Abstract
Bone does not turn over uniformly, and becomes susceptible to post-translational modification by non-enzymatic glycation (NEG). NEG of bone causes the formation of advanced glycation end-products (AGEs) and this process is accelerated with aging, diabetes and antiresorptive postmenopausal osteoporosis therapy. Due to the elevated incidence of fracture associated with aging and diabetes, several studies have attempted to measure and evaluate AGEs as biomarkers for fracture risk. Here current methods of estimating AGEs in bone by liquid chromatography and fluorometric assay are summarized and the relationships between AGEs and fracture properties at whole bone, apparent tissue and matrix levels are discussed.
Keywords: Advanced glycation end-products (AGEs), Bone, Mechanical properties, Non-enzymatic glycation, Pentosidine, Toughness
Introduction
Post-translational modification of proteins by the process of non-enzymatic glycation (NEG) occurs in tissues with limited turnover such as cartilage and tendon (1–2). The slow turnover process exposes matrix proteins to the extracellular environment for extended times, leading to modifications by NEG. NEG of tissues causes the formation and accumulation of advanced glycation end-products (AGEs) with age and disease, negatively impacting biomechanical properties (3–5).
Bone, in contrast, is considered to be a tissue with substantial turnover. Bone turnover rates of 5–25% per year have been reported in menopausal and postmenopausal women (6). Consequently, NEG was considered to occur in non-significant proportions and to be largely irrelevant for bone (7) except in diabetes where altered sugar metabolism caused the accumulation of AGEs (8). However, over the last decade additional evidence has emerged showing bone turnover to be a highly selective and a heterogeneous process. For example, the proportion of interstitial tissue in both cortical and cancellous bone compartments increases with chronological age and new bone formation, in the form of osteonal refilling and formation of trabecular packets, decreases with age (9–10). Also, some fragments of circumferential lamellar bone, formed during bone growth, remain unremodeled and survive well into old age (11). Thus, bone does not turn over uniformly and certain areas, analogous to tissues with slow turnover, become susceptible to post-translational modification by NEG.
In vitro cell culture studies show that NEG-modified tissue becomes more resistant to osteoclastic bone resorption (12) and causes a decrease in osteoblast proliferation and differentiation (13). Concomitant decreases in resorption and formation will decrease bone turnover, making NEG a widespread mechanism of protein modification in bone.
Several recent studies have analyzed NEG of bone under a variety of conditions in order to explain the increased fracture risk associated with aging and diabetes. In addition, since bisphosphonate therapy for postmenopausal osteoporosis slows down bone turnover, the connection between bisphosphonate therapy and NEG has also received significant attention. This Perspective will examine NEG of bone due to aging, diabetes and bisphosphonate treatment, with particular emphasis on AGEs and their influence on bone fracture.
Non-enzymatic Glycation (NEG) of Bone
NEG has been shown to modify both collagenous and non-collagenous matrix proteins in bone (14–17). However, due to the abundance of type I collagen in bone and its demonstrated role in bone fracture (18), AGEs in bone are reported in terms of collagen. Type I collagen in bone consists of tropocollagen molecules that contain three polypeptide chains. Each chain is a left-hand helix characterized by a unique amino acid sequence involving glycine-proline-X or glycine-X-hydroxyproline, where X is another amino acid (e.g., lysine or arginine). The unique amino acid sequence makes it possible for the three polypeptide chains to wrap around each other in a right-hand sense and form a triple helix with glycine sitting at the center. The other amino acids are present at the triple helix surface. The amino acids present on the triple helix surface and at the N- and C-telopeptide terminals participate in NEG to form covalent crosslinks with their neighboring tropocollagen molecules.
NEG-mediated crosslinking involves a reaction between an aldehyde of the open chain form of glucose and the e-amino group of lysine or hydroxylysine on collagen. The resultant aldimine (glucosyl-lysine) undergoes a rearrangement to form Schiff base adduct and/or the Amadori product (19). Both Schiff base adduct and Amadori product undergo further reactions with other amino groups to form AGEs.
