Abstract
Objective:
As glucagon-like peptide-1 receptor agonists lower blood pressure (BP) in type 2 diabetes mellitus (T2DM), we examined BP control in relation to targets set by international bodies prior to randomization in the Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) trial.
Methods:
We analyzed baseline data from LEADER (NCT01179048), an ongoing phase 3B, randomized, double-blind, placebo-controlled cardiovascular outcomes trial examining the cardiovascular safety of the glucagon-like peptide-1 receptor agonist liraglutide in 9340 people with T2DM from 32 countries [age (all mean ± SD) 64 ± 7.2 years, BMI 32.5 ± 6.3 kg/m2, duration of diabetes 12.7 ± 8.0 years], all of whom were at high risk for cardiovascular disease (CVD).
Results:
A total of 81% (n = 7592) of participants had prior CVD and 90% (n = 8408) had a prior history of hypertension. Despite prescription of multiple antihypertensive agents at baseline, only 51% were treated to a target BP of less than 140/85 mmHg and only 26% to the recommended baseline BP target of less than 130/80 mmHg. In univariate analyses, those with prior CVD were prescribed more agents (P < 0.001) and had lower BP than those without (137 ± 18.8/78 ± 10.6 mmHg versus 140 ± 17.7/80 ± 9.9 mmHg; P < 0.001). In logistic regression analyses, residency in North America (64% treated to <140/85 mmHg; 38% treated to <130/80 mmHg) was the strongest predictor of BP control.
Conclusion:
These contemporary data confirm that BP remains insufficiently controlled in a large proportion of individuals with T2DM at high cardiovascular risk, particularly outside North America. Longitudinal data from the LEADER trial may provide further insights into BP control in relation to cardiovascular outcomes in this condition.
Keywords: blood pressure, cardiovascular disease, glucagon-like peptide-1, regional differences, targets, type 2 diabetes mellitus
Abbreviations: CVD; cardiovascular disease; eGFR; estimated glomerular filtration rate; GLP-1; glucagon-like peptide-1; LEADER; liraglutide effect and action in diabetes, evaluation of cardiovascular outcome results; SGLT-2; sodium–glucose linked transporter-2
INTRODUCTION
As blood pressure (BP) control is an important strategy for preventing cardiovascular and renal complications in type 2 diabetes mellitus (T2DM) [1,2], some treatment guidelines include disease-specific BP targets [3,4]. However, these can be difficult to achieve, even with multiple antihypertensive agents [5,6].
Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results (LEADER) is an ongoing phase 3B global cardiovascular outcome trial examining the cardiovascular safety and potential benefits of the glucagon-like peptide-1 (GLP-1) analogue liraglutide in individuals with T2DM at high risk of cardiovascular disease (CVD) [7]. The primary objective is to assess the effect of treatment with liraglutide compared with placebo (for at least 3.5 years and up to 5 years) on the incidence of cardiovascular events, as defined by the primary end point of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke in adult patients with T2DM.
The efficacy of BP-lowering agents is not greatly influenced by BMI [8], but as BMI and BP are closely associated [9], more agents are required to achieve BP targets in obese individuals [8]. In T2DM, this therapeutic challenge is compounded by weight gain in association with many commonly used glucose-lowering agents (insulin, sulphonylureas, and thiazolidinediones) [10,11]. In the UK Prospective Diabetes Study, in which patients with T2DM had a BMI 29.8 ± 5.5 kg/m2 (mean ± SD) and a very conservative definition of ‘tight’ BP control (<150/85 mmHg), 29% still required three or more antihypertensive agents [1].
Glucose-lowering agents that have been introduced more recently either do not affect weight (e.g., dipeptidyl peptidase-4 inhibitors) [10,11] or are associated with weight reduction [e.g., GLP-1 receptor agonists [10,12]; sodium–glucose linked transporter-2 (SGLT-2) inhibitors] [13]. When added to another glucose-lowering therapy, liraglutide treatment is associated with weight reduction of 1.2–2.6 kg and SBP reduction of 2.3–6.7 mmHg [14–18]. It therefore has the potential to facilitate the achievement of BP targets in overweight and obese patients already being treated with multiple agents.
In the present study, we analyzed baseline data from the LEADER trial to: describe BP control at the time of randomization; report rates of nonachievement of BP targets in relation to contemporary and updated guidelines; and determine the factors predicting nonachievement of targets prior to randomization to liraglutide or placebo. During the trial recruitment period (2010–2012), international guidelines recommended a BP target of <130/80 mmHg in T2DM [3,4].
Prompted by initial analyses, we also examined BP control categorized according to the geographical regions represented in the trial.
RESEARCH DESIGN AND METHODS
The LEADER study design and baseline characteristics have been published previously [7]. Briefly, participants were enrolled between September 2010 and April 2012 at 410 sites in 32 countries according to the following inclusion criteria: patients with T2DM aged at least 50 years with concomitant CVD, cerebrovascular disease, peripheral vascular disease, chronic renal failure, or chronic heart failure; or patients aged at least 60 years with one or more of the following cardiovascular risk factors: microalbuminuria or proteinuria, hypertension, and left ventricular hypertrophy by ECG or imaging, left ventricular dysfunction by imaging, or ankle–brachial index less than 0.9. All patients had baseline HbA1c at least 7.0% (53 mmol/mol). Exclusion criteria included type 1 diabetes mellitus, calcitonin at least 50 ng/l, use of GLP-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, or insulin preparations other than human neutral protamine Hagedorn, long-acting analogs, or premixed preparations within 3 months prior to screening.
BP was measured at the screening visit following usual practice for clinical research at each individual trial center, with adherence to the following standards as a minimum. Participants were asked to avoid caffeine, smoking, and exercise at least 30 min before measurement. Measurements were taken with the participant in a sitting position, with the legs uncrossed, and the back and arm supported. Participants were sitting for at least 5 min before the first reading was taken. Two measurements at intervals of at least 2 min were performed. Participants and trial personnel were advised not to talk during the measurements. The manufacture and model of the semi-automated measurement devices and the cuff sizes used were not recorded. The mean of the two BP measurements was subsequently calculated for use in analyses. In some individuals (<1%) for whom measurements were not available from the screening visit, those from the randomization visit were used. Hypertension was defined as SBP at least 140 mmHg, a DBP at least 90 mmHg, or an existing prescription of antihypertensive medication.
