Abstract
Liposomal amphotericin B (AmBisome®; LAmB) is a unique lipid formulation of amphotericin B. LAmB is a standard of care for a wide range of medically important opportunistic fungal pathogens. LAmB has a significantly improved toxicity profile compared with conventional amphotericin B deoxycholate (DAmB). Despite nearly 20 years of clinical use, the pharmacokinetics and pharmacodynamics of this agent, which differ considerably from DAmB, remain relatively poorly understood and underutilized in the clinical setting.
The molecular pharmacology, preclinical and clinical pharmacokinetics, and clinical experience with LAmB for the most commonly encountered fungal pathogens are reviewed. In vitro, experimental animal models and human clinical trial data are summarized, and novel routes of administration and dosing schedules are discussed.
LAmB is a formulation that results in reduced toxicity as compared with DAmB while retaining the antifungal effect of the active agent. Its long terminal half-life and retention in tissues suggest that single or intermittent dosing regimens are feasible, and these should be actively investigated in both preclinical models and in clinical trials. Significant gaps remain in knowledge of pharmacokinetics and pharmacodynamics in special populations such as neonates and children, pregnant women and obese patients.
Keywords: liposomal amphotericin B, Ambisome, antifungal agents
1. Introduction
Amphotericin B is a polyene antifungal agent with a broad range of activity against yeasts and molds, as well as the protozoan parasite Leishmania spp. LAmB binds to ergosterol in the fungal cell membrane leading to ion leakage and cell death. The initial formulation was amphotericin B deoxycholate (DAmB), which was developed in the 1950s. For many decades DAmB was the only antifungal agent available for the treatment of invasive fungal diseases. However, the significant dose-limiting toxicity of DAmB (most notably nephrotoxicity and infusion-related reactions) provided the impetus to develop new less toxic formulations. Liposomal amphotericin B (AmBisome®; LAmB) is a unique lipid formulation of amphotericin B that has been used for nearly 20 years to treat a broad range of fungal infections. While the antifungal activity of amphotericin B is retained following its incorporation into a liposome bilayer, its toxicity is significantly reduced [1].
The drug exposure-effect relationships for LAmB differ significantly from DAmB and remain relatively poorly understood. This review summarizes the available information on the pharmacokinetic and pharmacodynamic relationships of LAmB from both a clinical and preclinical perspective. A comparative summary of LAmB and amphotericin B deoxycholate is shown in Table 1.
Table 1. “At-a-glance” comparison of characteristics of AmBisome as compared to Amphotericin B Deoxycholate.
| LAmB (AmBisome®) | DAmB (Fungizone®) | |
|---|---|---|
| MOLECULAR | ||
| Size(µm) | 0.08 | <0.04 |
| Molecular weight (particle size [µ m]) | 924 | 924 |
| PHARMACOKINETIC[28, 117– 119] | ||
| Cl (ml.kg/h) | 9.7 ± 5.4 | 13.1 ± 2.0 |
| Renal Clearance(ml h−1kg−1) | 0.495 ± 0.25 | 4.1 ± 0.68 |
| Vd(L/kg) | 0.2 - 1.6 | 2 - 2.3 |
| Cmax(mg/L) | 22.9 ± 10 (2mg/kg) | 1.43 ± 0.2 (0.6mg/kg) |
| Terminal half Life- second phase(h) | 6-23 | 10-24 |
| AUC(0–24) mg.h/L | 171+/126 | 1-30 |
| Distribution | spleen>liver>kidneys>lung | liver>spleen>lung>kidney |
| CLINICAL | ||
| FDA approved Indications | Aspergillosis, candidiasis, cryptococcal meningitis,visceral leishmaniasis,prolonged febrile neutropenia | Cryptococcosis, blastomycosis, candidiasis,coccidioidomycosis, histoplasmosis, mucormycosis, aspergillosis |
| Standard dosing in invasive mycosis | 3mg/kg | 1mg/kg |
| Nephroxicity risk[70] | Moderate | High |
| Cost[120] | High £82.19 per 50mg vial |
Low £3.88 per 50mg vial |
The findings and conclusions in this review are restricted specifically to the compound manufactured by Gilead Sciences, Foster City CA (AmBisome), and cannot be extrapolated to other lipid formulations of amphotericin B, including those that are also otherwise referred to as “liposomal amphotericin B”.