Advanced Glycation End-products (AGEs)
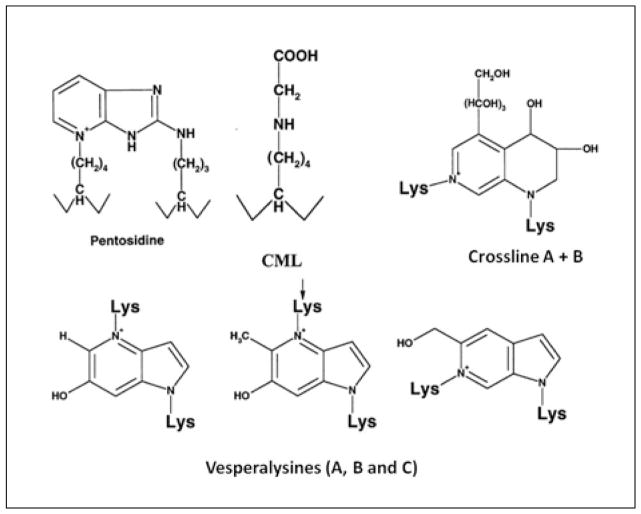
AGEs represent several intermolecular crosslinks that are formed as a result of NEG (20). To date a number of intermolecular crosslinks have been identified or proposed to occur in AGEs. These include pentosidine (21), vesperlysines (22), imidazolium compounds – methylimidazolium (MOLD (23)) and glyoxalimidazolium (GOLD (24)) – crossline (25), carboxymethyl- and carboxyethyllysine (CML and CEL (26)) and NFC-1 (non-fluorescent component-1) (27).
Out of the AGE crosslinks listed above only one, pentosidine, has been quantified in bone (15–17). However, because pentosidine is present at a low concentration of one crosslink per 200–300 collagen molecule in AGEs and bone (7;28), the estimation/measurement of bone’s total AGE content is somewhat of a challenge. Current methods are discussed below.
Measurement of AGEs in Bone
Two methods for estimating AGEs in bone have been used. Both of these methods are based on the fact that a majority of the crosslinks in AGEs are fluorescent. Furthermore, controlled in vitro NEG reactions (29) and naturally aged proteins including lens crystallins and collagenous connective tissue (30) show classical browning associated with an increase in fluorescence.
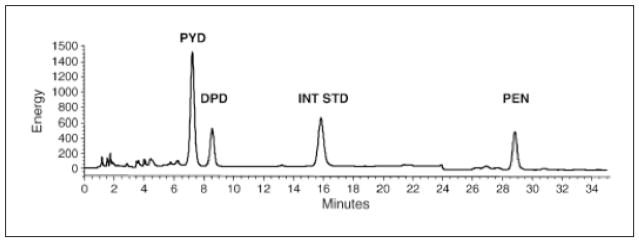
Based on the pioneering work of Monnier and coworkers, who isolated, purified, synthesized and elucidated the structure of pentosidine (21), the first method utilizes a single-column high-performance liquid chromatography (HPLC) for the quantification of pentosidine. Under this scheme, pentosidine is separated from enzymatic crosslinks (pyridinoline (PYD) and deoxypiridinoline (DPD)) in acid-hydrolyzed bone samples in a C18 column and quantified using fluorescence (335 nm excitation; 385 nm emission) and internal standards (16;31) (Fig. 1). The amount of pentosidine, estimated from the chromatogram, is normalized by the collagen amount present in the sample. The amount of collagen contained in each sample is approximated from its hydroxyproline content using an HPLC kit that was originally developed to estimate the amount of collagen in urine (Biorad, Germany). This method is expensive but provides rapid and precise measurement of pentosidine (the lowest detection limit is 0.02 pmol (31)). In addition, enzymatic crosslinks (PYD and DPD), providing an index of tissue maturity, can also be measured from the same HPLC run. Using this method, pentosidine can also be measured from the patient’s urine and serum and used as a biomarker in a clinical setting (32–33). Because pentosidine content in bone is minimal and represents a small fraction (<1%) of total AGEs (28), the utility of pentosidine as a reliable indicator of AGE content in bone needs further evaluation.
Fig. 1.
Chromatogram showing the fluorescence-based measurement of enzymatic (PYD and DPD; excitation/emission: 297/395 nm) and non-enzymatic (PEN; excitation/emission: 335/385 nm) crosslinks from a 54-year-old male donor (human vertebrae L3). With permission from Elsevier (45).