A target BP of less than 130/80 mmHg, as recommended by the international authorities cited above [3,4,19], was disseminated to all study sites as the LEADER Global Expert Panel's ‘Standard of Care’ guideline and was therefore the principal target used in this analysis [7]. However, owing to subsequent changes in BP target recommendations, we also report achievement of targets according to the updated European Societies of Hypertension and Cardiology 2013 target of 140/85 mmHg [20] and the American Diabetes Association 2015 target of less than 140/90 mmHg [21]. Resistant hypertension was defined as prescription of four or more antihypertensive agents (or for those with BP >140/90 mmHg, the prescription of three or more agents) [21].
Sites were grouped as being from: North America [USA and Canada (n = 2821)]; Europe (Czech Republic, Poland, Romania, Austria, Belgium, Denmark, Finland, France, Germany, UK, Greece, Ireland, Italy, the Netherlands, Spain, Sweden, Turkey, and Serbia; n = 3521); and other [i.e. countries not easily grouped into geographical regions (India, China, South Korea, Taiwan, South Africa, Australia, Brazil, Mexico, Arab Emirates, Russia, and Israel; n = 2998)].
All study participants gave written informed consent. The trial (NCT01179048) was approved by the relevant local ethical committees and is being conducted in conformity with the Declaration of Helsinki.
Statistical analysis
Distributions of continuous variables are described by mean ± SD whereas categorical variables are listed as numbers and percentages (%). Associations of prior CVD status and global region with covariates and factors of interest were screened using Fisher's exact test and F-test, respectively. Multiple logistic regression models were developed to assess the association between dichotomized uncontrolled hypertension end points and predefined explanatory factors. Variable selection was decided on before model fitting. Covariates were selected by consideration of potential impact on hypertension ‘off-target’ measurements, based on review of the literature and availability in the LEADER database. Main effects of all covariates/factors were retained in the model, as the ample sample size of the LEADER study allowed no need for further variable selection. Statistical analyses were performed using SAS 9.3 software (SAS Institute, Cary, North Carolina, USA). Analyses were performed using data available after the trial recruitment phase, which may be subject to minor changes following closure of the database at trial completion.
RESULTS
A total of 91% of LEADER participants, all of whom had T2DM, were also hypertensive. In all 61% (n = 5665) of LEADER participants were prescribed two or more, and 19% (n = 1811) three or more antihypertensive agents. However, at the time of the baseline visit, only 51% were treated to a target of less than 140/85 mmHg and 26% to a target of less than 130/80 mmHg.
BP was lower in those who had prior CVD (81% of the study population) in comparison with those who had not (137 ± 18.8/78 ± 10.6 mmHg vs. 140 ± 17.7/80 ± 9.9 mmHg; P<0.001). A higher proportion of these individuals achieved recommended targets: 52 versus 44% for a target BP of less than 140/85 mmHg and 28 versus 21% for a target BP of less than 130/80 mmHg (both P < 0.001), presumably reflecting the use of more antihypertensive agents (≥3 in 20.9 vs. 13.0%; P < 0.001). Those with prior CVD were younger (63.9 ± 7.6 vs. 65.8 ± 5.2 years; P < 0.001) with a higher proportion being men and White (Table 1, left hand panel); estimated glomerular filtration rate was lower (79.2 ± 28.5 vs. 91.6 ± 23.1 ml/min per 1.73 m2; P < 0.001) although positive microalbuminuria status was less prevalent (13.9 vs. 48.6%; P < 0.001). HbA1c, duration of diabetes, and BMI were similar between the groups with and without prior CVD.
TABLE 1.
Demographic and clinical characteristics and clinical history stratified by prior cardiovascular disease and region
| All N = 9340 | No prior CVD N = 1748 | Prior CVD N = 7592 | P value | North America N = 2821 | Europe N = 3521 | Other N = 2998 | P value | |
| Age (years) | 64.3 ± 7.2 | 65.8 ± 5.2 | 63.9 ± 7.6 | <0.001 | 64.3 ± 7.4 | 64.8 ± 7.2 | 63.4 ± 6.6 | <0.0001 |
| Diabetes duration (years) | 12.7 ± 8.0 | 12.3 ± 7.5 | 12.8 ± 8.1 | 0.023 | 13.5 ± 8.6 | 11.6 ± 7.4 | 13.3 ± 8.0 | <0.0001 |
| BMI (kg/m2) | 32.5 ± 6.3 | 32.4 ± 6.3 | 32.5 ± 6.3 | 0.45 | 34.5 ± 6.8 | 32.7 ± 5.7 | 30.4 ± 5.8 | <0.0001 |
| Female (n, %) | 3337 (35.7) | 793 (45.4) | 2544 (33.5) | <0.001 | 975 (34.6) | 1080 (30.7) | 1282 (45.7) | <0.0001 |
| Prior CVD (n, %) | 7592 (81.3) | - | - | - | 2342 (83.0) | 2922 (83.0) | 2328 (77.7) | <0.0001 |
| HbA1c (%) | 8.7 ± 1.5 | 8.8 ± 1.6 | 8.7 ± 1.5 | 0.012 | 8.7 ± 1.5 | 8.3 ± 1.3 | 9.0 ± 1.7 | <0.0001 |
