Abstract
Objectives:
Semen composition is influenced by HIV-1 infection, yet the impact of semen components on HIV infection of primary target cells has only been studied in samples from HIV-uninfected donors.
Design:
We compared the effect of seminal plasma (SP) from chronically HIV-infected (SP+) versus uninfected donors (SP–) on HIV-1 infection of peripheral blood mononuclear cells (PBMCs) and CD4+ T cells.
Methods:
Primary cells were infected with HIV-1 in the presence of SP+ or SP– and analyzed for infection level, metabolic activity, HIV receptor expression, proliferation and activation. SP+ and SP– were compared for infection-enhancing peptides, cytokines and prostaglandin E2 levels.
Results:
SP– efficiently enhanced HIV-1 R5 infection of CD4+ T cells, whereas SP+ enhancing activity was significantly reduced. RANTES (CCL5) concentrations were elevated in SP+ relative to SP–, whereas the concentrations of infectivity-enhancing peptides [semen-derived enhancer of viral infection (SEVI), SEM1, SEM2] were similar. CCR5 membrane expression levels were reduced on CD4+ T cells shortly postexposure to SP+ compared with SP– and correlated to R5-tropic HIV-1 infection levels, and CCR5 ligands’ concentrations in semen. SP+ and SP– displayed similar enhancing activity on PBMC infection by X4-tropic HIV-1. Addition/depletion of RANTES (regulated on activation, normal T-cell expressed and secreted) from SPs modulated their effect on PBMC infection by R5-tropic HIV-1.
Conclusion:
Semen from HIV-infected donors exhibits a significantly reduced enhancing potential on CD4+ T-cell infection by R5-tropic HIV-1 when compared with semen from uninfected donors. Our data indicate that elevated seminal concentrations of RANTES in HIV-infected men can influence the ability of semen to enhance infection.
Keywords: CCR5, cytokines, enhancing peptides, HIV-1, infected men, receptors, semen, transmission, RANTES/CCL5, CD4+ T cells
Introduction
Semen is the main vector for HIV transmission [1]. In addition to being a carrier of HIV, semen has been reported to influence the efficiency of HIV infection of target cells through the intrinsic properties of its a-cellular fraction, the seminal plasma (SP) [2–9]. The effects of SP from HIV-infected men on infection of primary target cells have not been investigated; all studies so far have focused on SP provided by uninfected donors and/or used nonprimary cells as target cells. Several elements (such as cytokine and microbial content, infection of semen-producing organs by HIV/SIV and lower ejaculate volumes in HIV-infected men) indicate that HIV infection triggers significant alterations in the composition of SP [3,10–20].
CD4+ T lymphocytes appear to be the primary target for HIV infection within the semen-exposed mucosa, where they are present within stratified squamous epithelia and in subepithelial tissues [21–24]. Breaches in the mucosal epithelial layer (due to micro-trauma during intercourse or ulcers resulting from local infections/inflammation) are frequent, which can bring HIV target cells into direct contact with semen [23]. In this context, we sought to compare: the effect of SP from HIV-infected men (SP+) versus SP from uninfected donors (SP–) on HIV-1 infection of primary cells; the composition of SP+ versus SP– in terms of relative levels of infectivity-enhancing amyloids, cytokines, and prostaglandins; the impact of SP+ and SP– on CD4+ T-cell surface receptor expression, activation state, and proliferation.
Methods
Semen collection and seminal plasma preparation
Samples and exposure data were provided by GERMETHEQUE biobank BB-0033-00081 (France). Semen was collected from antiretroviral therapy-naive HIV-infected men (clinical characteristics in Table S1) and uninfected healthy men of proven fertility who gave consent for semen donation within the framework of our research protocol, authorized by the French drug safety agency AFSSAPS (no B90850-30) and Ministère de l’enseignement supérieur et de la recherche (no DC-2010-1155). Individual semen samples from HIV-infected and uninfected donors were collected, processed and analyzed using strictly the same procedures at the center for the cryopreservation of ‘eggs and semen’ (CECOS), following World Health Organization (WHO) recommendations [25], as we described [20]. Donors completed a research questionnaire on uro-genital infections and infertility risk factors and underwent clinical examination, as described [20]. None of the HIV-infected and uninfected men recruited had either clinical evidence of urogenital infections or medical history of sexually transmitted infections (apart from HIV) or urinary infections within the last 5 years. The analysis of semen parameters (Table S2) showed no significant differences in polymorphonuclear cell concentrations (Mann–Whitney test, P = 0.77), spermatozoa concentrations (P = 0.61) or volume (P = 0.11) among semen samples from HIV-infected versus uninfected men. One HIV-infected donor had a polymorphonuclear cell count above 1 million cell/ml (leukocytospermia), which may be associated with genital infection or poor sperm quality [26]. A slightly higher pH was observed in semen from HIV-infected versus uninfected men (median of 8.1 versus 7.9, P = 0.02) (Table S2). Ejaculates were liquefied for 30 min at 37°C and centrifuged 10 min at 1000g at room temperature. SPs were stored at –80°C.
HIV-1 variants and virus stocks
HIV-1 clade B strains using as co-receptor for cell entry either CCR5 (R5 SF162) or CXCR4 (X4 IIIB) were obtained from the NIBSC (National Institute for Biological Standards and Control) Centralised Facility for AIDS Reagents. Viruses were grown in peripheral blood mononuclear cells (PBMCs) stimulated by phytohemagglutinin (PHA, 2 μg/ml; Sigma Aldrich, Saint Louis, Missouri, USA) and human recombinant interleukin-2 (IL-2, 5 ng/ml; Roche Applied Science, Basel, Switzerland) to provide viral stocks. The culture supernatants were ultracentrifuged for 1 h at 100 000g on a 20% sucrose pillow [27]. The resulting virus stocks were titrated on PBMCs by using the 50% infectivity end-point method (TCID50) of Reed and Muench [28] and by measuring p24 concentrations using ELISA. Virus stocks were stored in aliquot at –80°C.
Cell culture and infection
Human PBMCs were purified by ficoll density centrifugation and cultured in RPMI medium (Sigma) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol/l l-glutamine and 10% (v/v) fetal bovine serum. PBMCs were activated with PHA (2 μg/ml) for 72 h and cultured with IL-2 (5 ng/ml) for 24 h before and after infection. CD4+ T cells were purified from PHA-stimulated PBMC by negative selection (Dynabeads Untouched Human CD4; Life Technologies, Carlsbad, California, USA). Of the resulting cell population, 97% was CD3+CD4+ T lymphocytes, which underwent IL-2 (5 ng/ml) stimulation for 24 h before and after infection. PBMCs or purified CD4+ T cells stimulated with PHA and IL-2 were seeded at 2×105 cells/well in 96 well flat-bottom plates in 100 μl of medium. HIV-1 SF162 or IIIB strains (MOI 0.01, corresponding to 2 or 5 ng/ml p24, respectively) were mixed with serial dilutions of SP in 11 μl of inoculum to achieve final SP concentration on the cells of 1, 0.2, 0.04 or 0%. Each condition was tested in triplicate. Inoculums were removed by centrifugation (280g, 10 min) after 3 h of exposure (unless specified) and fresh medium added. After 3 days of culture, supernatants were collected and frozen for p24 viral protein assay by ELISA (Innotest HIV Antigen mAb; Innogenetics, Zwijnaarde, Belgium). Cell viability was systematically assessed in all the infectivity experiments. The number of viable PBMCs was evaluated as per the manufacturer's instructions using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, Wisconsin, USA). PBMC viability at different time points was also assessed using the amine-reactive red dye (Live/dead Fixable dead cell stain kit; Life Technologies). Acquisition was performed with a FACScalibur flow cytometer (Becton Dickinson) and CELLQuestPro Software was used for analysis.
