Abstract
Objectives:
A sizeable percentage of individuals infected by HIV and on antiretroviral therapy (ART) fail to increase their CD4+ T-cells to satisfactory levels. The percentage of regulatory T-cells (Tregs) has been suggested to contribute to this impairment. This study aimed to address this question and to expand the analysis of Tregs subpopulations during ART.
Design:
Longitudinal follow-up of 81 HIV-infected individuals during the first 24 months on ART.
Methods:
CD4+ T-cell counts, Tregs percentages, and specific Tregs subpopulations were evaluated at ART onset, 2, 6, 9, 12, 16, 20, and 24 months of ART (five individuals had no Tregs information at baseline).
Results:
The slope of CD4+ T-cell recovery was similar for individuals with moderate and with severe lymphopenia at ART onset. No evidence was found for a contribution of the baseline Tregs percentages on the CD4+ T-cell counts recovery throughout ART. In comparison to uninfected individuals, Tregs percentages were higher at ART onset only for patients with less than 200 cells/μl at baseline and decreased afterwards reaching normal values. Within Tregs, the percentage of naive cells remained low in these patients. Reduced thymic export and increased proliferation of Tregs vs. conventional CD4+ T cells might explain these persistent alterations.
Conclusion:
No effect of Tregs percentages at baseline was detected on CD4+ T-cell recovery. However, profound alterations on Tregs subpopulations were consistently observed throughout ART for patients with severe lymphopenia at ART onset.
Keywords: antiretroviral therapy, baseline CD4+ T-cell counts, HIV, immune reconstitution, regulatory T cells
Introduction
The emergence of drugs with potent antiretroviral activity and their combination for the treatment of HIV infection markedly reduced the morbidity and mortality associated with this infection. The antiretroviral therapy (ART) dampens viral replication, allowing the raise of the CD4+ T-cell counts, which culminates with immune reconstitution in most patients [1]. However, among the HIV-infected individuals that initiate ART and become virologically suppressed for several years (4–7 years), 15–40% fail to achieve a satisfactory CD4+ T-cell reconstitution. These individuals are referred to as immunological nonresponders [2]. The failure to reconstitute the CD4+ T-cell counts might result from insufficient production of new T cells and/or insufficient division of the peripheral T cells, which may be aggravated by continuous peripheral destruction of T cells [3]. Several conditions, individually or in combination, might contribute to this defect (reviewed by Gaardbo et al.[2] and by Corbeau et al.[4]): advanced age; long periods of time between HIV infection and ART initiation; bone marrow impairment to produce hematopoietic stem cells; impaired thymic function; enhanced T-cell activation and/or apoptosis (mainly because of residual viral replication, gut microbial translocation, or coinfections by hepatitis C virus, cytomegalovirus, or other herpes virus). Importantly, low CD4+ T-cell counts at ART onset have consistently been shown as the strongest predictor for poorer CD4+ T-cell recovery even for virologically suppressed individuals.
The role of regulatory T cells (Tregs) in HIV-infection pathogenesis has been extensively debated [5]. These cells may play a beneficial role by dampening the immune activation and suppressing HIV replication within conventional CD4+ T cells (Tconv) [6,7]. Still, Tregs may have a harmful role by impairing specific anti-HIV immune response [8–11], inhibiting lymphopenia-induced proliferation [12,13], contributing for gut microbial translocation [14,15] and for exacerbated lymphoid tissues’ fibrosis [16]. The dynamics of Tregs during HIV infection have been the subject of several studies. Most reports describe that Tregs percentages among CD4+ T cells in ART-naïve HIV-infected individuals is higher than in healthy controls [17–22], though others observed no differences [7,8,23]. The percentage of Tregs during ART is also a controversial issue, whereas most studies show that Tregs percentages during ART tend to reach values similar to those in HIV-uninfected controls [18,19,22,24–26], others claim its maintenance above what is considered normal for healthy controls [17,20,21,27]. The potential effect of Tregs on the immune recovery of individuals on ART has also been suggested. Although a few studies showed an association between high Tregs percentages and lower CD4+ T-cell numbers [3,12,25,28,29], the contribution of Tregs percentages, at ART onset, on the quality of the immune reconstitution has not been established. The lack of consensus between the various studies might relate to diverse factors including the study design (cross-sectional or longitudinal studies), the small number of patients in most studies, the heterogeneity on the baseline CD4+ T-cell counts at ART onset and the definition of Tregs (please see supplementary Table S1, for more detailed information on studies addressing these questions). In an attempt to bring some clarification to these important questions, we considered of relevance to perform a longitudinal study, encompassing a reasonable number of individuals, followed carefully from ART onset, presenting quite different levels of CD4+ T-cell counts at baseline and for whom all samples were analysed with the same protocol, being Tregs identified using the most updated available and comprehensive set of markers (CD25highCD127-FOXP3+ among CD4+CD3+ lymphocytes) [30]. In accordance, we followed a cohort of HIV-infected individuals from ART onset up to 24 months. In the analysis, several parameters that could be confounders for the association between Tregs at baseline and CD4+ T-cell recovery were considered.
Materials and methods
Study population
Individuals included in this study were on medical care at the Hospital Joaquim Urbano Unit/Centro Hospitalar do Porto (HJUU/CHP), Portugal. Individuals were provided an explanation of the study and, from those who agreed to participate, a signed informed consent was obtained (Hospital Joaquim Urbano's Ethical Committee approval reference 168/CES, 2 October, 2009). Inclusion criteria were: being over 18 years of age, infected with HIV-1 (referred to as HIV, from now on), and ART-naive but with criteria to initiate ART. Clinical information and blood samples were collected at baseline (i.e. immediately before ART initiation) and 2, 6, 9, 12, 16, 20, and 24 months afterwards. ART schemes chosen (Supplementary Table 2) for each individual took into consideration characteristics of each individual, scientific policy, and the national and international guidelines [31,32].
From the 96 individuals initially involved in the study, 81 (95% Caucasian) filled the requisites to be included in the present work as they were therapy compliant for the 24 months (cohort characterization depicted in Supplementary Table S2). From the 81 individuals included in the study (Supplementary Table S3), no information regarding Tregs and its subpopulations at baseline was available for five of them (AH006, AH037, AH091, AH092 and AH093), two individuals presented plasma viral loads above 50 copies/ml at 24 months after ART onset (AH054 and AH080) but were confirmed to be virologically suppressed at 28 months, for four individuals, the data relative to 28 months of follow-up were used instead of the 24 months (three did not attend the 24 months-visit – AH014, AH031, and AH085 – and one had pneumonia at this time point – AH030). A group of age and sex-matched HIV-uninfected study participants, living in the same region, was used as control (Supplementary Table S2).