| Insulin treatment (n, %) | 3905 (41.8) | 645 (36.9) | 3260 (42.9) | <0.001 | 1182 (41.9) | 1399 (39.7) | 1020 (44.6) | 0.0014 |
| SBP (mmHg) | 138 ± 18.6 | 140 ± 17.7 | 137 ± 18.8 | <0.001 | 133 ± 18.1 | 141 ± 18.3 | 139 ± 18.8 | <0.0001 |
| DBP (mmHg) | 78 ± 10.5 | 80 ± 9.9 | 78 ± 10.6 | <0.001 | 75 ± 10.5 | 79 ± 10.2 | 80 ± 10.2 | <0.0001 |
| BP <140 and <90 mmHg (n, %) | 5083 (54.5) | 842 (48.2) | 4241 (55.9) | <0.0001 | 1881 (66.7) | 1624 (46.2) | 1578 (52.6) | <0.0001 |
| BP <140 and <85 mmHg (n, %) | 4720 (50.5) | 772 (44.2) | 3939 (52.0) | <0.0001 | 1788 (63.4) | 1493 (42.4) | 1439 (48.0) | <0.0001 |
| BP <130 and <80 mmHg (n, %) | 2469 (26.4) | 372 (21.3) | 2097 (27.6) | <0.0001 | 1073 (38.0) | 715 (20.3) | 681 (22.7) | <0.0001 |
| Resistant hypertension (n, %) | 1112 (11.9) | 160 (9.2) | 952 (12.5) | <0.001 | 255 (9.0) | 552 (15.7) | 305 (10.2) | <0.0001 |
| Number of antihypertensive medications | <0.001 | <0.0001 | ||||||
| 0 (n, %) | 790 (8.5) | 275 (15.7) | 515 (6.8) | 213 (7.6) | 275 (7.8) | 302 (10.1) | ||
| 1–2 (n, %) | 6739 (72.1) | 1245 (71.2) | 5494 (72.4) | 2078 (73.6) | 2443 (69.4) | 2218 (74.0) | ||
| 3–4 (n, %) | 1811 (19.4) | 228 (13.0) | 1583 (20.9) | 530 (18.8) | 803 (22.8) | 478 (15.9) | ||
| Race | <0.001 | <0.0001 | ||||||
| Asian (n, %) | 922 (9.9) | 169 (9.7) | 753 (9.9) | 58 (2.1) | 28 (0.8) | 836 (27.9) | ||
| Black (n, %) | 775 (8.3) | 240 (13.7) | 535 (7.0) | 472 (16.7) | 16 (0.5) | 287 (9.6) | ||
| White (n, %) | 7237 (77.5) | 1263 (72.3) | 5974 (78.7) | 2217 (78.6) | 3463 (98.4) | 1557 (51.9) | ||
| Other (n, %) | 406 (4.3) | 76 (4.3) | 330 (4.3) | 74 (2.6) | 14 (0.4) | 318 (10.6) | ||
| Microalbuminuria (n, %) | 1907 20.4 | 849 (48.6) | 1058 (13.9) | <0.001 | 610 (21.6) | 593 (16.8) | 704 (23.5) | <0.0001 |
| Macroalbuminuria (n, %) | 493 (5.3) | 190 (10.9) | 303 (4.0) | <0.001 | 145 (5.1) | 108 (3.1) | 240 (8.0) | <0.0001 |
| eGFR (ml/min per 1.73m2) | 81.5 ± 28.0 | 91.6 ± 23.1 | 79.2 ± 28.5 | <0.001 | 77.5 ± 26.6 | 82.8 ± 26.6 | 83.9 ± 30.3 | <0.0001 |
| Current or previous smoker (n, %) | 5467 (58.5) | 870 (49.8) | 4597 (60.5) | <0.001 | 1792 (63.5) | 2327 (66.1) | 1348 (45.0) | <0.0001 |
| Statin (n, %) | 7038 (75.4) | 1038 (59.4) | 6000 (79.0) | <0.001 | 2313 (82.0) | 2731 (77.6) | 1994 (66.5) | <0.0001 |
| Aspirin (n, %) | 5798 (62.1) | 695 (39.8) | 5103 (67.2) | <0.001 | 1876 (66.5) | 2126 (60.4) | 1796 (59.9) | <0.0001 |
Data are shown as mean ± SD unless stated otherwise; P values for covariates by F-test and factors by Fisher's exact test. BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate.
BP was higher in European participants (141 ± 18.3/79 ± 10.2 mmHg) than in those from North America (133 ± 18.1/75 ± 10.5 mmHg; P < 0.001) and other countries (139 ± 18.8/80 ± 10.2 mmHg) (P < 0.0001 for both SBP and DBP), even though more were prescribed three or more antihypertensive agents (23.0 vs. 19.0 vs. 16.0%, respectively; P < 0.001) (Table 1). BP target was achieved in more participants in North America and other countries than in Europe, that is, 63 vs. 48 vs. 42%, respectively, for less than 140/85 mmHg (P < 0.0001) and 38 vs. 23 vs. 20%, respectively, for less than 130/80 mmHg (P < 0.0001). Rates of achievement of BP control in individual countries within the regional categories are shown in Supplementary Table 1, rates of SBP and DBP control individually are shown in Supplementary Table 2.
BMI was higher in participants from North America than in those from Europe and other countries (34.5 ± 6.8 vs. 32.7 ± 5.7 vs. 30.4 ± 5.8 kg/m2;P < 0.0001); estimated glomerular filtration rate was lower (78 ± 26.6 vs. 83 ± 26.6 vs. 84 ± 30.3 ml/min per 1.73 m2; P < 0.0001) and a higher proportion were Black (17 vs. 0.5 vs. 9.6%; P < 0.0001). However, resistant hypertension was present in a higher proportion of Europeans compared with those from North America and other countries (15.7 vs. 9.0 vs. 10.2%; P < 0.0001). Documented duration of diabetes was shorter in European than in North American and other participants (11.6 ± 7.4 vs. 13.5 ± 8.6 vs. 13.3 ± 8.0 years; P < 0.0001) and HbA1c was lower (8.3 ± 1.3 vs. 8.7 ± 1.5 vs. 9.0 ± 1.7%; P < 0.0001). The prevalence of prior CVD did not differ among participants recruited in North America and Europe and similar proportions within each region were receiving insulin treatment (Table 1).
In logistic regression analyses restricted to participants prescribed at least one antihypertensive agent (and therefore operationally defined as having hypertension), the strongest predictor of achievement of a BP target of either less than 130/80 mmHg (Fig. 1), less than 140/85 mmHg (Fig. 2), or less than 140/90 mmHg (Fig. 3) was residence in North America.
FIGURE 1.