Real-time PCR and RT-PCR
HIV-1 DNA and RNA and CCR5 mRNA were measured in PBMCs as previously described [16,29]. PBMCs infected in the presence of nevirapine (37.5 μmol/l) were used for negative control of DNA originating from the input virus.
Semen-derived enhancer of viral infection and semenogelin-derived fragments SEM1 (49–107) and SEM2 (49–107) ELISAs
Semen-derived enhancer of viral infection (SEVI), SEM1 and SEM2 ELISAs were performed as previously described [4,7].
Cytokines and prostaglandin E2 measurements
Cytokine concentrations were analyzed using the Bio-Plex Pro assay (Bio-Plex Pro Human Cytokine Group I [27-plex] and Group II [21-plex] panels; Bio-Rad, Hercules, California, USA). Total and active transforming growth factor beta (TGF-β) levels in SP were determined using the Quantikine human TGF-β kits (R&D Systems, Minneapolis, Minnesota, USA). Prostaglandin E2 (PGE2) levels were determined using the Prostaglandin E2 EIA kits (Cayman Chemical Company, Ann Arbor, Michigan, USA).
Flow cytometry experiments
Cells were stained using fluorescently conjugated monoclonal antibodies to CD3-PerCP (clone SP34–2), CD4-FITC (clone RPA-T4), in combination with either CCR5-PE (clone 3A9), CXCR4-PE (clone 12G5), or the activation marker CD69-PE (clone FN50), all from BD Biosciences (Franklin Lakes, New Jersey, USA). Corresponding fluorescent isotype controls were used at the same concentrations as the reference antibody. Cells were stained with antibodies by incubation for 30 min at 4°C, washed in PBS-1% FCS and fixed in 1.5% paraformaldehyde. Proliferation assays were performed using the Click-iT EdU Flow Cytometry Assay kit (Life Technologies), as per manufacturer instructions. A gate (PBMC gate) was defined in the analysis to exclude nonviable cells and debris. The percentage of live/dead cells in the PBMC gate and in the CD4+ cell population was analyzed using the Live/dead Fixable dead cell stain kit, as described above. Acquisition was performed with a FACScalibur flow cytometer (Becton Dickinson) and CELLQuestPro Software was used for analysis. The cell surface expression levels in the flow cytometry profiles are expressed as mean fluorescence intensity (MFI) indices. The percentage of stained cells is also presented.
RANTES addition and depletion experiments
To test the effect of RANTES (regulated on activation, normal T-cell expressed and secreted) on p24 release from HIV-1 R5SF162 infected PBMCs, human recombinant RANTES was added to diluted (1%) SP from uninfected donors to reach a final concentration of 5 or 10 pg/ml (corresponding to 500 and 1000 pg/ml, respectively, in undiluted SP). Conversely, SPs from infected donors were depleted of RANTES: after a preclearing of IgGs by incubation with an excess (1 mg/ml) of GammaBind Sepharose beads (GE Healthcare, Little Chalfont, Buckinghamshire, UK) for 1 h at 4°C with rotation, the beads were removed by centrifugation for 3 min at 1500 rpm, and anti-RANTES mAbs (10 mg/ml) were added for 1 h on ice. A second incubation with GammaBind Sepharose beads was performed, and immunocomplexes bound to beads removed by centrifugation. Treated samples were tested by ELISA (R&D Systems) to confirm RANTES depletion.
Statistical analyses
All data were analyzed with the nonparametric Wilcoxon–Mann–Whitney test. All tests involving more than two pairwise comparisons were corrected for multiple testing using Benjamini and Hochberg (FDR) correction [30]. Correlations were calculated using the Spearman test. Statistical analyses were performed using commercially available software (GraphPadPrism 6, GraphPad Software, Inc., La Jolla, California, USA).
Results
We observed that low concentrations of SP (≤1%), when incubated with PBMCs for 3 h, demonstrated a dose-dependent enhancement of HIV infection (Fig. S1A) without influencing cellular metabolism (Fig. S1B). This finding is consistent with previous reports [4,6,7]. In contrast, SP (1%) incubated with PBMCs for 24 h produced a lower level of HIV infection, and reduced cellular viability (Fig. S2). These findings are consistent with previous reports that SP is cytotoxic to PBMCs after prolonged exposure (>3 h), even when highly diluted [4,6,31–33]. The results also support a previous finding that the inhibitory activity of SP on CD4+ T cells’ infection is the result of toxicity [4].
Using the noncytotoxic conditions established above, we then compared SP collected from 20 chronically infected, therapy-naive HIV-infected donors (SP+) and 16 uninfected men (SP–) (see Tables S1 and S2 for clinical characteristics and semen analyses), alongside pooled SP from 50 additional uninfected donors (pool SP–).
SP+ (n = 20) displayed a significantly reduced enhancing effect on PBMC infection by R5-tropic HIV-1 when compared with SP– (n = 16), as measured by p24 ELISA on culture supernatants 72 h after a 3-h exposure (significant reduction in three independent experiments of 47, 35.5 and 24%, with a median enhancement of 1.7–2.9-fold for 1% SP–, donor range 0.98–6.92-fold versus median 1.5–1.54-fold for 1% SP+, donor range 0.4–2.8-fold) (Fig. 1a). No differences in PBMC metabolic activity were ever observed with SP+ or SP–, whether after the 3-h semen exposure or at the end of the 72-h culture, and with or without exogenous virus (Fig. 1b, and Fig. S3). In agreement with the p24 assay, the level of HIV gag RNA within PBMCs exposed to SP– (n = 10) was significantly higher than that in PBMCs exposed to SP+ (n = 10) or to virus only (Fig. 1c). Quantification of HIV DNA revealed a higher number of HIV DNA copies in PBMCs exposed to SP– (n = 10) versus SP+ (n = 10) from 24 h after the 3-h exposure duration onward (Fig. 1d). No correlation was found between semen or blood viral loads of the donors and the level of PBMC infection following SP+ exposure measured either by p24 ELISA, vRNA or vDNA levels (Spearman test). Similar to that which was observed in PBMCs, SP+ (n = 17) had a significantly reduced enhancing effect on purified CD4+ T-cell infection by R5-tropic HIV-1 when compared with SP– (n = 15) (reduction of 22%, with median enhancement of 2.2-fold for 1% SP–, donor range 1.5–3.5-fold versus median enhancement 1.7-fold for 1% SP+, donor range 0.26–2.88-fold), in the absence of cytotoxic effect (Fig. 1e and f). The viability of PBMCs assessed at different time points (24, 48 and 72 h) using flow cytometry was also similar between cells exposed to virus only and cells exposed to virus together with SP+ or SP– (Fig. S4).
Fig. 1.
Effect of SP+ versus SP– on R5-tropic HIV-1 infection of PBMCs and CD4+ T cells.