Sample processing and flow cytometry
Blood was collected and sent on the same day for routine measurements (by a reference laboratory to assess CD4+ T-cell count and HIV viral load) and for complementary multiparametric flow cytometry analyses. Quantification of Tregs was performed in peripheral blood mononuclear cells and the evaluation of overall CD4+ T-cell activation in whole blood, as previously described [25], using the following anti-human monoclonal antibodies: anti-CD3 (OKT3 or UCHT1), anti-CD4 (RPA-T4), anti-CD8 (RPA-T8), anti-CD25 (BC96), anti-CD31 (WM59), anti-CD45RA (HI100), anti-CD45RO (UCHL1), anti-CD127 (clone AO19D5), anti-HLA-DR (L243), anti-FOXP3 (PCH101), and anti-Ki67 (MOPC-21). Samples were acquired on a BD LSRII flow cytometer using the FACS DIVA v6.0 (BD Biosciences, San Jose, California, USA) and data analysed using FlowJo vX.07 (TreeStar, Ashland, Oregon, USA). The gating strategy is presented on Supplementary Figure S1.
Statistical analysis
To evaluate the distribution of the variables, skewness, and kurtosis values were calculated and normal distribution was considered for variables with absolute values of skewness below 3 and kurtosis below 8 [33]. Brown–Forsythe test was used to evaluate equality of variances. When the conditions of normality, equality of variances, and other assumptions specific for each test were observed, parametric tests were used; otherwise, the nonparametric counterpart was chosen. Repeated measures one-way analysis of variance (ANOVA) or Friedman's test, followed by Bonferroni's or Dunn's multiple comparison tests respectively, were used to perform repeated measures to evaluate the evolution of Tregs percentages and CD4+ T-cell counts throughout ART in comparison to baseline (Figs 1b and 2b). Two-way ANOVA repeated measures followed by Sidak's multiple comparison tests were used to compare the CD4+ T-cell counts throughout ART between individuals with less than or at least 10% Tregs among CD4+ T cells (Fig. 3). One-way ANOVA or Kurskall–Wallis test, followed by Bonferroni's or Dunn's multiple comparison tests respectively, were used to compare the CD4+ T-cell slopes between the different CD4+ T-cell strata at baseline (Fig. 1c), the Tregs percentages of HIV-uninfected individuals with that from the HIV-infected individuals at different times of ART (Fig. 2a) and the percentage of naïve and proliferating Tregs and the recent thymic emigrant Tregs counts (Fig. 4). As missing values are not allowed on repeated measurements analyses (Figs 1c, 2b, and 3), this limitation was surpassed by estimating the missing values as the mean of the neighbour values (estimated values represented 1.25 and 2.88% of all CD4+ T-cell counts and Tregs percentages measurements, respectively; Supplementary Table S3, depicts the raw data where estimated values are underlined). Importantly, the analyses performed including and excluding individuals with estimated values yielded similar results (Supplementary Figures S2 and S3, respectively.
Fig. 1.
CD4+ T-cell counts increase rate was similar between individuals with quite distinct CD4+ T-cell counts at baseline.
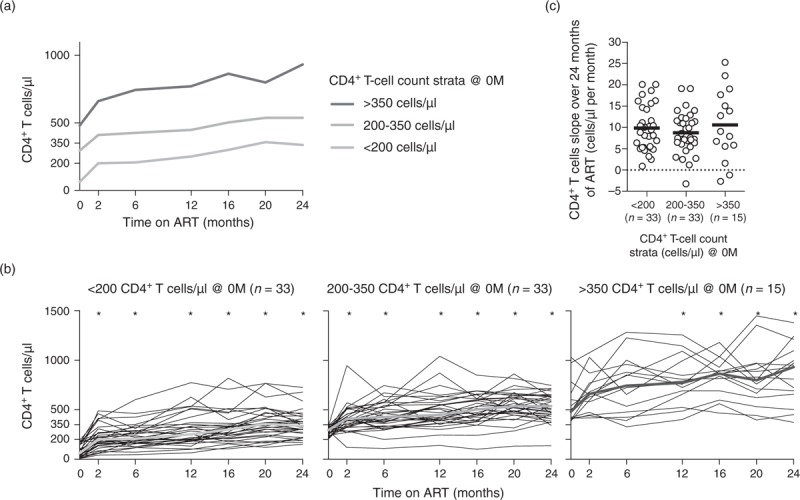
(a) CD4+ T-cell progression for three-defined CD4+ T-cell strata. The median of each time point is represented for the three strata independently. (b) The evolution of the CD4+ T-cell counts throughout ART is represented for each individual (thin black lines) and for the median of all the individuals (thick green line). The evolution of CD4+ T-cell counts with ART was evaluated using one-way ANOVA repeated measures followed by Bonferroni's multiple comparison tests using as reference group the ART-naive individuals: <200 F (32, 192) = 17.59, P < 0.0001, η2 = 0.326; 200–350: F (32, 192) = 10.83, P < 0.0001, η2 = 0.275; and >350: F (14, 84) = 17.64, P < 0.0001, η2 = 0.140. (c) Comparison of the rates of CD4+ T-cell recovery during the 24 months of ART between the three-defined strata. Slopes were calculated by the least squares estimation method [25]. Each dot represents a single individual and the solid horizontal line the mean. Comparisons were performed using a Kruskal–Wallis test followed by Dunn's multiple comparison tests; no statistical significant differences were observed (X2KW (2, 78) = 0.528 P = 0.7681, η2KW = 0.264). ART, antiretroviral therapy; M, months.
Fig. 2.
Immunosuppressed individuals at ART onset tend to show higher Tregs percentages, which declined with ART for most patients.
