Abstract
Aim
To determine whether the promoter polymorphism -203A>C of cholesterol-7α-hydroxylase encoding gene (CYP7A1) affects diurnal variation in CYP7A1 enzyme activity.
Methods
The study included 16 healthy male volunteers – 8 homozygous for -203A and 8 homozygous for the -203C allele of CYP7A1. Three 15-hour examinations (from 7am to 10pm) were carried out for each of the participants: after one-day treatment with cholestyramine; after one-day treatment with chenodeoxycholic acid (CDCA); and a control examination without any treatment. The plasma concentration of 7α-hydroxy-4-cholesten-3-one (C4), a marker of CYP7A1 activity, was determined in all the experiments at 90-min intervals.
Results
CYP7A1 activity was up-regulated after treatment with cholestyramine and suppressed after treatment with CDCA. There were no differences between -203A and -203C allele carriers in the response of enzyme activity to both drugs. In the control experiment, -203A allele carriers displayed diurnal variation in enzyme activity, whereas CYP7A1 activity did not change in -203C allele carriers. These results were confirmed by modeling the dynamics of C4 using polynomial regression.
Conclusion
The promoter polymorphism of the CYP7A1 gene has a pronounced impact on diurnal variation in CYP7A1 activity.
Cholesterol 7α- hydroxylase (CYP7A1) is the key regulatory enzyme of the classic bile acid biosynthetic pathway. Its activity is subjected to complex feedback regulation in order to maintain the bile acid pool (1,2). Enzyme activity has been shown to exhibit a distinct diurnal variation in rodents (3) and humans (4). The study in humans took advantage of the fact that CYP7A1 activity in the liver correlated with concentration of 7α-hydroxy-4-cholesten-3-one (C4) in plasma, which can be thus used as a reliable marker of CYP7A1 activity (5,6). The same authors demonstrated that there were two peaks in enzyme activity, the first at midday and the second around 10pm (4). In our previous study in healthy volunteers carried out between 7am and 10pm, we reproduced their findings for the midday peak (7). However, we noted that enzyme activity displayed very high inter-individual variability.
Several polymorphisms have been identified in the regulatory regions of the CYP7A1 gene. One of these polymorphisms, -203A>C (rs3808607), has been shown to affect cholesterolemia and responsiveness of cholesterolemia to diet as well as drug treatment (8-13). The mechanisms involved have not yet been explained and it is still not clear whether this promoter polymorphism can also affect diurnal variation in enzyme activity. Therefore, in this case-control study, we investigated whether the course of CYP7A1 diurnal activity differed between homozygous young healthy male carriers of -203A and -203C alleles of the CYP7A1 gene. We also analyzed the effect of short-term chenodeoxycholic acid (CDCA) and cholestyramine (a bile acid sequestrant) treatments on CYP7A1 activity throughout the day. These drugs down-regulate and up-regulate CYP7A1 activity, respectively.
Participants and methods
Participants and study design
Sixteen healthy male volunteers – 8 homozygous for the -203A CYP7A1 allele and 8 homozygous for the -203C allele – were recruited from among the personnel of both participating institutions. Both groups were matched with respect to age (mean±SD, 25.7 ± 3.4 vs 25.3 ± 3.8 years, respectively). The study design was exactly the same as in our previous study (7), consisting of three daylong examinations. One of these examinations served as a control examination (without any drug intervention), while the other two were used to investigate the effect of short-term administration of cholestyramine (Questran®, Bristol-Myers Squibb, Prague, Czech Republic, 16 g/d) and chenodeoxycholic acid (CDCA; Chenofalk®, Dr Falk Pharma GmbH, Freiburg, Germany, 1-1.5 g/d depending on the weight of the participant). One day before each of these examinations (Day 0), the first blood sample was drawn at 7am, after which participants received food for the whole day to standardize their intake before the study. On the day of examination (Day 1), the first blood sample was drawn again at 7am and the blood samples were then collected at 90-min intervals for 15 h until 10pm. Again, participants received food for the whole day and were required to eat at exactly defined intervals (breakfast at 7.15am, snack at 9.45am, lunch at 12.30pm, snack at 3.30pm, and dinner at 5.30pm). The amount of food was calculated to cover their energy requirements – ~ 160 kJ/kg/d – and the diet was relatively low in fat (25% of energy intake). When the examination included drug administration, the drugs were given on Day 0 and Day 1. Cholestyramine was given in two doses on both days – one with breakfast, the other with dinner. Due to differences in pharmacokinetics, CDCA treatment was started with dinner on Day 0, and on Day 1 was given in two doses at the same time as cholestyramine. The order of the examinations was randomized and carried out at three-week intervals at a minimum. The study protocol adhered to the principles of the Declaration of Helsinki, was approved by the Ethics Committee of the Institute for Clinical and Experimental Medicine, and all of the participants gave their informed consent.
