14 (RGS14), transmembrane AMPA receptor regulatory protein γ5 (TARP γ5; encoded by Cacng5) and others, show a high degree of overlap with one another and label a large proportion of CA2 pyramidal neurons, most of which do overlap with an area that contains DG axons9,15,19,27–31. Furthermore, a recent study in mice has shown unambiguously that DG axons form functional synapses on some CA2 pyramidal neurons, which were identifiable by RGS14 expression9, demonstrating that the largest segment of a molecularly defined CA2 receives DG input. Note that only a very small portion of the area staining for RGS14, closest to CA1, fits Lorente de Nó’s classic definition (but see BOX 1 and REF. 32).
Before the discovery of genes and proteins with expression patterns that delineate the hippocampal subfields, most investigators made subfield designations based solely on the presence or absence of mossy fibre axons (for examples, see REFS 33,34), and so several of them designated an area as CA3a that should now be considered part of area CA2 (REFS 35–37). In an attempt to communicate some of these anatomical observations, some investigators have assigned their own nomenclature to the subregions, such as CA3a’ in rats38, and CA2a, CA2b and CA2c in humans20. We propose that the identification of large pyramidal neurons lacking complex spines and/or expressing markers such as PCP4, STEP and RGS14 is sufficient to distinguish CA2 from its neighbouring areas in the dorsal hippocampus, and that subdivisions within CA2 may also be warranted (BOX 1; FIG. 2). Interestingly, many of these CA2-enriched genes are not expressed within the ventral-most end of the hippocampus in rodents39 (FIG. 1).
Inputs
In most species, area CA2 receives bilateral inputs from the Schaffer collaterals of area CA3 (REF. 6) in addition to recursive ipsilateral and commissural contralateral inputs29,40. Assuming that the definition of area CA2 proposed by Lorente de Nó is in need of revision, as discussed above, newborn DG neurons can also make connections with neurons in the molecularly defined area CA2 (REF. 41). Interestingly, the presence of these DG terminals from newborn neurons in area CA2, like the presence of such terminals in CA3, seems to be influenced by factors that positively or negatively affect granule cell neurogenesis, such as exercise and inflammation, respectively41,42. Although the mossy fibre projection from the DG to CA3 is known to run along the transverse axis of the hippocampus (perpendicular to the long axis)6,40, recent work has demonstrated that DG projections turn as they reach the CA2 and continue along the long axis, towards the ventral hippocampus. In this way, it is conceivable that information reaching the dorsal and intermediate DG can be transmitted caudally to the intermediate and ventral hippocampus, respectively9. Considering also that the pyramidal neurons in area CA2 (and part of CA3a) project to CA1 in this longitudinal axis for up to half the length of the rodent hippocampus38, this DG–CA2–CA1 circuit could provide information transfer in the dorsal-to-ventral direction in rodents that is greater than the transfer of information provided by a DG–CA2 circuit alone.
Extrahippocampal inputs to CA2 include those from vasopressinergic neurons in the PVN10,11 and from the median raphe, as well as reciprocal connectivity to and from the SuM36,37,43, medial septum and the diagonal bands of Broca10. In rats, SuM neurons appear to form synapses onto pyramidal neurons that are asymmetric in their appearance (that is, they are likely to be excitatory)33, and they provide the major contribution of the substance P-containing synaptic terminals to the hippocampus36,37,43. Inputs from the SuM and PVN heavily innervate CA2 and distal CA3a, and appear to delineate the molecularly defined CA2 (REF. 11) (FIG. 2).
Outputs
The major target of projections from area CA2, as with projections from area CA3, is the CA1. However, projections from areas CA2 and CA3 differ in three important ways. First, CA2 pyramidal neurons project primarily to the area covered by the basal dendrites of CA1 pyramidal neurons, known as stratum oriens (SO), forming almost 20% of the input there, whereas CA3 pyramidal neurons project primarily to the area containing the apical dendrites of CA1 pyramidal neurons, known as stratum radiatum (SR)6,10,29,38 (FIG. 2).
Second, a recent study has shown that within SO, CA2 axons appear to target dendrites from a distinct row of CA1 pyramidal neurons, adjacent to SO, whereas CA3 neurons target the row of CA1 pyramidal neurons adjacent to SR9. These distinct rows of CA1 neurons can be identified by calbindin 1 immunoreactivity, by the presence of zinc and by evidence of synaptic inhibition by parvalbumin-immunopositive basket cells44–46. In addition, a recent report suggests different targets of the superficial, calbindin 1 immunopositive CA1 pyramidal neurons adjacent to the SR, and the deep, CA2-targeted, calbindin 1-immunonegative CA1 pyramidal neurons adjacent to SO. The former appear to project to the amygdala and the latter to the medial prefrontal cortex46. Recordings from these deep, SO-adjacent CA1 neurons in vivo show that these neurons fire in a way that is distinct from the superficial, SR-adjacent CA1 neurons, particularly during rapid eye movement sleep and sharp wave–ripples47,48.
Third, as mentioned above, the projection from the CA2 to CA1 has a vast caudal spread along the longitudinal axis of the rat hippocampus38,49. However, the degree to which this longitudinal spread differs from CA3 projections is unclear50. These longitudinal projections from area CA2 may serve to integrate the information from the dorsal hippocampus into the more ventral regions. In addition to the CA1 projections, the axons from CA2 pyramidal neurons, like those from CA3 neurons, ramify heavily within area CA2 and the CA3, thus providing a high degree of local and recursive interconnectivity6,27,38. This pattern of connectivity may explain the delay in signal propagation through the transverse axis in some, but not all, hippocampal slice preparations, perhaps depending on the plane of sectioning51.
Projections from area CA2 that leave the hippocampus include axons that form reciprocal connections back to the SuM and septal nuclei10,38 (FIG. 2). CA2 pyramidal neurons have also been reported to project back to the medial entorhinal cortex52 (but see REFS 9,10,53), and given the potential impact of such projections on how the entorhinal cortex interacts with the hippocampus, further work on the role of this circuitry is clearly warranted. Taken together, these studies highlight that connectivity of the CA2 region of the hippocampus is indeed quite distinct from that of the CA1 and the CA3.
Properties of CA2 neurons
Anatomy
Pyramidal neurons in area CA2 have a morphology very similar to those in the CA3; they are larger and less densely packed than CA1 pyramidal neurons, and have fewer oblique dendrites (which branch from apical dendrites) than pyramidal neurons in the CA1 (REFS 6,54). Nonetheless, CA2 pyramidal neurons do have some distinct features. Four main subtypes of CA2 pyramidal neurons have been described in the guinea pig55. Curiously, the branches of these CA2 cells are sexually dimorphic: two of the pyramidal neuron types have a greater number of apical branches in males than in females, and females have more branches on the dendrites of biapical neurons55. The significance of these findings is not known, and more research on the relative function of apical and basal dendrites of the pyramidal cells in area CA2 will certainly be of interest.
Another notable anatomical feature of CA2 pyramidal neurons is that, in the mouse, their apical dendrites are branched in a way that may be particularly well suited for transmitting signals from the distal synapses in the stratum lacunosum-moleculare (SLM) to cell bodies54,56,57. These distal synapses arising from layer II of the entorhinal cortex onto CA2 pyramidal neurons would therefore be more effective at initiating firing of the postsynaptic pyramidal neurons than those synapses from layer III in the SLM of the mouse CA1 (REF. 58). However, the contribution of these inputs under different physiological conditions in vivo remains to be determined.
Inhibitory GABAergic interneurons in area CA2 may also be distinct from interneurons in other CA regions
In the rat and the mouse, parvalbumin-expressing interneurons are more numerous in CA2 than in CA1 or CA3 (REFS 59,60). Furthermore, the density of reelin- expressing interneurons in the rat CA2 stratum pyramidale (SP) is more than threefold and sixfold higher than that found in areas CA3 and CA1, respectively60 (FIG. 2). The number of calbindin 1-expressing interneurons is also higher in the area CA2 SP than in other CA regions (nearly tenfold higher than that of CA1 and CA3)60. The total number of reelin-positive interneurons, and their percentage of the total inhibitory neuron population, is also higher in CA2 SR than elsewhere in the hippocampus. These studies suggest that the inhibitory circuitry in CA2 is very different from that in the neighbouring areas CA1 and CA3, and thus may represent a powerful brake on CA2 pyramidal neuron firing.
Electrophysiology
Some disagreement in the literature exists regarding whether CA2 pyramidal neurons can be distinguished electrophysiologically from their neighbouring CA1 and CA3 neurons in in vitro hippocampal slice preparations. Nevertheless, CA2 pyramidal neurons do express several receptors and channels that would suggest that they have firing properties that are different from those of the other CA regions, making such a distinction possible in principle. For example, expression levels of members of the two-pore domain potassium channels can differ dramatically across the CA subfields, and TREK1 (also known as KCNK2), in particular, is highly expressed in CA2 compared with CA1 and CA3 (REF. 61). Indeed, consistent with this so-called leak channel being enriched in CA2, the resting membrane potential of CA2 pyramidal neurons was found to be slightly more negative than that of CA1 or CA3 neurons in some, but not all, studies9,15,53,58. Because TREK1 and other two-pore domain family members are influenced by a large number of factors such as pH, temperature and lipids62, further investigation into how these channels are regulated in CA2 will certainly be of interest. It should be noted, however, that membrane potentials are difficult to measure and compare accurately.
In some studies, a large capacitance and lower input resistance in CA2 pyramidal neurons were also observed9,53,58 but, as for resting membrane potentials, these findings are inconsistent among reports15,19. The large capacitances observed in some instances are most likely reflective of extensive dendritic and/or axonal arbors that may or may not have been preserved during tissue processing. Any one of these electrophysiological features may contribute to the delay in action potential firing in response to a depolarizing pulse that is observed in CA2 neurons in some studies9,15.
CA2 pyramidal neurons also demonstrate prominent dendritic sodium spikes57. It has been hypothesized that dendritic sodium spikes, in conjunction with particular branching patterns of the apical dendrites, provide a mechanism by which distal inputs from the entorhinal cortex layer II can be effectively transmitted to the cell body and cause CA2 pyramidal neurons to fire. Taken together, these electrophysiological characteristics may, in some cases, be used to identify CA2 pyramidal neurons in vitro, and perhaps in vivo.