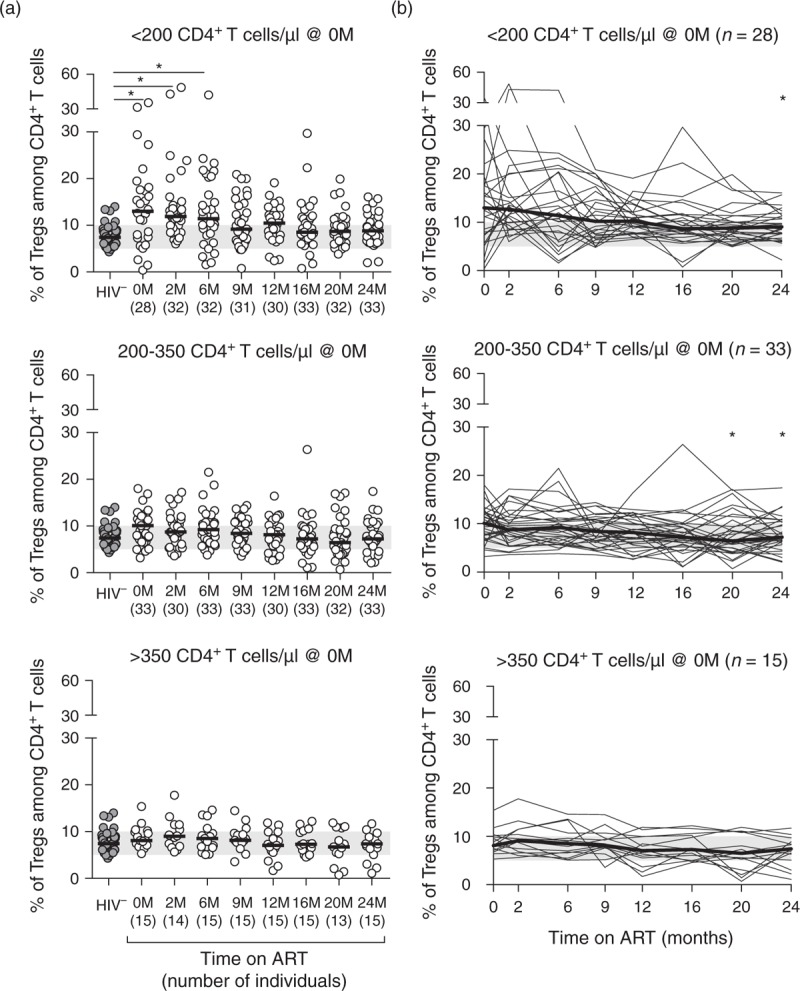
(a) Tregs percentages of healthy controls and HIV-infected individuals stratified according to their CD4+ T-cell counts at baseline. Each dot represents a single individual, the horizontal black line represents the group's median, and the shaded green horizontal bar represents the range of Tregs percentages among CD4+ T cells described for healthy individuals using similar markers [8]. Comparisons were performed using an one-way ANOVA or a Kruskal–Wallis test, followed by Bonferroni's or Dunn's multiple comparison tests, respectively, using as reference group the HIV-uninfected individuals: <200: X2KW (8, 286) = 34.590, P < 0.0001, η2KW = 0.118; 200–350: F (8,292) = 1.895, P = 0.0605, η2 = 0.049; and >350: F (8, 152) = 1.759, P = 0.0894, η2 = 0.085. (b) Stratification of HIV-infected individuals according to CD4+ T-cell counts at baseline and longitudinal evaluation of Tregs percentages throughout the first 24 months of ART. Each thin black line represents the Tregs percentages evolution of a single individual and the bold black line represents the median of the individuals for each time point. The evolution of Tregs percentages with ART was evaluated using Friedman's test followed by Dunn's multiple comparison tests using as reference group the ART-naive individuals:<200: X2F (7) = 27.91, P = 0.0002, n = 28, WK = 0.129; 200–350: X2F (7) = 23.19, P = 0.0016, n = 33, WK = 0.091; and >350: X2F (7) = 20.43, P = 0.0047, n = 15, WK = 0.182. ART, antiretroviral therapy; M, months.
Fig. 3.
Increase of CD4+ T-cell counts during ART was not associated with Tregs percentage at baseline.
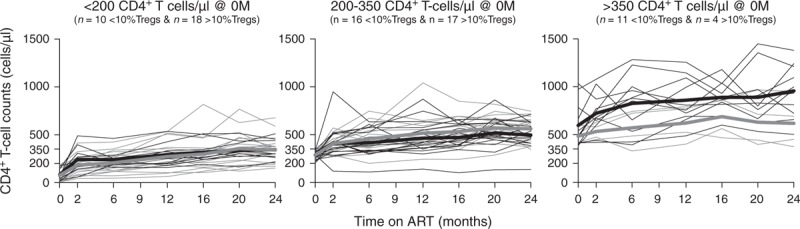
Stratification of HIV-infected individuals based on their CD4+ T-cell counts at baseline (<200, 200–350, and >350 cells/μl) and on their Tregs percentage at baseline (<10%, grey lines; ≥10%, green lines) and evaluation of the CD4+ T-cell reconstitution along ART. Each thin line represents a single individual and bold lines represent the median of all individuals from each group at each specific time point. A two-way ANOVA repeated measurements followed by Sidak's multiple comparison tests was performed to compare the CD4+ T-cell evolution of the two groups throughout ART: <200: F (1, 26) = 0.177, P = 0.6774, η2P = 0.017; 200–350: F (1, 31) = 1.017, P = 0.3211, η2P = 0.055; and F (1, 13) = 3.911, P = 0.0696, η2P = 0.419). ART, antiretroviral therapy; M, months.
Fig. 4.
Individuals with severe lymphopenia at baseline maintain throughout ART low percentage of Tregs with a naive phenotype and impaired production of Tregs by the thymus.
