Abstract
Urologic tissue engineering efforts have been largely focused on bladder and urethral defect repair. The current surgical gold standard for treatment of poorly compliant pathological bladders and severe urethral stricture disease is enterocystoplasty and onlay urethroplasty with autologous tissue, respectively. The complications associated with autologous tissue use and harvesting have led to efforts to develop tissue-engineered alternatives. Natural and synthetic materials have been used with varying degrees of success, but none has proved consistently reliable for urologic tissue defect repair in humans. Silk fibroin (SF) scaffolds have been tested in bladder and urethral repair because of their favorable biomechanical properties including structural strength, elasticity, biodegradability and biocompatibility. SF scaffolds have been used in multiple animal models, and have demonstrated robust regeneration of smooth muscle and urothelium. The pre-clinical data involving SF scaffolds in urologic defect repair are encouraging and suggest that they hold potential for future clinical use.
MeSH Keywords: Bladder, urethra, urinary tract, tissue engineering, silk, animal models
Introduction
Bladder and urethral defects secondary to congenital, malignant, traumatic, and infectious etiologies often necessitate surgical reconstruction with or without autologous tissue harvesting. The effective, yet imperfect functional outcomes and co-morbidities from autologous tissue use and harvest has led to significant research into the use of natural and synthetic biomaterials. This review will highlight the indications and current approaches to bladder and urethral reconstruction with an emphasis on the use of silk fibroin (SF) scaffolds as functional tissue engineered constructs.
Clinical Indications for Bladder or Urethral Defect Repair
The urinary bladder functions to store urine at low pressures and expel urine in a state of optimal compliance and contractility, thereby preserving renal function. In many conditions, the urinary tract is anatomically or functionally obstructed, potentially leading to renal dysfunction secondary to elevated urinary storage pressures. These conditions include posterior urethral valves, neurogenic bladder secondary to spina bifida or spinal cord injury, benign prostatic hyperplasia, bladder and cloacal exstrophy, severe voiding dysfunction, and malignancy. While these vary widely physiologically, they often require major surgical intervention with bladder augmentation or complete substitution with autologous tissue. The urethra is a urine and seminal fluid conduit that is compromised in a variety of congenital and acquired diseases. These include hypospadias, epispadias, strictures, fistulas, trauma, and malignancy. Any defect in the urethra’s mucosal barrier results in urinary extravasation, which can lead to spongiofibrosis and stricture disease. Urethral stricture disease often requires urethroplasty with or without autologous tissue augmentation.
Current Approaches for Bladder or Urethral Defect Repair
Patients with congenital bladder dysfunction frequently present early in the disease process, allowing clinicians to intervene and potentially prevent irreversible bladder decompensation. Management may include anticholinergic medications and/or intradetrusor botulinum toxin injections to abolish involuntary contractions, increase bladder volume, and reduce urine storage pressure. Anticholinergic drugs are associated with undesirable systemic adverse effects and their long-term efficacy is unpredictable and variable [1]. When medical management fails, patients may have progression of pathological bladder remodeling, which can result in urinary incontinence and renal damage. Bladder augmentation, commonly with small intestine, then becomes necessary to increase bladder capacity and decrease the high intravesical pressures. The use of pharmacological agents and clear intermittent catheterization has decreased the rate of augmentation cystoplasty in the myelomeningocele population from approximately 30 to 15% [2,3]. However, the long-term efficacy of this approach is hampered by frequent and severe complications associated with the use of gastrointestinal segments including chronic urinary tract infection, urinary calculi, bowel dysfunction, electrolyte abnormalities, bladder perforation, and neoplasms [4,5]. Urothelial carcinoma of the bladder necessitating cystectomy with bladder replacement has similar complications related to the use of a gastrointestinal segment [6,7].
Regarding urethral stricture disease, short, non-complex defects can be repaired with an end-to-end anastomosis. However, for long complex defects, urethroplasty is often performed in which a de novo urinary conduit is fashioned from a tissue graft and surgically integrated into the surrounding host tissue. Several types of autologous tissues have been explored including genital and extragenital skin flaps [8–10], buccal mucosa [9,11,12], bladder mucosa [13], and tunica vaginalis [14]. However, complications such as urethrocutaneous fistula, implant contracture, recurrent stricture, hair growth, stone formation, and diverticula are observed with these grafts [8–10,14]. Moreover, the harvesting of autologous tissues requires secondary surgical procedures, is routinely associated with morbidity at the donor site, and donor tissue volume is limited for reconstructive procedures [15,16]. Bladder and urethral substitution with autologous tissue often provides reliable function with minimal overall morbidity, but the complications associated with the use of these tissues can be catastrophic, which has led to investigatory efforts in urologic tissue engineering.
Conventional Biomaterials for Bladder and Urethral Defect Reconstruction
Both natural and synthetic biomaterials have been developed and investigated for their utility in bladder and urethral reconstruction. Natural matrices derived from small intestine submucosa (SIS) [17], decellularized bladder matrix (BAM) [18,19], omentum [20–22], pericardium [23,24], peritoneum [25], lyophilized dura [26], and amniotic membranes [27,28] have all been previously reported. In particular, decellularized collagen-based scaffolds including SIS and BAM have demonstrated marginal success in terms of restoring bladder functionality and capacity [17,29–31]. However, collagen matrices derived from native tissues require chemical processing for decellularization resulting in alterations in matrix biocompatibility and structural strength. These biomaterials have also been associated with in vivo fibrosis and contracture [32]. Synthetic biomaterials in the form of 3D porous foams, meshes, and hydrogels derived from poly-glycolic acid (PGA), poly-L-lactic acid (PLA), copolymers of poly-lactic and -glycolic acid (PLGA) [33–37]; PTFE Teflon [38]; polyether urethane [39]; silicone rubber [40]; and poly(ethylene) glycol (PEG) [41] have also been utilized as platforms for bladder reconstructive strategies. However, permanent synthetic biomaterials routinely demonstrate in vivo mechanical failure and urinary stone formation and thus are limited in clinical applications [42]. In addition, most synthetic polymers induce inflammation responses in vivo [43] and lack the structural integrity necessary to support long-term tissue replacement [44].
Pioneers in the field have provided the fundamental methodology and proof of the translational potential of bladder tissue engineering in humans [37,45–47]. Composite PGA and collagen based scaffolds seeded with autologous urothelial and smooth muscle cells were able to demonstrate histologically organized smooth muscle and urothelial compartments, but without an increase in bladder capacity or compliance [37]. Additionally, serious complications of bowel obstruction and/or bladder rupture were also seen using a similar scaffold in a phase II prospective study [45]. SIS use in humans has demonstrated smooth muscle and urothelium generation, but without improvement in compliance [46]. A recent study demonstrated that SIS use in neurogenic bladder patients had increases in compliance and capacity [47]. Although this report is very encouraging, long-term improvements in urodynamic function is warranted before wide scale translation of this technology can be considered.