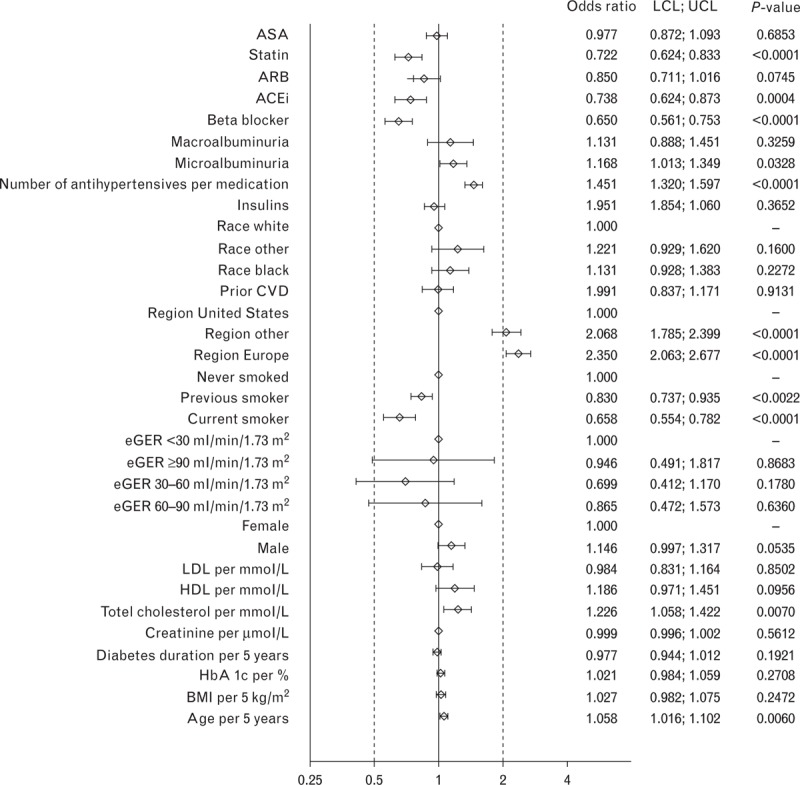
Odds ratios for characteristics associated with blood pressure target of >130/80 mmHg at baseline in the LEADER trial. ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LCL, lower confidence interval; LDL, low-density lipoprotein; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results; UCL, upper confidence interval.
FIGURE 2.
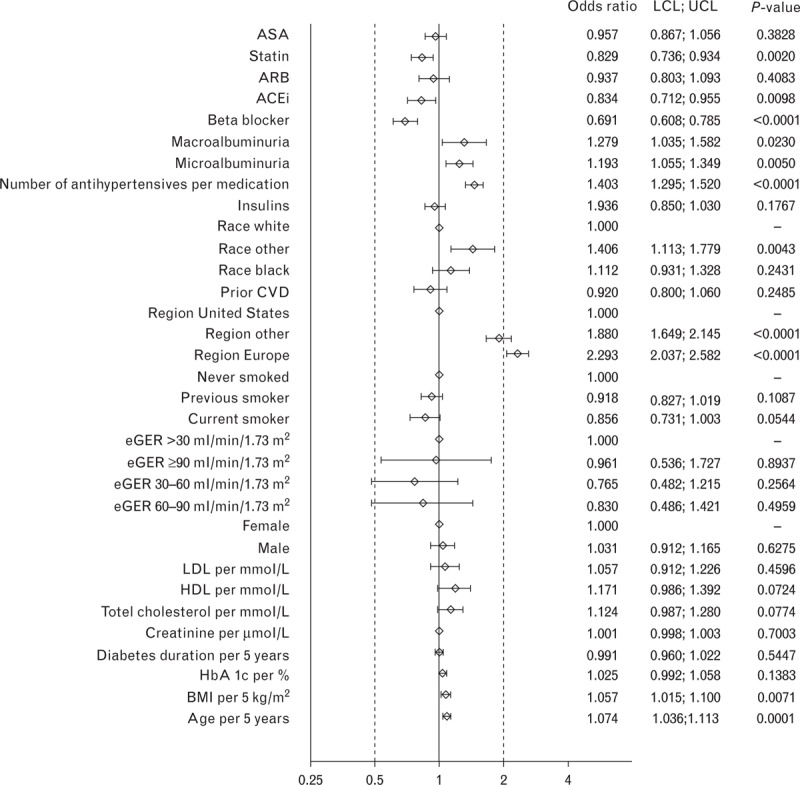
Odds ratios for characteristics associated with blood pressure target of >140/85 mmHg at baseline in the LEADER trial. ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LCL, lower confidence interval; LDL, low-density lipoprotein; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results; UCL, upper confidence interval.
FIGURE 3.
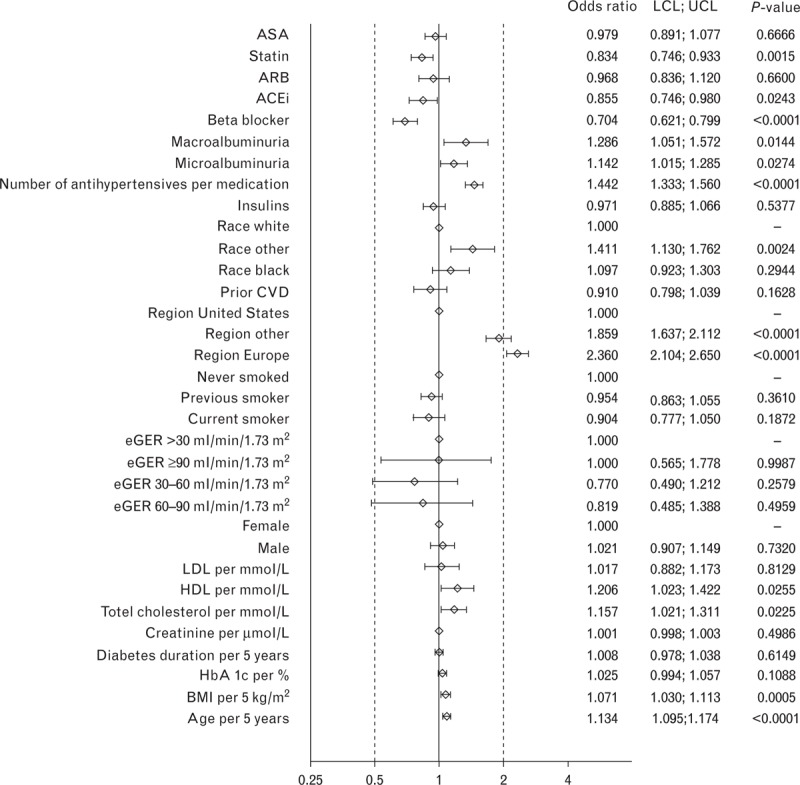
Odds ratios for characteristics associated with blood pressure target of >140/90 mmHg at baseline in the LEADER trial. ACEi, angiotensin-converting-enzyme inhibitor; ARB, angiotensin receptor blockers; ASA, acetylsalicylic acid; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LCL, lower confidence interval; LDL, low-density lipoprotein; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results; UCL, upper confidence interval.