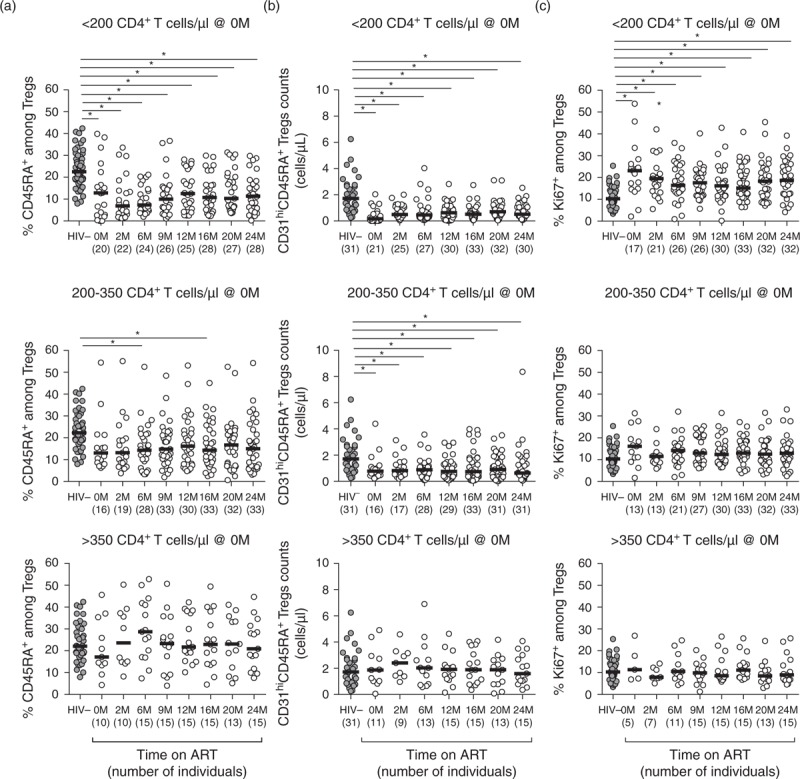
(a) Percentage of CD45RA+ cells among Tregs upon stratification of the individuals accordingly to their CD4+ T-cell counts at baseline. (b) Numbers of Tregs recent thymic emigrants (CD45RA+CD31hi) upon stratification of the individuals accordingly to their CD4+ T-cell counts at baseline. (c) Percentage of Ki67+ cells among Tregs upon stratification of the individuals accordingly to their CD4+ T-cell counts at baseline. In all graphs, each dot represents a single individual and the horizontal lines represent the median. Comparison between healthy controls and HIV-infected individuals at ART onset or on different time points upon ART initiation was performed using an one-way ANOVA or a Kruskal–Wallis test followed by Bonferroni's or Dunn's multiple comparison tests, using as reference group the uninfected individuals: (a) <200: F (8, 246) = 8.70, P < 0.0001, η2 = 0.221; 200–350: F (8, 262) = 1.91, P = 0.0585, η2 = 0.055; and >350: F (8, 144) = 0.63, P = 0.7554, η2 = 0.034; (b) <200: X2KW (7, 221) = 43.52, P < 0.0001, η2KW = 0.191; 200–350: F (7, 208) = 2.50, P = 0.0173, η2 = 0.078; and >350: F(7, 114) = 0.29, P = 0.9576, η2 = 0.017; (c) <200: X2KW (8, 152) = 37.64, P < 0.0001, η2KW = 0.145; 200–350: F (8, 237) = 1.32, P = 0.2362, η2 = 0.043; and >350: F (8, 131) = 0.61, P = 0.7714, η2 = 0.036. ART, antiretroviral therapy; M, months.
To evaluate the predictors for the CD4+ T-cell counts at 24 months of ART, hierarchical linear regression models were used. Independent variables were included in the model when P < 0.20 on a preliminary univariate analysis (Supplementary Table S4.
Overall, tests were considered significant when P < 0.05 and denoted with ∗; no intertest adjustments of the P-value were performed. As a measure of the magnitude of a difference, effect size (practical significance), was calculated as follows [34]: for the one-way ANOVA (both ordinary and repeated measures) the eta square (η2) was calculated as the ratio of the between-groups sum of squares (SSbtw) and the total SS (SStot; η2 = SSbtw/ SStot); for the Kruskal–Wallis test, η2 (η2KW) was calculated as the ratio of χ2 and the number of individuals (n) minus 1 [η2KW = χ2/(n − 1)]; for the two-way ANOVA repeated measures the partial η2 (η2P) was calculated as the ratio of the SSbtw and the sum of the SSbtw and the residual SS [SSres; η2P = SSbtw/(SSbtw + SSres)]; for the Friedman's test, Kendal's W (WK) was calculated as the ratio of χ2 and the number of groups (k) times n - 1 (WK = χ2/[k(n - 1)]); finally for the unpaired T-test Cohen's d was calculated as the ratio of the means’ difference (M) over the square root of the SD sum over two [d = (M1 − M2)/SQR[(SD1 + SD2)/2]]. For η2, η2P, and η2KW, a small effect size was considered for values between at least 0.01 and less than 0.06, medium between at least 0.06 and less than 0.14, and a large effect size at least 0.14; for the KW, a small effect size was considered for values between at least 0.1 and less than 0.3, medium between at least 0.3 and less than 0.5 and a large effect size when at least 0.5; for d, a small effect size was considered for values between at least 0.2 and less than 0.5, medium between at least 0.5 and less than 0.8, and a large effect size when at least 0.8.
Statistical analyses were performed using the SPSS v22 (IBM Corporation, Armonk, New York, USA) and the GraphPad Prism v6 (GraphPad Software, La Jolla, California, USA).
Results
Regulatory T-cell percentages dynamics during antiretroviral therapy varied accordingly to the baseline CD4+ T-cell counts
The ART regimens used resulted, as expected, in an overall rapid and strong decline of the plasma viral load for all individuals (Supplementary Figure S4) and in an overall significant increment of the CD4+ T-cell counts (Fig. 1a and b). No significant differences were observed on the CD4+ T-cell slope between individuals with distinct CD4+ T-cell count at ART onset (<200, 200–350, and >350 CD4+ T cells/μl; Fig. 1c), in accordance with previously published reports [31,35].
Tregs were defined as the CD127lowCD25highFOXP3+ cells among CD4+ T cells (CD3+CD4+), following the work of Sakaguchi and collaborators [30] and using the gating strategy previously described [25] depicted in Supplementary Figure S1. To determine whether Tregs percentages evolution throughout therapy differed accordingly to the disease stage at ART onset, individuals were stratified based on the baseline CD4+ T-cell counts (<200, 200–350, and >350 CD4+ T cells/μl). Interestingly, the differences observed on Tregs percentages between healthy controls and the overall HIV-infected individuals, or between baseline and the different time-points after ART onset, were particularly evident for individuals with very low CD4+ T-cell counts at baseline (<200 cells/μl; Fig. 2a and b). In addition, the group of patients with the lowest baseline CD4+ T-cell counts presented also a greater variability on the Tregs percentages, variance analysis revealed that it was unequal along the different time points among these individuals [F(8, 286) = 5.214, P < 0.0001, Brown–Forsythe test], but not for individuals with greater than 200 CD4+ T cells/μl at baseline [200–350: F(8, 292) = 1.895, P = 0.0605; >350: F(8, 152) = 0.866, P = 0.5467, Brown–Forsythe test (Fig. 2a)]. Notwithstanding the increased percentage of Tregs on HIV-infected individuals, the absolute numbers of Tregs were lower than in uninfected individuals throughout the whole follow-up period for individuals with less than 350 CD4+ T cells/μl at ART onset (Supplementary Figure S5A. Interestingly, at ART onset, the ratio between Tconv and Tregs was significantly lower for individuals with less than 200 CD4+ T cells/μl when compared to uninfected individuals (Supplementary Figure S5B, suggesting an increased loss of Tconv, in comparison to Tregs, in patients with severe lymphopenia.