Short-term clinical studies of urethral tissue engineering have investigated the utility of a number of biodegradable, natural and synthetic polymers either alone or seeded with autologous primary cells. Acellular collagen-derived scaffolds including SIS and BAM have been explored in onlay urethroplasty procedures for the repair of defects associated with hypospadias and urethral strictures [48–50]. These matrices have been shown to encourage urothelial regeneration and initial defect consolidation. However, complications including recurrent strictures, fistula formation, fibrosis, and graft contracture have limited their ability to promote restoration of normal urethral function in a significant cohort of patients [48–50].
Complications associated with tissue engineering strategies deploying decellularized collagen grafts and implants composed of synthetic polymers may be due in part to suboptimal structural properties of the scaffold material, which may not provide the appropriate elasticity, porosity, tensile strength, and degradation characteristics necessary to support long-term bladder or urethral function. These deficiencies therefore necessitate the evaluation of other novel biomaterials for urologic tissue engineering.
Novel Properties of Silk-based Biomaterials
Biomaterials must provide a defined microenvironment that promotes functional tissue regeneration. Ideally, scaffolds should: 1) support cell attachment, migration, cell-cell interactions, cell proliferation, and differentiation; 2) be compatible with the host immune system; 3) degrade at a controlled rate to match the rate of new tissue growth; 4) provide structural support for cells and new tissue formed in the scaffold during the initial stages of post-implantation; 5) have versatile processing options to alter structure and morphology related to tissue-specific needs.
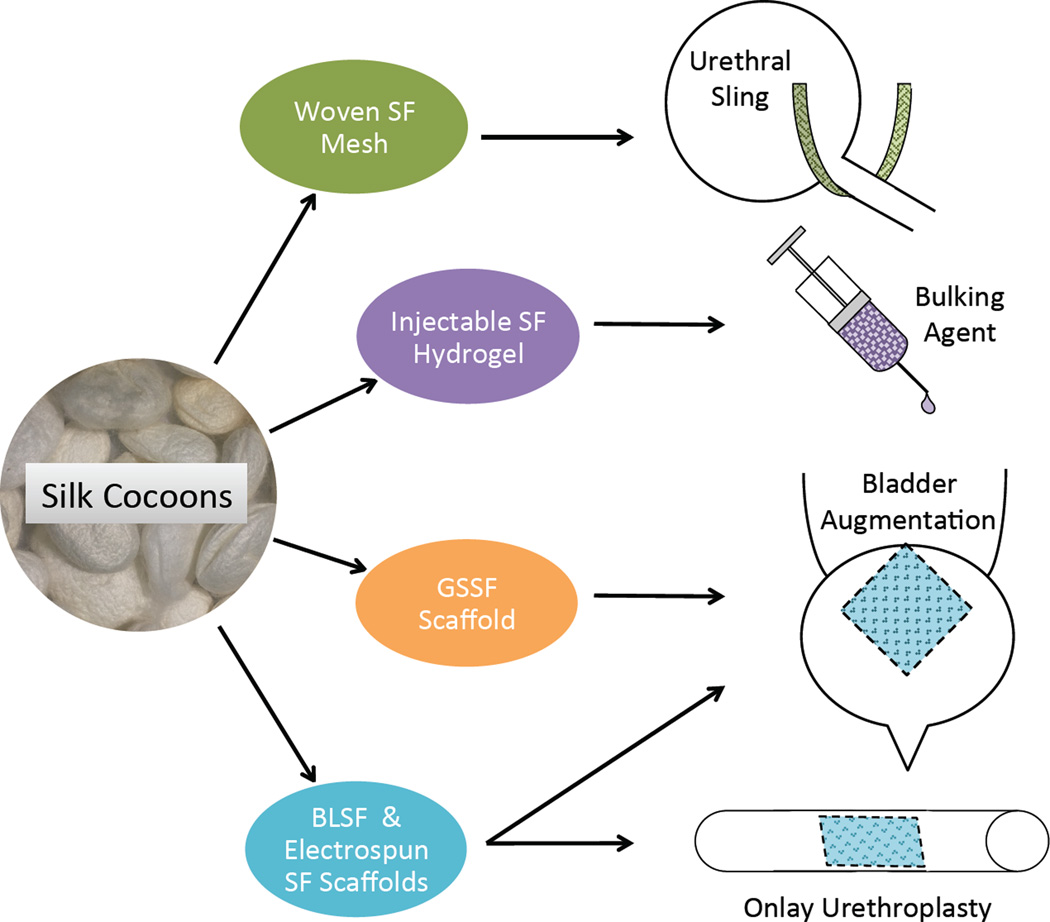
Silk fibroin (SF) is a protein-based polymer derived from Bombyx mori cocoons, which has properties that may address many of the needs for in situ bladder and urethral defect repair [51–53]. Historically, SF has been used clinically as sutures given their excellent tensile and elasticity characteristics compared to other nature and synthetic biomaterials [53,54]. These mechanical attributes make SF implants well suited for supporting bladder reconstruction where they must impart sufficient structural stability over defect sites during host tissue integration, but allow for organ flexibility necessary for micturition cycles. SF polymers exhibit diverse processing plasticity and through variations in fabrication techniques a multitude of matrix configurations can be achieved including 3D porous foams, nanodiameter fibers, hydrogels, tubes, and films which can be utilized for specific urologic applications [55–59]. In particular, tubular SF graft designs may have utility in restoration of complex urethral defects. SF matrices also exhibit tunable degradation rates dependent on factors such as scaffold pore size and SF content [60], which can be controlled during various stages of fabrication. Therefore, SF scaffolds can be engineered to maintain their 3D structure to support defect integrity, but gradually dissipate to allow for replacement of host tissues. Comparisons between SF biomaterials and other tissue engineered polymers such as PLGA and SIS have also demonstrated less immunogenic and inflammatory responses in the former suggesting higher degrees of biocompatibility can be achieved in respect to conventional urologic biomaterials [51,61]. Furthermore, SF constructs can also be functionalized with trophic factors using nontoxic and biocompatible chemistries and can be combined with native extracellular matrix components and motifs via surface coatings to enhance cell attachment and signaling [62–64] on host tissue-biomaterial interfaces. Lastly, silk constructs have stability of shape and lack of swelling under physiologic conditions due to its hydrophobic nature – a common problem with many other degradable biomaterials. These characteristics of silk have led to investigation of their utility in the bladder and urethra (Table 1), in addition to a number of non-genitourinary related medical specialties including gastroenterology [65–67], ophthalmology [68], orthopedic surgery [69–71], vascular surgery [72], neurology [73,74], plastic surgery [75], and otolaryngology [76].