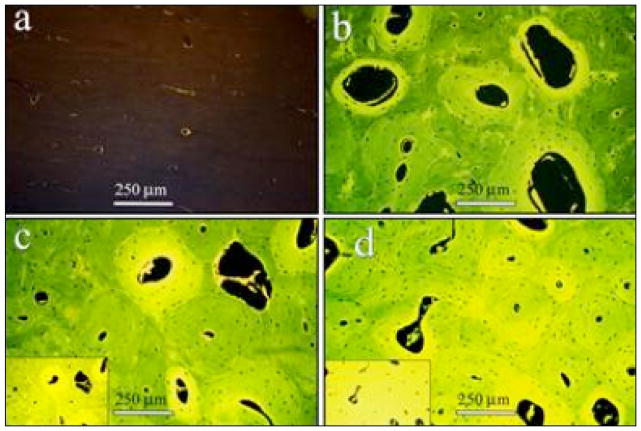
The second method of measuring AGEs is based on in vitro NEG reactions wherein the bulk fluorescence of glycated bone is observed to increase with the progressive browning of the tissue (34) (Fig. 2). This method is also used to measure AGEs in cartilage (5). Under this scheme, demineralized bone is subjected to papain digestion that preserves acid-stable as well as acid-soluble AGE crosslinks (34–36). AGE content is then determined from the digested sample using a microplate reader (370 nm excitation and 440 nm emission) and normalized to a quinine sulphate standard. The amount of collagen in the sample is estimated based on the amount of hydroxyproline that is also measured on the microplate reader against a hydroxyproline standard at a wavelength of 570 nm (21). AGE content is then expressed as ng of quinine sulphate fluorescence/mg of collagen.
Fig. 2.
Sections of in vitro glycated bone tissue showing a progressive increase in tissue fluorescence (excitation/emission: 370 nm/440 nm) with time of incubation (a: control; b: 3 days; c: 11 days; d: 38 days). Micrographs a–d, taken at auto-exposure, show that the increased incubation period leads to a more homogeneous glycation of cortical bone microstructure. For noting the increase in the level of fluorescence, indicative of AGE accumulation, compare to micrograph b and the insets in micrographs c and d. For the insets, the exposure was set at a fixed value (based on auto-exposure for section incubated for 3 days), and micrographs of the sections incubated for 11 and 38 days were taken at that fixed exposure. With permission from Elsevier (34).
Unlike the HPLC method, the fluorometric assay is inexpensive and relatively simple to execute. More importantly, both the excitation and emission wavelengths of six different fluorescent crosslinks including vesperlysines (A, B and C), CML, CEL, PEN and crossline (21–25) are fully or partially captured by the excitation/emission wavelength of the fluorometric assay (Fig. 3). Thus it is likely that the fluorometric assay gives a more comprehensive assessment of AGE content of bone compared to HPLC-based measurement of pentosidine alone. However, unlike the HPLC method, the fluorometric assay does not precisely identify collagen as the source of measured fluorescence and may contain contributions from non-collagenous matrix proteins and cell lysates. Given that collagen represents 90% of the organic matrix in bone and there are no reports of AGEs in the cells of bone matrix (predominantly osteocytes), this limitation is likely to be minor but requires further investigation.
Fig. 3.
Collagen-based AGE crosslinks that may contribute to the bulk fluorescence of the organic matrix that is measured using the fluorometric assay (excitation/emission: 370/440 nm). Excitation/emission wavelengths for the crosslinks shown are: pentosidine (335/385 nm), CEL/CML (340/455 nm), crossline (379/463 nm), vesperlysines A and B (366/442 nm) and vesperlysine C (345/405 nm). With permission from Elsevier (20).