2. Molecular Pharmacology of LAmB
Since their first description in 1965 [2], liposomes have been extensively investigated for use in drug delivery. They are spherical vesicles characterized by an aqueous core surrounded by a lipid bilayer. The composition of the liposome has a significant impact on the resultant pharmacokinetic properties. Liposomes can be engineered to maximize antifungal activity and minimize drug related toxicity. The liposome specifically used in LAmB was designed to enable parenteral administration, facilitate the stability of amphotericin B within the liposome, yet enable the active compound to engage with the fungus when encountered within various tissue sites [3].
The unilamellar lipid structure of LAmB has three major components. The first is hydrogenated soy phosphatidylcholine, which comprises the majority of the lipid bilayer. It has the advantage of a gel to liquid-crystal phase transition point of >37 °C[4] meaning it is not readily hydrolyzed at body temperature. Secondly, distearoylphosphatidyl glycerol was selected as its fatty acid chain is similar in length to that of the hydrophobic region of amphotericin B and has a net negative charge. Under the slightly acidic conditions used to prepare liposomes, the amino group of amphotericin B, with its net positive charge, forms an ionic complex with the disteareoylphosphatidyl glycerol thus promoting the retention of amphotericin B within the liposomal bilayer [5, 6]. The third component, cholesterol, was added as it binds amphotericin B and further facilitates the retention of amphotericin B within the liposome bilayer. Currently available lipid formulations of amphotericin B are not orally bioavailable, although early efforts to develop a lipid formulation suitable for oral administration are promising [7].
Other lipid formulations of amphotericin B in clinical use include amphotericin B lipid complex (ABLC) (Abelcet, Sigma Tau, Gaithersburg, MD) and amphotericin B colloidal dispersion (ABCD) (Amphocil/Amphotec, Three River Pharmaceuticals, Cranberry Township, PA). These formulations have significantly different compositions and therefore pharmacokinetic characteristics. ABLC is composed of flattened, ribbon-like multilamellar structures with particles 1600 - 11000 nm in size, resulting in a greater volume of distribution, perhaps from sequestration in the liver and spleen. Plasma concentrations of amphotericin B following ABLC are lower compared with LAmB. ABCD is a complex of amphotericin B and cholesteryl sulphate that forms thin disc shaped structures that are approximately 120 nm in diameter, which are rapidly removed from the circulation by the reticulendothelial system [8, 9]. Given the significant differences between the various formulations of amphotericin B conclusions from one compound cannot be necessarily extrapolated to another.
2.1. Mechanism of Action
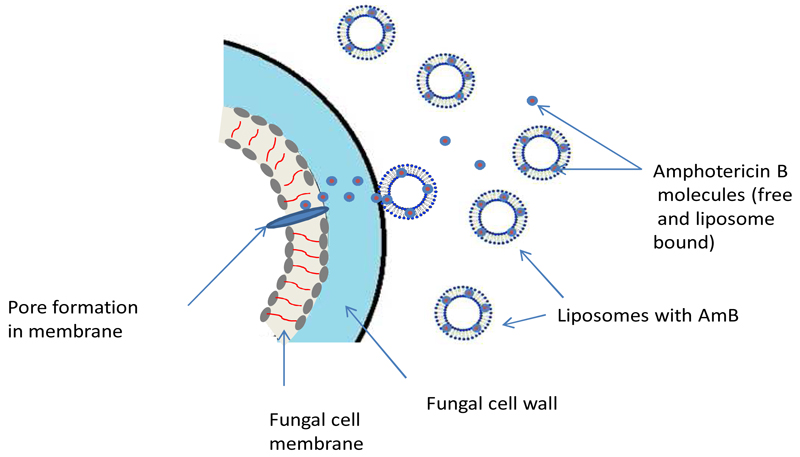
Amphotericin B binds to ergosterol in the fungal cell membrane, which leads to the formation of pores, ion leakage and ultimately fungal cell death. The binding of the liposome (both ‘loaded’ with amphotericin B and empty liposomes) to the cell wall of pathogenic yeasts and moulds has been demonstrated in vitro and in vivo using fluorescently labeled liposomes and gold-labeled liposomes [10–12]. Liposomes without AmB bind to the fungal cell wall, but both the ‘empty’ liposomes and the fungal cell remain intact. In contrast binding of amphotericin B containing liposomes results in fungal cell death [10]suggesting that binding results in liposomal disruption and release of amphotericin B, which is then free to exert its fungicidal activity by binding to ergosterol in the fungal cell membrane (Figure 1).
