Abstract
Glucocorticoids have gone unchallenged as an essential component of primary therapy for acute graft-versus-host disease (aGVHD) after allogeneic hematopoietic cell transplantation (HCT) despite limited complete response rates and adverse effects from this therapy. The role for alternate immunosuppressive agents as primary aGHVD treatment remains unexamined. In a series of 10 patients at high risk for corticosteroid toxicity or leukemia relapse who developed biopsy-proven grade II–III aGVHD after hematopoietic cell transplantation, we report that primary therapy with sirolimus resulted in durable complete remission of aGVHD in 5 (50%) without requirement for glucocorticoids. Mild chronic GVHD (cGVHD) developed in 4 (40%). Projected overall survival (OS) at 18 months is 79% (95% confidence interval [CI]: 38.1%–94.3%), and projected relapse-free survival (RFS) at 15 months is 70% (95% CI: 32.9%–89.2%). Sirolimus was well tolerated with mild and reversible thrombotic microangiopathy occurring in 2 patients. This experience provides preliminary evidence for the efficacy of sirolimus as a sole primary therapy in the treatment of aGVHD.
Keywords: Sirolimus, Acute graft-versus-host disease, Glucocorticoids
INTRODUCTION
Acute graft-versus-host disease (aGVHD) is an important complication of allogeneic hematopoietic cell transplantation (HCT). The historic first-line therapy for aGVHD has included 1–2 mg/kg of prednisone or equivalent dose of other glucocorticoids. Unfortunately, the complete response (CR) rate to this therapy is only 30% to 40% in several published series, with non-responders going on to additional immunosuppressive therapies for steroid-refractory disease [1–8]. Although previous attempts at combination therapy with additional agents added to glucocorticoids have produced mixed results [9,10], early reports from a CTN trial have suggested improved aGHVD response rates to a regimen of glucocorticoids and mycophenolate mofetil (MMF) [11]. In published series, overall survival (OS) for those with steroid responsive disease approaches 50% to 60%, with nonresponders realizing much worse outcomes because of competing threats from refractory aGVHD, toxicity, cumulative immunosuppression from additional therapies, primary disease relapse, and infectious complications. Additionally, up to 70% of patients will develop chronic GVHD (cGVHD). In the treatment of aGVHD and cGVHD, patients suffer numerous complications from glucocorticoids.
The primacy of glucocorticoids in the management of aGVHD has gone unchallenged. Conversely, alternative therapies that avoid complications of steroid exposure may offer promise for improved outcomes. Sirolimus exerts its immunosuppressive effect through inhibition of mTOR, or mammalian target of rapamycin, and by downstream effects that include inhibition of transcription and decreased kinase activity of cyclin enzymes involved in cell cycle progression; other postulated effects include inhibition of dendritic cell development and function, blockade of CD28-mediated costimulatory signaling on effector T cells, and a permissive effect on regulatory T cell expansion, proliferation, and survival [12–15]. Sirolimus has shown efficacy in the prevention [16,17] and treatment [18] of aGVHD, but some physicians find intolerable the risks of thrombotic microangiopathy (TMA) and hepatic veno-occlusive disease (VOD) in primary transplants. Sirolimus has not been examined as a sole primary therapy for aGVHD.
METHODS
A series of 10 recipients of HCT who developed aGVHD were treated with sirolimus as primary therapy; all cases of GVHD were biopsy confirmed. Primary treatment with glucocorticoids was avoided for the intolerance in older patients, and sirolimus was selected for its dual activity as immunosuppressant and anticancer drug for mitigating the exceedingly high risk for leukemia relapse in patients with active disease at the time of transplant. In all patients, tacrolimus target serum levels was decreased to 3–7 ng/mL while receiving concomitant sirolimus as an attempt to prevent TMA. Sirolimus was administered with a target serum level of 4–12 ng/mL. In the absence of ongoing aGVHD, tacrolimus was tapered with empiric dose reductions.
aGVHD was scored weekly per established consensus criteria [19]. CR was defined as sustained complete resolution of aGVHD without recurrence until death or last follow-up. Partial response was defined as an overall grade improvement of ≥1. cGVHD was scored according to the NIH consensus scoring criteria [20]. Indication, initial dose, and duration of any glucocorticoid therapy were recorded. Cumulative incidence of disease relapse, cGVHD, cytomegaolvirus (CMV) reactivation, and TMA are reported. OS, relapse-free survival (RFS), and failure-free survival were calculated by the Kaplan-Meier method, with failure defined as nonrelapse mortality (NRM) or requirement of glucocorticoid therapy after initial therapy with sirolimus. This study was approved as a retrospective review of a nonconsecutive patient series by the University of South Florida institutional review board.