Table 1.
Summary of Silk Fibroin Scaffold implementation in different Animal Models
| Animal (n) | Model Design | Silk Scaffold Design | Scaffold Size | Results | Ref |
|---|---|---|---|---|---|
| Murine (17) | Bladder Augmentation |
GSSF scaffold | 1 cm2 |
|
[77] |
| Rat (33) | Bladder Augmentation |
GSSF scaffold (n=25) vs. BLSF scaffold (n=8) |
0.7 × 0.7 cm2 |
|
[79] |
| Rat (12) | Neurogenic Bladder Secondary to Thoracic Spinal Cord Injury (SCI) |
BLSF scaffold | 1 × 1 cm2 |
|
[84] |
| Rat (30) | Suburethral Sling for Stress Urinary Incontinence |
Woven SF mesh alone (n=15) or seeded with mesenchymal stem cells (MSC, n=15) |
Not quantified |
|
[88] |
| Leporine (4) | Ventral Onlay Urethroplasty |
BLSF scaffold | 1 × 2 cm2 |
|
[87] |
| Leporine (30) | Bladder Implantation |
Electrospun SF scaffold assessed at 2, 4, and 8 weeks |
0.5 × 2 cm2 |
|
[81] |
| Canine (11) | Dorsal Onlay Urethroplasty |
Electrospun SF scaffold seeded with urothelial cells (n=6) or buccal mucosa (n=5) |
3 × 1 cm2 5 × 1.5 cm2 |
|
[85] [86] |
| Porcine (10) | Bladder Augmentation |
Two versions of BLSF: Group 1: Surface pore occlusions (n=6) vs. Group 2: Film casting (n=4) |
6 × 6 cm2 |
|
[80] |
GSSF = Gel spun silk fibroin BLSF = Bi-layer silk fibroin SF = Silk Fibroin
Silk Fibroin Scaffolds as a Bladder Substitute
The feasibility of acellular SF grafts for bladder reconstruction was first performed in a murine model of augmentation cystoplasty. In this study, a gel spun SF (GSSF) scaffold configuration was investigated which consisted of a 3D multi-laminate, nonporous matrix created via extrusion of aqueous SF solutions across a rotating mandrel [77]. SF grafts were evaluated in parallel with conventional biomaterial designs including SIS and PGA. Animals subjected to SF graft implantation demonstrated voluntary voiding and an 82% survival rate over the course of a 10 wk study period in comparison to 66% and 71% survival rates achieved with SIS and PGA, respectively. Following 10 wk of augmentation, the GSSF scaffolds were able to support regeneration of urothelial and smooth muscle components at the defect site based on histologic and immunohistochemical (IHC) staining of uroplakin and contractile protein expression (α-actin, calponin, and SM22α). In addition, there was minimal acute inflammatory reaction, in contrast to assessments of SIS and PGA matrices, which routinely promoted evidence of fibrosis and chronic inflammatory responses. Cystometric evaluations of the GSSF group demonstrated similar voiding patterns and increases in bladder capacity and voided volume while maintaining similar degrees of compliance relative to the control group [77]. This initial study provided proof of principle for the use of GSSF scaffolds in bladder reconstructive procedures. A follow-up report by Gomez et al. investigated the effect of manipulation of GSSF scaffold fabrication parameters on matrix performance in a murine model of augmentation cystoplasty [78]. The original GSSF prototype matrix was compared with two additional scaffold configurations with structurally and mechanically distinct properties created by altering initial winding and post-winding fabrication conditions. This study demonstrated that GSSF matrices with higher degrees of porosity displayed enhanced in vivo degradation rates relative to the original nonporous prototype while supporting similar rates of bladder tissue regeneration.
Next, the performance of various acellular SF graft configurations was studied in a rat model of bladder augmentation. Nonporous [77] and porous GSSF matrices [78], originally assessed in a murine model, were evaluated in parallel with a novel bi-layer SF (BLSF) design [79]. Histological/IHC and functional outcomes were compared to conventional SIS grafts. The bi-layer scaffold configuration was constructed from aqueous SF solutions by combining a solvent-casting/salt-leaching method with film casting to generate a porous scaffold compartment fused to an annealed film layer. This biodegradable matrix design was engineered to allow host tissue ingrowth to occur throughout the porous foam component while the film layer imparted a fluid-tight surgical seal for urine retention at the defect site. Following 10 wk of implantation, histological and IHC evaluations showed comparable extents of α-actin+, SM22α+ smooth muscle and uroplakin+, p63+ urothelial formation and maturation within all graft sites in respect to cystotomy controls. The incidence and size of urinary calculi was the highest in animals implanted with GSSF scaffolds and SIS matrices with frequencies ≥57% and stone diameters of 3–4 mm. In contrast, the BLSF group displayed substantially lower rates (20%) and smaller stone size (2 mm), similar to the levels observed in control animals (13%, 2 mm). Cystometric analyses at 10 weeks revealed that animals implanted with the BLSF grafts displayed superior urodynamic characteristics over GSSF graft designs including compliance, capacity, and spontaneous non-voiding contractions consistent with control levels [79]. Other research groups have also investigated the utility of BL grafts for bladder tissue engineering. Zhao et al., generated a composite biomaterial construct consisting of a porous SF foam surgically attached to an SIS matrix to provide a fluid-tight barrier layer and evaluated its performance in a rat model of bladder augmentation. Following 12 wk of implantation, this scaffold configuration demonstrated the ability to support smooth muscle regeneration without significant local tissue responses or systemic toxicity [81]. These reports show the efficacy of SF-based grafts for augmentation cystoplasty in rats and highlight the advantages of BLSF matrix configurations over GSSF scaffold designs.