Failure to display LTP at SR synapses
Glutamatergic synapses in the SR of CA2 are extremely resistant to long-term potentiation (LTP) induction by typical methods15,58,63,64. Surprisingly, however, many of the obvious candidate mechanisms do not seem to be responsible for this lack of LTP induction. For example, the high expression levels of TREKs in area CA2 (REF. 61) are unlikely to be involved, as inclusion of caesium in the electrode internal solution to block potassium channels, including TREKs, did not allow LTP induction in the CA2 SR15. Similarly, the failure to induce LTP at SR synapses is unlikely to be due to the high expression levels of the phosphatase STEP, a protein that has been shown to inhibit LTP induction in CA1 (REFS 65,66), as application of protein phosphatase inhibitors also failed to enable LTP induction in CA2 (REF. 15). Furthermore, although synaptic inhibition appears to be robust in area CA2 (REFS 59,60), application of picrotoxin, a type A GABA (GABAA) receptor antagonist, was also insufficient to facilitate LTP induction at CA2 excitatory SR synapses15,67, indicating that the failure to induce LTP at these synapses is due to cellular properties of the pyramidal neurons and not a consequence of robust inhibition. Other synaptic properties, such as AMPA receptor- and NMDA receptor-mediated synaptic responses, and presynaptic function, as assessed by measuring paired-pulse ratios and miniature excitatory postsynaptic current (EPSC) frequencies, are generally similar in pyramidal neurons of the CA1 and CA2 SR regions as well15, ruling out a number of other possible explanations for the resistance of CA2 neurons to LTP induction. Interestingly, however, this lack of LTP expression at excitatory synapses in area CA2 appears to be restricted to the SR region because synapses from the entorhinal cortex located in the SLM can readily express NMDA receptor-dependent LTP58.
By contrast, raising the extracellular calcium levels of the preparation well beyond normal levels and using a higher-than-typical frequency of tetanic stimulation allowed for LTP induction at CA2 SR synapses68. This finding that LTP can be induced in some circumstances indicated that although multiple proteins that are highly expressed in the CA2 region may contribute to the failure of LTP induction at CA2 SR synapses, these synapses nonetheless contain the necessary cellular machinery for LTP induction, and that high extracellular calcium levels can overcome the mechanisms preventing plasticity.
Consistent with this observation that calcium levels may be limited in CA2 pyramidal neurons, the buffering capacity and rate of calcium extrusion are nearly four times greater in CA2 dendrites and spines than in those in the CA1 region68. Furthermore, blockade of the plasma membrane ATPases (PMCAs), which slows calcium extrusion from neurons, was sufficient to enable LTP induction at CA2 synapses. One way by which the calmodulin-regulated PMCAs might be controlled in CA2 neurons is through PCP4 (REF. 68), a putative modulator of calmodulin that is highly expressed in CA2 pyramidal neurons69. Remarkably, CA2 pyramidal neurons also express high levels of an atypical TARP, TARP γ5, which has distinct glutamate receptor trafficking properties compared with other TARPs, and seems to primarily affect the calcium-permeable AMPA receptor subunits70–72. Although a role for TARP γ5 in limiting synaptic plasticity has not been tested, some evidence points to its ability to positively regulate the affinity of AMPA receptors for glutamate, as well as regulating the maximum current and desensitization rates of the receptor71,72.
Another protein highly enriched in CA2, RGS14, also has a crucial role in the suppression of LTP induction. Mice lacking RGS14 (Rgs14−/− mice) display robust LTP at SR synapses onto CA2 pyramidal neurons28. It is not known which G protein-coupled receptor (GPCR) is associated with RGS14 (REF. 73), but at least two families of GPCRs have been found to regulate excitatory CA2 synapses. Activation of the Gq-linked arginine vasopressin receptor 1B (AVPR1B) and oxytocin receptor (OXTR), both of which are highly expressed in CA2 pyramidal neurons74 (in the brain, AVPR1B is only expressed in CA2 (REF. 75)), enhances EPSCs in area CA2 (REF. 76). This effect could be due to a lowered threshold for LTP in response to activation of these receptors, as it is dependent on low-frequency (baseline) stimulation, activation of NMDA receptors, postsynaptic calcium signalling, and calcium/calmodulin-dependent protein kinase type II (CaMKII) activity. By contrast, activation of the Gi/o-linked adenosine A1 receptor (A1R), which is also enriched in CA2 (REF. 77), potently and tonically suppresses synaptic efficacy in the SR of CA2; antagonists of A1Rs, including caffeine, directly enhance EPSCs in a manner that does not require synaptic stimulation78. This is mediated by adenylyl cyclases and protein kinase A (PKA), but not by calcium or CaMKII78. Indeed, several of the adenylyl cyclases are highly expressed in area CA2 (REFS 79,80) (TABLE 1).
Table 1.
Genes differentially expressed in area CA2
| Gene* | Functions | Species | Refs |
|---|---|---|---|
| Cation-mediated signalling | |||
| CALB1 | Calcium binding | Human, monkey | 102,144 |
| CALB2 | Calcium binding | Monkey | 145 |
| Pcp4 | Calmodulin regulator | Rat, mouse, not in human | 19,69,146 |
| S100b | Calcium binding | Mouse | 39 |
| Necab2 | Calcium binding | Mouse | 147 |
| Camk4 | Kinase | Mouse | 39 |
| Camkk1 | Kinase | Mouse | 39 |
| Ryr1 | Calcium release | Mouse | 39 |
| Slc30a3 (also known as Znt3)‡ | Zinc transporter | Mouse | 148 |
| Extracellular matrix components | |||
| Acan | PNN component | Mouse, rat | 149–151 |
| Sdc2 (also known as Hspg) | PNN component | Rat | 152,153 |
| Ncam1 (also known as Ncam) | Cell adhesion, signalling | Rat | 154 |
| Amigo2 | Cell adhesion, signalling | Mouse | 39,155 |
| Dscam | Adhesion, signalling | Mouse | 156,157 |
| Sema7a | Adhesion, signalling | Mouse | 39 |
| Sparcl1 (also known as Sc1 and hevin) | Adhesion, signalling | Mouse | 158 |
| CDH10‡ | Adhesion, signalling | Human | 159 |
| CNTN3‡ | Adhesion, signalling | Human | 159 |
| Growth factor signalling | |||
| Fgf2 (also known as bFGF) | Growth factor | Rat | 24,160–162 |
| Fgf5§ | Growth factor | Rat, mouse | 39,163,164 |
| Ntf3 | Neurotrophin | Rat, mouse | 25,165–167 |
| Egfr | Growth factor receptor | Rat | 168 |
| Igf1r§ | Growth factor receptor | Rat, mouse | 116,169 |
| Igfbp4 | Growth factor binding | Rat | 170 |
| Signal transduction, cytoskeletal components and other functions | |||
| Actn2 | Cytoskeletal component | Rat | 31 |
| Step | Phosphatase | Rat | 65 |
| Map3k15 | Kinase | Mouse | 39 |
| C1qa | Complement cascade component | Mouse | 171,172 |
| Cacng5 | Regulator of GluA receptors | Mouse | 39,70 |
| Trek1 | Potassium channel | Rat, mouse | 61 |
| Nos1‡ | Nitric oxide synthesis | Mouse | 39 |
| Prkcb‡ | Kinase | Mouse | 39 |
| SHANK3‡ | Synaptic scaffold | Human | 159 |
| GPCR-related signalling | |||
| Rgs14 | RGS, scaffolding protein | Mouse | 28 |
| Rgs4 | RGS | Mouse | 39 |
| Adora1 | GPCR | Rat | 77 |
| ADORA2A | GPCR | Human | 159 |
| Sstr5 | GPCR | Rat, gerbil | 118,173 |
| Avpr1b | GPCR | Rat, mouse | 39,75,76 |
| Gpr12 | GPCR | Mouse | 39 |
| Adcy1, Adcy5 and Adcy6 | Adenylyl cyclases | Mouse | 79,80 |
| Pde4d | Phosphodiesterase | Mouse | 39 |
| Tiam2 | RACGEF | Mouse | 39 |
| Srgap2 | RHOGAP | Mouse | 39 |
Acan, aggrecan; Actn2, actinin, alpha 2; Adcy, adenylyl cyclase; Adora1, adenosine A1 receptor; ADORA2A, adenosine A2A receptor; Amigo2, adhesion molecule with Ig-like domain 2; Avpr1b, arginine vasopressin receptor 1B; CALB, calbindin; Camk4, calcium/calmodulin-dependent protein kinase 4; Camkk1, calcium/calmodulin-dependent protein kinase kinase 1, alpha; CDH10, cadherin 10; CNTN3, contactin 3; Dscam, Down syndrome cell adhesion molecule; Egfr, epidermal growth factor receptor; Fgf, fibroblast growth factor; GPCR, G protein-coupled receptor; Igf1r, insulin-like growth factor 1 receptor; Igfbp4, insulin-like growth factor-binding protein 4; Map3k15, mitogen-activated protein kinase kinase kinase 15; Ncam1, neural cell adhesion molecule 1; Necab2, N-terminal EF-hand calcium-binding protein 2; Nos1, nitric oxide synthase 1, neuronal; Ntf3, neurotrophin 3; Pcp4, Purkinje cell protein 4; Pde4d, phosphodiesterase 4D; PNN, perineuronal net component; Prkcb, protein kinase C, beta; RACGEF, RAC guanine nucleotide exchange factor; RGS, regulator of G protein signalling; RHOGAP, RHO GTPase-activating protein; Ryr1, ryanodine receptor 1; Sdc2, syndecan 2; Sema7a, semaphorin 7A; SHANK3, SH3 and multiple ankyrin repeat domains 3; Slc30a3, solute carrier family 30 (zinc transporter), member 3; Sparcl1, SPARC-like 1; Srgap2, SLIT ROBO RHO GTPase-activating protein 2; Sstr5, somatostatin receptor 5; Step, striatum-enriched protein-tyrosine phosphatase; Tiam2, T cell lymphoma invasion and metastasis 2.
Expression of an oligodendrocyte antigen is high in the rat CA2, but the gene encoding this protein is unknown174.
Low expression relative to CA1 and CA3.
Some expression in CA3a.
To summarize, the current understanding of excitatory synaptic plasticity in the SR of area CA2 is that it is suppressed under most conditions. However, rather than simply being absent, plasticity in the CA2 SR seems to be tightly regulated.