We next asked to which extent Tregs percentages at baseline were associated, per se, with the progression of CD4+ T-cell counts throughout ART. For that purpose, individuals were stratified according to their Tregs percentage at baseline (less than or at least 10%, as defined in [25]) and the CD4+ T-cell counts were evaluated throughout therapy. Globally, individuals with higher baseline Tregs percentages had lower CD4+ T-cell counts at baseline (209 ± 151 vs. 324 ± 237 cells/μl, t = 2.503, P = 0.0150, d = 0.578, unpaired T-test with Welch's correction), but no differences were detected on the overall CD4+ T-cell reconstitution between individuals with lower and higher Tregs percentage even when patients were stratified accordingly to their CD4+ T-cell counts at baseline (Fig. 3). As several factors are known to influence the immune reconstitution (e.g. age, sex, basal viral load, residual viral replication during ART, coinfections, immune activation status [4]), hierarchical linear regression models were computed to dissect whether Tregs percentages at baseline could represent an additional contributor to predict CD4+ T-cell recovery. Age, sex, coinfections, viral loads at baseline and at 24 months and immune activation status (HLA-DR+ cells among CD4+ T cells) at 24 months of ART, were able to significantly predict 25% of the CD4+ T-cell counts at 24 months of ART (Table 1, model 1). When the Tregs percentages at baseline were included in the model (Table 1, model 2) the predictive capacity of the model increased to 31%, with Tregs percentages at baseline being a significant predictor of CD4+ T-cell reconstitution. Taking all the aforementioned variables in consideration, for the gain of each percentage unit of Tregs at baseline, the predicted CD4+ T-cell counts at 24 months decreased by 12.7 cells/μl. When the CD4+ T-cell counts at baseline were included in the analysis (Table 1, model 3) the predictive value of the model raised to 66% and the contribution of the baseline Tregs percentages lost its significance. These analyses, considering several factors known to effect CD4+ T-cell counts reconstitution did not reveal an additional effect of Tregs percentages at baseline on CD4+ T-cell counts at 24 months of ART.
Table 1.
Hierarchical linear regression models to predict CD4+ T-cell counts at 24 months of antiretroviral therapy.
| Model 1 | Model 2 | Model 3 | ||||||||||
| B | SE | β | P | B | SE | β | P | B | SE | β | P | |
| Agea | −5.18 | 2.74 | −0.20 | 0.06 | −7.17 | 2.74 | −0.27 | 0.01* | −3.40 | 1.97 | −0.13 | 0.09 |
| Sexb | 44.35 | 66.28 | 0.07 | 0.51 | 79.59 | 64,87 | 0.13 | 0.22 | −27.57 | 47.27 | −0.04 | 0.56 |
| Log (HIV viral load) @ 0M | −113.09 | 43.82 | −0.28 | 0.01* | −97.49 | 42.39 | −0.24 | 0.02* | −23.36 | 31.03 | −0.06 | 0.45 |
| Log (HIV viral load) @ 24M | −393.25 | 184.99 | −0.16 | 0.12 | −331.55 | 177.75 | −0.19 | 0.07 | −48.42 | 129.18 | −0.03 | 0.71 |
| Chronic comorbilityc | −76.66 | 62.01 | −0.13 | 0.22 | −46.54 | 60.48 | −0.08 | 0.44 | −100.78 | 42.87 | −0.18 | 0.02* |
| HLA-DR+ among CD4+ T cells @ 24M | −7.00 | 1.81 | −0.43 | 0.00* | −7.14 | 1.73 | −0.43 | 0.00* | −2.72 | 1.33 | −0.17 | 0.04* |
| %Tregs cells @ 0M | − | − | − | − | −12.70 | 4.84 | −0.28 | 0.01* | 0.47 | 3.75 | 0.01 | 0.90 |
| CD4+ T-cell count @ 0M | − | − | − | − | − | − | − | − | 0.80 | 0.11 | 0.72 | 0.00* |
| F (dF1, dF2) | 4.86 (6, 65)* | 5.53 (7, 64)* | 18.29 (8, 63)* | |||||||||
| R2adjusted | 0.25 | 0.31 | 0.66 | |||||||||
| R2adjusted change | − | 0.06 | 0.41 | |||||||||
Selection of the variables to be included in the models was based on the literature [4] and on the univariate analysis (Table S4).
aAt baseline.
bReference category: women (n = 18).
cReference category: no chronic comorbidity (HCV infection/cancer/medical condition leading to recurrent infections; consider positive when at least one of them occurred during the 24 months of ART; n = 22).
Variables were considered to significantly contribute to the model when P < 0.05 and represented by *.
HIV-infected individuals presented regulatory T-cell subset disturbances that were not restored with antiretroviral therapy
Tregs functionality is assured not only by their numbers and proportions, in respect to the total CD4+ T cells, but also by the maintenance of the right proportion of their subpopulations. Tregs can be subdivided in naive (CD45RA+) and memory Tregs (CD45RA-), which are developmentally related subsets with distinct phenotypes [30,36]. We observed clear disturbances in Tregs subpopulations distribution; in ART-naive HIV-infected individuals the percentage of CD45RA+ cells among total Tregs was lower than in healthy individuals, and this difference persisted even after 24 months of ART especially for individuals with less than 200 CD4+ T cells/μl at baseline (Fig. 4a). As most naïve Tregs are generated in the thymus, they express high levels of CD31, which is a marker for recent thymic emigrants [30,37]. To understand whether the impairment on the recovery of the CD45RA+ Tregs population was associated with lower thymic output, we quantified CD45RA+ Tregs expressing high levels of CD31 (CD45RA+CD31hi Tregs), which should reflect blood's Tregs most recently exported from the thymus [38,39]. We observed that the CD45RA+CD31hi Tregs counts were consistently lower in HIV-infected patients with CD4+ T-cell counts less than 350 cells/μl, irrespective of their time on ART, in comparison to uninfected individuals (Fig. 4b). Interestingly, a significant increase from baseline was observed for CD45RA+CD31hi Tconv counts but not for CD45RA+CD31hi Tregs (Supplementary Figure S6, suggesting a stronger contribution of the thymus for the recovery of Tconv than for that of Tregs.
As for the percentage of Tregs undergoing proliferation, only patients with the lowest CD4+ T-cell counts at baseline (less than 200 cells/μl) presented significantly higher proportions of Ki67+ Tregs when compared to healthy individuals and these differences were maintained until the end of follow-up (Fig. 4c). Importantly, most of the Tregs undergoing proliferation had, as expected, a memory phenotype (Supplementary Figure S7. As shown by others, although in the context of allogeneic hematopoietic stem cell transplantation [40], we observed that the proportion of Ki67+ cells among Tregs was consistently higher than among Tconv, both for uninfected controls and for HIV-infected individuals, resulting in a ratio of Tregs Ki67+/Tconv Ki67+ cells around 5. Of relevance, this ratio was lower in HIV-uninfected vs. infected individuals with up to 6 months of ART (Supplementary Figure S8.