Genotyping and biochemical analyses
Determination of the -203A>C genotype of CYP7A1 was carried out as described earlier (14). Plasma cholesterol and triglyceride (TG) concentrations were determined using enzymatic kits from Roche Diagnostics GmbH, Mannheim, Germany. Concentration of C4 was determined by HPLC as described earlier (15).
Statistics
ANOVA for repeated measures was used to determine whether the concentrations of cholesterol, TG, and C4 varied throughout the experiments. ANOVA for repeated measures with one grouping factor (genotype) was used to determine whether there were any differences in the course of the concentration changes between carriers of -203A and -203C alleles. The data for analysis were logarithmically transformed if they did not pass the Shapiro-Wilk normality test. However, ANOVA results for logarithmically transformed and nontransformed data were comparable. Presented results of ANOVA are from nontransformed data. The dynamics of C4 concentration changes in the control experiment was modeled using polynomial regression of order 5. The JMP 10.0.0 statistical software (SAS Institute, Inc., Cary, NC, USA) was used for analyses. P-value lower than 0.05 was considered statistically significant.
Results
Homozygous carriers of A and C allele did not differ in plasma cholesterol (4.70 ± 0.91 vs 4.73 ± 0.65 mmol/L, respectively, P = 0.941, t test) and plasma TG levels (1.78 ± 1.03 vs 1.28 ± 0.39 mmol/L, respectively, P = 0.231, t test). The -203C allele carriers had significantly increased BMI (27.2 ± 3.3 vs 23.4 ± 3.5 kg/m2, P = 0.048, t test).
As expected, CYP7A1 activity – estimated on the basis of C4 concentration measurement at 7am – rose several-fold after one-day treatment with cholestyramine, dropped by a half after one-day treatment with CDCA, and did not change in the control experiment in all participants (Table 1).
Table 1.