DISCUSSION
These baseline data from the ongoing LEADER trial provide a cross-sectional description of BP control in a large cohort of individuals across global regions with long-standing T2DM and a high risk of CVD. At the time of randomization to liraglutide or placebo, the prevalence of hypertension (90%) was higher than in any other recent landmark trial of glucose-lowering therapy in T2DM (as shown in Table 2), though a proportion will have been misclassified as hypertensive because of prescription of antihypertensive agents for other indications. Nevertheless, despite this likely degree of misclassification, we report that BP measurements met the contemporary target set by most international bodies (<130/80 mmHg) in only one-quarter of participants.
TABLE 2.
Baseline characteristics of participants in the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results trial in relation to previous landmark trials
| Trial name | LEADER | EXAMINE [22] | SAVOR-TIMI 53 [23,24] | ACCORD [25] | RECORD [26] | ADVANCE [2] | PROACTIVE [27] | UKPDS Glucose [28] | UKPDS BP [1] | |
| Glucose | BP | |||||||||
| N | 9340 | 5380 | 16492 | 10251 | 4733 | 4447 | 11140 | 5238 | 3867 | 1148 |
| Age (years) | 64.3 ± 7.2 | 61.0 ± | 65 ± 8.5 | 62.2 ± 6.8 | 58.5 ± 8.3 | 66 ± 6.0 | 61.8 ± 7.7 | 53 ± 8.6 | 56.4 ± 8.1 | |
| Sex (% male) | 64.3 | 67.9 | 67.0 | 61.4 | 52.3 | 51.6 | 57 | 67 | 61 | 54 |
| Race (% white) | 77.5 | 72.7 | 75.3 | 64.5 | 60.5 | 98.9 | - | 99 | 81 | 87 |
| CVD (%) | 81 | 100 | 78 | 35 | 34 | 30 | 32 | 100 | – | – |
| Duration (years) | 12.7 ± 8.0 | 7.2 (2.6–13.8)* | 10.3 (5.2–16.7) | 10 (5–15) | 7.1 ± 5.0 | 8.0 ± 6.4 | 8 (4–14) | – | 2.6 (1–4.3) | |
| HbA1c (%) | 8.7 ± 1.5 | 8.0 ± 1.1 | 8.0 ± 1.4 | 8.3 ± 1.1 | 7.9 ± 0.7 | 7.5 ± 1.64 | 7.9 (7.0–8.9) | 6.2 ± 1.2 | 6.9 ± 1.6 | |
| BMI (kg/m2) | 32.5 ± 6.3 | 28.7 | 31.2 | 32.2 ± 5.5 | 31.6 ± 4.8 | 28 ± 5 | 31 ± 4.8 | 27.5 ± 5.2 | 29.6 ± 5.5 | |
| eGFR (ml/min per 1.73 m2) | 81.5 ± 28.0 | 71.2 | 72.6 ± 22.6 | 91.6 ± 28.8 | – | – | – | – | – | |
| eGFR <60 (%) | 21.8 | 29.1 | 72.6 | – | – | – | – | – | – | |
| Creatinine (μmol/l) | 86 ± 37.0 | – | – | 79 ± 17.7 | 65 | 87 ± 26 | 79 (68–92) | 81 (67–100) | – | |
| Microalbuminuria (%) | 20 | 19 | 27 | 46 | 7 | – | ||||
| ACR (mg/mmol) | 3.0 | – | 1.8 (0.7–7.7) | – | 14.3 (6.9–44.8) | – | 15 (7–40) | – | – | – |
| Hypertension (%) | 90.0 | 83.1 | 81.8 | – | 80 | 69 | 76 | – | – | |
| SBP (mmHg) | 138 ± 18.6 | – | – | 136 ± 17.1 | 139 ± 15.8 | 139 ± 15 | 145 ± 22 | 144 ± 18 | 135 ± 20 | 160 ± 19 |
| DBP (mmHg) | 78 ± 11 | – | – | 75 ± 11 | 76 ± 10 | 83 ± 8 | 81 ± 11 | 83 ± 10 | 82 ± 10 | 94 ± 10 |
| Antihypertensive medications (%) | 92 | – | – | 85 | 87 | – | 75 | 18 | 37 | |
| ACE inhibitor (%) | 49 | – | 54 | 53 | 52 | 43 (or ARB) | 43 | 63 | – | – |
| ARB (%) | 31.7 | – | 27.9 | – | 16.9 | – | 6 | 7 | – | – |
| β-blocker (%) | 55 | – | 62 | 29 | 26 | 21 | 25 | 55 | – | – |
| Smoker (%) | 12 | 14 | 13 | 14 | 13 | 16 | 14 | 13 | 31 | 23 |
| Insulin (%) | 42 | 30 | 41 | 35 | 37 | 0 | 2 | 42 | – | 24 |
| Statins (%) | 90 | 91 | 78 | 62 | 65 | 19 | 29 | 43 | – | – |
| Total cholesterol (mmol/l) | 4.4 ± 1.17 | 4.0 ± 1.13 | – | 4.7 ± 1.08 | 5.0 ± 1.16 | – | 5.2 ± 1.20 | 5.4 ± 1.10 | – | |
| LDL cholesterol (mmol/l) | 2.3 ± 0.92 | 2.04 | – | 2.7 ± 0.87 | 2.5 ± 1.01 | 3.30.9 | 3.1 ± 1.03 | 2.9 (2.3–3.5) | 3.5 ± 1.02 | – |
| HDL cholesterol (mmol/l) | 1.2 ± 0.32 | 1.12 | – | 1.1 ± 0.29 | 1.2 ± 0.37 | 1.2 ± 0.3 | 1.3 ± 1.03 | 1.1 (0.9–1.3) | 1.1 ± 0.24 | – |
*Data are shown as mean ± SD or median with interquartile ranges or range.
ACE, angiotensin-converting-enzyme; ACR, albumin:creatinine ratio; ARB, angiotensin receptor blockers; BP, blood pressure; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LEADER, Liraglutide Effect and Action in Diabetes: Evaluation of cardiovascular outcome Results.
LEADER was designed so that participants could be recruited in either primary or secondary CVD prevention categories: major baseline characteristics have already been reported separately for these groups [7]. The present analysis adds more detail to the previous report, giving BP measurements in relation to treatment and targets.