RESULTS
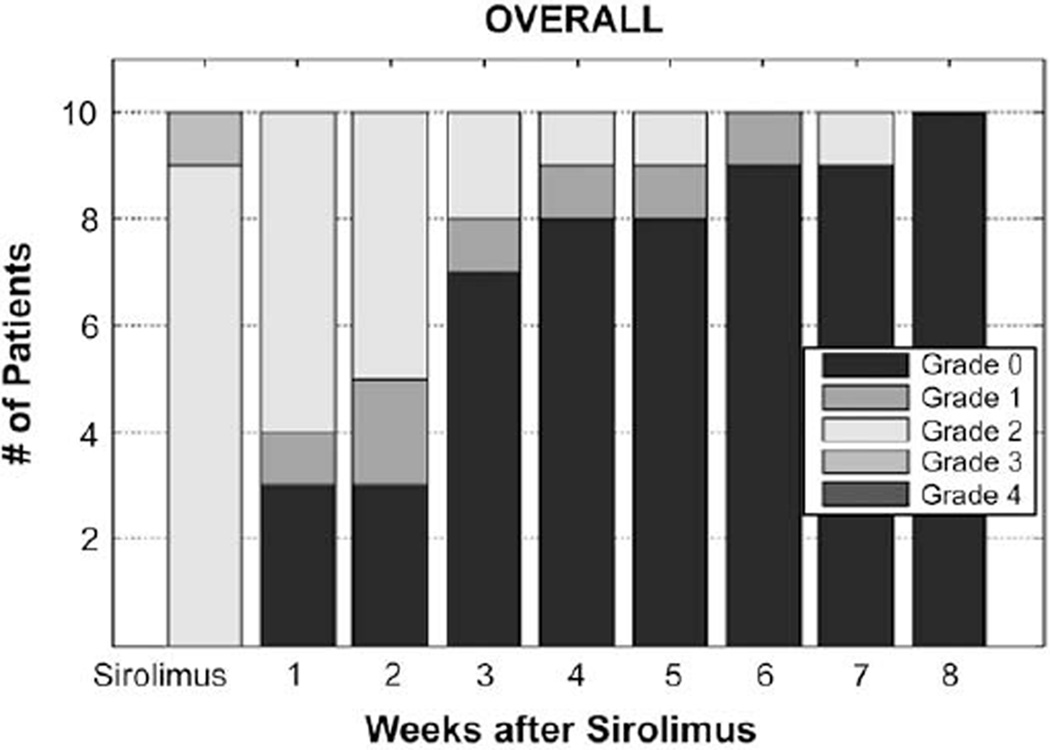
Ten patients were treated with sirolimus as the primary therapy for biopsy-proven aGVHD at a median of 27 days after HCT (range: 15–103 days) with primary aGHVD prophylaxis consisting of either tacrolimus plus methotrexate (MTX; n = 7) or MMF (n = 3). Medications used in prophylaxis of aGVHD, namely, tacrolimus and MMF, were continued with sirolimus. Sirolimus was administered orally, and therapeutic levels were achieved in all cases, including those with gastrointestinal (GI) involvement. aGVHD was treated at the earliest possible time of onset, and therefore was grade II overall in 9/10 patients. Baseline characteristics of this series are summarized in Table 1. These patients were at high risk for primary disease relapse at time of transplant, with only 2/10 in complete remission at the time of transplant. Those not in remission included secondary acute myelogenous leukemia (AML) responsive to hypomethylating agents but with persistent blasts (range: 8%–15%) and cytogenetic abnormalities (n = 3), persistent idiopathic myelofibrosis (n = 2), multiple myeloma (MM) in very good partial remission (VGPR) with persistent low-level serum monoclonal protein (n = 1), follicular cell lymphoma in partial remission after salvage therapy with persistent hypermetabolic adenopathy on computed tomography/positron emission tomography (CT/PET) and no morphologic or molecular bone marrow involvement (n = 1), and acute lymphobalstic leukemia (ALL) s/p induction therapy with morphologic remission, but persistent immunoglobulin gene rearrangement (n = 1). At a median follow-up of 6.5 months (range: 2.4–18.3 months), 5 (50%) patients achieved sustained CR of aGVHD with sirolimus without requiring any second-line therapy with glucocorticoids or other salvage therapy (Table 2). In 1 patient, a complete remission of aGVHD was attained with sirolimus, but upon a flare of aGVHD 51 days after initial remission, a second remission was achieved with the addition of 0.5 mg/kg of prednisone. In 1 patient, 1mg/kg of prednisone was initially utilized at the onset of aGVHD, but was rapidly tapered completely off given concern for toxicity for a total duration of corticosteroid therapy of 9 days with the addition of sirolimus; after complete remission was reached, no further glucocorticoids were needed. In 3 patients (30%), glucocorticoids were used as salvage