LTD at inhibitory synapses
The action potential output of CA2 pyramidal neurons may also be regulated by the actions of inhibitory networks67. In area CA2, like in area CA1, inhibitory synapses onto pyramidal neurons from parvalbumin-expressing interneurons can undergo long-term depression (LTD) in response to repetitive high-frequency stimulation of Schaffer collaterals59. This LTD of inhibitory synaptic transmission in area CA2, however, is mediated by δ-opioid receptors, which contribute to a presynaptic reduction in neuro-transmitter release. As of yet, however, no distinguishing cellular marker for this CA2-specific population of inhibitory synapses or neurons has been identified that might explain this mechanism of synaptic depression. It has been proposed that plasticity of inhibitory connections in area CA2 gates the excitatory output of the CA2 (REF. 67), and future work may provide further insight into the unique properties of synaptic inhibition in area CA2. Thus, novel and interesting regulatory mechanisms occur at inhibitory synapses of area CA2.
Gene expression
It is likely that the different functional characteristics of the hippocampal subfields are driven not only by a combination of specific sets of inputs and the differences in anatomical organization of the subfields but also by unique transcriptomes in each region7. Thus, examining the types of genes that are enriched in area CA2, compared with those in CA1 and CA3, may provide insights into its unique roles in hippocampal plasticity, behaviour and susceptibility to disease. New resources, such as the Allen Brain Atlas39 and databases for cell-specific transcriptomes, have now made it possible to define a molecular profile for CA2 (TABLE 1). The molecularly defined borders of CA2 have a high degree of overlap with the updated anatomical definition discussed above, which includes, for example, the input from the SuM6–9,19 (BOX 1). In the case of the Allen Brain Atlas resource, the quantification of gene expression and assignments of anatomical regions were automated and based on approximately 50 ‘enriched’ genes per region, all of which are determined by expert curators. Surprisingly, many of the genes identified as being enriched in area CA2 do not appear in the ventral hippocampus, clearly indicating a discrepancy between a molecular definition of area CA2 and a classic one based on neuroanatomy81 (FIG. 1).
Several different functional classes of proteins appear to be particularly enriched in area CA2. These include calcium regulators, extracellular matrix components, proteins involved in growth factor- and GPCR-mediated signalling, and intracellular signal transduction proteins (TABLE 1). As mentioned above, many of these proteins enriched in area CA2 are predicted to have roles both in limiting plasticity and in limiting damage from calcium-dependent pathological processes. However, an interesting question is why these proteins might be effective at limiting plasticity at Schaffer collateral synapses but not synapses from the entorhinal cortex. Many of the expression patterns of plasticity-limiting CA2-enriched proteins such as RGS14 do not show obvious exclusion from the distal dendrites82, which receive input from the entorhinal cortex layer II. As such, it may be necessary to investigate the intracellular trafficking of these individual proteins. Alternatively, the mechanisms underlying the plasticity observed at the distal synapses58 may differ from those in the more proximal dendrites in area CA2.
Role in behaviour
Spatial and contextual processing
The hippocampus is best known for its role in encoding episodic memory and spatial processing2. Much has been learned about how the hippocampus encodes information by assessing neuronal activity during hippocampal-dependent behavioural tasks. However, until only very recently, such direct studies have largely neglected the CA2 region, probably because of its small size. Some insight into the role of area CA2 in memory and other behaviours has come from studies using mice lacking particular genes that are enriched in area CA2. One such study used mice lacking RGS14 (Rgs14−/− mice), which, as described above, is a repressor of synaptic plasticity in CA2 pyramidal neurons. These mice showed enhanced novel object recognition memory and faster acquisition learning in the Morris water maze28. Because deletion of Rgs14 enabled plasticity in CA2, behavioural studies in these mice were, in effect, assessing the outcome of enhanced synaptic communication through CA2. These findings are therefore consistent with the idea that CA2 neurons, as part of the hippocampal circuitry, may be important for some canonical hippocampal-dependent behaviours such as declarative learning53. Furthermore, they suggest that Rgs14, a gene that is highly expressed in area CA2, seems to suppress memory, similar to a few other select ‘memory-inhibiting’ genes83.
More recently, one of the first studies to directly determine how CA2 neurons respond to changes in context found that area CA2 is very different from its neighbouring hippocampal subfields. Using compartment analysis of temporal activity fluorescence in situ hybridization (catFISH) in mice84, the authors of this study found that when subtle contextual changes were made to a familiar environment, such as replacing an object with a new one, the cell ensembles in CA2 changed dramatically, whereas changes to cell ensembles in areas CA1 and CA3 were closer to being proportional to the magnitude of the contextual change84. These data suggest that CA2 neurons are more sensitive to subtle changes in contexts than neurons in areas CA1 and CA3 (REF. 84).
Another recent study recorded neuronal activity in hippocampi of freely moving rats exploring an arena to compare spatial firing properties of CA2 neurons with those in CA1 and CA3 (REF. 85) (FIG. 3). Mankin et al.85 found that CA2 neurons, similar to those in the other CA fields, display place fields, consistent with a previous study that reported little difference in spatial firing between CA1 and CA2 neurons in rats exploring a radial arm maze86. However, CA2 place fields were later found to be slightly greater in number and larger in size than those in the CA1 and CA3 (REF. 85). CA2 neurons also had a higher mean firing rate than CA1 and CA3 neurons30,85. Together, these findings indicate that CA2 neurons carry less spatial information than those in the other CA regions. These findings are consistent with the idea that CA2 neurons play some part in spatial processing, although perhaps not to the level of those of its neighbouring CA1 and CA3 areas.
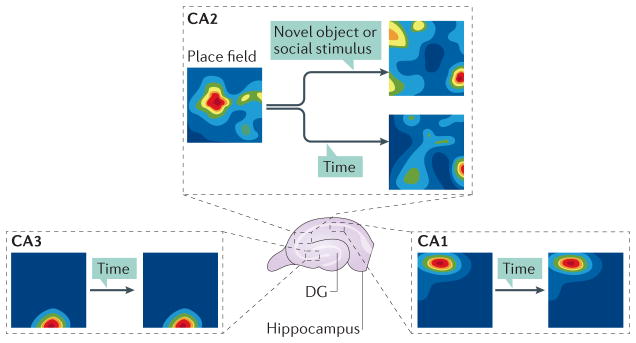
Figure 3. Comparison of neuron spatial firing in CA regions.
Neuronal recordings in awake behaving rats to characterize spatial firing in CA2(REF. 85) revealed that CA2 neurons fired in place fields, indicated by the warm colours. CA2 place fields differed from those in CA1 and proximal CA3 in that there were more of them and that they were larger. Not illustrated is that several properties of place fields in distal CA3 (closest to CA2; CA3a) closely resembled those in CA2 (REFS 87,88). Notably, place fields in CA2 changed with the passage of time (hours to days) and could shift significantly in response to social stimulation and novel objects (that is, global remapping)30. These results together suggest that CA2 is less sensitive to novel global contextual cues than to novel local cues and social stimuli. DG, dentate gyrus.
In subsequent studies87,88, it was found that most of these spatial properties gradually change along a gradient starting from the proximal CA3 (the part closest to the DG; also designated CA3c) to the distal CA3 (the part closest to CA2; also designated here as CA3a) and into CA2. Specifically, the mean firing rate and average place field size gradually increased from proximal CA3 to CA2 while rats explored a familiar open field; spatial information decreased along the same gradient87,88. Some of the most striking characteristics of CA2 neurons that are distinct from those in most of area CA3 are their lack of sensitivity to changes across different familiarized environments88 and their sensitivity to local cues87. In both studies87,88, neuron firing in the distal-most CA3a closely resembled that in CA2, further supporting the case for a portion of CA3a to be considered part of area CA2 (BOX 1).
Another particularly intriguing feature of area CA2 is that place fields in this region change more in response to the passage of time (hours to days) than in response to alterations in the shape of a context85. On the basis of these findings, the authors of the study suggested that CA2 neuronal ensembles code weakly for changes in context, and code more strongly for the passage of time. Thus, at the level of individual neurons recorded in vivo, area CA2 demonstrates properties consistent with a role in spatial coding, but at the population level area CA2 may participate in temporal coding to a greater extent than spatial contextual coding. Although the findings of Wintzer et al.84 and Mankin et al.85 seem to be contradictory with respect to contextual coding, we note experimental differences in how the context was changed in these studies; Wintzer et al. introduced a novel change to a familiar environment, whereas Mankin et al. repeatedly used a familiarized change in the shape of the box. The results of these studies imply that updates to a context can engage area CA2, which otherwise may be involved in temporal coding, and highlight how unique properties in area CA2 may play a part in encoding distinct hippocampal behaviours.
Social behaviour
There has been considerable recent interest from the neuroscience community in social behaviour. One study examined how social stimuli such as exposure to either novel or familiar animals would influence CA2 neuron firing in rats30. The authors of this study found that place fields shift significantly in response to social stimulation (that is, global remapping; see FIG. 3). This effect, however, was not specific to social stimulation; novel (inanimate) objects placed in the recording arena also elicited global remapping. Similar to their findings in other hippocampal subfields89, the authors of this study found no increase in CA2 neuron firing rates upon social stimulation. These results, together with the previous findings87,88, suggest that neuronal firing in area CA2 is less sensitive to novel global contextual cues than to novel local cues and social stimuli.
Further evidence suggestive of a specific role for area CA2 in social behaviour is that CA2 pyramidal neurons express high levels of receptors for the ‘social’ neuropeptides vasopressin and oxytocin74,75,90 and receive the majority of the vasopressinergic hippocampal PVN inputs10,11 (FIG. 4). Both mice lacking Avpr1b and mice lacking Oxtr have deficits in social recognition memory and social aggression behaviours91–93. In the mouse brain, Avpr1b expression is largely restricted to pyramidal neurons in hippocampal CA2 (REF. 75), and indeed re-expression of Avpr1b in the dorsal CA2 of Avpr1b−/− mice restored normal aggressive behaviours76. Notably, spatial memory, tested by performance on the Morris water maze task, was not affected in Avpr1b−/− mice, but these mice did have impairments in associative memory when a time delay was introduced between presentations of objects93. These findings have raised the intriguing possibility that area CA2 has a central role in the encoding or processing of temporal order, in addition to social memory processing.