The promising results of the acellular BLSF grafts in a rat model led to investigations in a porcine model of bladder reconstruction [80]. Tu et al. evaluated two versions of the BLSF matrix configuration: a porous SF foam buttressed by heterogeneous SF surface pore occlusions (Group 1) and the original prototype design consisting a porous SF foam annealed to a SF film (Group 2). Animals were augmented with a 6 × 6 cm2 scaffold and exhibited high rates of survival (Group 1: 5/6, 83%; Group 2: 4/4, 100%). The single mortality in the animal in Group 1 occurred on postoperative day two and revealed urinary ascites as the probable cause of death. Bladder distention during necropsy revealed prominent fluid leaks throughout the center of the scaffold. These results demonstrate the presence of the SF film is an essential feature in maintaining the fluid-tight seal during host tissue remodeling. At 3 m post-implantation, both groups showed comparable extents of smooth muscle and multi-layered urothelium regeneration histologically and levels of protein expression by IHC staining (urothelium: uroplakin, p63; smooth muscle: α-actin, SM22α) across the entire implant region. De novo innervation and vascularization processes were also evident in all regenerated tissues indicated by synaptophysin+ neuronal boutons and vessels lined with CD31 expressing endothelial cells, respectively. Additionally, intravesical urinary calculi formation was 55% higher in the Group 2 in comparison to the Group 1. This increase in calculi formation is presumably due to the relatively slow degradation kinetics of the SF film layer. Urodynamic evaluations demonstrated mean increases in bladder capacity over pre-operative levels (Group 1: 277%; Group 2: 153%) which exceeded nonsurgical control gains (144%) encountered due to animal growth. In addition, animals augmented with both matrix configurations displayed increases in bladder compliance over pre-operative levels (Group 1: 357%; Group 2: 338%) similar to growth-related elevations observed in non-surgical controls (354%). Furthermore, neotissues supported by both graft designs displayed ex vivo contractile responses to carbachol, α,β-methylene-ATP, KCl, and electrical field stimulation that were similar to control levels [80]. These data detailed the ability of acellular BLSF scaffolds to support regeneration of innervated, vascularized smooth muscle and urothelial tissues within 3 m with structural and functional properties comparable to native tissue in a porcine model of bladder repair.
Electrospun SF scaffolds have also been investigated in rabbit model of bladder reconstruction [81]. These biomaterials are composed of nonwoven SF nanofibers wherein porosity and fiber diameter can be manipulated via processing conditions to emulate structural characteristics of native extracellular matrix [81]. Huang et al., performed histological and functional comparisons of electrospun SF scaffolds and bladder acellular matrix (BAM) constructs in full thickness defects within the leporine bladder. Over the course of an 8 wk of implantation period, both constructs supported the formation of cytokeratin+ (CK) multi-layered urothelia, however higher degrees of α-actin+, SM22α+ smooth muscle formation was observed with the use of electrospun SF matrices. In addition, ex vivo organ bath studies demonstrated that neotissues supported by electrospun SF grafts generated elevated levels of contractile force in response to carbachol, KCl, and phenylephrine in respect to the BAM group [81]. These study results demonstrate that electrospun SF scaffolds are more efficient at generating functional smooth muscle regeneration than BAM in a rabbit model of augmentation cystoplasty.
Validation of prospective implant designs in animal models of bladder disease is a crucial step toward clinical translation of tissue engineered grafts given that previous studies have shown that underlying urologic pathologies can influence regenerative outcomes [82,83]. Chung et al. performed a comparative assessment of SIS and BLSF grafts in a rat model of neurogenic bladder secondary to spinal cord injury (SCI). Following 6 wk of thoracic spinal cord injury, bladders were augmented with scaffold groups and maintained for a 10 wk implantation period. Rats subjected to SCI alone exhibited a 72% survival rate (13/18) while SCI rats receiving SIS and BLSF scaffolds displayed respective survival rates of 83% (10/12) and 75% (9/12) over the course of the study period. Histological and IHC evaluations demonstrated both implants supported de novo formation of smooth muscle layers with contractile protein expression [α-SMA and SM22α] as well as maturation of multi-layer urothelia expressing CK and uroplakin proteins. In addition, de novo innervation processes were apparent in neotissues supported by both grafts indicated by the presence of synaptophysin+ neuronal boutons, however the density of these synaptic areas was significantly lower in comparison to non-injured animals. Discrete areas of mild fibrosis within the lamina propria were also present in both implant groups accompanied by chronic inflammatory reactions consisting of follicular aggregates of mononuclear cell infiltrates [84]. These results are in contrast to previous findings in the non-diseased rat model wherein no chronic inflammatory events were observed following bladder augmentation with these scaffold configurations [79]. Improvements in certain urodynamic parameters in SCI animals, such as decreased peak intravesical pressure, following implantation with both matrix configurations was also observed. This study highlights the ability of both SIS and BLSF scaffolds to promote de novo tissue formation as well as improved cystometric performance of the neurogenic bladder. However modifications in graft designs are still needed to restore aberrant innervation and mitigate host tissue responses in this pathological setting. The versatility of SF matrix processing techniques may offer advantages over SIS constructs for graft optimization due the ability of structural, mechanical, and degradative parameters to be easily modified.
Silk Fibroin Scaffolds as a Urethral Substitute
Various tissue-engineering approaches with SF scaffolds have been explored for urethral tissue reconstruction. Xie et al. first described the use of electrospun SF scaffolds seeded with ex vivo expanded, bladder urothelial cells in a canine model of dorsal onlay urethroplasty [85]. Urethras implanted with cell seeded SF grafts were capable of supporting voluntary voiding for up to 6 m post-op with no evidence of stricture formation observed throughout the study period. Histological evaluation of the original implantation sites at both 2 and 6 m post-op demonstrated reconstitution of native urethral architecture with a stratified urothelium present and no evidence of fibrosis observed. In contrast, control animals wherein excision of urethral tissue was performed in the absence of scaffold integration, presented with urothelial stricture disease in combination with aberrant tissue formation with significant inflammatory reactions. A follow-up study by Xie and colleagues assessed the performance of electrospun SF scaffolds seeded with ex vivo expanded oral fibroblasts and keratinocytes for dorsal onlay urethroplasty in canines [86]. This cell seeded construct was engineered to mimic buccal mucosa, a popular choice for urethral repair in humans [11,12]. Similar histological and functional outcomes were achieved as observed with the use of SF implants seeded with bladder-derived cell populations. The authors concluded that although cell seeded strategies are labor and resource intensive, requiring donor tissue harvest and ex vivo culture techniques, the use of SF scaffolds in combination with seeded bladder or mucosal cell populations is a promising strategy for engineering functional urethral tissues.