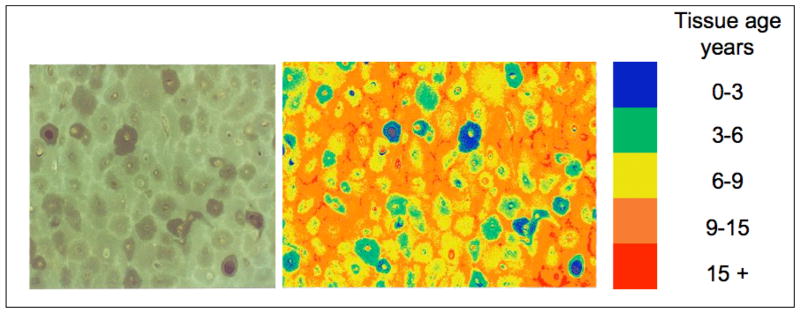
Because fluorescence imaging is commonly used for bone, images collected at wavelengths corresponding to the fluorometric assay can also be used to provide a tissue map of the relative AGE content of bone compartments. In pioneering work Gibson et al. (37) applied this technique to show that, consistent with the expected tissue age in bone, AGE content was highest in circumferential lamellar bone followed by interstitial and osteonal bone. An example of cortical bone’s AGE map created by this technique is shown in Fig. 4.
Fig. 4.
Fluorescence map of a cortical bone cross-section indicating the distribution of AGEs in bone. The micrograph on the left shows an image collected using the excitation/emission settings corresponding to the fluorometric assay (excitation/emission: 370/440 nm). The micrograph on the right shows a false color image based on the fluorescence intensity of interstitial bone in bones of different ages. Similar to the fluorometric assay (34), this technique provides the measurement of AGEs within individual microstructural components of bone in terms of fluorescence normalized to a quinine sulphate standard (37). Images and analysis provided by Gary Gibson, PhD (Henry Ford Hospital) based on Gibson et al. (37).
Bone Fracture Properties
In order to understand the relationship between AGEs and bone fracture, it is important to note a few fundamental concepts relevant to AGEs and bone biomechanics.
First, because NEG modifies only the proteins, AGEs accumulate in the organic matrix of bone. The organic matrix of bone contributes predominantly to the plastic or post-yield properties of bone. In a classic study, Burstein et al. (38) demonstrated that the removal of organic matrix did not affect the elastic properties of bone but completely eliminated the post-yield deformation – a major source of energy dissipation and fracture resistance in bone (39). Consistent with this finding, several studies using in vitro glycated bone and its matched control have demonstrated that accumulation of AGEs in bone reduces the post-yield properties of bone without altering the elastic properties (34–35;40). Thus the contributions of AGEs to bone fracture should be evaluated from post-yield properties.
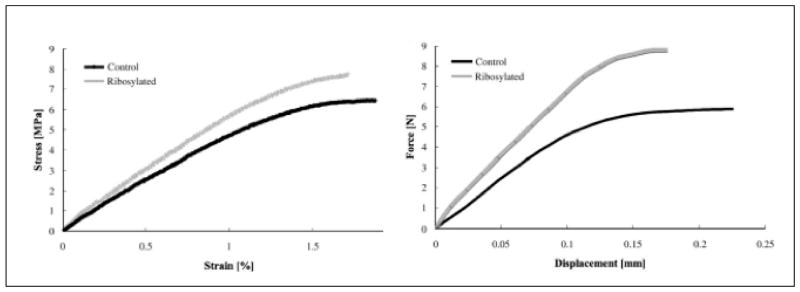
Second, there are inherent differences between the two most commonly reported bone fracture properties, strength and toughness (41). Strength (measured as maximum force normalized by bone area) represents the maximum load-carrying capacity of bone while toughness (measured as area under the load-deformation or stress-strain curve) represents a combination of the maximum load-carrying capacity as well as the maximum deformation before fracture. Because fracture in bone is strain-controlled (42) and AGEs affect the organic matrix (a known contributor to post-yield deformation), the inclusion of deformation with load-carrying capacity produces a more relevant parameter of bone fracture property. An example to support this concept is shown in Fig. 5 where the strength of in vitro glycated cancellous bone is higher than its matched control but the toughness is lower.
Fig. 5.
Representative curves of mechanical tests conducted at apparent tissue (left) and matrix (trabecular) levels of human cancellous bone. Note that the average strength of in vitro ribosylated cancellous bone is 7% higher than its matched control but the average toughness is 48% lower (35). With permission from Elsevier (35).