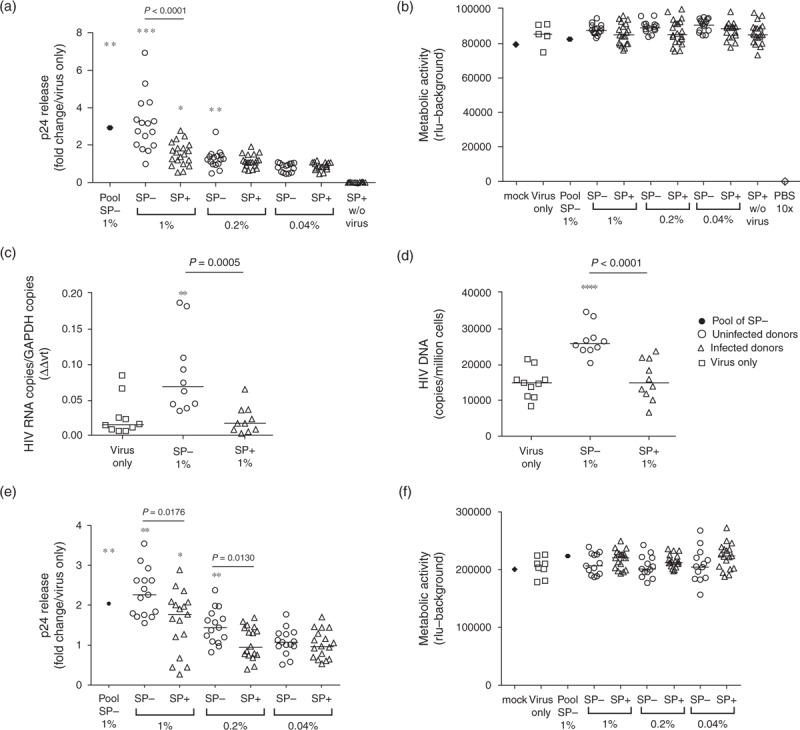
PBMCs (a–d) or isolated CD4+ T cells (e and f) were exposed to the indicated dilutions of SP+ or SP– from individual donors (for each condition, each symbol represents one donor) for 3 h in presence of HIV-1 R5 SF162 strain and then cultured in fresh culture medium. Pooled SP– from 50 additional uninfected donors was tested in parallel (pool of SP–). Infectivity was measured in PBMCs after 72 h of culture by measuring p24 content in the supernatants (a), by real-time RT-PCR quantification of HIV-1 gag transcripts (c) and by real-time PCR quantification of HIV-1 long terminal repeat (LTR) DNA in one million PBMCs after 24 h (d). HIV-1 p24 release was measured by ELISA in CD4+ T-cell supernatants after 72 h of culture (e). (a and e) Results are expressed as fold change relative to virus only for p24 release. (c) Results are shown as relative expression of HIV gag RNA standardized to GAPDH mRNA expression and (d) as HIV LTR DNA copies/million cells based on albumin gene quantification. Results shown are representative of two (c, d) or three (a, b) independent experiments for each donor. SP+ were also tested in the absence of exogenous virus at the final dilution of 1% (SP+ 1% w/o virus). (b and f) The viability of PBMCs and CD4+ T cells exposed to HIV-1 R5-tropic SF162 in presence of SP from uninfected or infected donors was evaluated by measuring cellular metabolic activity (ATP levels). Results shown are the mean of triplicate wells (except mock). The metabolic activity of mock sample (no virus, no seminal plasma) was measured in triplicate wells in each plate and used as a reference to correct for variations amongst the plates. The result shown for mock condition is the mean of the different plates. As a positive control for the assay, cells were incubated for 3 h with cytotoxic concentrations of PBS (10×) instead of the inoculum. Statistical analysis with nonparametric test: Wilcoxon–Mann–Whitney test. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared with virus only.
The infection-enhancing properties of semen have been attributed to seminal amyloid fibrils (SEVI, SEM1 and SEM2), which promote the attachment of HIV to target cells [6,7]. The median level of SEVI was similar between SP+ and SP– (Fig. 2a), whereas slightly higher levels of SEM1 and SEM2 fragments were observed in SP+ compared with SP– (Fig. 2b and c). These results indicate that the lower level of PBMC infection observed with SP+ compared with SP– was not associated with lower concentrations of these peptides. There were no correlations between the seminal concentrations of those enhancing peptides and PBMC or purified CD4+ T-cell infection levels post exposure to SP– or SP+.
Fig. 2.
Levels of enhancing peptides and cytokines in SP+ and SP–.
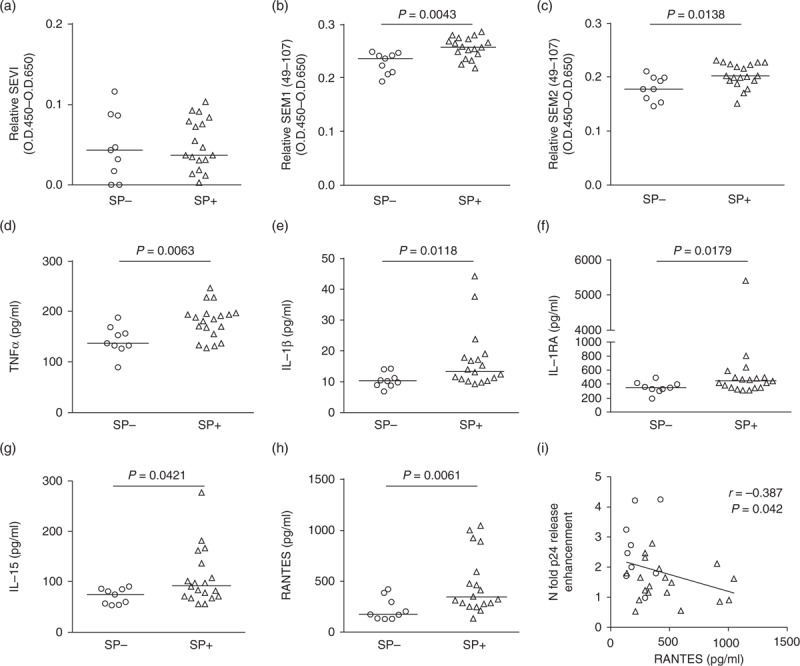
Relative SEVI (a), SEM 1 (b) and SEM 2 fragments (c) levels in SP from HIV-infected or uninfected donors, as determined by ELISA. (d–h) Levels of TNFα, IL-1β, IL-RA, IL-15 and RANTES concentrations in SP from infected and uninfected donors are shown as determined by Luminex. (a–h) Statistical analysis was conducted with the nonparametric Wilcoxon–Mann–Whitney test. (i) RANTES concentrations in SP– and SP+ negatively correlated with infection enhancement activity in PBMCs. Statistical analysis with nonparametric test: Spearman test. Circles represent SP– and triangles SP+.