In unadjusted analyses, BP was better controlled in secondary prevention participants (who formed 81% of the total) than in those in the primary prevention category; this was in keeping with apparently more intensive pharmacological management. These findings are consistent with recently-reported baseline data from the secondary prevention study entitled Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS study) in which a higher proportion of participants who met the inclusion criteria because of previous myocardial infarction were treated to a target BP of less than 140/80 mmHg than in those with peripheral arterial disease but no prior cardiovascular event [29]. In LEADER, hypertension was one of the comorbidities specified for inclusion in the primary prevention category; this was one of the criteria selected to ensure that those randomized were at sufficient risk of CVD to provide adequate statistical power for the safety and efficacy end points. This may account, at least in part, for the apparently higher levels of baseline BP observed in this group. It should be noted that the presence or absence of CVD was not a significant determinant of BP in fully adjusted analyses. Nevertheless, in both primary and secondary prevention groups, there remained considerable scope for treatment intensification, as only 21 and 13%, respectively, were prescribed three or more antihypertensive agents.
Participants recruited in European centers and other countries had higher BP than those recruited in North America (by mean SBP and DBP 8/4 mmHg and 6/5 mmHg, respectively), although they were prescribed more antihypertensive agents, had lower BMI (by 2 kg/m2 and 4 kg/m2, respectively), and included fewer Black participants (usually considered to be more likely to be treatment resistant) [30]. Differences according to region of recruitment did not appear to be accounted for by differences in the demographic factors recorded.
Regional differences in control of BP have previously been reported from population-based surveys. In general, these have been consistent with the present observations, namely that BP levels in Europe are on average higher than in North America, particularly the USA [31,32]. These differences are not considered an artifact of method of measurement or case selection in that higher prevalence of hypertension at the population level is closely correlated with higher rates of stroke mortality [31]. In surveys of individuals without diabetes there can be higher rates of case-finding in some countries (e.g. the US) than others, biasing the mean BP of the treated population toward a lower level; this is unlikely to explain the present results, as all participants were known cases of T2DM under routine monitoring of BP. Neither can the differences be readily explained by treatment intensity as the number of antihypertensive agents prescribed per individual was similar in Europe and the US [33].
Higher BP at the population level in Europe may not be explained entirely by treatment effects, in that differences can be observed even in young adults (few of whom have a diagnosis of hypertension or are on BP-lowering treatment): therefore, other environmental and/or genetic factors must also play a role [31]. Differences in BP in the background populations from which participants were drawn may have been reflected in the cohort randomized in LEADER, but we acknowledge that the differences we report may also, at least in part, have reflected differences in sampling strategies among recruitment centers in European, North American, and other countries. Moreover, we cannot exclude a higher prevalence of nonadherence to therapy, more frequent prescription of less-effective therapy, or more frequent use of subtherapeutic dosages in European LEADER participants. There may also have been a lower prevalence of treatment-refractory hypertension amongst North American participants.
After the completion of recruitment into LEADER, BP targets were relaxed to less than 140/85 mmHg by the European Societies of Hypertension and Cardiology in 2013 [34]; they were then revised to less than 140/90 mmHg by the US Joint National Committee (JNC 8) [35] and the American Diabetes Association [36]. Even before recruitment into LEADER began, discussions had been initiated in Europe on potentially relaxing BP treatment targets [37]. It is possible that clinical practice began to change in European centers involved in LEADER prior to the publication of revised targets and that early implementation of this change in practice contributed to the higher mean BP levels we observed in European participants. This explanation has recently been invoked by the Trial Evaluating Cardiovascular Outcomes with Sitagliptin investigators to explain a similar mean difference in SBP and DBP (8/4 mmHg) at baseline between participants with T2DM from North America versus Western Europe [29].
Even with revised targets, rates of BP control cannot be considered satisfactory at baseline in LEADER, as these were achieved only in 50–54% of trial participants. Although these rates may at first sight seem low, they are in keeping with a contemporary pooled analysis of longitudinal data from individuals with and without diabetes [38]; the literature suggests they are likely to be an improvement on those that would have been observed a decade ago [38–40]. The predictors of nonachievement of BP targets in our analyses were broadly similar, independently of the target figures used. The observation that BP control was markedly better in the United States than in Europe in the large samples of patients with T2DM and high cardiovascular risk recruited into the LEADER trial prompts us to reflect on the potential public health effects of the recent global (European-led) relaxation of BP targets in terms of hypertension-related cardiovascular outcomes, particularly given that targets are infrequently achieved and BP lowering using currently available pharmacological agents is well established to reduce rates of mortality and other complications [41]. The SPRINT trial has recently highlighted the benefits of BP-lowering in nondiabetic individuals and hence rekindled enthusiasm for more intensive BP targets in diabetes [42]. This has reopened discussion of the results of the ACCORD trial [25] which provided a key stimulus for relaxation of previous BP targets in individuals with T2DM.
The strength of the present analysis is the large number of patients studied at baseline in a clinical trial setting, with data collection according to a standard protocol and use of a central laboratory. Moreover, multiracial sampling of individuals from a global population favors the generalizability of our findings. However, as acknowledged above, selection bias at study sites could have influenced our results. Furthermore, measurement of BP was cross-sectional rather than longitudinal and some details of the methods of measurement within individual sites were not available.
It is clear from the present analysis that BP targets are too infrequently achieved in many individuals with T2DM. Many mechanisms for resistance to therapy have been identified and reviewed, including higher baseline BP because of obesity, activation of the sympathetic nervous system, changes in central hemodynamics [43], volume overload, and renin–angiotensin system activation [41]. Glucose-lowering therapies that also possess BP-lowering effects may therefore have an attractive profile from a CVD prevention perspective, as demonstrated by a reduction in cardiovascular death with the SGLT-2 inhibitor with empagliflozin as recently reported in the EMPA-REG trial [44].
GLP-1 receptor agonists and SGLT-2 inhibitors reduce BP in part via weight reduction, but also influence renal sodium handling [12,45], whereas GLP-1 receptor agonists may also have direct effects on the vasculature [12]. It is reasonable to speculate that such agents may facilitate the long-term achievement of BP targets in people with hypertension and T2DM treated with multiple agents, although as shown in the recent ELIXA trial, this will not necessarily translate into cardiovascular benefit [46]. Moreover, it should also be noted that in the LEADER trial, a ‘standard of care’ approach was used, that is, any effect of GLP-1 therapy on BP between the groups as randomized over the full duration of the trial may ultimately be offset by additional therapy in the placebo arm.