Third, bone fracture tests are generally reported at whole bone, apparent tissue and material (ECM) levels. Testing at these three levels provides variables that determine the propensity of bone to fracture. At the whole bone or organ level, the femur, radius or vertebra is subjected to non-cyclic loading until fracture and the resulting load and deformation values, representative of the bone’s structural and material characteristics, are used to provide an estimate of strength or toughness (43). In the context of AGEs, whole bone tests are more common for small animal studies (44) but are also reported for humans (45). At the apparent tissue level, cores of cancellous bone harvested from the vertebrae, proximal femur, and tibia are loaded to fracture and the resulting load and deformation values, representative of the core’s microarchitectural and material characteristics, are converted to stress and strain and used to provide estimates of strength and toughness. At the material or matrix level, a variety of tests are available that utilize machined specimens with or without notches. The testing of specimens without a notch relies on the presence of a random flaw in bone that grows into a fracture and the resulting load and deformation values are converted into stress and strain to provide measures of strength and toughness (46). The testing of the notched specimen relies on a machined flaw in bone, representing a microcrack or other weakness. Under loading the flaw grows into a fracture and the resulting load, crack length and displacement data are used to provide measures of bone’s resistance to initiation and propagation of fracture (39). As whole bone strength is considered to be a measure of fracture risk in bone (43), the material level tests may seem irrelevant while assessing the effects of AGEs on bone fracture. However, AGEs are material level modifications and the experience from the aircraft industry and engineering materials shows that material level changes can cause a crack to grow into a full-scale failure of the entire structure including bridges and fuselages.
Contribution of AGEs to Bone Fracture
Accumulation of AGEs in bone with aging (15;17;40) and diabetes (8;44) and the concurrent increase in bone fracture risk (47–48) have led to a number of investigations of the contributions of AGEs to bone fracture. These investigations have either tested the correlation between AGE levels and bone fracture or have used in vitro models to evaluate the causality between accumulation of AGEs and bone fracture.
Correlation approaches relate AGEs to clinically assessed bone fracture or biomechanically determined bone fracture properties. For example, pentosidine, measured from urine or serum, predicts vertebral fractures in postmenopausal women and older adults with diabetes (33;48). Furthermore, after adjustment for traditional risk factors, urinary pentosidine predicts vertebral fracture in the general population (49). However the level of correlation and its significance varies among different cohorts due to differences in dietary habits, HPLC methods and the contribution of other tissues to secreted pentosidine (32).
In contrast to clinical data, cadaveric models are equivocal on the strong correlation between increased AGEs and bone fracture. Saito et al. (50;51) have shown that, compared to age-matched controls, pentosidine levels are elevated in cortical and cancellous bone tissue excised from hip fracture patients. Viguet-Carrin et al. (45) have shown that bone’s pentosidine predicts the whole bone fracture properties of human vertebrae independently of BMD. At the apparent level, AGE content, measured by the fluorometric assay (35) and HPLC (52), predicts the post-yield fracture properties of cancellous bone obtained from the human femoral head and vertebral bodies (age range: 42 to 97 years). Similar reports are available at the matrix level where post-yield fracture properties measured from individual trabeculae (35;52) and cortical bone specimens (40;53) (age range 42 to 90 years) were predicted by AGEs.
In vitro NEG and animal models showing the accumulation of AGEs in bone have more directly implicated AGEs as one of the causes of increased bone fragility. The induction of NEG via in vitro ribosylation resulted in dose-dependent AGE accumulation in bone and caused a consequent reduction in bone’s matrix- and apparent-level fracture properties (34;35). Furthermore, AGE-induced stiffening of the organic matrix was established as one of the mechanisms of AGE-induced bone fragility (34). Similar findings were reported from animal models where the accumulation of AGEs in type 2 (adult) diabetes led to a consequent increase in bone fragility (8;44).
More importantly, some of the changes in bone matrix due to antiresorptive postmenopausal osteoporosis therapy with bisphosphonates mirror the results presented above. In particular, bisphosphonate therapy results in the accumulation of AGEs in bone in dogs (54) and postmenopausal women (55). Furthermore, accumulated AGEs due to bisphosphonate therapy correlate with reduced bone turnover rates and increased bone fragility (36;55). Because a number of bone matrix changes accompany bisphosphonate therapy (56), further work is necessary to determine the mechanism of AGE accumulation with bisphosphonate therapy and the extent to which bisphosphonate-induced increases in AGEs contribute to bone fragility.