Figure 1 : Schematic representation of the postulated mode of action of liposomal Amphotericin B.
Free, protein bound and liposome-associated amphotericin B circulate in the bloodstream following the administration of LAmB. The liposomes preferentially attach to the fungal cell wall. The active amphotericin B molecule is released and transfers to the cell membrane where it can exert its activity, forming pores and leading to ion leakage and cell death.
NB The precise mechanism of this transfer remains unknown
The precise mechanism by which amphotericin B is transferred from the liposome through the fungal cell wall to the fungal membrane is not known. It is likely that the process is facilitated by the higher binding affinity of amphotericin B for ergosterol (the sterol present in fungal cell membranes) compared with cholesterol, which is the principal lipid component of the liposome [13]. Temperature also appears to be important in the transfer of amphotericin B between the liposome and the fungus and occurs most efficiently at body temperature [14].
3. PHARMACOKINETICS
3.1. Bioanalytical Issues
Concentrations of LAmB can be measured using high performance liquid chromatography (HPLC), liquid chromatography – mass spectrometry (LC/MS/MS), or bioassay. The assay has a significant impact on what exactly is being measured (i.e. total amphotericin B, protein bound drug, liposome associated drug and freely circulating drug). Caution, therefore, is required with the interpretation of drug concentrations. Extraction of amphotericin B from the liposome is a critical step in the bioanalysis of LAmB. Destruction of the liposome with release of active drug can be achieved with organic solvents such as methanol or DMSO. Assays have been developed to measure both free and liposome-bound amphotericin B. Failure to completely disrupt the liposome results in an underestimation of the total concentration of amphotericin B within the matrix.
The current understanding of the pharmacokinetic and pharmacodynamic properties of LAmB is largely based on measurement of total concentrations of amphotericin B in both plasma and tissues. However, only a fraction of total amphotericin B concentrations in any matrix is biologically active: some is liposome-associated, and fractions that are not liposome-associated may be bound (to plasma proteins or tissues) or exist as free drug. Moreover, measuring concentrations from tissue homogenates has the inherent limitation of being unable to distinguish which specific sub-compartment the drug is residing in, and therefore how much biologically active drug is available at the site of infection [15].
3.2. Preclinical Data
The preclinical pharmacokinetics of LAmB have been extensively characterized in a number of experimental model systems. This body of work can be summarized as follows:
Studies in mice, rats, and rabbits all suggest that the plasma pharmacokinetics of LAmB are linear [16, 17], however tissue pharmacokinetics of LAmB are more complex. Generally, there is a dose-dependent increase in tissue concentrations, although there also appear to be situations where changes in tissue concentrations do not appear to be linear. This nonlinearity is dependent on the organ and the species of laboratory animal [17, 18]. For example, in one murine study, hepatosplenic uptake at 12 hours following 0.3 mg/kg of LAmB accounted for approximately 80% of the injected drug [19]. However, following administration of a higher dosages of 7 mg/kg, there was a disproportionately greater concentration of LAmB in plasma, and hepatosplenic uptake decreased to 64% of the total injected dose [19].
The rank order of (total) tissue concentrations of amphotericin B following treatment of mice with LAmB is consistent between many studies and is in the order: spleen>liver>kidneys>lung [20].
The reticuloendothelial system (RES) is important for determining the shape of the LAmB concentration-time profile. The importance of the reticuloendothelial system (RES) has been confirmed in a number of laboratory animal species including rats [21] rabbits [22] and canines [23]. A possible explanation for accumulation of LAmB within the liver and spleen is the large numbers of macrophages in these organs. Macrophages readily phagocytose liposomes, which may also be beneficial in treating fungi residing within macrophages, such as Cryptococcus neoformans [24].
LAmB exhibits prolonged mean residence times in tissues, with some variation according to the tissue bed. In one study in rats much of the drug remained in the organs of uninfected animals at 72h post dosing. The majority of LAmB remains concentrated in the spleen and liver following intravenous administration; whereas, LAmB concentrations in the kidneys and lungs declines [25].
LAmB penetrates the central nervous system. As with DAmB, low concentrations of amphotericin B are found in CSF, while concentrations in cerebrum are approximately 100-fold higher. The ratio of plasma:cerebral tissue (estimated using a single time-point) is approximately 3% in a rabbit model [26].