Using Luminex and ELISA, we next investigated the concentrations of 46 cytokines and that of the main immunosuppressive seminal prostaglandin, PGE2 (Table S3). We found a significant increase in the median concentrations of five cytokines in SP+ versus SP–: TNFα (1.3-fold), IL-1β (1.3-fold), IL-1RA (1.2-fold), IL-15 (1.2-fold) and RANTES (CCL5) (1.9-fold) (Fig. 2d–h, and Table S3). Apart from IL-1RA, a positive correlation was observed between the levels of those cytokines and the semen viral loads (Table S4), as previously described for RANTES [34], IL-1β [15,35] and TNFα [14]. RANTES was the only cytokine for which a consistent (negative) correlation was observed between its concentrations in semen and the relative ability of the semen sample to enhance R5-tropic HIV-1 infection of PBMCs (Spearman test, r = –0.387, P = 0.042) (Fig. 2i). We next compared the effect of SP+ versus SP– on CD4+ T cells’ HIV receptor expression, activation status and proliferation. We did not observe any differential effect of SP+ versus SP– on CD4 or CXCR4 expression: CD4 expression on CD3+ T cells was similarly decreased at 6 and 24 h postexposure to SP– or SP+, when compared with infected cells without SP (Fig. 3a and b). CXCR4 expression on CD4+ T cells was unchanged at 6 h, and significantly increased at 24 h after SP– or SP+ exposure (Fig. 3a and c). A positive correlation with PGE2 concentrations, known to upregulate CXCR4 [36,37], was observed (Spearman test, P < 0.001, r = 0.68). In contrast, CCR5 expression was significantly decreased 6 h postexposure to SP+ when compared with SP– (Fig. 3a and d). This decrease was transient, as CCR5 membrane expression was elevated at 24 h postexposure to both SP– and SP+ when compared with the virus only control (Fig. 3d). No significant changes in CCR5 mRNA copy numbers were observed at any time points tested, indicating posttranscriptional modulation of CCR5 surface expression by SP (Fig. S5). The percentage of CCR5+ CD4+ T cells at 6 h positively correlated with the magnitude of infection of PBMCs (Spearman test, r = 0.711, P = 0.0025) (Fig. 3e). A negative correlation was observed between SP-induced alterations of CCR5 expression level and the added concentrations of seminal CCR5 ligands RANTES, MIP1α (CCL3) and MIP1β (CCL4) (Spearman test, r = –0.556, P = 0.039) (Fig. 3f). No correlations were found when RANTES, MIP1α and MIP1β concentrations were taken individually. Exposure of PBMCs to SP+ or SP– triggered a similar decrease of the expression of the activation marker CD69 on CD4+ T cells as early as 6 h post exposure (Fig. S6A). CD4+ T-cell proliferation was not significantly different between SP– and SP+ at 24 h and was slightly higher in presence of SP+ compared with SP– at 48 h (Fig. S6B). The high concentrations of the antiproliferative and immunosuppressive molecules PGE2 and TGFβ measured in both SP+ and SP– and previously described in semen [38,39] may contribute to the decreased proliferation of CD4+ T cells and their reduced expression of the activation marker CD69 following exposure to SP.
Fig. 3.
Effect of SP+ versus SP– on HIV receptor expression by CD4+ T cells.
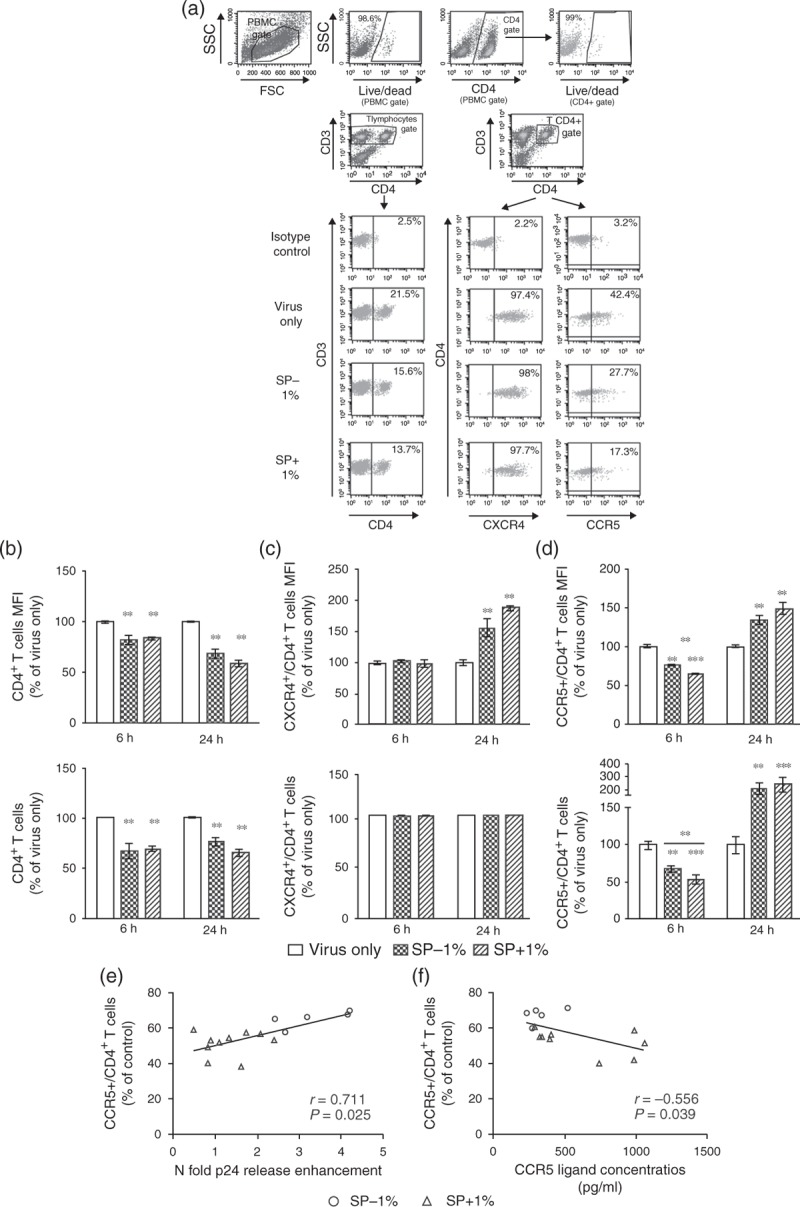
(a) The gating strategy is shown on representative flow cytometry plots: PBMCs exposed 3 h to HIV-1 SF162 and 0 or 1% SP– or SP+ were gated to exclude debris (PBMCs gate) and analyzed for the percentage of live PBMCs using Live/Dead dye, and percentage of live CD4+ cells by double labeling with Live/Dead and CD4+ antibody. PBMCs were further analyzed for their surface expression of CD3, CD4, CXCR4 and CCR5. The analysis of CD4+ expression was done on the CD3+ PBMC population (T lymphocytes gate). The analysis of CXCR4 and CCR5 expression was done on the CD3+/CD4+ population (CD4+ T lymphocytes gate). Representative results are shown. (b) The measure of cell-surface CD4+ mean fluorescence intensity (MFI) and percentage of CD4+ T cells at different time points following exposure to either SP+ or SP–, together with HIV-1 R5 SF162, showed a decrease of CD4+ expression as compared with control (cells exposed to virus only). (c) CXCR4 MFI with SP+ or SP– was similar to that in the absence of SP at 6 h. In contrast, CXCR4 MFI was increased 24 h post SP– or SP+ exposure. The percentage of CXCR4 positive cells was unchanged for all conditions and time points. (d) The CCR5 MFI and the percentage of CD4+ T cells expressing CCR5 was significantly decreased 6 h postexposure to SP+ and SP–, as compared with control (cells exposed to virus only). The extent of this decrease was significantly more pronounced with SP+ than with SP. In contrast, an increase in CCR5 MFI and percentage of CCR5+ cells was similarly observed at 24 h for both SP+ and SP– as compared with control. Control represents PBMCs cells exposed to virus (HIV-1 R5, 2 ng/ml p24) for 3 h in absence of SP. SP was used at a final dilution of 1%. (e) The magnitude of infection-enhancing activity in PBMCs following exposure to SP is correlated with the percentage of CD4+ T cells expressing CCR5 at 6 h. (f) The proportion of CD4+ T cells expressing CCR5 at 6 h is inversely correlated with the cumulative concentrations in SP of the CCR5 ligands RANTES, MIP1α and MIP1β. Shown is the mean of 5–10 wells/condition, each corresponding to different donors for SP– and SP+ ± SEM. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared with virus only. Statistical analysis with nonparametric test: Wilcoxon–Mann–Whitney test and Spearman test.