Fasting concentrations and areas under curve (AUC) of cholesterol, triglyceride (TG), and 7α-hydroxy-4-cholesten-3-one (C4) in homozygous -203A and -203C CYP7A1 allele carriers (mean ± standard deviation). 0 – control experiment, CDCA – experiment with short-term treatment using chenodeoxycholic acid, Q – experiment with short-term treatment using cholestyramine
| -203AA carriers |
-203CC carriers |
||||||
|---|---|---|---|---|---|---|---|
| 0 | CDCA | Q | 0 | CDCA | Q | ||
|
Cholesterol |
Day 0
[mmol/L] |
4.69
±1.08 |
4.88
±0.95 |
4.83
±0.96 |
4.70
±0.65 |
4.44
±0.75 |
4.81
±0.44 |
|
Day 1
[mmol/L] |
4.56
±1.10 |
4.98
±1.09 |
4.89
±1.10 |
4.71
±0.62 |
4.63
±0.61 |
4.44***
±0.42 |
|
|
AUC
[mmol*h/L] |
65.7
±15.6 |
70.2
±14.6 |
67.9
±15.0 |
68.0
±9.2 |
67.7
±9.4 |
63.2
±7.0 |
|
|
TG |
Day 0
[mmol/L] |
1.48
±0.84 |
1.58
±0.82 |
1.71
±1.26 |
1.54
±0.65 |
1.40
±0.73 |
1.33
±0.41 |
|
Day 1
[mmol/L] |
1.39
±0.82 |
1.66
±0.96 |
1.65
±0.85 |
1.70
±1.03 |
1.70
±0.72 |
1.85*
±0.65 |
|
|
AUC
[mmol*h/L] |
24.9
±13.3 |
28.9
±15.0 |
24.0
±12.9 |
29.0
±16.9 |
27.4
±9.2 |
28.1
±14.3 |
|
|
C4 |
Day 0
[μg/L] |
16.0
±9.1 |
19.9
±13.2 |
13.9
±8.7 |
22.9
±16.6 |
36.1
±32,8 |
28.2
±21.1 |
|
Day 1
[μg/L] |
20.3 a,b
±15.9 |
9.6 b,**
±9.6 |
70.2 a,***
±27.3 |
24.7 d,e
±23.1 |
15.6 e,**
±18.8 |
111.3 d,**
±70.1 |
|
| AUC [μg*h/L] | 326 b ±94 | 131 c ±65 | 1317 a ±312 | 270 e ±168 | 112 e ±71 | 1830 d ±808 | |
*,**,***P < 0.05, P < 0.01, P < 0.001 for Day 1 vs Day 0; a,b,c the same letters are assigned to the experiments that do not differ in -203A allele carriers where differences between experiments are detected by ANOVA; d,e,f the same letters are assigned to the experiments that do not differ in -203C allele carriers, where differences between experiments are detected by ANOVA.
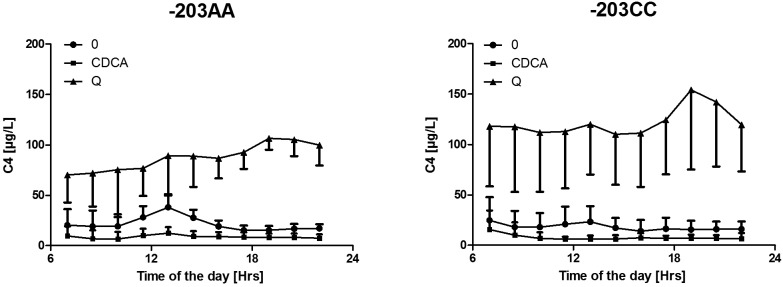
During the CDCA treatment, there were no changes in plasma C4 concentrations on the day of the study in both -203A and -203C allele carriers (Figure 1) (P = 0.373 and 0.208, respectively). Importantly, no effect of the genotype on the course of C4 concentrations was detected (P = 0.221).
Figure 1.
The course of 7α-hydroxy-4-cholesten-3-one (C4) concentrations throughout Day 1 after short-term administration of cholestyramine and CDCA and in the control experiment. 0 – control experiment, CDCA – experiment with short-term treatment using chenodeoxycholic acid, Q – experiment with short-term treatment using cholestyramine; -203AA, -203CC – homozygous carriers of the -203A and -203C allele of the CYP7A1 gene, respectively.
During the cholestyramine treatment, C4 concentrations significantly increased during the day in -203A allele carriers (P = 0.004). A similar but not significant trend was observed in -203C allele carriers (P = 0.074). However, the genotype had no effect on the course of C4 concentrations throughout the day (P = 0.659).