LAmB penetrates various sub-compartments of the lung in an rabbit model, in which measurement of intrapulmonary concentrations were taken 24 hours after the last dose of an 8 day treatment course of 5 mg/kg/day LAmB. [27] LAmB achieves quantifiable concentrations in lung tissue (i.e. whole homogenized lung), epithelial lining fluid and pulmonary alveolar macrophages. The extent and rate of penetration into these sub-compartments has not been rigorously quantified (i.e. the time-course of tissue concentrations has not been determined, but rather estimated using the single time-points in the study). Estimates from paired plasma and tissue concentrations suggest the overall extent of penetration into various sub-compartments of the lung is approximately 10%.
3.3. Pharmacokinetics in humans
As in laboratory animals, circulating LAmB probably largely consists of intact liposomes [28]. There are also likely to be pools of relatively low concentrations of non-liposomal associated drug that is bound to human serum albumin (HSA) and alpha 1-acid glycoprotein (AAG) as well as smaller pool of free drug.
Several population pharmacokinetic models have been developed to describe the pharmacokinetics of LAmB in immunocompromised patients [29–31]. These models provide measures of central tendency for the model parameter values, such as volume and clearance, as well as the extent of inter-individual variability in drug exposure for patients receiving various regimens of LAmB. A bi-or triphasic decline in total amphotericin B concentrations is observed. These models in different patient populations (some receiving a standard regimen of 3 mg/kg/day, while another receiving an intermittent regimen of 10 mg/kg, followed by two dosages of 5 mg/kg/day) provide comparable estimates for clearance and volume [31].
The key findings from human pharmacokinetic studies are as follows:
Clearance is approximately 1-2 L/h and the volume of the central compartment is approximately 20 litres, which is significantly less than estimates for members of the triazole class of antifungal [31].
LAmB has a long terminal half-life in plasma (approx. 152 h in one study) [28]
Total plasma concentrations of amphotericin B are higher than observed with amphotericin B deoxycholate, even following correction for the higher weight-based dosages that are used for LAmB. The majority of circulating drug is likely to be biologically inactive. The liposome acts as a “pool” or “sump” of drug. Biologically active drug is not released until there is direct contact with the fungus [28].
Urinary clearance of LAmB is 4.5% of the dose after the end of the first week, which is significantly lower than for DAmB. Similarly, fecal clearance is significantly lower than observed with DAmB. Both these observations suggest that the amphotericin B in the liposome is not “seen” or “available” to these clearance mechanisms [28]. As with DAmB, LAmB is predominantly excreted as unchanged drug, via urinary and biliary excretion. Intact liposomes are not excreted [32].
LAmB is not found in significant concentration in urine, meaning it is not a suitable agent for treating lower urinary tract infections (urethritis or cystitis) caused by Candida species. This is in contrast to the significant concentration found in the kidney parenchyma [20], which makes it useful in the treatment of renal candidiasis (i.e. pyelonephritis which often complicates systemic candidiasis).
As in experimental models, the distribution of LAmB, rather than its metabolism, is the primary determinant of the shape of the concentration-time profile—studies in patients have consistently failed to detect any amphotericin B metabolites [28].
The uptake of drug by the reticulendothelial system may be non-linear, with dosages ≥7.5 mg/kg resulting in lower drug exposures than predicted on the basis of the pharmacokinetics from studying lower dosages. In this case, tissue uptake is not saturated. Rather, dosage escalation results in activation or induction of an additional pathway that leads to accelerated clearance and lower-than-expected drug exposure [33].
Urinary excretion of free drug occurs rather than secretion, reabsorption or metabolism. Active excretion of non-liposomal amphotericin B into bile does occur, but to a lesser extent than amphotericin B deoxycholate. Amphotericin B undergoes moderate hepatic extraction and is unlikely to be affected by changes in hepatic blood flow. Excretion of intact liposomes into the bile does not occur [28].
There are few tissue pharmacokinetic data from patients. One study reported pleural fluid concentrations of approximately 10% of plasma levels in a patient with pulmonary mucormycosis and empyema [34]. LAmB has been quantified in liver and spleen, but also the kidney, lung, myocardium and brain in patients undergoing autopsy after receiving LAmB [35]. In one study of 14 paediatric oncology patients, amphotericin B concentrations in the CSF were approximately 0.1% of concurrent serum levels, which is consistent with the preclinical data [36].