To investigate whether decreased CCR5 expression was involved in the reduced infection of PBMC exposed to SP+ compared with SP–, we analyzed the impact of SP+ versus SP– on X4-tropic HIV-1 infection. Our results show that SP– and SP+ had similar enhancing effects on PBMCs infection by HIV-1 X4 (Fig. 4a). Cell viability was not affected by SP exposure (Fig. 4b).
Fig. 4.
Effect of SP+ versus SP– on X4-tropic HIV-1 infection of PBMCs.
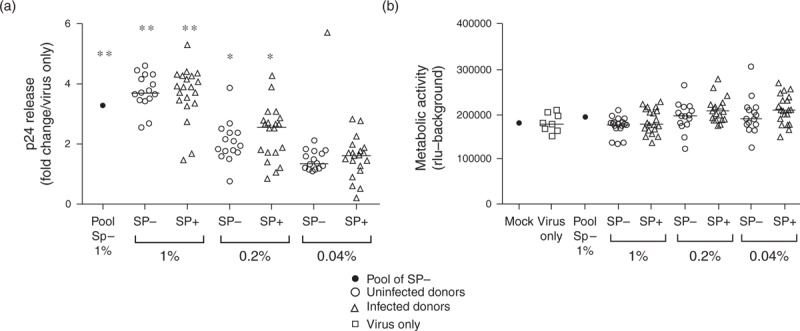
PBMCs (a) were exposed to the indicated dilutions of SP+ or SP– for 3 h in presence of HIV-1 X4 strain (for each condition, each symbol represents 1 donor). Pooled SP– from 50 additional uninfected donors was tested in parallel. Infectivity of PBMCs was measured after 72 h of culture in fresh medium following a 3-h exposure to SP and virus by p24 ELISA of culture supernatants. Results are expressed in fold change compared with virus only. The viability of PBMCs (b) exposed to HIV-1 X4-tropic IIIB in presence of SP from uninfected or infected donors was evaluated by measuring cellular metabolic activity (ATP levels). Results shown are the mean of triplicate wells (except mock) and are representative of three independent experiments for each donor (n = 15–20). The metabolic activity of mock condition (no virus, no SP) was measured in triplicate wells in each plate and used as a reference amongst the plates. The result shown for mock condition is the mean of the different plates. As a positive control for the assay, cells were incubated for 3 h with cytotoxic concentrations of PBS (10×) instead of the inoculum. Results shown are representative of three independent experiments for each donor. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared with virus only. Statistical analysis with nonparametric test: Wilcoxon–Mann–Whitney test.
We then spiked two SP– pools (from 50 donors each) as well as SP– from five separate donors with recombinant RANTES at concentrations similar to those measured in SP+ (5 and 10 pg/ml in 1% SP, corresponding to 500 and 1000 pg/ml in undiluted SP). The median SP-enhancing activity of HIV-1 R5-tropic infection of PBMCs decreased from 2.6 to 1.4-fold in the presence of 5 pg/ml RANTES, and was lost (1-fold) with 10 pg/ml RANTES (P = 0.047) (Fig. 5a). Interestingly, whereas the addition of RANTES decreased the SP-enhancing activity of two pools of SP– and three individual SP– from different donors, the same concentrations of RANTES had no inhibitory effect in SP– from two donors (Fig. 5a).
Fig. 5.
Effect of SPs added or depleted of RANTES on R5-tropic HIV-1 infection of PBMCs.
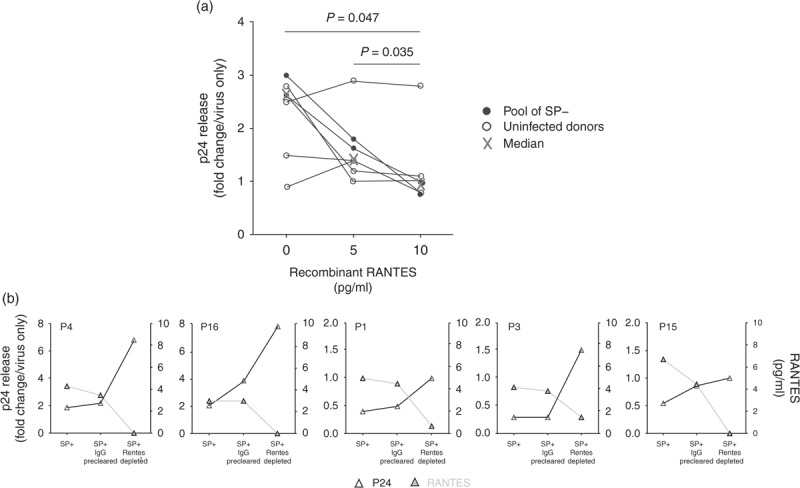
PBMCs were exposed for 3 h to HIV-1 R5-tropic SF162 strain in the presence or absence of SPs (diluted to 1%) and infectivity of PBMCs measured after 72 h of culture in fresh medium by p24 ELISA on culture supernatants. (a) Human recombinant RANTES was added to 1% SP from two pools of SP– (each pool derived from 50 uninfected donors) and from five additional uninfected donors. Results representing the mean of triplicate wells are expressed in fold change compared with virus only. Statistical analysis with nonparametric test: Wilcoxon matched-pairs signed-rank test. (b) SPs from five HIV-infected donors (SP+) were first precleared of IgGs (SP+ IgG precleared) before being depleted of RANTES (SP+ RANTES depleted), as described in methods. Graphs display for each donor both p24 release (expressed in fold change compared with virus only), and RANTES concentrations in 1% SP, before and after IgG preclearing and RANTES depletion.
SP+ depleted from RANTES consistently increased HIV-1 infection levels by a median of 3.6-fold (range 1.8–5.8) that of untouched SP+ in five separate donors (Fig. 5b). The IgG preclearing step (Fig. 5b), performed prior to antibody-specific RANTES depletion, only led to a minor median enhancement of 1.2-fold that of untouched SP+ (range 1–1.8), together with a slight decrease in RANTES concentrations in 4/5 samples.
Discussion
Understanding the role of semen in HIV transmission is crucial to the design of effective prevention strategies. Because cell-free virus was found to infect cervicovaginal and rectal target cells within 1–4 h postexposure in macaques [40,41], the most relevant time frame for studying SP's impact on HIV transmission is during the few hours following the intercourse. In addition, short SP exposure is necessary to avoid SP-induced cytotoxic effect on CD4+ T cells in culture. Our results show that under noncytotoxic conditions mimicking rapid infection of the recipient's target cells through contaminated semen, SP from uninfected men enhanced HIV-1 infection of PBMCs. In agreement with Kim et al.[4], our results suggest that the reported inhibitory effect of prolonged exposure (>24 h) to SP from uninfected men of CD4+ T cells [3,5] was due to cytotoxicity.