Conclusions
Similar to tissues with slow turnover, NEG occurs in bone leading to the accumulation of AGEs. Out of several potential intermolecular AGE crosslinks only one, pentosidine, has been identified in bone. Thus current methods of estimating AGEs in bone use either an HPLC technique to measure pentosidine or a fluorometric assay to measure the normalized bulk fluorescence of the organic matrix. Studies done at whole bone, apparent tissue and matrix levels have shown a negative relationship between AGE content and bone fragility. The use of in vitro glycation models and diabetic animals has helped to establish causality between the accumulation of AGEs and the increased propensity of bone to fracture. Information obtained from cadaveric and animal models on the mechanism of AGE accumulation and its effects on bone fragility is already proving to be relevant and useful in a clinical setting. Several approaches are currently being tested to determine the efficacy of AGEs, measured from serum or urine, as valid markers of bone fracture risk. Accumulation of AGEs and their correlation with increased bone fragility has also been shown to occur during bisphosphonate therapy for postmenopausal osteoporosis.
Acknowledgments
NIH grant AG20618.
Footnotes
Conflict of Interest: None reported.
Peer Review: This article has been peer-reviewed.
References
- 1.Monnier VM, Sell DR, Abdul-Karim FW, Emancipator SN. Collagen browing and cross-linking are increased in chronic experimental hyperglycemia. Relevance to diabetes and aging. Diabetes. 1988 Jul;37(7):867–72. doi: 10.2337/diab.37.7.867. [DOI] [PubMed] [Google Scholar]
- 2.Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thrope SR, Baynes JW, Bayliss MT, Bijlsma JW, Lafeber FP, Tekoppele JM. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000 Sep 1;350(Pt 2):381–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experimental measurements and theoretical predictions. J Biomech. 2006;39(6):1021–9. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Hunter SA, Noyes FR, Haridas B, Levy MS, Butler DL. Meniscal material properties are minimally affected by matrix stabilization using glutaraldehyde and glycation with ribose. J Orthop Res. 2005 May;23(3):555–61. doi: 10.1016/j.orthres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Bank RA, Bayliss MT, Lafeber FP, Maroudas A, Tekoppele JM. Aging and zonal variation in post-translational modification of collagen in normal human articular cartilage. The age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998 Feb 15;330(Pt 1):345–51. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996 Mar;11(3):337–49. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 7.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance. Bone. 1998 Mar;22(3):181–7. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 8.Tomasek JJ, Meyers SW, Basinger JB, Green DT, Shew RL. Diabetic and age-related enhancement of collagen-linked fluorescence in cortical bones of rats. Life Sci. 1994;55(11):855–61. doi: 10.1016/0024-3205(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 9.Ural A, Vashishth D. Interactions between microstructural and geometrical adaptation in human cortical bone. J Orthop Res. 2006 Jul;24(7):1489–98. doi: 10.1002/jor.20159. [DOI] [PubMed] [Google Scholar]
- 10.Chavassieux P, Meunier PJ. Histomorphometric approach of bone loss in men. Calcif Tissue Int. 2001 Oct;69(4):209–13. doi: 10.1007/s00223-001-1047-5. [DOI] [PubMed] [Google Scholar]
- 11.Akkus O, Polyakova-Akkus A, Adar F, Schaffler MB. Aging of microstructural compartments in human compact bone. J Bone Miner Res. 2003 Jun;18(6):1012–9. doi: 10.1359/jbmr.2003.18.6.1012. [DOI] [PubMed] [Google Scholar]
- 12.Valcourt U, Merle B, Gineyts E, Viguet-Carrin S, Delmas PD, Garnero P. Non-enzymatic glycation of bone collagen modifies osteoclastic activity and differentiation. J Biol Chem. 2007 Feb 23;282(8):5691–703. doi: 10.1074/jbc.M610536200. [DOI] [PubMed] [Google Scholar]
- 13.Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci. 2008 Apr;1126:166–72. doi: 10.1196/annals.1433.044. [DOI] [PubMed] [Google Scholar]