In summary, the present data highlight that, even with modern antihypertensive therapy, adequate control of BP is not achieved in many high-risk individuals with T2DM, particularly outside North America. Longitudinal data from the LEADER trial may provide further insights into BP control in relation to cardiovascular outcomes in this condition.
ACKNOWLEDGEMENTS
The authors wish to thank David Dynnes Ørsted and Henrik Wachmann from Novo Nordisk for support with statistical analysis and coordination in the preparation of this study, and Watermeadow Medical (supported by Novo Nordisk) for editorial assistance. We are also greatly indebted to the many people with type 2 diabetes worldwide who agreed to participate in the LEADER trial.
Prior presentation: Abstracts based on this study were presented as posters at the 51st European Association for the Study of Diabetes Annual Meeting (EASD Stockholm, 14–18 September 2015; A-15-713) and the International Diabetes Federation Meeting (IDF Vancouver, 30 November – 4 December 2015; 0113-PD).
Source of funding: Editorial assistance, provided by Watermeadow Medical, was funded by Novo Nordisk.
Conflicts of interest
J.R.P. is a member of the LEADER Global Expert Panel for which he has received honoraria and meeting expenses from Novo Nordisk. He has also acted in an advisory capacity under contracts between his employer and the following companies: Alere, AstraZeneca, Boehringer-Ingleheim, Eli-Lilly, GlaxoSmithKline, Janssen, Merck Sharpe & Dohme, and Sanofi. He receives support-in-kind for an investigator-led trial from Merck Group (Germany).
S.P.M. has received research grants and or consulting fees from Amylin, Novo Nordisk, St. Jude Medical, Terumo, The Medicines Company, and Volcano Corp. S.C.B. has served in advisory panels for Astra-Zeneca, Boehringer Ingelheim; Bristol-Meyers Squibb, Janssen, Eli Lilly, Merck Sharpe & Dohme, Novo Nordisk and Sanofi, and as board member for Glycosmedia. E.F. has served in advisory panels for AstraZeneca/Bristol-Meyers Squibb, Bioton, Boehringer Ingelheim, Johnson & Johnson, Novartis and Novo Nordisk, and attended speaker's bureau for AstraZeneca/Bristol-Meyers Squibb, Bioton, Boehringer Ingelheim, Merck, Novo Nordisk and Servier. S.J. has served in advisory panel/speaker's bureau/as consultant, and received research support from Abbott, Astra Zeneca, Bayer, Bristol-Meyers Squibb, Boehringer Ingelheim, Daiichi-Sankyo, Essex, GlaxoSmithKline, Janssen, Johnson & Johnson, Eli Lilly, Merck, Merck Sharp & Dohme, Novo Nordisk, Novartis, Pfizer, Roche, Sanofi-Aventis, Solvay, Takeda and Viatris. L.M. has served in advisory panels for Janssen and Novo Nordisk, received research support from Sanofi-Aventis, attended speaker's bureau for Abbott, Merck Sharp & Dohme, Eli Lilly, Novo Nordisk, Sanofi-Aventis and AstraZeneca, and has been involved in clinical trials sponsored by Novo Nordisk, Sanofi-Aventis, GlaxoSmithKline and Boehringer-Ingelheim. L.A.L. has served on advisory panels for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Merck, Novo Nordisk, Sanofi and Servier; received research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Pfizer, Sanofi and Servier; attended speaker's bureau for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk and Sanofi. M.H. has served in advisory panels for Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novo Nordisk and Sanofi; attended speaker's bureau for Eli Lilly and Novo Nordisk; and received research support from AstraZeneca/Bristol-Meyers Squibb and Eli Lilly. I.S. is an investigator and/or a consultant without any direct financial benefit under contracts between her employer and the following companies: AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk and Sanofi. M.O. has served in advisory panels for Novo Nordisk, AstraZeneca, Bristol-Meyers Squibb, Novartis, Eli Lilly, Servier, Sanofi, and Merck Sharp & Dohme, and attended speaker's bureau for Eli Lilly, Novo Nordisk, Sanofi, Servier, Novartis, Merck, Merck Sharp & Dohme and Medtronic. M.S. has served in advisory panels for Novo Nordisk, received research support from Novartis, and attended speaker's bureau AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck Sharp & Dohme, Novartis, Novo Nordisk, and Servier. L.V.G. has served on advisory panels for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi U.S; and attended speaker's bureau for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk and Sanofi U.S. J.F.M. is an investigator and/or consultant receiving honoraria from Abbott, Bayer, Boehringer Ingelheim, Novo Nordisk, Roche and Vifor. F.M.M.B. is a full-time employee and stock holder of Novo Nordisk A/S. B.Z. has received honoraria from AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk, Merck, Sanofi, Eli Lilly and Takeda for scientific advisory board meetings and presentations. His institution has received research support from Novo Nordisk, Boehringer Ingelheim and Merck. N.R.P. has received speaker's fees from Novo Nordisk, Servier and Takeda. He has also received fees to attend advisory board meetings from Takeda and Novo Nordisk, and consultancy fees regarding the conduct of randomized controlled trials from Astra Zeneca and Novo Nordisk.
Supplementary Material
Reviewers’ Summary Evaluations
Reviewer 1
The study illustrates baseline characteristics of type 2 diabetic patients included in the LEADER 4 trial, thus reporting that the overall blood pressure control rate (<140/90 mmHg) is relatively poorly achieved, particularly when considering the more intensive blood pressure targets (<130/80 mmHg). This is of potential clinical relevance, since the issue concerning the most appropriate blood pressure targets to be achieved in high-risk hypertensive patients with diabetes is still actively debated. For these reasons, a closer analysis of baseline cardiovascular risk stratification might be of help for properly interpreting the future outcomes of this trial.
Reviewer 2
This study presents data from the LEADER trial, confirming the relatively poor levels of blood pressure control achieved in type 2 diabetes patients at high cardiovascular risk, especially according to more strict BP targets and in countries other than North America. Despite the fact that comparability is limited by the nature of a multicenter trial, with different sampling schemes, this study provides an informative contemporary, inter-region perspective on BP control among diabetes patients.