When comparing SP from infected versus uninfected men, we found that the enhancement activity mediated by SP+ was significantly reduced compared with SP– when R5-tropic infection of PBMCs and CD4+ T cells was carried out. In contrast, enhancement activity was similar between SP– and SP+ when PBMCs were infected with an HIV-1 X4-tropic virus. The viral-enhancing activity of semen was previously attributed to positively charged amyloids including SEVI, SEM1 and SEM2 fibrils which capture virions and promote their attachment to cells [6,7,42]. Our data set of SP+ displayed similar or even slightly higher concentrations of these peptides compared with our SP– samples, suggesting that the lower level of PBMC infection by R5-tropic HIV-1 following exposure to SP+ cannot be explained by a decreased level of those enhancing peptides.
The comparison of the seminal cytokine contents between our cohort of infected and uninfected men showed five significantly elevated cytokines in SP+. Of these the CCR5 ligand RANTES showed the highest increase (∼2 versus 1.2–1.3-fold for TNFα, IL-1β, IL-15 and IL-1RA). Considerable heterogeneity in semen cytokine levels exists between cohorts of uninfected individuals [3,10,38,43] as well as between cohorts of HIV-infected men [12–14,34,43,44], which likely reflects both inter-cohorts differences (e.g. number of individual tested, geographical origins, detection kits used, etc.) and the wide range of concentrations found for most cytokines between individuals, which may be linked to the semen microbiome, HIV load, or genital infections [3,10,12–14,38,43]. However, the analysis of published studies on cytokine content in semen from HIV+ men revealed that RANTES was also elevated in several other cohorts [13,34,44]. RANTES inhibits infection by R5-tropic strains through receptor downregulation and competitive binding to CCR5 [45]. A negative correlation was consistently and exclusively found between seminal RANTES concentrations and the SP infection-enhancing activity during R5-tropic HIV-1 infection of PBMCs. When examining CD4+ T-cells HIV receptor expression, activation and proliferation at different time points, the only difference found between the effects of SP– versus SP+ was a significantly more pronounced initial decrease of CCR5 surface expression. This decreased expression most likely reflected down-modulation of CCR5 rather than a binding competition between the CCR5 antibody and CCR5 ligands as the binding site of the CCR5 antibody used (3A9) is different from that of RANTES, MIP1α and MIP1β [46]. The percentage of CD4+ T cells expressing CCR5 positively correlated the magnitude of PBMCs infection and negatively correlated with the added concentrations of the CCR5 ligands RANTES, MIP-1α and MIP-1β in semen. In addition to this, our finding that SP+ and SP– similarly enhanced HIV-1 IIIB infection of PBMCs further points to the decreased CCR5 surface expression by SP+ as a key factor in the lower level of infection activity in SP+ when compared with that of SP– when an R5-tropic virus is used in the inoculum. The unchanged CCR5 mRNA levels post SP– or SP+ exposure at all-time points suggests modifications of the intracellular trafficking of the receptor (e.g. enhanced internalization) compatible with ligand binding. The addition of recombinant RANTES to SPs from uninfected donors led to a decrease in the enhancing activity of two different pools of SP– from 50 donors each, and three out of five SP– from separate donors, demonstrating that RANTES in SP can indeed trigger an inhibitory effect. Interestingly, our results also suggest that other semen's factors (such as the concentrations of enhancing factors and other CCR5 ligands) may modulate RANTES inhibitory effect on HIV infection. RANTES depletion in SP+ samples increased HIV replication, leading to the suppression of SP+ mediated inhibitory activity in three of three donors and to an increase of SP+ mediated enhancing activity in two of two donors. By contrast, the IgG preclearing step only had a very modest effect on SP+ activity and, in four of five samples, led to a slight decrease in RANTES concentrations. These results indicate that RANTES concentrations in SPs from different HIV-infected donors indeed influence SP effect on HIV infection.
Semen from HIV+ individuals may contain soluble HIV proteins, as well as other pathogens, which could also directly affect HIV infection of target cells. For instance, soluble gp120 was shown in vitro to either inhibit or enhance HIV replication, by competing with virions for CD4+ and co-receptor binding, or inducing cell signaling, respectively [47–49]. Importantly however, the concentrations of soluble gp120 in body fluids are thought to be insufficient to trigger these effects [50]. Thus, a virus-soluble gp120 paired competitive assay study showed that the effect of the soluble gp120 on virion entry efficiency could only be seen with high amount of protein, probably beyond the range of that found in body fluids [47]. Moreover, soluble gp120-mediated cross linking to CD4+ receptor was shown to down-modulate the membrane expression of CD4+[51] whereas in our study, we did not observe any specific effect of SP+ on CD4+ receptor expression when compared with SP–. As for other soluble viral proteins, they would potentially affect not only R5 strains, but also X4 strains, whereas the reduced enhancing activity of SP+ was specific for R5 strain. Altogether, these elements argue against a role of soluble HIV proteins in SP+ effect.
Regarding other pathogens, there were no clinical signs of active co-infections in any of the semen donors, and except for one HIV+ donor, leukocyte concentrations in semen were in the normal range, namely below 1 million/ml [25]. However, a large proportion of genital tract infection in men is asymptomatic and a normal leukocyte cell count in semen does not exclude the possibility of an infection [26]. Therefore the presence of other semen-contaminating pathogens cannot be ruled out. Among those, CMV and HSV-2 have been shown to directly stimulate HIV-1 R5 entry and replication in CD4+ cells, notably through increased CCR5 expression and cell activation [52,53]. This is opposite to the SP+ effect evidenced in our study, as SP+ decreased HIV R5 infection and CCR5 expression, and both SP+ and SP– exposure diminished proliferation of CD4+ T cells and reduced the expression of the activation marker CD69. Although several other semen-contaminating pathogens may enhance or inhibit HIV replication in CD4+ cells, their effects are not specific for HIV R5 strains [54–58]. Thus it is unlikely that other pathogens in semen directly contributed to the R5 strain-specific reduced enhancing effect of SP+.
Overall, we showed that the effect of SP on HIV-1 infection varies depending on the donor status (HIV infected or not) and viral tropism (R5 versus X4). Our results indicate that HIV infection significantly modifies SP composition, and suggest that a balance of stimulatory (e.g. enhancing amyloids and HIV replication enhancing cytokines such as IL-1β, TNFα) and inhibitory molecules (e.g. cytokines like RANTES that decrease HIV-1 infection) are at play, to which various viral strains and target cells will be differently susceptible. For instance, SP from infected and uninfected men were recently reported to enhance R5-tropic HIV-1 infection of TZM-bl to a similar level [2]. Previous reports have described various effects of SP from uninfected men depending on the cells, for example, cell lines or different primary cell types [4,8,9,59–64]. Importantly, to date, all the in-vivo studies on the impact of SP on HIV transmission [65–67] have been performed with semen from uninfected donors. Our results emphasize the importance of testing semen from infected donors, as well as whole SP instead of purified seminal factors, to account for the balance between inhibitory and stimulatory factors.
In conclusion, this study is the first to directly compare the effect of SP from HIV-infected and uninfected men on HIV-1 infection of primary CD4+ T cells. Although SP from uninfected men enhanced HIV-1 infection of PBMCs, consistent with previous reports [4,6,7], SP from infected individuals showed a significantly reduced enhancing activity when an R5-tropic HIV-1 was used. Our results suggest that RANTES in semen is at least in part responsible for the decreased enhancing activity of SP from infected men. These results highlight the complex effects of semen on HIV infection and point to the importance of total consideration of the experimental system (including the status of semen donor, the target cell types, and the duration of exposure to semen) when assessing semen modulating effects. In-vivo experiments in animal models using semen from infected individuals are urgently needed to better understand the role of this complex fluid on HIV transmission.