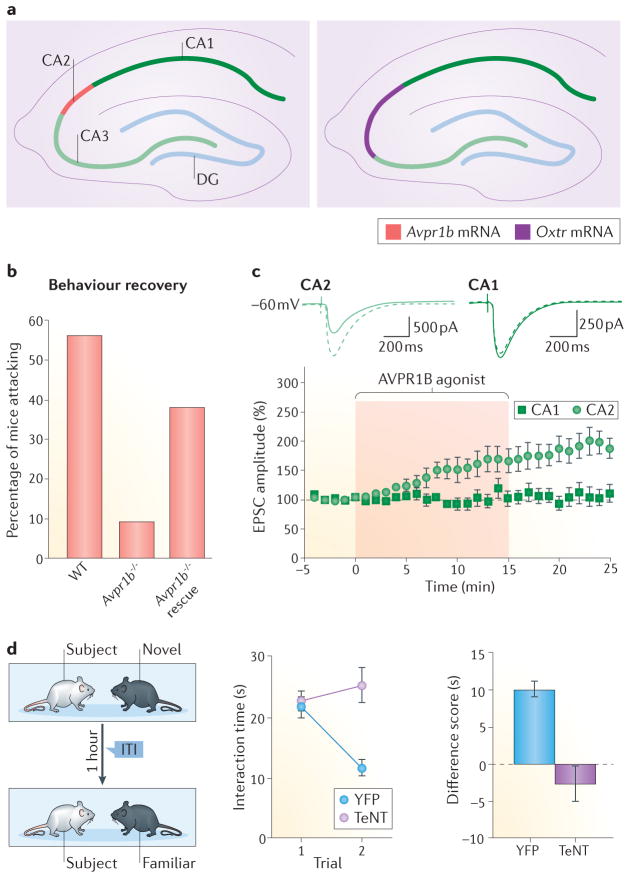
Figure 4. A role for CA2 in social memory.
a | The receptors for ‘social’ neuropeptides vasopressin and oxytocin (arginine vasopressin receptor 1B (AVPR1B) and oxytocin receptor (OXTR)) are highly expressed in CA2 pyramidal neurons. BothAvpr1b−/− and Oxtr−/− mice have deficits in social recognition memory and social aggression91,92. b | Virus-mediated re-expression of Avpr1b in the dorsal CA2 of Avpr1b−/− mice rescued social aggression behaviours76. c | An AVPR1B agonist, as well as oxytocin (not illustrated), induces a slowly developing synaptic potentiation in CA2, but not in CA1, neurons76. d | Silencing of CA2 pyramidal neuron output in mice impairs social recognition memory, but not sociability53. Control animals spent less time investigating familiar mice than novel mice, but the animals with tetanus toxin (TeNT) expressed in CA2 were unable to differentiate between novel and familiar mice. EPSC, excitatory postsynaptic current; ITI, inter-trial interval; WT, wild type; YFP, yellow fluorescent protein. Partsb and c are from REF. 76, Nature Publishing Group. Part d is from REF. 53, Nature Publishing Group.
New tools to genetically manipulate specific populations of neurons have allowed researchers to address, in a precise manner, the role of area CA2 in behaviour. For example, using a CA2-specific Cre-expressing mouse strain, Hitti and Siegelbaum53 silenced CA2 synaptic output using a Cre-dependent construct encoding tetanus toxin light chain to inhibit neurotransmission. This CA2-specific neuronal silencing resulted in a selective impairment of social recognition memory but did not impair other hippocampus-dependent memory tasks, such as novel object recognition or spatial memory. Specifically, the mice were unable to differentiate between novel and familiar mice, which demonstrates that synaptic transmission from CA2 pyramidal neurons is essential for encoding of social information into memories. These data are consistent with work showing that excitotoxic lesions of area CA2 impaired social recognition memory94. However, it remains unclear how CA2 neurons integrate social processing with other aspects of episodic memory, such as time and space. Global remapping of CA2 neuron spatial firing may be one mechanism by which CA2 could encode social aspects of a memory30. Further, some evidence suggests that CA2 has a role in diseases associated with impaired social behaviour (BOX 2).
Box 2. Possible roles of area CA2 in disease.
In mice, lesions of area CA2 and silencing of CA2 pyramidal neurons result in impaired social recognition memory53,94, suggesting that this region is important for normal social behaviour. As impairments in social behaviour are one of the core symptoms observed in autism spectrum disorder (ASD), this suggests that CA2 dysfunction may mediate part of its aetiology. Although there is currently no consensus on specific brain structures or functions that underlie the pathophysiology of ASD129, a role for the hippocampus in symptoms of ASD has been hypothesized; impairments in memory (in particular, social memory130) and a propensity for seizures131 have been reported in individuals with ASD. Interestingly, several genes that have been linked to ASD in humans are expressed at lower levels in area CA2 than in other hippocampal subregions in the rodent and human brain (TABLE 1), including CDH10 (which encodes cadherin 10), CNTN3 (which encodes contactin 3) and SHANK3 (which encodes SH3 and multiple ankyrin repeat domains 3)132. Future studies on the expression of these genes during development in humans, and the role of CA2 in rodent social behaviour, will provide insight into whether this region of the hippocampus has an important role in some of the symptoms of ASD.
Among the hippocampal subfields, area CA2 also appears to be strikingly affected in some psychiatric disorders. A post-mortem study found that the number of non-pyramidal neurons in area CA2 was approximately 40% lower in tissue from individuals with schizophrenia or bipolar disorder than in tissue from controls, whereas the number and density of pyramidal neurons were unaffected133. Subsequent studies confirmed that the loss of interneurons, particularly the parvalbumin-positive interneurons, and of glutamate decarboxylase 67 (GAD67; also known as GAD1) expression (a marker of GABAergic neurons) within the hippocampus was greatest in area CA2 in tissue from individuals with schizophrenia134,135. Loss of staining for kainate receptor subunits 5, 6 and 7 was also greatest in area CA2 in hippocampi from individuals with schizophrenia136. Finally, increased NMDA receptor subunit NR2B (also known as GluN2B) expression137, but decreased histamine H3 receptor binding138, has been reported in the CA2 subfield of tissue from individuals with either bipolar disorder or schizophrenia. Taken together, many of these findings, particularly the loss of inhibitory function in CA2, might partly explain the hypermetabolism observed in the CA1 of individuals with schizophrenia using functional imaging139.
Several animal models of schizophrenia recapitulate many of the symptoms of schizophrenia seen in humans. For example, reelin haploinsufficient mice display behavioural impairments that are consistent with the types of behavioural deficits seen in patients with schizophrenia140 and have a significant decrease in the number of parvalbumin-immunopositive interneurons in area CA2. Interestingly, among the hippocampal subfields in the rat, the highest density of reelin-immunopositive interneurons is found in area CA2 (REF. 60) (FIG. 2). In another example, infusion of a type A (GABAA) receptor antagonist into the rat basolateral amygdala, which mimics one aspect of circuit disruption in individuals with schizophrenia, resulted in decreased numbers of CA2 stratum oriens (SO) interneurons141. One study has shown that there are projections from the parvocellular and magnocellular divisions of the basal nucleus of the amygdala to the SO and the stratum radiatum (SR) of area CA2 (REF. 142) (but see also REF. 10). Although it is proposed that widespread dysfunction throughout several corticolimbic structures contributes to the pathophysiology of schizophrenia, the striking abnormalities observed in interneuron density and receptor expression in area CA2 suggest this hippocampal subregion may have a role in some of the symptoms of schizophrenia, particularly cognitive dysfunction.
Resistance to injury
Neuronal cell death can occur in response to various insults, including reduced oxygen and glucose transport to neurons, physical shearing or compression of the brain tissue and parenchyma, or abnormal electrical activity in the form of seizures. These insults are followed by processes such as membrane depolarization and activation of voltage-gated calcium channels and excitotoxicity ensuing from excessive glutamate release (with subsequent further increases in intracellular calcium levels) and release of free radicals, all of which can initiate necrotic and/or apoptotic signalling pathways95. Given the wide range of processes that can contribute to cell death, it comes as some surprise that CA2 pyramidal neurons seem to be highly resistant to several different causes of neuronal injury.
Soon after it was discovered that hippocampal neurons in general are extremely vulnerable to seizure activity, ischaemic insult and trauma, it was noted that neurons of the CA2 region are relatively resistant to these processes14,96. Temporal lobe epilepsy (TLE), for example, is commonly associated with hippocampal sclerosis, which includes severe loss of pyramidal cells from the hippocampal structure97,98. However, CA2 neurons do not often display this pathology99. A more recent meta-analysis of several human studies that quantified neuronal loss in the CA regions of individuals with chronic epilepsy revealed that area CA1 is most susceptible to neuron loss, that areas CA3 and CA4 show intermediate levels of cell loss and that area CA2 displays the least cell loss100. Indeed, examination of hippocampi from patients with TLE and/or status epilepticus similarly demonstrated that among the CA fields, area CA2 is the most resistant to cell loss98–100. In humans, area CA2 is also resistant to damage caused by hypoxia and traumatic brain injury101,102. Strikingly similar findings have been reported in animal models of hypoxia, ischaemia and traumatic brain injury14,103–108 (see also REFS 109,110 for examples).
Several mechanisms may underlie the resistance of CA2 neurons to ischaemia- and trauma-induced cell death, some of which may overlap with the mechanisms underlying the resistance of the CA2 region to excitatory synaptic plasticity. These possible mechanisms include the superior calcium-handling capacity of CA2 pyramidal neurons compared with that of neurons in other CA areas68 and the high expression levels of molecules associated with neuroprotection, such A1Rs77,111 and receptors and binding partners for growth factors, which have been shown to promote neuronal survival and reduce seizure activity in animal models112,113. Indeed, local administration of insulin-like growth factor 1 (IGF1) has been shown to reduce seizure severity and decrease neuronal loss in animal and cell culture models of epilepsy114,115, which may be attributable to the high expression levels of the IGF1 receptor and IGF-binding protein 4 in rodent CA2 neurons116,117. In addition, somatostatin receptor 5, which negatively couples to voltage-gated calcium channels, is highly expressed in CA2 neurons, as shown in gerbils118, and may contribute to protection of CA2 neurons from insults that trigger calcium influx.
Differences in the intracellular signalling response to seizure activity are apparent in area CA2; even minutes after the onset of pilocarpine-induced seizure activity in mice, levels of phosphorylated (active) extracellular signal-regulated kinase (ERK) are substantially lower in area CA2 than in neighbouring areas of the hippocampus (for further information, see supplemental information of REF. 15). Thus, area CA2 is exceptionally resistant to damage even early in the biochemical pathways initiated in response to seizures. Interestingly, Proechimys guyannensis, a rodent native to the Amazon region, shows distinctly dispersed neurons in the CA2 SP and increased resistance to experimental epileptogenesis compared with Wistar rats119. In addition, compared with Wistar rats, P. guyannensis shows high basal expression of calbindin 1 and calbindin 2 (also known as calretinin) within the CA2 region, which are likely to be expressed in interneurons120.