- 14.Gundberg CM, Anderson M, Dickson I, Gallop PM. “Glycated” osteocalcin in human and bovine bone. The effect of age. J Biol Chem. 1986 Nov 5;261(31):14557–61. [PubMed] [Google Scholar]
- 15.Takahashi M, Hoshino H, Kushida K, Inoue T. Direct measurement of crosslinks, pyridinoline, deoxypyridinoline, and pentosidine, in the hydrolysate of tissues using high-performance liquid chromatography. Anal Biochem. 1995 Dec 10;232(2):158–62. doi: 10.1006/abio.1995.0002. [DOI] [PubMed] [Google Scholar]
- 16.Saito M, Marumo K, Fujii K, Ishioka N. Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal Biochem. 1997 Nov 1;253(1):26–32. doi: 10.1006/abio.1997.2350. [DOI] [PubMed] [Google Scholar]
- 17.Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro F, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005 Jun;1043:710–7. doi: 10.1196/annals.1333.082. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JB, Kindt JH, Drake B, Hansma HG, Morse DE, Hansma PK. Bone indentation recovery time correlates with bond reforming time. Nature. 2001 Dec 13;414(6865):773–6. doi: 10.1038/414773a. [DOI] [PubMed] [Google Scholar]
- 19.Robins SP, Bailey AJ. Age-related changes in collagen: the identification of reducible lysine-carbohydrate condensation products. Biochem Biophys Res Commun. 1972 Jul 11;48(1):76–84. doi: 10.1016/0006-291x(72)90346-4. [DOI] [PubMed] [Google Scholar]
- 20.Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998 Dec 1;106(1–2):1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 21.Sell DR, Monnier VM. Isolation, purification and partial characterization of novel fluorophores from aging human insoluble collagen-rich tissue. Connect Tissue Res. 1989;19(1):77–92. doi: 10.3109/03008208909016816. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Nakazawa Y, Ienaga K. Acid-stable fluorescent advanced glycation end products: vesperlysines A, B, and C are formed as crosslinked products in the Maillard reaction between lysine or proteins with glucose. Biochem Biophys Res Commun. 1997 Mar 6;232(1):227–30. doi: 10.1006/bbrc.1997.6262. [DOI] [PubMed] [Google Scholar]
- 23.Nagaraj RH, Shipanova IN, Faust FM. Protein cross-linking by the Maillard reaction. Isolation, characterization, and in vivo detection of a lysine-lysine cross-link derived from methylglyoxal. J Biol Chem. 1996 Aug 9;271(32):19338–45. doi: 10.1074/jbc.271.32.19338. [DOI] [PubMed] [Google Scholar]
- 24.Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995 Mar 21;34(11):3702–9. doi: 10.1021/bi00011a027. [DOI] [PubMed] [Google Scholar]
- 25.Obayashi H, Nakano K, Shigeta H, Yamaguchi M, Yoshimori K, Fukui M, Fujii M, Kitagawa Y, Nakamura N, Nakamura K, Nakazawa Y, Ienaga K, Ohta M, Nishimura M, Fukui I, Kondo M. Formation of crossline as a fluorescent advanced glycation end product in vitro and in vivo. Biochem Biophys Res Commun. 1996 Sep 4;226(1):37–41. doi: 10.1006/bbrc.1996.1308. [DOI] [PubMed] [Google Scholar]
- 26.Nagai R, Araki T, Hayashi CM, Hayase F, Horiuchi S. Identification of N epsilon-(carboxyethyl)lysine, one of the methylglyoxal-derived AGE structures, in glucose-modified protein: mechanism for protein modification by reactive aldehydes. J Chromatogr B Analyt Technol Biomed Life Sci. 2003 May 5;788(1):75–84. doi: 10.1016/s1570-0232(02)01019-x. [DOI] [PubMed] [Google Scholar]
- 27.Bailey AJ, Sims TJ, Avery NC, Halligan EP. Non-enzymic glycation of fibrous collagen: reaction products of glucose and ribose. Biochem J. 1995 Jan 15;305(Pt 2):385–90. doi: 10.1042/bj3050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991 Jun 25;266(18):11654–60. [PubMed] [Google Scholar]