therapy after sirolimus for persistent aGVHD. Two required 0.5 mg/kg of prednisone for persistent upper (n = 1) or lower (n = 1) symptoms (aGVHD: GI grade 1, overall grade II); these were similar in baseline characteristics with those who achieved complete resolution of aGVHD with sirolimus alone. The other required 1 mg/kg of prednisone for persistent aGVHD (skin grade 3, overall grade II); this case differed in having a DRB1-incompatible, 9/10 matched unrelated donor. With persistent aGVHD after 1 mg/kg of glucocorticoids, MMF was successful in inducing durable complete remission of aGVHD. Allowing for primary therapy with sirolimus and salvage steroids, all had reached CR of aGVHD by 8 weeks after initiation of sirolimus (Figure 1).
Table 1 .
Baseline Characteristics
| Frequency | |
|---|---|
| Median age | 57.5 (range: 28–68) |
| Condition | |
| ALL | 1 |
| AML | 4 |
| MDS | 1 |
| MM | 1 |
| MPD | 2 |
| NHL | 1 |
| Remission status | |
| Complete remission (CR) | 2 |
| Not in CR | 8 |
| Cell source | |
| PBSCT | 10 |
| BMT | 0 |
| Donor relation | |
| Related donor | 4 |
| Unrelated donor | 6 |
| HLA matching | |
| 10/10 | 8 |
| 9/10 | 2 |
| Recipient/donor sex | |
| Female/female | 3 |
| Female/male | 1 |
| Male/female | 1 |
| Male/male | 5 |
| Conditioning regimen | |
| Flu/Bu | 5 |
| Flu/Bu/ATG | 2 |
| Flu/Bu/Rituxan | 1 |
| Flu/Mel | 1 |
| Pento/BU/Rituxan | 1 |
| aGVHD prophylaxis | |
| TAC/MTX | 7 |
| TAC/MMF | 3 |
| Donor/recipient CMV | |
| Neg/neg | 4 |
| Neg/pos | 3 |
| Pos/pos | 3 |
| aGVHD onset date (median) | 3.86 weeks (range: 2.14–14.71) |
| Overall aGVHD onset grade* | |
| I | 0 |
| II | 9 |
| III | 1 |
| IV | 0 |
| aGVHD onset organ stage | |
| Skin | |
| 1 | 2 |
| 2 | 1 |
| 3 | 2 |
| 4 | 0 |
| GI | |
| 1 | 7 |
| 2 | 1 |
| 3 | 0 |
| 4 | 0 |
| Liver | |
| 1 | 1 |
| 2 | 0 |
| 3 | 0 |
| 4 | 0 |
ALL indicates acute lymphoblastic leikemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndromes; MM, multiple myeloma; MPD, myeloproliferative disorder; NHL, non-Hodgkin’s lymphoma; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; GI, gastrointestinal; CMV, cytomegalovirus; PBSCT, peripheral blood stem cell transplantation; BMT, bone marrow transplantation; Tac/MMF, tacrolimus/mycophenolate mofetil; Tac/MTX, tacrolimus/methotrexate; Flu/Mel, fludarabine and melphalan; Flu/Bu, fludarabine and busulfan; Flu/Bu/ATG, fludarabine, busulfan, and antithymocyte globulin.