However, the general resistance to damage of neurons in CA2 is not necessarily maintained in hippocampal slices in culture, as in such preparations the CA2 region seems to be the most sensitive to excitotoxic cell death induced by blocking inhibitory transmission121. This increased sensitivity to excessive excitatory activity is not likely to be simply an artefact of cultures per se, because it was found that dissociated CA2 neurons in culture were the least sensitive to excitatory amino acid-induced excitotoxicity, as would be expected from in vivo studies122. However because expression levels of PCP4 and RGS14 change dramatically during the first 2 postnatal weeks19,82, the timing of which may or may not be recapitulated in vitro, the reason for the selective vulnerability of CA2 neurons in some organotypic slice cultures remains enigmatic.
Interestingly, despite its resistance to cell death, abnormal activity in area CA2 may have a role in the progression of TLE in patients. Two studies have examined the electrophysiological properties of CA2 neurons in tissue surgically resected from individuals with medically refractory TLE. Using field potential recordings, Wittner et al. 123 found interictal-like spike activity in area CA2 of individuals with TLE. Whether similar spikes occurred in CA1 and CA3 neurons of this tissue was not investigated, but in tissue resected from patients with TLE these regions do show significant cell loss and in vitro recordings from these tissues show that interictal spikes rarely occur in these regions124. Excitatory synaptic events detected with whole-cell recordings were observed in the majority of CA2 pyramidal cells, but inhibitory synaptic events were seen in only a small number of these cells123. This work is consistent with data from a previous study indicating a lack of inhibitory synaptic events recorded from CA2 neurons from tissue of individuals with TLE125. Interestingly, one study, using voltage imaging in normal rat hippocampal slices, bathed in a solution with a high potassium concentration to elicit epileptiform activity, demonstrated that epileptic discharges originated at the border between areas CA1 and CA2 (REF. 63), and another study demonstrated bursting of CA2 neurons in disinhibited hippocampal slices from guinea pigs that led to synchronized bursting in all CA subfields126.
Finally, a recent report has detailed another possible mechanism underlying epileptic activity in a mouse model of TLE127. Following kainate injection into the hippocampus, CA2 pyramidal neurons not only survived in far greater numbers than CA3 and CA1 pyramidal neurons, but they also appeared to disperse and take over territory once occupied by neurons in areas CA1 and CA3. In addition, the mossy fibres were found to sprout into the CA2 SP, forming synapses on CA2 pyramidal neuron somata127. This study further demonstrates that neurons in the DG and area CA2 support epileptic activity, suggesting that growth of this aberrant circuitry might underlie, in part, the development of TLE.
On the basis of these findings and the selective resistance of CA2 neurons to cell death, we believe that further exploration of the role of CA2 neurons in epileptogenesis will prove to be fruitful in both basic and clinical research realms.
Concluding remarks
Despite the fact that more than 70 years have passed since Lorente de Nó originally described the CA2 subfield, the significance of the many unique morphological and physiological properties of area CA2 is only beginning to be understood. In addition to participating in canonical hippocampal circuits known to underlie spatial processing, area CA2 is crucial for the consolidation of socially relevant information into long-term memory, and may also play a part in temporal encoding. With the advent of tools that enable targeting of genetically defined cell populations, we expect rapid progress to be made in further characterizing the functions of area CA2. Finally, given the findings that CA2 is particularly resistant to certain types of damage, area CA2 has emerged as an important region to study in models of disease characterized by impairments in spatial, social, temporal and memory processing.
Acknowledgments
The authors thank D. Lustberg and other members of the Dudek laboratory for comments on the manuscript. This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, US National Institutes of Health (Z01 ES100221).
Glossary
- Cornu Ammonis (CA)
Latin for Ammon’s horn, CA is now known as the hippocampus proper and was used by Rafael Lorente de Nó when subdividing the regio superior and regio inferior into CA1, CA2, CA3 and CA4
- Mossy fibres
The axons of the dentate gyrus granule neurons most noted for forming very large synapses on the thorny excrescences on proximal dendrites of CA3 neurons, and now known to also form synapses on many CA2 neurons in rats and mice
- Long-term potentiation (LTP)
A lasting increase in the effectiveness of synaptic potentials induced by high-frequency afferent stimulation
- Cell ensembles
A collection of cells that show coordinated firing activity and are thought to encode a particular memory trace or engram
- Place fields
Locations (or places) within a two-dimensional arena at which a particular neuron will fire while the animal traverses it
- Global remapping
Changes in the primary place field location of a neuron in response to a change in the environment — for example, the context or the passage of time
- Excitotoxicity
The property of excitatory amino acids such as glutamate to cause neuron death, beginning with massive depolarization of the cell membrane and influx of calcium
- Status epilepticus
A state of prolonged seizure activity lasting for more than 5 minutes, or multiple seizure events without returning to normal consciousness
Footnotes
Competing interests statement
The authors declare no competing interests.
DATABASES
Mouse.brain-map.org: http://mouse.brain-map.org/search/show?page_num=0&page_size=20&no_paging=false&search_term=446&search_type=fine_structure
Hippocampome.org: http://www.hippocampome.org/php/index.php
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Note added in proof
Two papers that further support a role for area CA2 in social memory have been published since this Review was submitted. The first presents evidence that optical stimulation of PVN axons in CA2 enhances social recognition memory through AVPR1B receptors175. The second reports that there are age-dependent changes in excitability and synaptic inhibition in CA2 in a mouse model of 22q11.2 deletion syndrome that is associated with social memory impairment176.
References
- 1.van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat Rev Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 2.Buzsaki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geva-Sagiv M, Las L, Yovel Y, Ulanovsky N. Spatial cognition in bats and rats: from sensory acquisition to multiscale maps and navigation. Nat Rev Neurosci. 2015;16:94–108. doi: 10.1038/nrn3888. [DOI] [PubMed] [Google Scholar]
- 4.Goosens KA. Hippocampal regulation of aversive memories. Curr Opin Neurobiol. 2011;21:460–466. doi: 10.1016/j.conb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. This paper introduced the hippocampal CA subfield terminology and defined CA2 as a region that is distinct from area CA3. [Google Scholar]
- 7.Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24:3879–3889. doi: 10.1523/JNEUROSCI.4710-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lein ES, Callaway EM, Albright TD, Gage FH. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J Comp Neurol. 2005;485:1–10. doi: 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- 9.Kohara K, et al. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat Neurosci. 2014;17:269–279. doi: 10.1038/nn.3614. This is the first description of CA1 ‘deep’ neurons as the main target of CA2 pyramidal neurons, which contrast with the calbindin 1-immunopositive CA1 ‘superficial’ neurons primarily targeted by CA3 neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui Z, Gerfen CR, Young WS. Hypothalamic and other connections with dorsal CA2 area of the mouse hippocampus. J Comp Neurol. 2013;521:1844–1866. doi: 10.1002/cne.23263. This is one of the first studies to specifically examine the synaptic input to and output from area CA2, reporting connections from the PVN and to the SuM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Hernández VS. Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience. 2013;228:139–162. doi: 10.1016/j.neuroscience.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Yanagihara M, Niimi K. Substance P-like immunoreactive projection to the hippocampal formation from the posterior hypothalamus in the cat. Brain Res Bull. 1989;22:689–694. doi: 10.1016/0361-9230(89)90088-9. [DOI] [PubMed] [Google Scholar]
- 13.Nadler JV, Perry BW, Cotman CW. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. This paper contained the first report that CA2 is resistant to cell death in an animal model of epilepsy. [DOI] [PubMed] [Google Scholar]
- 14.Sloviter RS, Damiano BP. Sustained electrical stimulation of the perforant path duplicates kainate-induced electrophysiological effects and hippocampal damage in rats. Neurosci Lett. 1981;24:279–284. doi: 10.1016/0304-3940(81)90171-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Choi Y-S, Obrietan K, Dudek SM. Synaptic plasticity (and the lack thereof) in hippocampal CA2 neurons. J Neurosci. 2007;27:12025–12032. doi: 10.1523/JNEUROSCI.4094-07.2007. This is the first report of the lack of typical LTP induction in CA2 pyramidal neurons in the SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLardy T. Some cell and fibre peculiarities of uncal hippocampus. Prog Brain Res. 1963;3:71–78. [Google Scholar]
- 17.Laurberg S, Zimmer J. Aberrant hippocampal mossy fibers in cats. Brain Res. 1980;188:555–559. doi: 10.1016/0006-8993(80)90054-2. [DOI] [PubMed] [Google Scholar]
- 18.Hirama J, Shoumura K, Ichinohe N, You S, Yonekura H. Cornu ammonis of the cat: lack of a separate field of CA2. J Hirnforsch. 1997;38:487–493. [PubMed] [Google Scholar]
- 19.San Antonio A, Liban K, Ikrar T, Tsyganovskiy E, Xu X. Distinct physiological and developmental properties of hippocampal CA2 subfield revealed by using anti-Purkinje cell protein 4 (PCP4) immunostaining. J Comp Neurol. 2014;522:1333–1354. doi: 10.1002/cne.23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz DG. The distribution of chromogranin A-like immunoreactivity in the human hippocampus coincides with the pattern of resistance to epilepsy-induced neuronal damage. Ann Neurol. 1990;27:266–275. doi: 10.1002/ana.410270308. [DOI] [PubMed] [Google Scholar]