Discussion
The evolution of the percentage of total Tregs and that of Tregs subpopulations during ART, as well as their association with the immune reconstitution of HIV-infected individuals, are still controversial issues that merits attention. As an attempt to add evidence that could bring consensus to this matter, we performed a longitudinal study of HIV-infected individuals followed at specific time points for 24 months upon ART onset. This study is unique and important to complement previous published work, since it is longitudinal and encompasses 81 individuals, all followed from the day they initiated ART, and from whom Tregs were identified as CD127lowCD25highFOXP3+ cells among CD4+CD3+ lymphocytes (for comparison with previous studies please see Supplementary Table S1). If we take into consideration that a key feature of HIV infection is the immune hyperactivation [41], the usage of this complete set of markers to identify Tregs is pivotal to clearly discriminate Tregs from activated Tconv given that activation of Tconv might lead to the upregulation of CD25 and downregulation of CD127; FOXP3 is not exclusive from Tregs; and CD127 and FOXP3 allow a better definition of the boundary between CD25int and CD25hi cells [30]. To our knowledge, only one additional longitudinal study to date used this complete set of Tregs markers though with a somewhat smaller sample size (n = 11 patients on ART) [22]. An additional advantage of the present study relies on the fact that the enrolled patients presented quite distinct baseline CD4+ T-cell counts. This allowed us to follow, for distinct baseline CD4+ T-cell counts the dynamics of Tregs and of its subpopulations throughout ART, and to evaluate the potential combined effect of baseline Tregs percentages and CD4+ T-cell counts on the overall immune recovery.
The Tregs percentage of ART-naive HIV-infected individuals was found to be higher than that of healthy controls, as reported previously by others [17–22]. Our study, by including a greater number of individuals with quite varied baseline CD4+ T-cell counts at ART onset, clearly shows that, despite the overall higher percentage of Tregs, there is a substantial heterogeneity in the Tregs percentages among HIV-infected patients. Interestingly, the group of individuals with the lowest CD4+ T counts at baseline was noticeably enriched with patients with the higher Tregs percentages. These findings may justify why some previous studies suggested that Tregs percentages are similar between ART-naive HIV-infected individuals and healthy controls [8,23], since they encompassed mostly patients with moderate lymphopenia.
Several reports indicated a role for Tregs on the accomplishment of a successful immune reconstitution [3,12,25,28,29]. Individuals with impaired immune reconstitution (i.e., less than 500 CD4+ T cells/μl after 1–15 years of ART) were shown to have the highest Tregs percentages (>10%) and lowest nadir values (the lowest CD4+ T-cell count achieved by each HIV-infected individual) [25]. Another study, that included multivariate analysis adjusted for age, nadir, Tregs percentages and CD8+ T-cell activation, suggested that low nadirs and high Tregs percentages during ART were associated with inadequate immunological response [28]. All the above mentioned studies evaluated the impact of Tregs percentages during ART. The hierarchical linear regression model presented herein showed that the percentage of Tregs at baseline had no predictable impact on the CD4+ T-cell recovery until up to 24 months of ART. This observation contrasts with the recent work published by Saison and collaborators [26]. In that study, the authors claim that the Tregs percentages at baseline are a strong independent prognostic factor of immune recovery, particularly for the individuals with low nadir [26]. Part of these discrepancies may be explained by the used of different criteria to define Tregs, whereas, Saison and collaborators define Tregs as CD4+CD25+CD127-, and we defined these cells as CD127lowCD25highFOXP3+ cells among CD4+ T cells. Additionally, the model in the present study also adjusts for peripheral T-cell activation (HLA-DR+ among CD4+ T cells), which, as expected, was a strong predictor of immune reconstitution.
Despite the fact that some reports showed no alterations on the suppressive capacity of Tregs during HIV-infection [42], recent data reported that Tregs infected in vitro by HIV have impaired suppressive capacity and decreased expression of critical genes for Tregs function [43,44]. Though no information on individuals’ Tregs suppressive function is available in the present study, which is a weakness, one could envisage that the previously reported impaired suppressive capacity could explain why individuals with high Tregs percentages at baseline had no increased difficulties in recovering their CD4+ T-cell counts. In accordance, we observed that Tregs percentages at baseline positively correlates with the percentage of CD4+ T cells with an activated phenotype (HLA-DR+) and undergoing proliferation (Ki67+; data not shown). Future in-vitro studies are needed to evaluate Tregs’ suppressive capacity from individuals with high and low baseline Tregs percentages.
Disturbances on Tregs subsets homeostasis in ART-naive HIV-infected individuals have been described in several studies [3,8,29,45–47]. Some of these evaluated the percentage of Tregs subsets among CD4+ T cells [8,46]. However, because the percentage of total Tregs among CD4+ T cells is highly variable within HIV-infected individuals, looking at Tregs subsets percentages in respect to total CD4+ T cells, and not specifically in the context of Tregs, might limit data interpretation. Taking into account only the studies that measured naive cells among Tregs cells, the one by Gaardbo and collaborators showed a lower percentage of naive cells in HIV-infected individuals on ART, when compared to uninfected individuals, irrespectively from the patients’ immune reconstitution outcome [47]. On the contrary, Serana and collaborators observed no significant differences on this Tregs subset percentage in healthy controls vs. individuals on ART for more than 6 years [45]. The discrepancy between these results might now be justified, as we observed that the evolution of the naive cells proportion among Tregs varies according to the CD4+ T-cell counts at baseline.
Naive Tregs are mostly composed by natural Tregs, which are generated in the thymus [30,37] that is known to be affected by HIV infection [39,48,49]. The proportion of naive Tregs depends on the balance between the newly generated naive Tregs and those that through activation/proliferation are converted into activated Tregs. By quantifying recent thymic emigrant Tregs (i.e. Tregs that express CD45RA+CD31hi), we show here that, while there was a general increase in the number of CD45RA+CD31hi Tconv, the recent thymic emigrant Tregs counts in HIV-infected individuals on ART was consistently lower than the one of uninfected controls, and remained unaltered throughout ART. These results suggest that the decreased proportion of Tregs, observed during ART, might in part result from a proportionally decreased production of Tregs by the thymus, which might also impact on the decreased percentage of naive Tregs. Notwithstanding, we observed, as others [40], higher proportions of Ki67+ cells among Tregs in comparison to Tconv. Of interest, the ratio Tregs Ki67+/Tconv Ki67+ was found decreased in HIV-infected patients from baseline up to 6 months on ART. The fact that the number of CD45RA+CD31hi Tregs was maintained consistently lower suggests that an impaired production of Tregs by the thymus is, at least in part, responsible for the sustained decreased proportion of naïve Tregs among total Tregs, although cell division might also contribute.