Acknowledgements
C.C., O.B., D.M., G.M., F.A., N.R., J.M., and O.Z. have performed experiments and analyzed data. L.B., C.P., C.R., P.M., C.P. have contributed samples, data analyses and study design. N.D.R has conceived, designed and supervised the work. All authors read and approved the final manuscript. We are most grateful to Christine Monfort and Emmanuelle Becker for assistance with statistical analyses, and to the Service des Maladies infectieuses et tropicales from Toulouse Universitary Hospital for HIV patients recruitment, in particular Dr Lise Cusin, Martine Obadia and Prof. Bruno Marchou. We also thank the team of CECOS Midi-Pyrénées for seminal plasma preparation and particularly Drs Myriam Daudin, Nathalie Moinard, and all the laboratory technicians.
Sources of support: this work was funded by ANRS, Sidaction and Inserm. C.C. was supported by the French ministry of research and by ANRS. O.B. was the recipient of a Sidaction fellowship. G.M. was supported by ANRS.
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol 2008; 8:447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usmani SM, Zirafi O, Muller JA, Sandi-Monroy NL, Yadav JK, Meier C, et al. Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen. Nat Commun 2014; 5:3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balandya E, Sheth S, Sanders K, Wieland-Alter W, Lahey T. Semen protects CD4+ target cells from HIV infection but promotes the preferential transmission of R5 tropic HIV. J Immunol 2010; 185:7596–7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KA, Yolamanova M, Zirafi O, Roan NR, Staendker L, Forssmann WG, et al. Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI. Retrovirology 2010; 7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martellini JA, Cole AL, Venkataraman N, Quinn GA, Svoboda P, Gangrade BK, et al. Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma. FASEB J 2009; 23:3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munch J, Rucker E, Standker L, Adermann K, Goffinet C, Schindler M, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell 2007; 131:1059–1071. [DOI] [PubMed] [Google Scholar]
- 7.Roan NR, Muller JA, Liu H, Chu S, Arnold F, Sturzel CM, et al. Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection. Cell Host Microbe 2011; 10:541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabatte J, Ceballos A, Raiden S, Vermeulen M, Nahmod K, Maggini J, et al. Human seminal plasma abrogates the capture and transmission of human immunodeficiency virus type 1 to CD4+ T cells mediated by DC-SIGN. J Virol 2007; 81:13723–13734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabatte J, Faigle W, Ceballos A, Morelle W, Rodrigues CR, Lenicov FR, et al. Semen clusterin is a novel DC-SIGN ligand. J Immunol 2011; 187:5299–5309. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DJ, Politch JA, Tucker LD, Fichorova R, Haimovici F, Tuomala RE, et al. Quantitation of mediators of inflammation and immunity in genital tract secretions and their relevance to HIV type 1 transmission. AIDS Res Hum Retroviruses 1998; 14 Suppl 1:S43–S49. [PubMed] [Google Scholar]
- 11.Anderson JA, Ping LH, Dibben O, Jabara CB, Arney L, Kincer L, et al. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog 2010; 6:e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kafka JK, Sheth PM, Nazli A, Osborne BJ, Kovacs C, Kaul R, et al. Endometrial epithelial cell response to semen from HIV-infected men during different stages of infection is distinct and can drive HIV-1-long terminal repeat. AIDS 2012; 26:27–36. [DOI] [PubMed] [Google Scholar]
- 13.Lisco A, Munawwar A, Introini A, Vanpouille C, Saba E, Feng X, et al. Semen of HIV-1-infected individuals: local shedding of herpesviruses and reprogrammed cytokine network. J Infect Dis 2011; 205:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivier AJ, Masson L, Ronacher K, Walzl G, Coetzee D, Lewis DA, et al. Distinct cytokine patterns in semen influence local HIV shedding and HIV target cell activation. J Infect Dis 2014; 209:1174–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu CM, Osborne BJ, Hungate BA, Shahabi K, Huibner S, Lester R, et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathog 2014; 10:e1004262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deleage C, Moreau M, Rioux-Leclercq N, Ruffault A, Jegou B, Dejucq-Rainsford N. Human immunodeficiency virus infects human seminal vesicles in vitro and in vivo. Am J Pathol 2011; 179:2397–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Tortorec A, Le Grand R, Denis H, Satie AP, Mannioui K, Roques P, et al. Infection of semen-producing organs by SIV during the acute and chronic stages of the disease. PLoS One 2008; 3:e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Tortorec A, Satie AP, Denis H, Rioux-Leclercq N, Havard L, Ruffault A, et al. Human prostate supports more efficient replication of HIV-1 R5 than X4 strains ex vivo. Retrovirology 2008; 5:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roulet V, Satie AP, Ruffault A, Le Tortorec A, Denis H, Guist’hau O, et al. Susceptibility of human testis to human immunodeficiency virus-1 infection in situ and in vitro. Am J Pathol 2006; 169:2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bujan L, Sergerie M, Moinard N, Martinet S, Porte L, Massip P, et al. Decreased semen volume and spermatozoa motility in HIV-1 infected patients under antiretroviral treatment. J Androl 2007; 28:444–452. [DOI] [PubMed] [Google Scholar]
- 21.Saba E, Grivel JC, Vanpouille C, Brichacek B, Fitzgerald W, Margolis L, et al. HIV-1 sexual transmission: early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol 2010; 3:280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 2007; 26:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 2011; 62:127–139. [DOI] [PubMed] [Google Scholar]
- 24.Shen R, Richter HE, Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol 2010; 65:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed.Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 26.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed.Geneva: WHO; 2010. [Google Scholar]
- 27.Papkalla A, Munch J, Otto C, Kirchhoff F. Nef enhances human immunodeficiency virus type 1 infectivity and replication independently of viral coreceptor tropism. J Virol 2002; 76:8455–8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown WF. Variance estimation in the Reed-Muench fifty per cent end-point determination. Am J Hyg 1964; 79:37–46. [DOI] [PubMed] [Google Scholar]
- 29.Roulet V, Denis H, Staub C, Le Tortorec A, Delaleu B, Satie AP, et al. Human testis in organotypic culture: application for basic or clinical research. Hum Reprod 2006; 21:1564–1575. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B (Methodol) 1995; 57:289–300. [Google Scholar]
- 31.Fiore JR, La Grasta L, Di Stefano M, Buccoliero G, Pastore G, Angarano G. The use of serum-free medium delays, but does not prevent, the cytotoxic effects of seminal plasma in lymphocyte cultures: implications for studies on HIV infection. New Microbiol 1997; 20:339–344. [PubMed] [Google Scholar]
- 32.Okamoto M, Byrn R, Eyre RC, Mullen T, Church P, Kiessling AA. Seminal plasma induces programmed cell death in cultured peripheral blood mononuclear cells. AIDS Res Hum Retroviruses 2002; 18:797–803. [DOI] [PubMed] [Google Scholar]
- 33.Allen RD, Roberts TK. The relationship between the immunosuppressive and cytotoxic effects of human seminal plasma. Am J Reprod Immunol Microbiol 1986; 11:59–64. [DOI] [PubMed] [Google Scholar]