- 21.Gaarskjaer FB. The organization and development of the hippocampal mossy fiber system. Brain Res. 1986;396:335–357. doi: 10.1016/0165-0173(86)90004-4. [DOI] [PubMed] [Google Scholar]
- 22.Woodhams PL, Celio MR, Ulfig N, Witter MP. Morphological and functional correlates of borders in the entorhinal cortex and hippocampus. Hippocampus. 1993;3:303–311. [PubMed] [Google Scholar]
- 23.Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- 24.Emoto N, et al. Basic fibroblast growth factor (FGF) in the central nervous system: identification of specific loci of basic FGF expression in the rat brain. Growth Factors. 1989;2:21–29. doi: 10.3109/08977198909069078. [DOI] [PubMed] [Google Scholar]
- 25.Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- 26.Zhao X, et al. Transcriptional profiling reveals strict boundaries between hippocampal subregions. J Comp Neurol. 2001;441:187–196. doi: 10.1002/cne.1406. [DOI] [PubMed] [Google Scholar]
- 27.Mercer A, Trigg HL, Thomson AM. Characterization of neurons in the CA2 subfield of the adult rat hippocampus. J Neurosci. 2007;27:7329–7338. doi: 10.1523/JNEUROSCI.1829-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SE, et al. RGS14 is a natural suppressor of both synaptic plasticity in CA2 neurons and hippocampal-based learning and memory. Proc Natl Acad Sci USA. 2010;107:16994–16998. doi: 10.1073/pnas.1005362107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shinohara Y, et al. Hippocampal CA3 and CA2 have distinct bilateral innervation patterns to CA1 in rodents. Eur J Neurosci. 2012;35:702–710. doi: 10.1111/j.1460-9568.2012.07993.x. [DOI] [PubMed] [Google Scholar]
- 30.Alexander GM, et al. Social and novel contexts modify hippocampal CA2 representations of space. Nat Commun. 2016;7:10300. doi: 10.1038/ncomms10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyszynski M, et al. Differential regional expression and ultrastructural localization of α-actinin-2, a putative NMDA receptor-anchoring protein, in rat brain. J Neurosci. 1998;18:1383–1392. doi: 10.1523/JNEUROSCI.18-04-01383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tóth K, Freund TF. Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience. 1992;49:793–805. doi: 10.1016/0306-4522(92)90357-8. [DOI] [PubMed] [Google Scholar]
- 33.Maglóczky Z, Acsády L, Freund TF. Principal cells are the postsynaptic targets of supramammillary afferents in the hippocampus of the rat. Hippocampus. 1994;4:322–334. doi: 10.1002/hipo.450040316. [DOI] [PubMed] [Google Scholar]
- 34.Haglund L, Swanson LW, Köhler C. The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat. J Comp Neurol. 1984;229:171–185. doi: 10.1002/cne.902290204. [DOI] [PubMed] [Google Scholar]
- 35.Nitsch R, Leranth C. Substance P-containing hypothalamic afferents to the monkey hippocampus: an immunocytochemical, tracing, and coexistence study. Exp Brain Res. 1994;101:231–240. doi: 10.1007/BF00228743. [DOI] [PubMed] [Google Scholar]
- 36.Gall C, Selawski L. Supramammillary afferents to guinea pig hippocampus contain substance P-like immunoreactivity. Neurosci Lett. 1984;51:171–176. doi: 10.1016/0304-3940(84)90546-9. [DOI] [PubMed] [Google Scholar]
- 37.Borhegyi Z, Leranth C. Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats; a species difference between rats and monkeys. Exp Brain Res. 1997;115:369–374. doi: 10.1007/pl00005706. [DOI] [PubMed] [Google Scholar]
- 38.Tamamaki N, Abe K, Nojyo Y. Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Res. 1988;452:255–272. doi: 10.1016/0006-8993(88)90030-3. [DOI] [PubMed] [Google Scholar]
- 39.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2006;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LW, Wyss JM, Cowan WM. An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol. 1978;181:681–715. doi: 10.1002/cne.901810402. [DOI] [PubMed] [Google Scholar]
- 41.Llorens-Martín M, Jurado-Arjona J, Avila J, Hernández F. Novel connection between newborn granule neurons and the hippocampal CA2 field. Exp Neurol. 2015;263:285–292. doi: 10.1016/j.expneurol.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci. 2015;19:151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Borhegyi Z, Leranth C. Substance P innervation of the rat hippocampal formation. J Comp Neurol. 1997;384:41–58. doi: 10.1002/(sici)1096-9861(19970721)384:1<41::aid-cne3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 44.Dong HW, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic–anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci USA. 2009;106:11794–11799. doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slomianka L, Amrein I, Knuesel I, Sørensen JC, Wolfer DP. Hippocampal pyramidal cells: the reemergence of cortical lamination. Brain Struct Funct. 2011;216:301–317. doi: 10.1007/s00429-011-0322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee SH, et al. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron. 2014;82:1129–1144. doi: 10.1016/j.neuron.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizuseki K, Diba K, Pastalkova E, Buzsáki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14:1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valero M, et al. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat Neurosci. 2015;18:1281–1290. doi: 10.1038/nn.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ropireddy D, Bachus SE, Ascoli GA. Non-homogeneous stereological properties of the rat hippocampus from high-resolution 3D serial reconstruction of thin histological sections. Neuroscience. 2012;205:91–111. doi: 10.1016/j.neuroscience.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andersen P, Bliss TVP, Skrede KK. Lamellar organization of hippocampal excitatory pathways. Exp Brain Res. 1971;13:222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- 51.Sekino Y, Obata K, Tanifuji M, Mizuno M, Murayama J. Delayed signal propagation via CA2 in rat hippocampal slices revealed by optical recording. J Neurophysiol. 1997;78:1662–1668. doi: 10.1152/jn.1997.78.3.1662. [DOI] [PubMed] [Google Scholar]
- 52.Rowland DC, et al. Transgenically targeted rabies virus demonstrates a major monosynaptic projection from hippocampal area CA2 to medial entorhinal layer II neurons. J Neurosci. 2013;33:14889–14898. doi: 10.1523/JNEUROSCI.1046-13.2013. This study found that some CA2 neurons project back to the entorhinal cortex, which is one of the sources of input to CA2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. This study was the first to silence CA2 synapses in mice, which resulted in a deficit in social recognition memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishizuka N, Cowan WM, Amaral DG. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. J Comp Neurol. 1995;362:17–45. doi: 10.1002/cne.903620103. [DOI] [PubMed] [Google Scholar]
- 55.Bartesaghi R, Ravasi L. Pyramidal neuron types in field CA2 of the guinea pig. Brain Res Bull. 1999;50:263–273. doi: 10.1016/s0361-9230(99)00198-7. [DOI] [PubMed] [Google Scholar]
- 56.Piskorowski RA, Chevaleyre V. Synaptic integration by different dendritic compartments of hippocampal CA1 and CA2 pyramidal neurons. Cell Mol Life Sci. 2011;69:75–88. doi: 10.1007/s00018-011-0769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Q, Srinivas KV, Sotayo A, Siegelbaum SA. Dendritic Na+ spikes enable cortical input to drive action potential output from hippocampal CA2 pyramidal neurons. eLife. 2014;3:e04551. doi: 10.7554/eLife.04551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chevaleyre V, Siegelbaum SA. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron. 2010;66:560–572. doi: 10.1016/j.neuron.2010.04.013. The paper provides the first electrophysiological characterization of the entorhinal cortex layer II synapses formed onto CA2 pyramidal neurons in a slice preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piskorowski RA, Chevaleyre V. Delta-opioid receptors mediate unique plasticity onto parvalbumin-expressing interneurons in area CA2 of the hippocampus. J Neurosci. 2013;33:14567–14578. doi: 10.1523/JNEUROSCI.0649-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Botcher NA, Falck JE, Thomson AM, Mercer A. Distribution of interneurons in the CA2 region of the rat hippocampus. Front Neuroanat. 2014;8:104. doi: 10.3389/fnana.2014.00104. This is a comprehensive study of the different types of interneurons enriched in area CA2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mathie A, Al-Moubarak E, Veale EL. Gating of two pore domain potassium channels. J Physiol (Lond) 2010;588:3149–3156. doi: 10.1113/jphysiol.2010.192344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chang PY, Taylor PE, Jackson MB. Voltage imaging reveals the CA1 region at the CA2 border as a focus for epileptiform discharges and long-term potentiation in hippocampal slices. J Neurophysiol. 2007;98:1309–1322. doi: 10.1152/jn.00532.2007. [DOI] [PubMed] [Google Scholar]
- 64.Caruana DA, Alexander GM, Dudek SM. New insights into the regulation of synaptic plasticity from an unexpected place: hippocampal area CA2. Learn Mem. 2012;19:391–400. doi: 10.1101/lm.025304.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boulanger LM, et al. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pelkey KA, et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 67.Nasrallah K, Piskorowski RA, Chevaleyre V. Inhibitory plasticity permits the recruitment of CA2 pyramidal neurons by CA3. eNeuro. 2015 doi: 10.1523/ENEURO.0049-15.2015. http://dx.doi.org/10.1523/ENEURO.0049-15.2015. [DOI] [PMC free article] [PubMed]
- 68.Simons SB, Escobedo Y, Yasuda R, Dudek SM. Regional differences in hippocampal calcium handling provide a cellular mechanism for limiting plasticity. Proc Natl Acad Sci USA. 2009;106:14080–14084. doi: 10.1073/pnas.0904775106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sangameswaran L, Hempstead J, Morgan JI. Molecular cloning of a neuron-specific transcript and its regulation during normal and aberrant cerebellar development. Proc Natl Acad Sci USA. 1989;86:5651–5655. doi: 10.1073/pnas.86.14.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCγ subunit family in developing and adult mouse brains. Neurosci Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Kato AS, Siuda ER, Nisenbaum ES, Bredt DS. AMPA receptor subunit-specific regulation by a distinct family of type II TARPs. Neuron. 2008;59:986–996. doi: 10.1016/j.neuron.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 72.Soto D, et al. Selective regulation of long-form calcium-permeable AMPA receptors by an atypical TARP, γ-5. Nat Neurosci. 2009;12:277–285. doi: 10.1038/nn.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vellano CP, Lee SE, Dudek SM, Hepler JR. RGS14 at the interface of hippocampal signaling and synaptic plasticity. Trends Pharmacol Sci. 2011;32:666–674. doi: 10.1016/j.tips.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS. A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. This is the first report of expression of a receptor for a social neuropeptide in hippocampal area CA2, which prompted interest in area CA2 in the realm of social behaviour. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pagani JH, et al. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry. 2015;20:490–499. doi: 10.1038/mp.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ochiishi T, et al. High level of adenosine A1 receptor-like immunoreactivity in the CA2/CA3a region of the adult rat hippocampus. Neuroscience. 1999;93:955–967. doi: 10.1016/s0306-4522(99)00179-7. [DOI] [PubMed] [Google Scholar]