All cases of aGVHD were biopsy confirmed.
Table 2.
Summary of Individual Patient Outcomes
| Age | Disease | Disease remission at HCT |
aGVHD prophylaxis |
Overall onset grade |
Skin/GI/Liver stage |
CR with Sirolimus |
Required steroids |
Malignancy Relapse |
Death |
|---|---|---|---|---|---|---|---|---|---|
| 28 | ALL | No | Tac/MTX | II | 2/0/1 | Yes | No | No | No |
| 56 | MM | No | Tac/MTX | II | 0/1/0 | Yes | No | No | No |
| 68 | AML | No | Tac/MTX | II | 0/1/0 | Yes | No | Yes | Yes |
| 52 | MPD | No | Tac/MTX | II | 3/1/0 | Yes | Yes 0.5 mg/kg (recurrent) | No | No |
| 58 | MPD | No | Tac/MTX | II | 1/1/0 | No | Yes 0.5 mg/kg (persistent) | No | No |
| 34 | NHL | No | Tac/MTX | II | 0/1/0 | No | Yes 0.5 mg/kg (persistent) | No | No |
| 57 | AML | Yes | Tac/MTX | II | 3/0/0 | No | Yes 1 mg/kg and MMF (persistent) | No | No |
| 66 | MDS | Yes | Tac/MMF | II | 0/1/0 | * | No | Yes | Yes |
| 63 | AML | No | Tac/MMF | II | 0/1/0 | Yes | No | Yes | No |
| 67 | AML | No | Tac/MMF | III | 1/2/0 | Yes | No | Yes | No |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; MDS, myelodysplastic syndromes; MM, multiple myeloma; MPD, myeloproliferative disorder; NHL, non-Hodgkin lymphoma; aGVHD, acute graft-versus-host disease; cGVHD, chronic graft-versus-host disease; GI, gastrointestinal; Tac/MMF, tacrolimus/mycophenolate mofetil; Tac/MTX, tacrolimus/methotrxate; HCT, hematopoietic cell transplant.
1 mg/kg of prednisone utilized at the onset of aGVHD, but then rapidly tapered off after addition of sirolimus with total duration steroid treatment of 9 days; maintained CR of aGVHD with no further steroids.
Figure 1.
Number of patients with weekly overall aGVHD scores after initiation of sirolimus.
The cumulative incidence of cGVHD in this group was 40% with maximal cGVHD grade of mild, which did not require escalation of immunosuppressive therapy. The primary malignancy relapsed in 4 patients. Two died of recurrent AML, the other 2 patients are alive after relapse, 1 undergoing therapy and the other with sustained remission. With a median follow up of 6.5 months (range: 2.4–18.3 months) after transplant, projected OS at 18 months is 79% (95% CI: 38.1%–94.3%), and projected RFS at 15 months is 70% (95% CI: 32.9%–89.2%). Projected failure-free survival, as defined above, is 51% (95% CI: 16%–78%) at 18 months after transplant.
Sirolimus was overall well tolerated, with 2 cases of mild TMA that resolved without end-organ compromise after dose reduction (1) or discontinuation (1) of tacrolimus. By time of death or last follow up, 2 had immunosuppression tapered (n = 1) or entirely withdrawn (n = 1) for primary disease relapse. None otherwise had liberated from immunosuppression by median follow-up of 6.5 months (range: 2.4–18.3 months).