3.4. Special populations
LAmB is not removed by hemodialysis [37] and does not require dosage adjustment in this setting. The impact of weight on clearance is unclear. One study suggested that weight did not improve the fit of a model to PK data [29], while another suggested a statistically weak, but significant relationship between weight and clearance [31]. Further studies are required and studies in obese patients would be especially helpful. The effect of obesity on the pharmacokinetics of LAmB is not well characterized and it is unclear whether dosing should be calculated using total or lean body weight. Dosing obese patients on total body weight results in a higher incidence of nephrotoxicity compared with patients with normal body weight [38], which may be due to low accumulation in fat tissue. Dosing based on total body weight may be inappropriate and lead to relative overdosing [39].
There are only relatively limited PK data in children [30, 40], especially for younger and smaller children (<10 kg). Despite extensive use in neonates and premature infants with suspected or proven invasive fungal infection, there are no robust PK data in this population, and no good information on optimal dosing regimens.
Although LAmB can cause hepatotoxicity, there is no current recommendation to reduce dosing in patients with pre-existing hepatic failure and its effects in this population remain unknown. A single autopsy study reported enhanced pulmonary deposition in a patient with a failed liver transplant who had received LAmB for aspergillosis [37]. There are very few data regarding the use of LAmB in pregnancy. Small case series of patients with visceral leishmaniasis in pregnancy treated with LAmB have reported positive clinical outcomes without evidence of any adverse effects to the fetus [41].
4. Pharmacodynamics
LAmB is less potent on a mg-per-mg basis compared with amphotericin B deoxycholate. Differences in exposure-response relationships are most obvious from an in vitro model of the human alveolus, where the effective dose for 50% effect (ED50) was 1.03 and 0.12 mg/L for LAmB and DAmB, respectively [11]. Similar findings are apparent in other animal models of invasive fungal infection including a murine aspergillosis model [42] and a rabbit aspergillosis study [43]. These results suggest that much of the active compound is ‘locked’ in the liposome and is in effect biologically inert.
LAmB has antifungal activity in the central nervous system of a rabbit model of Candida meningoencephalitis and leads to complete sterilization within the cerebrum [26]. LAmB is also effective in a murine model of CNS aspergillosis [44]. In preclinical studies of disseminated candidiasis [45, 46], blastomycosis [47], mucormycosis [48] histoplasmosis [16, 49] and cryptococcosis [50] dose-dependent antifungal activity is consistently observed.
5. Toxicity
5.1. Nephrotoxicity
LAmB is consistently the least nephrotoxic of all commercially available lipid formulations of amphotericin B. At regimens as high as 10 to 15 mg/kg/day, LAmB is associated with significantly less renal toxicity in infected animals compared with amphotericin B deoxycholate [18, 22, 23].
These preclinical findings have been confirmed in numerous clinical studies, where LAmB is consistently less nephrotoxic than amphotericin B deoxycholate [51]. This may result from fewer HDL receptors in the kidney, which are preferential receptors for the binding of LAmB [52]. The preferential distribution of LAmB to the liver and spleen as compared to the renal tract may also lead to relatively lower concentrations in the kidneys and therefore less renal toxicity [53]. Renal toxicity is likely to result from free or readily diffusible amphotericin B interacting with the renal distal tubules. The active drug in LAmB is locked into the liposome and not free to engage with various sub-compartments within the kidney. There is no glomerulofiltration due to the size of the liposomes, which may explain the lower renal toxicity of LAmB [21].
5.2. Infusion reactions
Infusion related toxicity is a recognized side effect of DAmB, causing acute fevers and chills, possibly due to a proinflammatory cytokine response mediated by TLR2 and CD14 cells [54]. The infusion of LAmB may result in an idiosyncratic reaction that manifests as a classic triad of chest pain/ discomfort, flank/abdominal pain, and dyspnea in the first few minutes of infusion. These symptoms resolve with cessation of the infusion and administration of an anti-histamine agent [55]. The reaction is not a dose-dependent phenomenon. As the clinical syndrome of infusion reaction is more similar to other liposome-associated drugs (such as liposomal doxorubicin) than to DAmB, the reaction may be to the liposome rather than the active drug. The mechanism remains unclear but is postulated to be complement mediated [56].