Recently, Chung and colleagues compared the performance of acellular BLSF and SIS matrices in a ventral onlay urethroplasty model in rabbits [87]. Both scaffold configurations supported voluntary voiding throughout the study period and retrograde urethrography at 3 m post-op revealed wide urethral calibers similar to pre-operative assessments with no evidence of urethral abnormalities or stone formation. Histological and IHC evaluations at 3 m post-implantation demonstrated that BLSF and SIS grafts supported the formation of α- actin+, SM22α+ smooth muscle bundles as well as de novo CK+ urothelium throughout the original repair site. Evidence of innervation and vascularization was also observed in both scaffold groups to similar extents as seen in urethrotomy controls. In contrast to BLSF scaffolds, SIS matrices produced chronic inflammatory responses throughout the graft site. The results of this study demonstrate that BLSF scaffolds represent promising biomaterials for onlay urethroplasty, capable of promoting similar degrees of tissue regeneration in comparison to conventional SIS scaffolds, but with reduced immunogenicity.
Silk Fibroin Scaffolds for Stress Urinary Incontinence
Silk fibroin platforms have also been studied as treatments for stress urinary incontinence (SUI). In particular, Zou and colleagues investigated the utility of a woven SF mesh either alone or seeded with bone marrow-derived mesenchymal stem cells (MSC) in a rat SUI model secondary to bilateral sciatic nerve transection [88]. Both MSC-seeded and acellular grafts were capable of rescuing leak point pressures to levels observed in sham controls, in contrast to injured animals without sling treatment. In addition, MSC-seeded implants demonstrated higher collagen content and failure force compared to unseeded grafts suggesting that stem cell populations promote extracellular matrix deposition leading to increased mechanical robustness. In addition to woven SF constructs, SF hydrogel formulations produced by ultrasonication or vortexing [89] have shown utility as injectable bulking agents [90] and therefore may have the potential to reinforce the bladder neck and serve as a therapy for SUI.
Conclusion
SF biomaterials represent emerging medical device technologies for urinary tract reconstruction. The ability of SF polymers to be fabricated into a multitude of biocompatible graft designs with tunable structural, mechanical, and degradative properties has led to the development of scaffold configurations capable of supporting functional urologic tissue formation in a variety of bladder and urethral defect models (Figure 1). In vivo analyses have shown that SF matrices offer a number of advantages over conventional decellularized tissue constructs and synthetic biomaterials including reduced immunogenicity and enhanced functional performance. Further preclinical studies focusing on long-term evaluations in urologic disease settings will be necessary to determine their suitability for human clinical trials.
Figure 1.
Footnotes
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent: All studies by Joshua R. Mauney and Carlos R. Estrada Jr. involving animal subjects were performed after approval by the appropriate institutional review boards.
Conflict of Interest: None
References
Papers of particular interest, published recently, have been highlighted as:
** Of major importance (80,84)
- 1.Hegde SS. Muscarinic receptors in the bladder: from basic research to therapeutics. Br J Pharmacol. 2006;147(Suppl 2):S80–S87. doi: 10.1038/sj.bjp.0706560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edelstein RA, Bauer SB, Kelly MD, et al. The long-term urological response of neonates with myelodysplasia treated proactively with intermittent catheterization and anticholinergic therapy. J Urol. 1995;154(4):1500–1504. [PubMed] [Google Scholar]
- 3.Kaefer M, Pabby A, Kelly M, Darbey M, Bauer SB. Improved bladder function after prophylactic treatment of the high risk neurogenic bladder in newborns with myelomentingocele. J Urol. 1999;162(3 Pt 2):1068–1071. doi: 10.1016/S0022-5347(01)68069-8. [DOI] [PubMed] [Google Scholar]
- 4.Hensle TW, Gilbert SM. A review of metabolic consequences and long-term complications of enterocystoplasty in children. Curr Urol Rep. 2007;8(2):157–162. doi: 10.1007/s11934-007-0066-9. [DOI] [PubMed] [Google Scholar]
- 5.Somani BK, Kumar V, Wong S, et al. Bowel dysfunction after transposition of intestinal segments into the urinary tract: 8-year prospective cohort study. J Urol. 2007;177(5):1793–1798. doi: 10.1016/j.juro.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Amini E, Djaladat H. Long-term complications of urinary diversion. Curr Opin Urol. 2015;25(6):570–577. doi: 10.1097/MOU.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 7.Berger I, Wehrberger C, Ponholzer A, et al. Impact of the use of bowel for urinary diversion on perioperative complications and 90-day mortality in patients aged 75 years or older. Urol Int. 2015;94(4):394–400. doi: 10.1159/000367853. [DOI] [PubMed] [Google Scholar]
- 8.Kim KR, Suh JG, Paick JS, Kim SW. Surgical outcome of urethroplasty using penile circular fasciocutaneous flap for anterior urethral stricture. World J Mens Health. 2014;32(2):87–92. doi: 10.5534/wjmh.2014.32.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding J, Li Q, Li S, et al. Ten years' experience for hypospadias repair: combined buccal mucosa graft and local flap for urethral reconstruction. Urol Int. 2014;93(4):454–459. doi: 10.1159/000360796. [DOI] [PubMed] [Google Scholar]
- 10.Son le T, Hung le T, Thang le C, Linh NT. The use of dermal graft in severe chordee hypospadias repair: experience from Vietnam. Pediatr Surg Int. 2015;31(3):291–295. doi: 10.1007/s00383-015-3656-5. [DOI] [PubMed] [Google Scholar]
- 11.Caldamone AA, Edstrom LE, Koyle MA, Rabinowitz R, Hulbert WC. Buccal mucosal grafts for urethral reconstruction. Urology. 1998;51(5A Suppl):15–19. doi: 10.1016/s0090-4295(98)00088-0. [DOI] [PubMed] [Google Scholar]
- 12.Barbagli G, Palminteri E, Guazzoni G, Montorsi F, Turini D, Lazzeri M. Bulbar urethroplasty using buccal mucosa grafts placed on the ventral, dorsal or lateral surface of the urethra: are results affected by the surgical technique? J Urol. 2005;174(3):955–957. doi: 10.1097/01.ju.0000169422.46721.d7. discussion 957–958. [DOI] [PubMed] [Google Scholar]