- 34.Storey DF, Dolan MJ, Anderson SA, Meier PA, Walter EA. Seminal plasma RANTES levels positively correlate with seminal plasma HIV-1 RNA levels. AIDS 1999; 13:2169–2171. [DOI] [PubMed] [Google Scholar]
- 35.Berlier W, Cremel M, Hamzeh H, Levy R, Lucht F, Bourlet T, et al. Seminal plasma promotes the attraction of Langerhans cells via the secretion of CCL20 by vaginal epithelial cells: involvement in the sexual transmission of HIV. Hum Reprod 2006; 21:1135–1142. [DOI] [PubMed] [Google Scholar]
- 36.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res 2011; 71:7463–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salcedo R, Zhang X, Young HA, Michael N, Wasserman K, Ma WH, et al. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood 2003; 102:1966–1977. [DOI] [PubMed] [Google Scholar]
- 38.Politch JA, Tucker L, Bowman FP, Anderson DJ. Concentrations and significance of cytokines and other immunologic factors in semen of healthy fertile men. Hum Reprod 2007; 22:2928–2935. [DOI] [PubMed] [Google Scholar]
- 39.Robertson SA, Ingman WV, O’Leary S, Sharkey DJ, Tremellen KP. Transforming growth factor beta--a mediator of immune deviation in seminal plasma. J Reprod Immunol 2002; 57:109–128. [DOI] [PubMed] [Google Scholar]
- 40.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol 2000; 74:6087–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro Dos Santos P, Rancez M, Pretet JL, Michel-Salzat A, Messent V, Bogdanova A, et al. Rapid dissemination of SIV follows multisite entry after rectal inoculation. PLoS One 2011; 6:e19493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roan NR, Munch J, Arhel N, Mothes W, Neidleman J, Kobayashi A, et al. The cationic properties of SEVI underlie its ability to enhance human immunodeficiency virus infection. J Virol 2009; 83:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berlier W, Bourlet T, Levy R, Lucht F, Pozzetto B, Delezay O. Amount of seminal IL-1beta positively correlates to HIV-1 load in the semen of infected patients. J Clin Virol 2006; 36:204–207. [DOI] [PubMed] [Google Scholar]
- 44.Hoffman JC, Anton PA, Baldwin GC, Elliott J, Anisman-Posner D, Tanner K, et al. Seminal plasma HIV-1 RNA concentration is strongly associated with altered levels of seminal plasma interferon-gamma, interleukin-17, and interleukin-5. AIDS Res Hum Retroviruses 2014; 30:1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alfano M, Poli G. Role of cytokines and chemokines in the regulation of innate immunity and HIV infection. Mol Immunol 2005; 42:161–182. [DOI] [PubMed] [Google Scholar]
- 46.Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, Ruffing N, et al. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J Exp Med 1997; 186:1373–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwalbe B, Hauser H, Schreiber M. A virus-envelope paired competitive assay to study entry efficiency of human immunodeficiency virus type 1 in vitro. J Virol Methods 2014; 205C:91–98. [DOI] [PubMed] [Google Scholar]
- 48.Bren GD, A. TS, Whitman J, Shepard B, Badley AD. HIV gp120 induces, NF-kappaB dependent, HIV replication that requires procaspase 8. PLoS One 2009; 4:e4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misse D, Gajardo J, Oblet C, Religa A, Riquet N, Mathieu D, et al. Soluble HIV-1 gp120 enhances HIV-1 replication in nondividing CD4+ T cells, mediated via cell signaling and Tat cofactor overexpression. AIDS 2005; 19:897–905. [DOI] [PubMed] [Google Scholar]
- 50.Klasse PJ, Moore JP. Is there enough gp120 in the body fluids of HIV-1-infected individuals to have biologically significant effects?. Virology 2004; 32:1–8. [DOI] [PubMed] [Google Scholar]
- 51.Cefai D, Ferrer M, Serpente N, Idziorek T, Dautry-Varsat A, Debre P, et al. Internalization of HIV glycoprotein gp120 is associated with down-modulation of membrane CD4 and p56lck together with impairment of T cell activation. J Immunol 1992; 149:285–294. [PubMed] [Google Scholar]
- 52.Johnson EL, Howard CL, Thurman J, Pontiff K, Johnson ES, Chakraborty R. Cytomegalovirus upregulates expression of CCR5 in central memory cord blood mononuclear cells, which may facilitate in utero HIV type 1 transmission. J Infect Dis 2015; 211:187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rollenhagen C, Lathrop MJ, Macura SL, Doncel GF, Asin SN. Herpes simplex virus type-2 stimulates HIV-1 replication in cervical tissues: implications for HIV-1 transmission and efficacy of anti-HIV-1 microbicides. Mucosal Immunol 2014; 7:1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guan M, Zhang RD, Wu B, Henderson EE. Infection of primary CD4+ and CD8+ T lymphocytes by Epstein-Barr virus enhances human immunodeficiency virus expression. J Virol 1996; 70:7341–7346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ensoli B, Lusso P, Schachter F, Josephs SF, Rappaport J, Negro F, et al. Human herpes virus-6 increases HIV-1 expression in co-infected T cells via nuclear factors binding to the HIV-1 enhancer. EMBO J 1989; 8:3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding J, Rapista A, Teleshova N, Mosoyan G, Jarvis GA, Klotman ME, et al. Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation. J Immunol 2010; 184:2814–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fakruddin JM, Lempicki RA, Gorelick RJ, Yang J, Adelsberger JW, Garcia-Pineres AJ, et al. Noninfectious papilloma virus-like particles inhibit HIV-1 replication: implications for immune control of HIV-1 infection by IL-27. Blood 2007; 109:1841–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lusso P, Secchiero P, Crowley RW, Garzino-Demo A, Berneman ZN, Gallo RC. CD4 is a critical component of the receptor for human herpesvirus 7: interference with human immunodeficiency virus. PNAS 1994; 91:3872–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stax MJ, van Montfort T, Sprenger RR, Melchers M, Sanders RW, van Leeuwen E, et al. Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes. Virology 2009; 391:203–211. [DOI] [PubMed] [Google Scholar]
- 60.Madison MN, Roller RJ, Okeoma CM. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014; 11:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thurman AR, Anderson S, Doncel GF. Effects of hormonal contraception on antiretroviral drug metabolism, pharmacokinetics and pharmacodynamics. Am J Reprod Immunol 2014; 71:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doncel GF, Joseph T, Thurman AR. Role of semen in HIV-1 transmission: inhibitor or facilitator?. Am J Reprod Immunol 2010; 65:292–301. [DOI] [PubMed] [Google Scholar]
- 63.Doncel GF, Clark MR. Preclinical evaluation of anti-HIV microbicide products: new models and biomarkers. Antiviral Res 2010; 88 Suppl 1:S10–S18. [DOI] [PubMed] [Google Scholar]
- 64.Lawrence P, Portran D, Terrasse R, Palle S, Olivier T, Fantini J, et al. Selective transmigration of monocyte-associated HIV-1 across a human cervical monolayer and its modulation by seminal plasma. AIDS 2012; 26:785–796. [DOI] [PubMed] [Google Scholar]
- 65.Miller CJ, Marthas M, Torten J, Alexander NJ, Moore JP, Doncel GF, et al. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol 1994; 68:6391–6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neildez O, Le Grand R, Caufour P, Vaslin B, Cheret A, Matheux F, et al. Selective quasispecies transmission after systemic or mucosal exposure of macaques to simian immunodeficiency virus. Virology 1998; 243:12–20. [DOI] [PubMed] [Google Scholar]
- 67.Munch J, Sauermann U, Yolamanova M, Raue K, Stahl-Hennig C, Kirchhoff F. Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus. Retrovirology 2013; 10:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