- 78.Simons SB, Caruana DA, Zhao M, Dudek SM. Caffeine-induced synaptic potentiation in hippocampal CA2 neurons. Nat Neurosci. 2012;15:23–25. doi: 10.1038/nn.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mons N, Segu L, Nogues X, Buhot MC. Effects of age and spatial learning on adenylyl cyclase mRNA expression in the mouse hippocampus. Neurobiol Aging. 2004;25:1095–1106. doi: 10.1016/j.neurobiolaging.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 80.Visel A, Alvarez-Bolado G, Thaller C, Eichele G. Comprehensive analysis of the expression patterns of the adenylate cyclase gene family in the developing and adult mouse brain. J Comp Neurol. 2006;496:684–697. doi: 10.1002/cne.20953. [DOI] [PubMed] [Google Scholar]
- 81.Watson C, Paxinos G. Chemoarchitecton Atlas Mouse Brain. Academic Press; 2009. [Google Scholar]
- 82.Evans PR, Lee SE, Smith Y, Hepler JR. Postnatal developmental expression of regulator of G protein signaling 14 (RGS14) in the mouse brain. J Comp Neurol. 2013;522:186–203. doi: 10.1002/cne.23395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wintzer ME, Boehringer R, Polygalov D, McHugh TJ. The hippocampal CA2 ensemble is sensitive to contextual change. J Neurosci. 2014;34:3056–3066. doi: 10.1523/JNEUROSCI.2563-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mankin EA, Diehl GW, Sparks FT, Leutgeb S, Leutgeb JK. Hippocampal CA2 activity patterns change over time to a larger extent than between spatial contexts. Neuron. 2015;85:190–201. doi: 10.1016/j.neuron.2014.12.001. The paper provides the first electrophysiological characterization of rat CA2 neuron firing in vivo. The authors note that place fields are unstable and so may provide a substrate for time coding in the hippocampus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martig AK, Mizumori SJY. Ventral tegmental area disruption selectively affects CA1/CA2 but not CA3 place fields during a differential reward working memory task. Hippocampus. 2011;21:172–184. doi: 10.1002/hipo.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee H, Wang C, Deshmukh SS, Knierim JJ. Neural population evidence of functional heterogeneity along the CA3 transverse axis: pattern completion versus pattern separation. Neuron. 2015;87:1093–1105. doi: 10.1016/j.neuron.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu L, Igarashi KM, Witter MP, Moser EI, Moser MB. Topography of place maps along the CA3-to-CA2 axis of the hippocampus. Neuron. 2015;87:1078–1092. doi: 10.1016/j.neuron.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 89.von Heimendahl M, Rao RP, Brecht M. Weak and nondiscriminative responses to conspecifics in the rat hippocampus. J Neurosci. 2012;32:2129–2141. doi: 10.1523/JNEUROSCI.3812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee HJ, Macbeth AH, Pagani JH, Young WS. Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 92.Wersinger SR, Temple JL, Caldwell HK, Young WS. Inactivation of the oxytocin and the vasopressin (Avp) 1b receptor genes, but not the Avp 1a receptor gene, differentially impairs the Bruce effect in laboratory mice (Mus musculus) Endocrinology. 2008;149:116–121. doi: 10.1210/en.2007-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.DeVito LM, et al. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci. 2009;29:2676–2683. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014;40:3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tymianski M. Emerging mechanisms of disrupted cellular signaling in brain ischemia. Nat Neurosci. 2011;14:1369–1373. doi: 10.1038/nn.2951. [DOI] [PubMed] [Google Scholar]
- 96.Hatanpaa KJ, et al. Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: differential vulnerability of hippocampal subfields. J Neuropathol Exp Neurol. 2014;73:136–142. doi: 10.1097/OPX.0000000000000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sommer W. Erkrankung des ammonshorns als aetiologisches moment der epilepsie. Arch Psychiat Nervenkr. 1880;10:631–675. in German. [Google Scholar]
- 98.Corsellis JA, Bruton CJ. Neuropathology of status epilepticus in humans. Adv Neurol. 1983;34:129–139. [PubMed] [Google Scholar]
- 99.Bratz E. Ammonshornbefunde bei epileptikern. Arch Psychiat Nervenkr. 1899;31:820–836. in German. [Google Scholar]
- 100.Steve TA, Jirsch JD, Gross DW. Quantification of subfield pathology in hippocampal sclerosis: a systematic review and meta-analysis. Epilepsy Res. 2014;108:1279–1285. doi: 10.1016/j.eplepsyres.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 101.Maxwell WL, et al. There is differential loss of pyramidal cells from the human hippocampus with survival after blunt head injury. J Neuropathol Exp Neurol. 2003;62:272–279. doi: 10.1093/jnen/62.3.272. [DOI] [PubMed] [Google Scholar]
- 102.Sloviter RS, Sollas AL, Barbaro NM, Laxer KD. Calcium-binding protein (calbindin-D28K) and parvalbumin immunocytochemistry in the normal and epileptic human hippocampus. J Comp Neurol. 1991;308:381–396. doi: 10.1002/cne.903080306. [DOI] [PubMed] [Google Scholar]
- 103.Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- 104.Kotapka MJ, Graham DI, Adams JH, Gennarelli TA. Hippocampal pathology in fatal non-missile human head injury. Acta Neuropathol. 1992;83:530–534. doi: 10.1007/BF00310031. [DOI] [PubMed] [Google Scholar]
- 105.Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miyamoto O, et al. Neurotoxicity of Clostridium perfringens epsilon-toxin for the rat hippocampus via the glutamatergic system. Infect Immun. 1998;66:2501–2508. doi: 10.1128/iai.66.6.2501-2508.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sadowski M, et al. Pattern of neuronal loss in the rat hippocampus following experimental cardiac arrest-induced ischemia. J Neurol Sci. 1999;168:13–20. doi: 10.1016/s0022-510x(99)00159-8. [DOI] [PubMed] [Google Scholar]
- 108.Yang G, et al. Regional difference of neuronal vulnerability in the murine hippocampus after transient forebrain ischemia. Brain Res. 2000;870:195–198. doi: 10.1016/s0006-8993(00)02319-2. [DOI] [PubMed] [Google Scholar]
- 109.Bothe HW, Bosma HJ, Hofer H, Hossmann KA, Angermeier WF. Selective vulnerability of hippocampus and disturbances of memory storage after mild unilateral ischemia of gerbil brain. Stroke. 1986;17:1160–1163. doi: 10.1161/01.str.17.6.1160. [DOI] [PubMed] [Google Scholar]
- 110.Buckmaster PS, Wen X, Toyoda I, Gulland FMD, Van Bonn W. Hippocampal neuropathology of domoic acid-induced epilepsy in California sea lions (Zalophus californianus) J Comp Neurol. 2014;522:1691–1706. doi: 10.1002/cne.23509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Young D, Dragunow M. Neuronal injury following electrically induced status epilepticus with and without adenosine receptor antagonism. Exp Neurol. 1995;133:125–137. doi: 10.1006/exnr.1995.1015. [DOI] [PubMed] [Google Scholar]
- 112.Xu B, Michalski B, Racine RJ, Fahnestock M. Continuous infusion of neurotrophin-3 triggers sprouting, decreases the levels of TrkA and TrkC, and inhibits epileptogenesis and activity-dependent axonal growth in adult rats. Neuroscience. 2002;115:1295–1308. doi: 10.1016/s0306-4522(02)00384-6. [DOI] [PubMed] [Google Scholar]
- 113.Gluckman P, et al. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 114.Miltiadous P, Stamatakis A, Stylianopoulou F. Neuroprotective effects of IGF-I following kainic acid-induced hippocampal degeneration in the rat. Cell Mol Neurobiol. 2009;30:347–360. doi: 10.1007/s10571-009-9457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sun X, et al. Insulin/PI3K signaling protects dentate neurons from oxygen-glucose deprivation in organotypic slice cultures. J Neurochem. 2010;112:377–388. doi: 10.1111/j.1471-4159.2009.06450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chung YH, et al. Region-specific alterations in insulin-like growth factor-I receptor in the central nervous system of nNOS knockout mice. Brain Res. 2004;1021:132–139. doi: 10.1016/j.brainres.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 117.Beilharz EJ, et al. Differential expression of insulin-like growth factor binding proteins (IGFBP) 4 and 5 mRNA in the rat brain after transient hypoxic-ischemic injury. Brain Res Mol Brain Res. 1993;18:209–215. doi: 10.1016/0169-328x(93)90191-q. [DOI] [PubMed] [Google Scholar]
- 118.Kang TC, et al. The somatostatin receptors in the normal and epileptic hippocampus of the gerbil: subtype-specific localization and its alteration. Brain Res. 2003;986:91–102. doi: 10.1016/s0006-8993(03)03192-5. [DOI] [PubMed] [Google Scholar]
- 119.Arida RM, Scorza FA, de Amorim Carvalho R, Cavalheiro E. A Proechimys guyannensis: an animal model of resistance to epilepsy. Epilepsia. 2005;46(Suppl 5):189–197. doi: 10.1111/j.1528-1167.2005.01065.x. [DOI] [PubMed] [Google Scholar]
- 120.Scorza CA, et al. Distinctive hippocampal CA2 subfield of the Amazon rodent Proechimys. Neuroscience. 2010;169:965–973. doi: 10.1016/j.neuroscience.2010.05.079. [DOI] [PubMed] [Google Scholar]
- 121.Han C, Kasai N, Torimitsu K. CA2: the most vulnerable sector to bicuculline exposure in rat hippocampal slice cultures. Neuroreport. 2005;16:333–336. doi: 10.1097/00001756-200503150-00006. [DOI] [PubMed] [Google Scholar]
- 122.Mattson MP, Kater SB. Development and selective neurodegeneration in cell cultures from different hippocampal regions. Brain Res. 1989;490:110–125. doi: 10.1016/0006-8993(89)90436-8. [DOI] [PubMed] [Google Scholar]
- 123.Wittner L, et al. The epileptic human hippocampal cornu ammonis 2 region generates spontaneous interictal-like activity in vitro. Brain. 2009;132:3032–3046. doi: 10.1093/brain/awp238. [DOI] [PubMed] [Google Scholar]
- 124.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 125.Williamson A, Spencer DD. Electrophysiological characterization of CA2 pyramidal cells from epileptic humans. Hippocampus. 1994;4:226–237. doi: 10.1002/hipo.450040213. [DOI] [PubMed] [Google Scholar]
- 126.Wong RK, Traub RD. Synchronized burst discharge in disinhibited hippocampal slice. I Initiation in CA2-CA3 region. J Neurophysiol. 1983;49:442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]