- 13.Monfort G, Bretheau D, Di Benedetto V, Bankole R. Urethral stricture in children: treatment by urethroplasty with bladder mucosa graft. J Urol. 1992;148(5):1504–1506. doi: 10.1016/s0022-5347(17)36950-1. [DOI] [PubMed] [Google Scholar]
- 14.Foinquinos RC, Calado AA, Janio R, Griz A, Macedo A, Jr, Ortiz V. The tunica vaginalis dorsal graft urethroplasty: initial experience. Int Braz J Urol. 2007;33(4):523–529. doi: 10.1590/s1677-55382007000400011. discussion 529–531. [DOI] [PubMed] [Google Scholar]
- 15.Sinha RJ, Singh V, Sankhwar SN, Dalela D. Donor site morbidity in oral mucosa graft urethroplasty: implications of tobacco consumption. BMC Urol. 2009;9:15. doi: 10.1186/1471-2490-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fasolis M, Zavattero E, Sedigh O, et al. Oral mucosa harvest for urologic reconstruction: role of maxillofacial surgeon and donor-site morbidity evaluation. J Craniofac Surg. 2014;25(2):604–606. doi: 10.1097/SCS.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 17.Kropp BP, Sawyer BD, Shannon HE, et al. Characterization of small intestinal submucosa regenerated canine detrusor: assessment of reinnervation, in vitro compliance and contractility. J Urol. 1996;156(2 Pt 2):599–607. doi: 10.1097/00005392-199608001-00008. [DOI] [PubMed] [Google Scholar]
- 18.Wefer J, Sievert KD, Schlote N, et al. Time dependent smooth muscle regeneration and maturation in a bladder acellular matrix graft: histological studies and in vivo functional evaluation. J Urol. 2001;165(5):1755–1759. [PubMed] [Google Scholar]
- 19.Cartwright LM, Shou Z, Yeger H, Farhat WA. Porcine bladder acellular matrix porosity: impact of hyaluronic acid and lyophilization. J Biomed Mater Res A. 2006;77(1):180–184. doi: 10.1002/jbm.a.30652. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein MB, Dearden LC, Gualtieri V. Regeneration of subtotally cystectomized bladder patched with omentum: an experimental study in rabbits. J Urol. 1967;97(4):664–668. doi: 10.1016/S0022-5347(17)63095-7. [DOI] [PubMed] [Google Scholar]
- 21.Baumert H, Simon P, Hekmati M, et al. Development of a seeded scaffold in the great omentum: feasibility of an in vivo bioreactor for bladder tissue engineering. Eur Urol. 2007;52(3):884–890. doi: 10.1016/j.eururo.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 22.Hattori K, Joraku A, Miyagawa T, Kawai K, Oyasu R, Akaza H. Bladder reconstruction using a collagen patch prefabricated within the omentum. Int J Urol. 2006;13(5):529–537. doi: 10.1111/j.1442-2042.2006.01351.x. [DOI] [PubMed] [Google Scholar]
- 23.Portis AJ, Elbahnasy AM, Shalhav AL, et al. Laparoscopic augmentation cystoplasty with different biodegradable grafts in an animal model. J Urol. 2000;164(4):1405–1411. [PubMed] [Google Scholar]
- 24.Kambic H, Kay R, Chen JF, Matsushita M, Harasaki H, Zilber S. Biodegradable pericardial implants for bladder augmentation: a 2.5-year study in dogs. J Urol. 1992;148(2 Pt 2):539–543. doi: 10.1016/s0022-5347(17)36649-1. [DOI] [PubMed] [Google Scholar]
- 25.Jelly O. Segmental cystectomy with peritoneoplasty. Urol Int. 1970;25(3):236–244. doi: 10.1159/000279676. [DOI] [PubMed] [Google Scholar]
- 26.Kelami A. Lyophilized human dura as a bladder wall substitute: experimental and clinical results. J Urol. 1971;105(4):518–522. doi: 10.1016/s0022-5347(17)61563-5. [DOI] [PubMed] [Google Scholar]
- 27.Iijima K, Igawa Y, Imamura T, et al. Transplantation of preserved human amniotic membrane for bladder augmentation in rats. Tissue Eng. 2007;13(3):513–524. doi: 10.1089/ten.2006.0170. [DOI] [PubMed] [Google Scholar]
- 28.Fishman IJ, Flores FN, Scott FB, Spjut HJ, Morrow B. Use of fresh placental membranes for bladder reconstruction. J Urol. 1987;138(5):1291–1294. doi: 10.1016/s0022-5347(17)43586-5. [DOI] [PubMed] [Google Scholar]
- 29.Reddy PP, Barrieras DJ, Wilson G, et al. Regeneration of functional bladder substitutes using large segment acellular matrix allografts in a porcine model. J Urol. 2000;164(3 Pt 2):936–941. doi: 10.1097/00005392-200009020-00005. [DOI] [PubMed] [Google Scholar]
- 30.Merguerian PA, Reddy PP, Barrieras DJ, et al. Acellular bladder matrix allografts in the regeneration of functional bladders: evaluation of large-segment (>24 cm) substitution in a porcine model. BJU Int. 2000;85(7):894–898. doi: 10.1046/j.1464-410x.2000.00513.x. [DOI] [PubMed] [Google Scholar]
- 31.Probst M, Piechota HJ, Dahiya R, Tanagho EA. Homologous bladder augmentation in dog with the bladder acellular matrix graft. BJU Int. 2000;85(3):362–371. doi: 10.1046/j.1464-410x.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 32.Brown AL, Farhat W, Merguerian PA, Wilson GJ, Khoury AE, Woodhouse KA. 22 week assessment of bladder acellular matrix as a bladder augmentation material in a porcine model. Biomaterials. 2002;23(10):2179–2190. doi: 10.1016/s0142-9612(01)00350-7. [DOI] [PubMed] [Google Scholar]
- 33.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17(2):149–155. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 34.Lai JY, Yoon CY, Yoo JJ, Wulf T, Atala A. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J Urol. 2002;168(4 Pt 2):1853–1857. doi: 10.1097/01.ju.0000030040.76258.5a. discussion 1858. [DOI] [PubMed] [Google Scholar]
- 35.Nakanishi Y, Chen G, Komuro H, et al. Tissue-engineered urinary bladder wall using PLGA mesh-collagen hybrid scaffolds: a comparison study of collagen sponge and gel as a scaffold. J Pediatr Surg. 2003;38(12):1781–1784. doi: 10.1016/j.jpedsurg.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 36.Atala A, Freeman MR, Vacanti JP, Shepard J, Retik AB. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J Urol. 1993;150(2 Pt 2):608–612. doi: 10.1016/s0022-5347(17)35561-1. [DOI] [PubMed] [Google Scholar]
- 37.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 38.Godbole P, Mackinnon AE. Expanded PTFE bladder neck slings for incontinence in children: the long-term outcome. BJU Int. 2004;93(1):139–141. doi: 10.1111/j.1464-410x.2004.04573.x. [DOI] [PubMed] [Google Scholar]