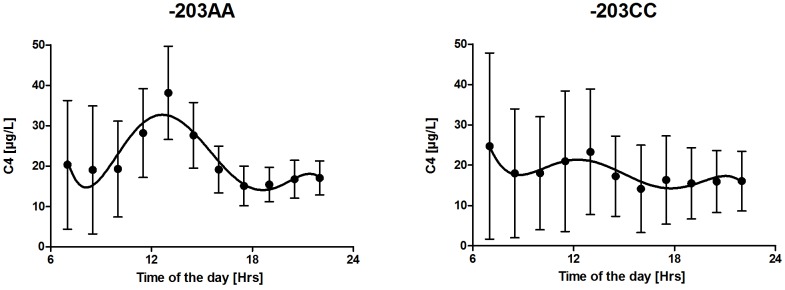
In the control experiment, plasma C4 concentrations significantly varied during the day in -203A allele carriers (P = 0.001) with a peak around 1pm. On the other hand, C4 concentrations did not change during the day in -203C allele carriers. Moreover, there was a trend for the effect of the genotype on the course of C4 concentrations (P = 0.092). These results suggest that there may be differences in the diurnal variation in CYP7A1 activity between homozygous -203A and -203C allele carriers. To test such a hypothesis, we modeled the dynamics of C4 concentration changes using fifth order polynomial regression (Figure 2, Table 2). The regression equation coefficient of order 2 significantly differed from zero only in -203A allele carriers, but not in those carrying the -203C allele. Importantly, the coefficients of the second and fourth order of corresponding regression equations were different between carriers of -203A and -203C alleles.
Figure 2.
The course of 7α-hydroxy-4-cholesten-3-one (C4) concentrations throughout Day 1 as modeled using polynomial regression of order 5. -203AA, -203CC – homozygous carriers of the -203A and -203C allele of the CYP7A1 gene, respectively.
Table 2.
Coefficients of the polynomial regression equation (a0, a1, a2, a3, a4, a5) of order 5 for the relationship between 7α-hydroxy-4-cholesten-3-one (C4, µg/L) concentration and time of day (t, hours) in -203A and -203C allele carriers. C4 = a0 + a1*(t-7) + a2*(t-14.5)2 + a3*(t-14.5)3 + a4*(t-14.5)4 + a5*(t-14.5)5. Estimates are mean (SE).
| -203AA carriers | -203CC carriers | |
|---|---|---|
| Coefficient |
Coefficient estimate |
Coefficient estimate |
| a0 |
60.20 (8.96)** |
33.19 (4.36)*** |
| a1 |
-4.258 (1.158)* |
-1.918 (0.563)* |
| a2 |
-0.6564 (0.2174)* |
-0.1563 (0.1058)† |
| a3 |
0.2135 (0.0733)* |
0.1021 (0.0357)* |
| a4 |
0.0088 (0.0037) |
0.0032 (0.0018)† |
| a5 | -0.0025 (0.0010) | -0.0014 (0.0005)* |
*,**,***P < 0.05, P < 0.01, P < 0.001 for the coefficient estimate being equal to zero.
†P < 0.05 for coefficient estimates being equal for homozygous carriers of the -203A and -203C alleles. The equation is valid for the interval between 7am until 10pm.
CDCA treatment had no effect on cholesterol and TG concentrations for all of the participants. Administration of cholestyramine resulted in an 8% decrease in cholesterol concentration and a 39% increase in triglyceridemia in homozygous -203C allele carriers. No such effect was observed in -203A allele carriers. However, the AUCs of both cholesterol and TG on Day 1 did not differ between -203A and -203C allele carriers (Table 1).
Discussion
This small study of healthy volunteers showed that homozygous participants carrying the -203A allele of the CYP7A1 displayed diurnal variation in CYP7A1 activity, peaking at midday. No diurnal variation in CYP7A1 activity was observed in homozygous carriers of the -203C allele.
Such findings were further corroborated by modeling the dynamics of C4 concentration changes using polynomial regression of order 5. Importantly, we found that the coefficients of the second and fourth order (a2 and a4) of the corresponding regression equations were different in the carriers of -203A and -203C alleles. It should be pointed out that only functions with nonzero coefficients of odd order (2 and 4 in our case) display the presence of a peak, while coefficients of even order do not. This strongly supports the idea that the course of C4 concentrations differs between homozygous carriers of the two alleles – the peak of CYP7A1 activity can be observed only in A allele carriers.