Taken together, our data shows that the evolution of Tregs percentages and of Tregs subpopulations during ART varies greatly according to the lymphopenia level at baseline. Whereas the Tregs percentages were significantly higher than that of the control individuals for more lymphopenic patients, this was not observed for individuals initiating ART with CD4+ T-cell counts greater than 350 cells/μl. In addition, individuals with very low CD4+ T-cell counts at baseline displayed high diversity on the Tregs percentages. These observations conciliate some controversial data reported in the literature. The present study also shows that, throughout ART, although Tregs percentages may reach values similar to uninfected individuals, the proportion of naïve cells among Tregs tend to be consistently low. A reduced export of Tregs by the thymus might be responsible for this, though a role for the increased activation/turnover of naive and the consequent acquisition of a memory phenotype should also be considered.
The reported results strengthen the idea that very low baseline CD4+ T-cell counts leads to disruptions of different immune populations, in addition to other severe conditions induced by low CD4+ T-cell counts [50], and that ART cannot counteract part of these alterations. The individuals analysed in the present work should be followed for longer periods to clearly define the potential role of Tregs, and of other cell subsets, on the quality of the immune reconstitution of HIV-infected individuals under ART.
Acknowledgements
The authors acknowledge Dr Gaetano Marrone for the critical reading of the manuscript and to Dr Ana E. Sousa for encouraging discussions.
A.H., V.C.T., and A.M.-R. performed experiments; C.N., A.H., A.B., R.Rb-S., and C.L.S. analysed the data; C.N. and A.B. made the figures; C.N., A.H., R.S.-C., and M.C.-N. designed the research; C.N., A.H. and M.C.-N. wrote the paper; all authors approved the final version of the manuscript.
The work was funded by the Portuguese Foundation for Science and Technology (FCT; PIC/IC/83313/2007) and cofinanced by the Programa Operacional Novo Norte (ON.2 – O Novo Norte) under the Quadro de Referencia Estratégica Nacional (QREN) through the Fundo Europeu de Desenvolvimento Regional (FEDER). A FCT fellowship, under the Programa Operacional Potencial Humano (POPH) through the Fundo Social Europeu (FSE), was given to C.N. (SFRH/BPD/65380/2009) and to R.Rb-S. (PD/BD/106047/2015; fellowship in the context of the Inter-University Doctoral Programme in Ageing and Chronic Disease, a FCT PhD Program).
Conflicts of interest
A.H. and R.S-C. are consultants for Abbvie LDA, Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, Merck Sharp, and Dohme and ViiV Healthcare. All the remaining authors report no conflict of interests.
Supplementary Material
References
- 1.Maartens G, Celum C, Lewin SR. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet 2014; 384:258–271. [DOI] [PubMed] [Google Scholar]
- 2.Gaardbo JC, Hartling HJ, Gerstoft J, Nielsen SD. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol 2012; 2012:670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, et al. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS 2010; 24:1991–2000. [DOI] [PubMed] [Google Scholar]
- 4.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood 2011; 117:5582–5590. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood 2013; 121:29–37. [DOI] [PubMed] [Google Scholar]
- 6.Eggena MP, Barugahare B, Jones N, Okello M, Mutalya S, Kityo C, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol 2005; 174:4407–4414. [DOI] [PubMed] [Google Scholar]
- 7.Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis 2009; 199:1318–1322. [DOI] [PubMed] [Google Scholar]
- 8.Simonetta F, Lecuroux C, Girault I, Goujard C, Sinet M, Lambotte O, et al. Early and long-lasting alteration of effector CD45RA(-)Foxp3(high) regulatory T-cell homeostasis during HIV infection. J Infect Dis 2012; 205:1510–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss L, Donkova-Petrini V, Caccavelli L, Balbo M, Carbonneil C, Levy Y. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 2004; 104:3249–3256. [DOI] [PubMed] [Google Scholar]
- 10.Andersson J, Boasso A, Nilsson J, Zhang R, Shire NJ, Lindback S, et al. The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J Immunol 2005; 174:3143–3147. [DOI] [PubMed] [Google Scholar]
- 11.Kinter A, McNally J, Riggin L, Jackson R, Roby G, Fauci AS. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proc Natl Acad Sci U S A 2007; 104:3390–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendez-Lagares G, Pozo-Balado MM, Genebat M, Garcia Perganeda A, Leal M, Pacheco YM. Severe immune dysregulation affects CD4(+)CD25(hi)FoxP3(+) regulatory T cells in HIV-infected patients with low-level CD4 T-cell repopulation despite suppressive highly active antiretroviral therapy. J Infect Dis 2012; 205:1501–1509. [DOI] [PubMed] [Google Scholar]
- 13.Winstead CJ, Reilly CS, Moon JJ, Jenkins MK, Hamilton SE, Jameson SC, et al. CD4+CD25+Foxp3+ regulatory T cells optimize diversity of the conventional T cell repertoire during reconstitution from lymphopenia. J Immunol 2010; 184:4749–4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw JM, Hunt PW, Critchfield JW, McConnell DH, Garcia JC, Pollard RB, et al. Increased frequency of regulatory T cells accompanies increased immune activation in rectal mucosae of HIV-positive noncontrollers. J Virol 2011; 85:11422–11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med 2010; 2:32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estes JD, Wietgrefe S, Schacker T, Southern P, Beilman G, Reilly C, et al. Simian immunodeficiency virus-induced lymphatic tissue fibrosis is mediated by transforming growth factor beta 1-positive regulatory T cells and begins in early infection. J Infect Dis 2007; 195:551–561. [DOI] [PubMed] [Google Scholar]