- 39.Pattison M, Webster TJ, Leslie J, Kaefer M, Haberstroh KM. Evaluating the in vitro and in vivo efficacy of nano-structured polymers for bladder tissue replacement applications. Macromol Biosci. 2007;7(5):690–700. doi: 10.1002/mabi.200600297. [DOI] [PubMed] [Google Scholar]
- 40.Rohrmann D, Albrecht D, Hannappel J, Gerlach R, Schwarzkopp G, Lutzeyer W. Alloplastic replacement of the urinary bladder. J Urol. 1996;156(6):2094–2097. [PubMed] [Google Scholar]
- 41.Adelow CA, Frey P. Synthetic hydrogel matrices for guided bladder tissue regeneration. Methods Mol Med. 2007;140:125–140. doi: 10.1007/978-1-59745-443-8_7. [DOI] [PubMed] [Google Scholar]
- 42.Falke G, Caffaratti J, Atala A. Tissue engineering of the bladder. World J Urol. 2000;18(1):36–43. doi: 10.1007/s003450050007. [DOI] [PubMed] [Google Scholar]
- 43.Athanasiou KA, Niederauer GG, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102. doi: 10.1016/0142-9612(96)85754-1. [DOI] [PubMed] [Google Scholar]
- 44.Mauney JR, Nguyen T, Gillen K, Kirker-Head C, Gimble JM, Kaplan DL. Engineering adipose-like tissue in vitro and in vivo utilizing human bone marrow and adipose-derived mesenchymal stem cells with silk fibroin 3D scaffolds. Biomaterials. 2007;28(35):5280–5290. doi: 10.1016/j.biomaterials.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joseph DB, Borer JG, De Filippo RE, Hodges SJ, McLorie GA. Autologous cell seeded biodegradable scaffold for augmentation cystoplasty: phase II study in children and adolescents with spina bifida. J Urol. 2014;191(5):1389–1395. doi: 10.1016/j.juro.2013.10.103. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer M, Kaiser A, Stehr M, Beyer HJ. Bladder augmentation with small intestinal submucosa leads to unsatisfactory long-term results. J Pediatr Urol. 2013;9(6 Pt A):878–883. doi: 10.1016/j.jpurol.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Liao L. Tissue engineered cystoplasty augmentation for treatment of neurogenic bladder using small intestinal submucosa: an exploratory study. J Urol. 2014;192(2):544–550. doi: 10.1016/j.juro.2014.01.116. [DOI] [PubMed] [Google Scholar]
- 48.Palminteri E, Berdondini E, Colombo F, Austoni E. Small intestinal submucosa (SIS) graft urethroplasty: short-term results. Eur Urol. 2007;51(6):1695–1701. doi: 10.1016/j.eururo.2006.12.016. discussion 1701. [DOI] [PubMed] [Google Scholar]
- 49.Fiala R, Vidlar A, Vrtal R, Belej K, Student V. Porcine small intestinal submucosa graft for repair of anterior urethral strictures. Eur Urol. 2007;51(6):1702–1708. doi: 10.1016/j.eururo.2007.01.099. discussion 1708. [DOI] [PubMed] [Google Scholar]
- 50.Farahat YA, Elbahnasy AM, El-Gamal OM, Ramadan AR, El-Abd SA, Taha MR. Endoscopic urethroplasty using small intestinal submucosal patch in cases of recurrent urethral stricture: a preliminary study. J Endourol. 2009;23(12):2001–2005. doi: 10.1089/end.2009.0074. [DOI] [PubMed] [Google Scholar]
- 51.Altman GH, Diaz F, Jakuba C, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 52.Wong Po Foo C, Kaplan DL. Genetic engineering of fibrous proteins: spider dragline silk and collagen. Adv Drug Deliv Rev. 2002;54(8):1131–1143. doi: 10.1016/s0169-409x(02)00061-3. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan D. Silk polymers : materials science and biotechnology. Washington, DC: American Chemical Society; 1994. [Google Scholar]
- 54.Cunniff PM, Fossey SA, Auerbach MA, et al. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polymers for Advanced Technologies. 1994;5(8):401–410. [Google Scholar]
- 55.Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424(6952):1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- 56.Jin HJ, Fridrikh SV, Rutledge GC, Kaplan DL. Electrospinning Bombyx mori silk with poly(ethylene oxide) Biomacromolecules. 2002;3(6):1233–1239. doi: 10.1021/bm025581u. [DOI] [PubMed] [Google Scholar]
- 57.Nazarov R, Jin HJ, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5(3):718–726. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 58.Kim UJ, Park J, Li C, Jin HJ, Valluzzi R, Kaplan DL. Structure and properties of silk hydrogels. Biomacromolecules. 2004;5(3):786–792. doi: 10.1021/bm0345460. [DOI] [PubMed] [Google Scholar]
- 59.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26(15):2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y, Rudym DD, Walsh A, et al. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29(24–25):3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panilaitis B, Altman GH, Chen J, Jin HJ, Karageorgiou V, Kaplan DL. Macrophage responses to silk. Biomaterials. 2003;24(18):3079–3085. doi: 10.1016/s0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 62.Franck D, Gil ES, Adam RM, et al. Evaluation of silk biomaterials in combination with extracellular matrix coatings for bladder tissue engineering with primary and pluripotent cells. PLoS One. 2013;8(2):e56237. doi: 10.1371/journal.pone.0056237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J Biomed Mater Res A. 2004;71(3):528–537. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 64.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54(1):139–148. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 65.Algarrahi K, Franck D, Ghezzi CE, et al. Acellular bi-layer silk fibroin scaffolds support functional tissue regeneration in a rat model of onlay esophagoplasty. Biomaterials. 2015;53:149–159. doi: 10.1016/j.biomaterials.2015.02.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung EJ, Ju HW, Park HJ, Park CH. Three-layered scaffolds for artificial esophagus using poly(varepsilon-caprolactone) nanofibers and silk fibroin: An experimental study in a rat model. J Biomed Mater Res A. 2015;103(6):2057–2065. doi: 10.1002/jbm.a.35347. [DOI] [PubMed] [Google Scholar]
- 67.Franck D, Chung YG, Coburn J, Kaplan DL, Estrada CR, Jr, Mauney JR. In vitro evaluation of bi-layer silk fibroin scaffolds for gastrointestinal tissue engineering. J Tissue Eng. 2014;5 doi: 10.1177/2041731414556849. 2041731414556849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jia L, Ghezzi CE, Kaplan DL. Optimization of silk films as substrate for functional corneal epithelium growth. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33408. [DOI] [PubMed] [Google Scholar]