The infusion-related toxicity of LAmB is consistently lower than other polyene formulations such as DAmB and amphotericin B lipid complex (ABLC) [57].
5.3. Hepatotoxicity
LAmB may result in deranged liver function tests. There is no evidence that this phenomenon is dose-dependent [58]. A retrospective case controlled study of 587 bone marrow transplant patients found that one-third of patients receiving LAmB had an increase in serum bilirubin, and that LAmB therapy was independently associated with a rise in transaminases; whereas, treatment with DAmB was not. However this has to be interpreted with caution due to confounding factors such as the co-administration of other hepatotoxic agents and the retrospective study design [59]. A study of tolerability of 141 treatment courses of LAmB in paediatric patients (median dose 2.8 mg/kg, median duration 13 days) observed mild to moderate increases in hepatic transaminases in 59% of cases, but this only resulted in cessation of the treatment course in a single patient [60]. The mechanism of hepatotoxicity with LAmB remains unknown. One autopsy study of 64 patients who had received LAmB and had deranged LFTs did not reveal any direct histopathological evidence of toxicity [61].
6. Clinical Experience with LAmB
AmBisome is used in wide variety of clinical scenarios. At the time of writing, Federal Drug Administration (FDA) approval for specific indications with recommended dosing includes empiric therapy in febrile neutropenia (3mg/kg/day), systemic aspergillosis, candidiasis, (both 3 – 5 mg/kg/day), visceral leishmaniasis (3mg/kg/day first 5 days then on days 14 and 21) and more recently cryptococcal meningitis(6mg/kg/day), as well as for patients for whom DAmB is not appropriate due to the risk of renal toxicity [62]. In Europe, approval is granted by individual countries rather than the European Medicines Agency (EMA). For example, in the United Kingdom approved indications by the Medicines and Healthcare Products Regulatory Agency (MHRA) is for visceral leishmaniasis, empiric therapy in febrile neutropenia, and the broad indication of severe systemic and/or deep mycoses, with no specific dosing guidelines. Key clinical trial data is summarized in Table 2
Table 2: Selected randomized clinical trials of Ambisome (LAmB).
| Clinical setting | Year of Study | Treatment arms | Total number of patients |
Key findings |
|---|---|---|---|---|
| Invasive aspergillosis [79] | 2007 | LAmB 3mg/kg vs LAmB 10mg/kg (for 14 days followed by 3mg/kg) |
201 | 12 week survival 72% in 3mg group v 59% in 10mg group(p >0.05) More toxicity with 10mg/kg |
| Invasive aspergillosis[76] | 1998 | LAmB 5mg/kg vs DAmB 1mg/kg |
64 | 14/32 complete response LAmB 6/34 complete response DamB (p=0.09) |
| Antifungal prophylaxis in neutropenia[64] | 2006 | 50mg LAmB alternate days vs control (no prophylaxis) |
132 | 5% (LAmB group) v 35% (control group) proven or probable IFI (NB not placebo controlled) |
| Persistent fever in neutropenia[70] | 1999 | LAmB 3mg/kg vs DAmB 0.6mg/kg |
687 | Survival similar (93% vs 90%). Fewer Confirmed breakthrough invasive fungal disease in LAmB group 3.2% vs 7.8%, (p=0.009) |
| Invasive candidiasis[82] | 2007 | Micafungin 100mg/day vs LAmB 3mg/kg/day |
392 | 181 (89.6%) micafungin and 170 (89.5%) with LAmB. Fewer adverse events in micafungin group |
| Cryptococcal[86] meningitis | 2010 | DAmB0.7mg/kg/day vs LAmB 3mg/kg/day vs LAmB 6mg/kg/day |
173 | Overall mortality at 10 weeks 11.6%,no sig. difference between groups. Less nephrotoxicity in LAmB 3mg/kg (p = 0.004) |
| Visceral Leishmaniasis[111] | 2011 | 1mg/kg DAmB (30 days) vs 5mg/kg single dose LAmB + miltefosine or paromomycin vs miltefosine + paromomycin |
475 | All combination LAmB groups non-inferior to standard 30 day DAmB therapy |
6.1. Prophylaxis for invasive fungal infection
Invasive fungal infection (IFI), most commonly caused by Aspergillus spp., is a devastating and often fatal complication in patients receiving immunosuppressive therapy. Therefore, prevention is a key clinical priority.