- 127.Häussler U, Rinas K, Kilias A, Egert U, Haas CA. Mossy fiber sprouting and pyramidal cell dispersion in the hippocampal CA2 region in a mouse model of temporal lobe epilepsy. Hippocampus. 2015 doi: 10.1002/hipo.22543. http://dx.doi.org/10.1002/hipo.22543. [DOI] [PubMed]
- 128.Lavenex P, Lavenex PB, Bennett JL, Amaral DG. Postmortem changes in the neuroanatomical characteristics of the primate brain: hippocampal formation. J Comp Neurol. 2009;512:27–51. doi: 10.1002/cne.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23:183–187. doi: 10.1016/j.ijdevneu.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 130.Boucher J, Mayes A, Bigham S. Memory in autistic spectrum disorder. Psychol Bull. 2012;138:458–496. doi: 10.1037/a0026869. [DOI] [PubMed] [Google Scholar]
- 131.Deykin EY, MacMahon B. The incidence of seizures among children with autistic symptoms. Am J Psychiatry. 1979;136:1310–1312. doi: 10.1176/ajp.136.10.1310. [DOI] [PubMed] [Google Scholar]
- 132.Shen EH, Overly CC, Jones AR. The Allen Human Brain Atlas: comprehensive gene expression mapping of the human brain. Trends Neurosci. 2012;35:711–714. doi: 10.1016/j.tins.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 133.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. This is the first report of disruption of CA2 inhibitory circuitry in a psychiatric disease state. [DOI] [PubMed] [Google Scholar]
- 134.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 135.Benes FM, et al. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Benes FM, Todtenkopf MS, Kostoulakos P. GluR5,6,7 subunit immunoreactivity on apical pyramidal cell dendrites in hippocampus of schizophrenics and manic depressives. Hippocampus. 2001;11:482–491. doi: 10.1002/hipo.1065. [DOI] [PubMed] [Google Scholar]
- 137.Gao XM, et al. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 138.Jin CY, Anichtchik O, Panula P. Altered histamine H3 receptor radioligand binding in post-mortem brain samples from subjects with psychiatric diseases. Br J Pharmacol. 2009;157:118–129. doi: 10.1111/j.1476-5381.2009.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schobel SA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–663. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 141.Berretta S, Gisabella B, Benes FM. A rodent model of schizophrenia derived from postmortem studies. Behav Brain Res. 2009;204:363–368. doi: 10.1016/j.bbr.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 142.Pikkarainen M, Rönkkö S, Savander V, Insausti R, Pitkänen A. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the hippocampal formation in rat. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- 143.Ding SL, Haber SN, Van Hoesen GW. Stratum radiatum of CA2 is an additional target of the perforant path in humans and monkeys. Neuroreport. 2010;21:245–249. doi: 10.1097/WNR.0b013e328333d690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leranth C, Ribak CE. Calcium-binding proteins are concentrated in the CA2 field of the monkey hippocampus: a possible key to this region’s resistance to epileptic damage. Exp Brain Res. 1991;85:129–136. doi: 10.1007/BF00229993. [DOI] [PubMed] [Google Scholar]
- 145.Seress L, Nitsch R, Leranth C. Calretinin immunoreactivity in the monkey hippocampal formation — I. Light and electron microscopic characteristics and co-localization with other calcium-binding proteins. Neuroscience. 1993;55:775–796. doi: 10.1016/0306-4522(93)90441-h. [DOI] [PubMed] [Google Scholar]
- 146.Utal AK, Stopka AL, Roy M, Coleman PD. PEP-19 immunohistochemistry defines the basal ganglia and associated structures in the adult human brain, and is dramatically reduced in Huntington’s disease. Neuroscience. 1998;86:1055–1063. doi: 10.1016/s0306-4522(98)00130-4. [DOI] [PubMed] [Google Scholar]
- 147.Zimmermann B, Girard F, Mészàr Z, Celio MR. Expression of the calcium binding proteins Necab-1,-2 and -3 in the adult mouse hippocampus and dentate gyrus. Brain Res. 2013;1528:1–7. doi: 10.1016/j.brainres.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 148.Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamamoto M, Marshall P, Hemmendinger LM, Boyer AB, Caviness VS. Distribution of glucuronic acid-and-sulfate-containing glycoproteins in the central nervous system of the adult mouse. Neurosci Res. 1988;5:273–298. doi: 10.1016/0168-0102(88)90031-4. [DOI] [PubMed] [Google Scholar]
- 150.Costa C, et al. Mapping of aggrecan, hyaluronic acid, heparan sulphate proteoglycans and aquaporin 4 in the central nervous system of the mouse. J Chem Neuroanat. 2007;33:111–123. doi: 10.1016/j.jchemneu.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 151.McRae PA, Baranov E, Sarode S, Brooks-Kayal AR, Porter BE. Aggrecan expression, a component of the inhibitory interneuron perineuronal net, is altered following an early-life seizure. Neurobiol Dis. 2010;39:439–448. doi: 10.1016/j.nbd.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fuxe K, et al. On the regional distribution of heparan sulfate proteoglycan immunoreactivity in the rat brain. Brain Res. 1994;636:131–138. doi: 10.1016/0006-8993(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 153.Brückner G, Grosche J, Hartlage-Rübsamen M, Schmidt S, Schachner M. Region and lamina-specific distribution of extracellular matrix proteoglycans, hyaluronan and tenascin-R in the mouse hippocampal formation. J Chem Neuroanat. 2003;26:37–50. doi: 10.1016/s0891-0618(03)00036-x. [DOI] [PubMed] [Google Scholar]
- 154.Fuxe K, Tinner B, Staines W, David G, Agnati LF. Regional distribution of neural cell adhesion molecule immunoreactivity in the adult rat telencephalon and diencephalon. Partial colocalization with heparan sulfate proteoglycan immunoreactivity. Brain Res. 1997;746:25–33. doi: 10.1016/s0006-8993(96)01130-4. [DOI] [PubMed] [Google Scholar]
- 155.Laeremans A, et al. AMIGO2 mRNA expression in hippocampal CA2 and CA3a. Brain Struct Funct. 2012;218:123–130. doi: 10.1007/s00429-012-0387-4. [DOI] [PubMed] [Google Scholar]
- 156.Barlow GM, Micales B, Lyons GE, Korenberg JR. Down syndrome cell adhesion molecule is conserved in mouse and highly expressed in the adult mouse brain. Cytogenet Cell Genet. 2001;94:155–162. doi: 10.1159/000048808. [DOI] [PubMed] [Google Scholar]
- 157.Shen L, et al. Altered expression of Dscam in temporal lobe tissue from human and experimental animals. Synapse. 2011;65:975–982. doi: 10.1002/syn.20924. [DOI] [PubMed] [Google Scholar]
- 158.Eroglu C. The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J Cell Commun Signal. 2009;3:167–176. doi: 10.1007/s12079-009-0078-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hawrylycz MJ, et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489:391–399. doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Woodward WR, et al. Nuclear and cytoplasmic localization of basic fibroblast growth factor in astrocytes and CA2 hippocampal neurons. J Neurosci. 1992;12:142–152. doi: 10.1523/JNEUROSCI.12-01-00142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eckenstein FP, Andersson C, Kuzis K, Woodward WR. Distribution of acidic and basic fibroblast growth factors in the mature, injured and developing rat nervous system. Prog Brain Res. 1994;103:55–64. doi: 10.1016/s0079-6123(08)61126-7. [DOI] [PubMed] [Google Scholar]
- 162.Williams TE, et al. Characterization and distribution of basic fibroblast growth factor-containing cells in the rat hippocampus. J Comp Neurol. 1996;370:147–158. doi: 10.1002/(SICI)1096-9861(19960624)370:2<147::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 163.Gómez-Pinilla F, van der Wal EA, Cotman CW. Possible coordinated gene expressions for FGF receptor, FGF-5, and FGF-2 following seizures. Exp Neurol. 1995;133:164–174. doi: 10.1006/exnr.1995.1019. [DOI] [PubMed] [Google Scholar]
- 164.Gonzalez AM, Berry M, Maher PA, Logan A, Baird A. A comprehensive analysis of the distribution of FGF-2 and FGFR1 in the rat brain. Brain Res. 1995;701:201–226. doi: 10.1016/0006-8993(95)01002-x. [DOI] [PubMed] [Google Scholar]
- 165.Ernfors P, Ibáñez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc Natl Acad Sci USA. 1990;87:5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vigers AJ, Baquet ZC, Jones KR. Expression of neurotrophin-3 in the mouse forebrain: insights from a targeted LacZ reporter. J Comp Neurol. 2000;416:398–415. doi: 10.1002/(sici)1096-9861(20000117)416:3<398::aid-cne10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 167.Friedman WJ, Ernfors P, Persson H. Transient and persistent expression of NT-3/HDNF mRNA in the rat brain during postnatal development. J Neurosci. 1991;11:1577–1584. doi: 10.1523/JNEUROSCI.11-06-01577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tucker MS, et al. Localization of immunoreactive epidermal growth factor receptor in neonatal and adult rat hippocampus. Brain Res. 1993;631:65–71. doi: 10.1016/0006-8993(93)91187-w. [DOI] [PubMed] [Google Scholar]
- 169.Bondy C, Werner H, Roberts CT, LeRoith D. Cellular pattern of type-I insulin-like growth factor receptor gene expression during maturation of the rat brain: comparison with insulin-like growth factors I and II. Neuroscience. 1992;46:909–923. doi: 10.1016/0306-4522(92)90193-6. [DOI] [PubMed] [Google Scholar]
- 170.Stenvers KL, Zimmermann EM, Gallagher M, Lund PK. Expression of insulin-like growth factor binding protein-4 and -5 mRNAs in adult rat forebrain. J Comp Neurol. 1994;339:91–105. doi: 10.1002/cne.903390109. [DOI] [PubMed] [Google Scholar]
- 171.Stephan AH, et al. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013;33:13460–13474. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lopez ME, Klein AD, Scott MP. Complement is dispensable for neurodegeneration in Niemann–Pick disease type C. J Neuroinflammation. 2012;9:216. doi: 10.1186/1742-2094-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kwak SE, et al. The expression of somatostatin receptors in the hippocampus of pilocarpine-induced rat epilepsy model. Neuropeptides. 2008;42:569–583. doi: 10.1016/j.npep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 174.Berger T, Frotscher M. Distribution and morphological characteristics of oligodendrocytes in the rat hippocampus in situ and in vitro: an immunocytochemical study with the monoclonal Rip antibody. J Neurocytol. 1994;23:61–74. doi: 10.1007/BF01189817. [DOI] [PubMed] [Google Scholar]
- 175.Smith AS, Williams Avram SK, Cymerblit-Sabba A, Song J, Young WS. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol Psychiatry. 2016 doi: 10.1038/mp.2015.189. http://dx.doi.org/10.1038/mp.2015.189. [DOI] [PMC free article] [PubMed]
- 176.Piskorowski RA, et al. Age-dependent specific changes in area CA2 of the hippocampus and social memory deficit in a mouse model of the 22q11.2 deletion syndrome. Neuron. 2016;89:163–176. doi: 10.1016/j.neuron.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]