It has been shown that CYP7A1 activity in humans has two peaks (4) – one in the early afternoon and the second before midnight – but due to the design of our study, we can make conclusions only about the first peak. Interestingly, there is no clear mechanistic explanation for the midday peak. It has been demonstrated that such a peak appears both in participants who eat normally and those who are fasting before midday (4). Such an increase is thus unlikely to be associated with food intake. Interestingly, CYP7A1 activity then decreases rapidly only in subjects who eat normally and not in those who are fasting. This may suggest that increased intrahepatic flux of bile acids, and especially intestinal fibroblast growth factor-19 (FGF-19) secretion, after a meal are involved in dampening the peak of CYP7A1 activity (16).
Using a dual luciferase reporter assay with promoter fragments (-716 to +14) of both variants of the CYP7A1, we confirmed the previous findings that in HepG2 cells the -203C allele was expressed five times more than the -203A allele (17). However, we did not see any differences in the effects of bile acids, cortisol and dexamethasone, insulin, and peroxisome proliferator-activated receptor α (PPARα) agonists (fenofibrate and WY-14643) on the expression of both promoter variants (Vlachová M, Blahová T, unpublished data). This may suggest that factors other than those associated with food intake may play a role in the regulation of CYP7A1 activity throughout the day. Furthermore, we tried to find whether any transcription factor binds differently to both variants of the promoter surrounding -203 position using in silico analysis (Transcription Element Search System, Vlachová M, unpublished data). Except of putative new binding site for a glucocorticoid receptor [AGAA-203CT] in -203A allele (which does not seem to be functional based on dual luciferase data), no other differences between the promoter variants were found. No differences in the presence of putative binding sites were found also in five other polymorphisms (rs1023652, rs1023649, rs3903445, and rs3824260) that are in a tight linkage disequilibrium with -203A>C variant (rs3808607).
The diurnal variation in CYP7A1 activity and Cyp7a1 gene expression has been extensively studied in mice and rats and it was demonstrated that Cyp7a1 expression in mice was controlled by several clock genes (18-22). It remains to be determined whether clock genes also play a role in the regulation of bile acid synthesis in humans.
Given that some participants do not display diurnal variation, it is surprising that this has remained unnoticed so far. This is likely due to the small sample size of studies investigating circadian variation in CYP7A1 activity (4,16), and it cannot be excluded that -203C allele carriers were not studied. This can even suggest that the effect of more prevalent -203A allele is dominant and heterozygous carriers display diurnal variation in CYP7A1 activity.
It must be also stressed that the -203A>C polymorphism may not be the one responsible for observed differences in circadian changes in CYP7A1 activity. It has been demonstrated that this polymorphism is in close linkage disequilibrium with several other polymorphisms in the CYP7A1 (23). In the Caucasian population (the only population in which studies of diurnal variation in CYP7A1 have been carried out), the -203A and -203C alleles are considered to be markers of haplotype blocks spanning 14 kb from the proximal promoter to the 3′-downstream of the CYP7A1. Therefore, either of the polymorphisms included in these haplotype blocks may be responsible for the observed differences in diurnal variation in the enzyme activity.
The fact that C allele carriers cannot increase the CYP7A1 diurnal activity might explain their hyperresponsiveness to dietary cholesterol and saturated fat as observed in previous studies (10-12). They might be unable to eliminate the excess of dietary cholesterol through its transformation into bile acids as efficiently as A allele carriers, and their cholesterolemia might be augmented.
The major limitation of this study, apart from the small sample size, is the lack of night blood sampling. In future studies, 24-hour monitoring and inclusion of heterozygous individuals should be undertaken. It should be kept in mind that C4 is only a surrogate marker of CYP7A1 activity – however, it is hard to overcome such a limitation in human studies. Additionally, -203C carriers in our cohort had higher BMI.