REFERENCES
- 1.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 2.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, et al. ADVANCE Collaborative Group. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840. [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). 2007 Guidelines for the management of arterial hypertension. Eur Heart J 2007; 28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003; 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 5.Bakris G, Sarafidis P, Agarwal R, Ruilope L. Review of blood pressure control rates and outcomes. J Am Soc Hypertens 2014; 8:127–141. [DOI] [PubMed] [Google Scholar]
- 6.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian cardiac outcomes trial-blood pressure lowering arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005; 366:895–906. [DOI] [PubMed] [Google Scholar]
- 7.Marso SP, Poulter NR, Nissen SE, Nauck MA, Zinman B, Daniels GH, et al. Design of the liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J 2013; 166:823–830. [DOI] [PubMed] [Google Scholar]
- 8.Reisin E, Graves JW, Yamal JM, Barzilay JI, Pressel SL, Einhorn PT, et al. Blood pressure control and cardiovascular outcomes in normal-weight, overweight, and obese hypertensive patients treated with three different antihypertensives in ALLHAT. J Hypertens 2014; 32:1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stamler R, Stamler J, Riedlinger WF, Algera G, Roberts RH. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA 1978; 240:1607–1610. [DOI] [PubMed] [Google Scholar]
- 10.Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med 2012; 366:1319–1327. [DOI] [PubMed] [Google Scholar]
- 11.Petrie JR, Adler A, Vella S. What to add in with metformin in type 2 diabetes? QJM 2011; 104:185–192. [DOI] [PubMed] [Google Scholar]
- 12.Petrie JR. The cardiovascular safety of incretin-based therapies: a review of the evidence. Cardiovasc Diabetol 2013; 12:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clar C, Gill JA, Court R, Waugh N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2: pii e001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU). Diabet Med 2009; 26:268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care 2009; 32:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373:473–481. [DOI] [PubMed] [Google Scholar]
- 17.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 2009; 32:1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia 2009; 52:2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens 2009; 27:2121–2158. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes – 2015. Diabetes Care 2015; 38 Suppl. 1:S1–S93. [PubMed] [Google Scholar]
- 22.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013; 369:1327–1335. [DOI] [PubMed] [Google Scholar]
- 23.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013; 369:1317–1326. [DOI] [PubMed] [Google Scholar]
- 24.Mosenzon O, Raz I, Scirica BM, Hirshberg B, Stahre CI, Steg PG, et al. Baseline characteristics of the patient population in the Saxagliptin Assessment of Vascular Outcomes Recorded in patients with diabetes mellitus (SAVOR)-TIMI 53 trial. Diabetes Metab Res Rev 2013; 29:417–426. [DOI] [PubMed] [Google Scholar]
- 25.ACCORD study group. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 2009; 373:2125–2135. [DOI] [PubMed] [Google Scholar]
- 27.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (prospective pioglitazone clinical trial in macrovascular events): a randomised controlled trial. Lancet 2005; 366:1279–1289. [DOI] [PubMed] [Google Scholar]
- 28.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352:837–853. [PubMed] [Google Scholar]
- 29.Bethel MA, Green JB, Milton J, Tajar A, Engel SS, Califf RM, Holman RR. TECOS Executive Committee. Regional, age and sex differences in baseline characteristics of patients enrolled in the trial evaluating cardiovascular outcomes with sitagliptin (TECOS). Diabetes Obes Metab 2015; 17:395–402. [DOI] [PubMed] [Google Scholar]
- 30.White WB, Turner JR, Sica DA, Bisognano JD, Calhoun DA, Townsend RR, et al. Detection, evaluation, and treatment of severe and resistant hypertension: proceedings from an American Society of Hypertension Interactive forum held in Bethesda, MD, U.S.A. J Am Soc Hypertens 2014; 8:743–757. [DOI] [PubMed] [Google Scholar]
- 31.Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United States. JAMA 2003; 289:2363–2369. [DOI] [PubMed] [Google Scholar]
- 32.Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure). National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet 2011; 377:568–577. [DOI] [PubMed] [Google Scholar]
- 33.Wolf-Maier K, Cooper RS, Kramer H, Banegas JR, Giampaoli S, Joffres MR, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension 2004; 43:10–17. [DOI] [PubMed] [Google Scholar]
- 34.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 2013; 31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 35.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. Standards of medical care in diabetes 2014. Diabetes Care 2014; 37 Suppl 1:S14–S80. [DOI] [PubMed] [Google Scholar]
- 37.Mancia G, Laurent S, Agabiti-Rosei E, Ambrosioni E, Burnier M, Caulfield MJ, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. Blood Press 2009; 18:308–347. [DOI] [PubMed] [Google Scholar]
- 38.Rückert IM, Baumert J, Schunk M, Holle R, Schipf S, Völzke H, et al. Blood pressure control has improved in people with and without type 2 diabetes but remains suboptimal: a longitudinal study based on the German DIAB-CORE consortium. PLoS One 2015; 10:e0133493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mourad JJ, Le Jeune S. Blood pressure control, risk factors and cardiovascular prognosis in patients with diabetes: 30 years of progress. J Hypertens Suppl 2008; 26:S7–13. [PubMed] [Google Scholar]
- 40.Borzecki AM, Berlowitz DR. Management of hypertension and diabetes: treatment goals, drug choices, current practice, and strategies for improving care. Curr Hypertens Rep 2005; 7:439–449. [DOI] [PubMed] [Google Scholar]
- 41.Eguchi K, Pickering TG, Kario K. Why is blood pressure so hard to control in patients with type 2 diabetes? J Cardiometab Syndr 2007; 2:114–118. [DOI] [PubMed] [Google Scholar]
- 42.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agnoletti D, Lieber A, Zhang Y, Protogerou AD, Borghi C, Blacher J, Safar ME. Central hemodynamic modifications in diabetes mellitus. Atherosclerosis 2013; 230:315–321. [DOI] [PubMed] [Google Scholar]
- 44.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 45.Wilding JP. The role of the kidneys in glucose homeostasis in type 2 diabetes: clinical implications and therapeutic significance through sodium glucose co-transporter 2 inhibitors. Metabolism 2014; 63:1228–1237. [DOI] [PubMed] [Google Scholar]
- 46.Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med 2015; 373:2247–2257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