- 69.Bi F, Shi Z, Liu A, Guo P, Yan S. Anterior cruciate ligament reconstruction in a rabbit model using silk-collagen scaffold and comparison with autograft. PLoS One. 2015;10(5):e0125900. doi: 10.1371/journal.pone.0125900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li JJ, Kim K, Roohani-Esfahani SI, Guo J, Kaplan DL, Zreiqat H. A biphasic scaffold based on silk and bioactive ceramic with stratified properties for osteochondral tissue regeneration. J Mater Chem B Mater Biol Med. 2015;3(26):5361–5376. doi: 10.1039/C5TB00353A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang W, Yang Y, Zhang K, Li Y, Fang G. Weft-knitted silk-poly(lactide-co-glycolide) mesh scaffold combined with collagen matrix and seeded with mesenchymal stem cells for rabbit Achilles tendon repair. Connect Tissue Res. 2015;56(1):25–34. doi: 10.3109/03008207.2014.976309. [DOI] [PubMed] [Google Scholar]
- 72.Wang D, Liu H, Fan Y. Silk fibroin for vascular regeneration. Microsc Res Tech. 2015 doi: 10.1002/jemt.22532. [DOI] [PubMed] [Google Scholar]
- 73.Teuschl AH, Schuh C, Halbweis R, et al. A New Preparation Method for Anisotropic Silk Fibroin Nerve Guidance Conduits and Its Evaluation In Vitro and in a Rat Sciatic Nerve Defect Model. Tissue Eng Part C Methods. 2015;21(9):945–957. doi: 10.1089/ten.tec.2014.0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flanagan KE, Tien LW, Elia R, Wu J, Kaplan D. Development of a sutureless dural substitute from Bombyx mori silk fibroin. J Biomed Mater Res B Appl Biomater. 2015;103(3):485–494. doi: 10.1002/jbm.b.33217. [DOI] [PubMed] [Google Scholar]
- 75.Clemens MW, Downey S, Agullo F, et al. Clinical application of a silk fibroin protein biologic scaffold for abdominal wall fascial reinforcement. Plast Reconstr Surg Glob Open. 2014;2(11):e246. doi: 10.1097/GOX.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen Y, Redmond SL, Papadimitriou JM, et al. The biocompatibility of silk fibroin and acellular collagen scaffolds for tissue engineering in the ear. Biomed Mater. 2014;9(1):015015. doi: 10.1088/1748-6041/9/1/015015. [DOI] [PubMed] [Google Scholar]
- 77.Mauney JR, Cannon GM, Lovett ML, et al. Evaluation of gel spun silk-based biomaterials in a murine model of bladder augmentation. Biomaterials. 2011;32(3):808–818. doi: 10.1016/j.biomaterials.2010.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gomez P, 3rd, Gil ES, Lovett ML, et al. The effect of manipulation of silk scaffold fabrication parameters on matrix performance in a murine model of bladder augmentation. Biomaterials. 2011;32(30):7562–7570. doi: 10.1016/j.biomaterials.2011.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seth A, Chung YG, Gil ES, et al. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials. 2013;34(20):4758–4765. doi: 10.1016/j.biomaterials.2013.03.038. Implemented a novel bi-layer scaffold with film casting design that demonstrated decreased intravesical calculus formation and superior urodynamic parameters in a rat animal model of bladder augmentation.
- 80. Tu DD, Chung YG, Gil ES, et al. Bladder tissue regeneration using acellular bi-layer silk scaffolds in a large animal model of augmentation cystoplasty. Biomaterials. 2013;34(34):8681–8689. doi: 10.1016/j.biomaterials.2013.08.001. Bi-layer silk fibroin scaffold use in a large animal model of bladder augmentaiton proved to be safe and regenerated smooth muscle and a multi-layered urothelium across the entire 6 cm scaffold.
- 81.Huang JW, Xu YM, Li ZB, et al. Tissue performance of bladder following stretched electrospun silk fibroin matrix and bladder acellular matrix implantation in a rabbit model. J Biomed Mater Res A. 2015 doi: 10.1002/jbm.a.35535. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, Frimberger D, Cheng EY, Lin HK, Kropp BP. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int. 2006;98(5):1100–1105. doi: 10.1111/j.1464-410X.2006.06447.x. [DOI] [PubMed] [Google Scholar]
- 83.Akbal C, Lee SD, Packer SC, Davis MM, Rink RC, Kaefer M. Bladder augmentation with acellular dermal biomatrix in a diseased animal model. J Urol. 2006;176(4 Pt 2):1706–1711. doi: 10.1016/j.juro.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 84. Chung YG, Algarrahi K, Franck D, et al. The use of bi-layer silk fibroin scaffolds and small intestinal submucosa matrices to support bladder tissue regeneration in a rat model of spinal cord injury. Biomaterials. 2014;35(26):7452–7459. doi: 10.1016/j.biomaterials.2014.05.044. Silk scaffold integration into an animal model with neurogenic bladder secondary to spinal cord injury promoted de novo tissue formation. However, modifications in graft design are still needed to restore aberrant innervation and mitigate host tissue responses in this pathological setting.
- 85.Xie M, Song L, Wang J, Fan S, Zhang Y, Xu Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J Surg Res. 2013;184(2):774–781. doi: 10.1016/j.jss.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 86.Xie M, Xu Y, Song L, Wang J, Lv X, Zhang Y. Tissue-engineered buccal mucosa using silk fibroin matrices for urethral reconstruction in a canine model. J Surg Res. 2014;188(1):1–7. doi: 10.1016/j.jss.2013.11.1102. [DOI] [PubMed] [Google Scholar]
- 87. Chung YG, Tu D, Franck D, et al. Acellular bi-layer silk fibroin scaffolds support tissue regeneration in a rabbit model of onlay urethroplasty. PLoS One. 2014;9(3):e91592. doi: 10.1371/journal.pone.0091592. Bi-layer silk fibroin scaffold for onlay urethroplasty in a rabbit model is capable of promoting similar degrees of tissue regeneration in comparison to conventional SIS scaffolds, but with reduced immunogenicity.
- 88.Zou XH, Zhi YL, Chen X, et al. Mesenchymal stem cell seeded knitted silk sling for the treatment of stress urinary incontinence. Biomaterials. 2010;31(18):4872–4879. doi: 10.1016/j.biomaterials.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 89.Yucel T, Cebe P, Kaplan DL. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009;97(7):2044–2050. doi: 10.1016/j.bpj.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brown JE, Partlow BP, Berman AM, House MD, Kaplan DL. Injectable silk-based biomaterials for cervical tissue augmentation: an in vitro study. Am J Obstet Gynecol. 2016;214(1):118 e111–118 e119. doi: 10.1016/j.ajog.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]