In conclusion, we demonstrated that the -203A allele of the CYP7A1 was associated with pronounced diurnal variation in CYP7A1 activity, whereas the -203C allele was not. It remains to be determined whether differences in diurnal variation in enzyme activity between carriers of the -203A and -203C alleles can explain the differential effects of these variants on cholesterolemia and its responsiveness to diet.
Acknowledgments
We thank Cedars Sinai Medical Center’s International Research and Innovation in Medicine Program, the Association for Regional Cooperation in the Fields of Health, Science and Technology (RECOOP HST Association) for their support.
Funding The project was supported by grant No. NT 13151-4/2012 from Internal Grant Agency of Ministry of Health of the Czech Republic.
Ethical approval The study protocol was approved by the Ethics Committee of the Institute for Clinical and Experimental Medicine and all participants gave their informed consent.
Declaration of authorship MV was responsible for genotyping the participants, conducting the study, data acquisition and analysis, and finalization of the manuscript. TB was responsible for conducting the study, data acquisition and analysis, and finalization of the manuscript. VL carried out the statistical analyses, ML, JP, and LV contributed to data acquisition and analysis. JK conceived the research question and protocol design, contributed to data acquisition, analysis and interpretation, and drafted the manuscript. All the authors contributed to the writing of the manuscript and approved the final submitted version.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–66. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrell JM, Chiang JY. Circadian rhythms in liver metabolism and disease. Acta Pharm Sin B. 2015;5:113–22. doi: 10.1016/j.apsb.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galman C, Angelin B, Rudling M. Bile acid synthesis in humans has a rapid diurnal variation that is asynchronous with cholesterol synthesis. Gastroenterology. 2005;129:1445–53. doi: 10.1053/j.gastro.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Axelson M, Bjorkhem I, Reihner E, Einarsson K. The plasma level of 7 alpha-hydroxy-4-cholesten-3-one reflects the activity of hepatic cholesterol 7 alpha-hydroxylase in man. FEBS Lett. 1991;284:216–8. doi: 10.1016/0014-5793(91)80688-Y. [DOI] [PubMed] [Google Scholar]
- 6.Sauter G, Berr F, Beuers U, Fischer S, Paumgartner G. Serum concentrations of 7alpha-hydroxy-4-cholesten-3-one reflect bile acid synthesis in humans. Hepatology. 1996;24:123–6. doi: 10.1053/jhep.1996.v24.pm0008707250. [DOI] [PubMed] [Google Scholar]
- 7.Kovar J, Lenicek M, Zimolova M, Vitek L, Jirsa M, Pitha J. Regulation of diurnal variation of cholesterol 7alpha-hydroxylase (CYP7A1) activity in healthy subjects. Physiol Res. 2010;59:233–8. doi: 10.33549/physiolres.931753. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Freeman DJ, Grundy SM, Levine DM, Guerra R, Cohen JC. Linkage between cholesterol 7alpha-hydroxylase and high plasma low-density lipoprotein cholesterol concentrations. J Clin Invest. 1998;101:1283–91. doi: 10.1172/JCI1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couture P, Otvos JD, Cupples LA, Wilson PW, Schaefer EJ, Ordovas JM. Association of the A-204C polymorphism in the cholesterol 7alpha-hydroxylase gene with variations in plasma low density lipoprotein cholesterol levels in the Framingham Offspring Study. J Lipid Res. 1999;40:1883–9. [PubMed] [Google Scholar]