DISCUSSION
We report here a series of patients who were treated with sirolimus as a first-line therapy of biopsy proven aGVHD. In this group with primarily overall grade II aGVHD and skin or gut involvement, a CR rate of 50% was reached with sirolimus alone, which is comparable to that seen with glucocorticoids. Additionally, those who required glucocorticoids after primary treatment with sirolimus achieved CR with only 0.5–1 mg/kg of glucocorticoids, suggesting a potential steroid-sparing effect. Only 1 patient had aGVHD refractory to glucocorticoids, which was salvaged with MMF.
The use of sirolimus as a steroid-free primary therapy for aGVHD in these patients was driven by both concern for intolerance of steroid adverse effects, but also that of primary disease relapse after transplantation. The potential antimalignancy effect of sirolimus motivated this approach [21–24]. The subjects represented here largely had high-disease risk, as evidenced by only 2 of 10 being in complete remission at the time of transplant. In this setting, the projected 15-month RFS of 70% compares favorably with what would otherwise be expected, given the high risk nature of these patients.
Although this limited series provides early evidence to support a clinical trial of this novel approach, several questions remain. First, the effectiveness of this approach needs validation in a larger series; selection bias poses a potential threat to the internal validity of this retrospective review. Second, sirolimus was successful in inducing complete remission of aGVHD in this series largely comprised of overall grade II disease, but further work remains to be done to evaluate the effectiveness of this therapy in more advanced grade disease. Additionally, although therapeutic levels of sirolimus were reached in cases with GI involvement, more advanced vomiting and/or large volume diarrhea could preclude achieving consistent therapeutic levels, as sirolimus is only available as an oral formulation. Next, although there is a theoretic rationale that sirolimus may decrease the risk of disease relapse, this needs to be further examined in sufficiently large series. Further work also needs to be done to evaluate this approach in standard risk patients. Finally, although 50% achieved complete remission of aGVHD without glucocorticoids here, the magnitude of this steroid sparing effect would be better borne out with examination of cumulative burden of steroid exposure in larger series.
Footnotes
Financial disclosure: The authors have nothing to disclose.
REFERENCES
- 1.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 2.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 3.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 4.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk factors and outcome. Blood. 1990;75:1024. [PubMed] [Google Scholar]
- 6.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 7.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(15):2062–2068. [PubMed] [Google Scholar]
- 8.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease:Initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 9.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 10.Levine JE, Paczesny S, Mineishi S, et al. Etanercept plus methylprednisolone as initial therapy for acute graft-versus-host disease. Blood. 2008;111:2470–2475. doi: 10.1182/blood-2007-09-112987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alousi A, Weisdorf DJ, Logan BR, et al. BMT CTN 0302: A phase II randomized trial evaluating etanercept, mycophenolate mofetil (MMF), denileukin diftitox, and pentostatin in combination with corticosteroids in 180 patients (pts) with newly diagnosed acute graft vs. host disease (aGVHD) Presented at American Society of Hematology meeting. 2008 [Google Scholar]
- 12.Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- 13.Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB, Joosten I. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood. 2006;107:1018–1023. doi: 10.1182/blood-2005-07-3032. [DOI] [PubMed] [Google Scholar]
- 15.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 16.Cutler C, Li S, Ho VT, et al. Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood. 2007;109:3108–3114. doi: 10.1182/blood-2006-09-046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alyea EP, Li S, Kim HT, et al. Sirolimus, tacrolimus, and low-dose methotrexate as graft-versus-host disease prophylaxis in related and unrelated donor reduced-intensity conditioning allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:920–926. doi: 10.1016/j.bbmt.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benito AI, Furlong T, Martin PJ, et al. Sirolimus (rapamycin) for the treatment of steroid-refractory acute graft-versus-host disease. Transplantation. 2001;72:1924–1929. doi: 10.1097/00007890-200112270-00010. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825. [PubMed] [Google Scholar]
- 20.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Armand P, Gannamaneni S, Kim HT, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26:5767–5774. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armand P, Kim HT, Cutler CS, et al. A prognostic score for patients with acute leukemia or myelodysplastic syndromes undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:28–35. doi: 10.1016/j.bbmt.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol. 2004;22:4217–4226. doi: 10.1200/JCO.2004.01.103. [DOI] [PubMed] [Google Scholar]
- 24.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]