- 29.Monnier VM, Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–3. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- 30.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):583–7. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viguet-Carrin S, Gineyts E, Bertholon C, Delmas PD. Simple and sensitive method for quantification of fluorescent enzymatic mature and senescent crosslinks of collagen in bone hydrolysate using single-column high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2009 Jan 1;877(1–2):1–7. doi: 10.1016/j.jchromb.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Gineyts E, Munoz F, Bertholon C, Sornay-Rendu E, Chapurlat R. Urinary levels of pentosidine and the risk of fracture in postmenopausal women: the OFELY study. Osteoporos Int. 2009 May 7; doi: 10.1007/s00198-009-0939-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008 Mar;93(3):1013–9. doi: 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- 34.Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenyzmatic glycation on biomechanical properties of cortical bone. Bone. 2001 Feb;28(2):195–201. doi: 10.1016/s8756-3282(00)00434-8. [DOI] [PubMed] [Google Scholar]
- 35.Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007 Apr;40(4):1144–51. doi: 10.1016/j.bone.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009 Jun;20(6):887–94. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibson G, Glotkowski M, Fyhrie D, Schaffler M, Les C, Tashman S. Fluorescence provides a measure of local tissue age and remodeling history in human compact bone. Trans Orthop Res Soc. 2000;25:691. [Google Scholar]
- 38.Burstein AH, Zika JM, Heiple KG, Klein L. Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg Am. 1975 Oct;57(7):956–61. [PubMed] [Google Scholar]
- 39.Vashishth D. Rising crack-growth-resistance behavior in cortical bone: Implications for toughness measurements. J Biomech. 2004 Jun;37(6):943–6. doi: 10.1016/j.jbiomech.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Shen X, Li X, Agrawal CM. Age-related changes in the collagen network and toughness of bone. Bone. 2002 Jul;31(1):1–7. doi: 10.1016/s8756-3282(01)00697-4. [DOI] [PubMed] [Google Scholar]
- 41.Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):509–18. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 42.Hazenberg JG, Taylor D, Lee TC. Dynamic short crack growth in cortical bone. Technol Health Care. 2006;14(4–5):393–402. [PubMed] [Google Scholar]
- 43.Keaveny TM, Bouxsein ML. Theoretical implications of the biomechanical fracture threshold. J Bone Miner Res. 2008 Oct;23(10):1541–7. doi: 10.1359/JBMR.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006 Oct;17(10):1514–23. doi: 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- 45.Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006 Nov;39(5):1073–9. doi: 10.1016/j.bone.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 46.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993 Jul-Aug;14(4):595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 47.Hui SL, Slemenda CW, Johnston CC., Jr Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988 Jun;81(6):1804–9. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC for the Health, Aging, and Body Composition study. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009 Apr 21; doi: 10.1210/jc.2008-2498. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab. 2008;26(1):93–100. doi: 10.1007/s00774-007-0784-6. [DOI] [PubMed] [Google Scholar]
- 50.Saito M, Fujii K, Marumo K. Degree of mineralization-related collagen crosslinking in the femoral neck cancellous bone in cases of hip fracture and controls. Calcif Tissue Int. 2006 Sep;79(3):160–8. doi: 10.1007/s00223-006-0035-1. [DOI] [PubMed] [Google Scholar]
- 51.Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17(7):986–95. doi: 10.1007/s00198-006-0087-0. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005 Dec;37(6):825–32. doi: 10.1016/j.bone.2005.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res. 2007 May;25(5):646–55. doi: 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008 Mar;19(3):329–37. doi: 10.1007/s00198-007-0533-7. [DOI] [PubMed] [Google Scholar]
- 55.Vashishth D, Bertholon C, Gineyts E, Chavassieux P, Boivin G, Delmas PD. Increased non-enzymatic glycation of cancellous bone due to decrease in remodeling during alendronate therapy of osteoporotic women. J Bone Miner Res. 2008 Sep;23(Suppl 1):S22. [Google Scholar]
- 56.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006 Oct;39(4):872–9. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]