- 17.Angin M, Kwon DS, Streeck H, Wen F, King M, Rezai A, et al. Preserved function of regulatory T cells in chronic HIV-1 infection despite decreased numbers in blood and tissue. J Infect Dis 2012; 205:1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulze Zur Wiesch J, Thomssen A, Hartjen P, Toth I, Lehmann C, Meyer-Olson D, et al. Comprehensive analysis of frequency and phenotype of T regulatory cells in HIV infection: CD39 expression of FoxP3+ T regulatory cells correlates with progressive disease. J Virol 2011; 85:1287–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montes M, Sanchez C, Lewis DE, Graviss EA, Seas C, Gotuzzo E, et al. Normalization of FoxP3(+) regulatory T cells in response to effective antiretroviral therapy. J Infect Dis 2011; 203:496–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaardbo JC, Nielsen SD, Vedel SJ, Ersboll AK, Harritshoj L, Ryder LP, et al. Regulatory T cells in human immunodeficiency virus-infected patients are elevated and independent of immunological and virological status, as well as initiation of highly active antiretroviral therapy. Clin Exp Immunol 2008; 154:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolte L, Gaardbo JC, Skogstrand K, Ryder LP, Ersboll AK, Nielsen SD. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active antiretroviral therapy may be due to increased thymic production of naive Tregs. Clin Exp Immunol 2009; 155:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Presicce P, Orsborn K, King E, Pratt J, Fichtenbaum CJ, Chougnet CA. Frequency of circulating regulatory T cells increases during chronic HIV infection and is largely controlled by highly active antiretroviral therapy. PLoS One 2011; 6:e28118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chevalier MF, Didier C, Petitjean G, Karmochkine M, Girard PM, Barre-Sinoussi F, et al. Phenotype alterations in regulatory T-cell subsets in primary HIV infection and identification of Tr1-like cells as the main interleukin 10-producing CD4+ T cells. J Infect Dis 2015; 211:769–779. [DOI] [PubMed] [Google Scholar]
- 24.Lim A, Tan D, Price P, Kamarulzaman A, Tan HY, James I, et al. Proportions of circulating T cells with a regulatory cell phenotype increase with HIV-associated immune activation and remain high on antiretroviral therapy. AIDS 2007; 21:1525–1534. [DOI] [PubMed] [Google Scholar]
- 25.Horta A, Nobrega C, Amorim-Machado P, Coutinho-Teixeira V, Barreira-Silva P, Boavida S, et al. Poor immune reconstitution in HIV-infected patients associates with high percentage of regulatory CD4+ T cells. PLoS One 2013; 8:e57336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saison J, Maucort Boulch D, Chidiac C, Demaret J, Malcus C, Cotte L, et al. Increased regulatory T-cell percentage contributes to poor CD4(+) lymphocytes recovery: a 2-year prospective study after introduction of antiretroviral therapy. Open Forum Infect Dis 2015; 2:ofv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Zhang H, Zhang T, Ji Y, Jiao Y, Wu H. Longitudinal changes of peripheral blood DC subsets and regulatory T cells in Chinese chronic HIV-1-infected patients during antiretroviral therapy. PLoS One 2012; 7:e37966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saison J, Ferry T, Demaret J, Maucort Boulch D, Venet F, Perpoint T, et al. Association between discordant immunological response to highly active antiretroviral therapy, regulatory T cell percentage, immune cell activation and very low-level viraemia in HIV-infected patients. Clin Exp Immunol 2014; 176:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foxall RB, Albuquerque AS, Soares RS, Baptista AP, Cavaleiro R, Tendeiro R, et al. Memory and naive-like regulatory CD4+ T cells expand during HIV-2 infection in direct association with CD4+ T-cell depletion irrespectively of viremia. AIDS 2011; 25:1961–1970. [DOI] [PubMed] [Google Scholar]
- 30.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10:490–500. [DOI] [PubMed] [Google Scholar]
- 31.AIDSinfo. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf [Assessed 31 January 2015] [Google Scholar]
- 32.CDC. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 Years. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a1.htm [Assessed 31 January 2015] [PubMed] [Google Scholar]
- 33.Kline RB. Principles and practice of structural equations modeling. 3rd edLondon, UK: Guilford Press; 2011. [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2nd edHillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 35.Sabin CA, Phillips AN. Should HIV therapy be started at a CD4 cell count above 350 cells/microl in asymptomatic HIV-1-infected patients?. Curr Opin Infect Dis 2009; 22:191–197. [DOI] [PubMed] [Google Scholar]
- 36.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30:899–911. [DOI] [PubMed] [Google Scholar]
- 37.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 1999; 162:5317–5326. [PubMed] [Google Scholar]
- 38.Rickabaugh TM, Kilpatrick RD, Hultin LE, Hultin PM, Hausner MA, Sugar CA, et al. The dual impact of HIV-1 infection and aging on naive CD4 T-cells: additive and distinct patterns of impairment. PLoS One 2011; 6:e16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fabre-Mersseman V, Dutrieux J, Louise A, Rozlan S, Lamine A, Parker R, et al. CD4(+) recent thymic emigrants are infected by HIV in vivo, implication for pathogenesis. AIDS 2011; 25:1153–1162. [DOI] [PubMed] [Google Scholar]
- 40.Matsuoka K, Kim HT, McDonough S, Bascug G, Warshauer B, Koreth J, et al. Altered regulatory T cell homeostasis in patients with CD4+ lymphopenia following allogeneic hematopoietic stem cell transplantation. J Clin Invest 2010; 120:1479–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol 2011; 6:223–248. [DOI] [PubMed] [Google Scholar]
- 42.Moreno-Fernandez ME, Zapata W, Blackard JT, Franchini G, Chougnet CA. Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol 2009; 83:12925–12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pion M, Jaramillo-Ruiz D, Martinez A, Munoz-Fernandez MA, Correa-Rocha R. HIV infection of human regulatory T cells downregulates Foxp3 expression by increasing DNMT3b levels and DNA methylation in the FOXP3 gene. AIDS 2013; 27:2019–2029. [DOI] [PubMed] [Google Scholar]
- 44.Angin M, Sharma S, King M, Murooka TT, Ghebremichael M, Mempel TR, et al. HIV-1 infection impairs regulatory T-cell suppressive capacity on a per-cell basis. J Infect Dis 2014; 210:899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serana F, Chiarini M, Quiros-Roldan E, Gotti D, Zanotti C, Sottini A, et al. Modulation of regulatory T-cell subsets in very long-term treated aviremic HIV(+) patients and untreated viremic patients. Open AIDS J 2014; 8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou H, Zhao H, Hao Y, Song C, Han J, Zhang J, et al. Excessive conversion and impaired thymic output contribute to disturbed regulatory T-cell homeostasis in AIDS patients with low CD4 cell counts. AIDS 2013; 27:1059–1069. [DOI] [PubMed] [Google Scholar]
- 47.Gaardbo JC, Hartling HJ, Ronit A, Springborg K, Gjerdrum LM, Ralfkiaer E, et al. Regulatory T cells in HIV-infected immunological nonresponders are increased in blood but depleted in lymphoid tissue and predict immunological reconstitution. J Acquir Immune Defic Syndr 2014; 66:349–357. [DOI] [PubMed] [Google Scholar]
- 48.Kolte L. Thymic function in HIV-infection. Dan Med J 2013; 60:B4622. [PubMed] [Google Scholar]
- 49.Dion ML, Poulin JF, Bordi R, Sylvestre M, Corsini R, Kettaf N, et al. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 2004; 21:757–768. [DOI] [PubMed] [Google Scholar]
- 50.Walker NF, Scriven J, Meintjes G, Wilkinson RJ. Immune reconstitution inflammatory syndrome in HIV-infected patients. HIV AIDS (Auckl) 2015; 7:49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