- 10.Hubacek JA, Pitha J, Skodova Z, Poledne R, Lanska V, Waterworth DM, et al. Polymorphisms in CYP-7A1, not APOE, influence the change in plasma lipids in response to population dietary change in an 8 year follow-up; results from the Czech MONICA study. Clin Biochem. 2003;36:263–7. doi: 10.1016/S0009-9120(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 11.Hofman MK, Weggemans RM, Zock PL, Schouten EG, Katan MB, Princen HM. CYP7A1 A-278C polymorphism affects the response of plasma lipids after dietary cholesterol or cafestol interventions in humans. J Nutr. 2004;134:2200–4. doi: 10.1093/jn/134.9.2200. [DOI] [PubMed] [Google Scholar]
- 12.Kovar J, Suchanek P, Hubacek JA, Poledne R. The A-204C polymorphism in the cholesterol 7alpha-hydroxylase (CYP7A1) gene determines the cholesterolemia responsiveness to a high-fat diet. Physiol Res. 2004;53:565–8. [PubMed] [Google Scholar]
- 13.Kajinami K, Brousseau ME, Ordovas JM, Schaefer EJ. Interactions between common genetic polymorphisms in ABCG5/G8 and CYP7A1 on LDL cholesterol-lowering response to atorvastatin. Atherosclerosis. 2004;175:287–93. doi: 10.1016/j.atherosclerosis.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Lenicek M, Komarek V, Zimolova M, Kovar J, Jirsa M, Lukas M, et al. CYP7A1 promoter polymorphism -203A>C affects bile salt synthesis rate in patients after ileal resection. J Lipid Res. 2008;49:2664–7. doi: 10.1194/jlr.M800364-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Lenicek M, Juklova M, Zelenka J, Kovar J, Lukas M, Bortlik M, et al. Improved HPLC Analysis of Serum 7{alpha}-Hydroxycholest-4-en-3-one, a Marker of Bile Acid Malabsorption. Clin Chem. 2008;54:1087–8. doi: 10.1373/clinchem.2007.100107. [DOI] [PubMed] [Google Scholar]
- 16.Lundasen T, Galman C. Angelin, Rudling M. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260:530–6. doi: 10.1111/j.1365-2796.2006.01731.x. [DOI] [PubMed] [Google Scholar]
- 17.De Castro-Oros I, Pampin S, Cofan M, Mozas P, Pinto X, Salas-Salvado J, et al. Promoter variant -204A > C of the cholesterol 7alpha-hydroxylase gene: association with response to plant sterols in humans and increased transcriptional activity in transfected HepG2 cells. Clin Nutr. 2011;30:239–46. doi: 10.1016/j.clnu.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Ferrell JM, Chiang JY. Short-term circadian disruption impairs bile acid and lipid homeostasis in mice. Cell Mol Gastroenterol Hepatol. 2015;1:664–77. doi: 10.1016/j.jcmgh.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Rhee JC, Yeo S, Shen R, Lee SK, Lee JW, et al. Crucial roles of mixed-lineage leukemia 3 and 4 as epigenetic switches of the hepatic circadian clock controlling bile acid homeostasis in mice. Hepatology. 2015;61:1012–23. doi: 10.1002/hep.27578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noshiro M, Kawamoto T, Furukawa M, Fujimoto K, Yoshida Y, Sasabe E, et al. Rhythmic expression of DEC1 and DEC2 in peripheral tissues: DEC2 is a potent suppressor for hepatic cytochrome P450s opposing DBP. Genes Cells. 2004;9:317–29. doi: 10.1111/j.1356-9597.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- 21.Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology. 2008;135:689–98. doi: 10.1053/j.gastro.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 22.Ma K, Xiao R, Tseng HT, Shan L, Fu L, Moore DD. Circadian dysregulation disrupts bile acid homeostasis. PLoS ONE. 2009;4:e6843. doi: 10.1371/journal.pone.0006843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamoto K, Wang S, Jenison RD, Guo GL, Klaassen CD, Wan YJ, et al. Linkage disequilibrium blocks, haplotype structure, and htSNPs of human CYP7A1 gene. BMC Genet. 2006;7:29. doi: 10.1186/1471-2156-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]