Several studies using laboratory animal models of infection have demonstrated the ability of LAmB to prevent or minimise invasive fungal infection caused by Candida albicans [20]. Similar studies have been performed for moulds such as Aspergillus spp. [63], as well as the dimorphic fungus Histoplasma capsulatum [16]. Collectively, these data demonstrate the potential efficacy of LAmB for preventing invasive infections caused by yeasts and molds, however the minimum effective concentration required for effective prophylaxis is not known.
The majority of clinical studies of LAmB for primary prophylaxis are either underpowered or have a number of flaws in study design. A possible exception is a study of LAmB (50 mg on alternate days) for the prevention of IFIs in neutropenic patients and autologous stem cell transplant recipients [64]. The incidence of IFI after the first neutropenic episode was 6.7% versus 35% in the intervention and control groups, respectively (p=0.001). The use of LAmB as prophylaxis in children is associated with fewer cases of IFI compared with historical controls (1.8 vs. 7.4%, respectively) and an apparent survival advantage [65]. LAmB can be used for prevention of IFIs in liver transplant recipients [66], and has an advantage over the triazoles of a relative lack of severe hepatotoxicity, and absence of interactions with immunosuppressive agents such as cyclosporine, tacrolimus and sirolimus. Importantly, however, demonstrating therapeutic efficacy is extremely difficult because of the relatively low incidence of IFIs. A number of regimens have been studied (e.g. 50 mg/day [67]; 100 mg day up to a 1-1.5g cumulative dosage [68]), but there is no consensus as to which is optimal.
6.2. Empiric therapy in prolonged febrile neutropenia
Persistent fever in neutropenic patients that is refractory to antibacterial agents is often treated with antifungal agents because of concerns of underlying undiagnosed invasive fungal infection. An early randomized trial compared DAmB (1mg/kg/day) with LAmB at doses of 1 mg or 3mg/kg/day for patients with febrile neutropenia unresponsive to antibacterials. [69] Treatment success in each group was 49%, 58%, 64% respectively, although a Kaplan-Meier analysis of time to defervescence did not reach statistical significance. Significantly fewer severe toxicity related events were observed in LAmB treated patients (1%) compared with 12% in the DAmB group (p<0.01). Another randomized trial compared DAmB (0.6 mg/kg) with LAmB (3 mg/kg) (Table 1). Survival was similar in both groups (93% vs. 90%) but there were fewer confirmed cases of breakthrough invasive fungal disease in the LAmB group (3.2% vs 7.8%, p =0.009) [70]. When LAmB at a dose of 3 mg/kg was compared to caspofungin in a similar setting, caspofungin was non-inferior in terms of treatment success, with significantly less nephrotoxicity than LAmB (2.6 percent vs. 11.5 percent, p<0.001) [71]. In a large study (>400 patients per arm) comparing voriconazole with LAmB at 3mg/kg/day for the treatment of persistent neutropenic fever, success rates were 26% for voriconazole and 30.6% for LAmB using a composite endpoint including breakthrough confirmed fungal infection, mortality and resolution of fever, (95% CI of -10.6 – 1.6.) There were however, more confirmed breakthrough fungal infections in the LAmB group (21 vs. 8, p <0.02) [72].
6.3. Invasive aspergillosis
There are multiple preclinical studies that suggest LAmB is an effective agent for the treatment of invasive aspergillosis [17, 73–75]. Dosages of 5 mg/kg in a rabbit model of IPA result in dose-dependent improvement in a number of measures of efficacy, including lung weight, fungal density, infarct score and galactomannan concentrations [43].
The clinical efficacy of LAmB for invasive aspergillosis was initially established in Phase II clinical trials [76, 77]. Given the high mortality associated with invasive aspergillosis, the clinical efficacy of higher dosages of LAmB was of significant interest. A Phase II study with a PK sub-study did not suggest that higher dosages (up to 15 mg/kg) resulted in improved clinical outcomes [78]. These findings were confirmed in a randomized Phase III clinical trial that compared LAmB 3 mg/kg with 10 mg/kg/day [79]. There was no difference in clinical response and survival between the two study arms. Mortality was higher in patients receiving the higher dosage, which may reflect the higher incidence of renal impairment in this study arm. Based on these studies, a dosage of LAmB 3 mg/kg is generally recommended for the treatment of invasive aspergillosis [80].
6.4. Invasive Candidiasis
Invasive candidiasis is a growing clinical problem due to increasing use of indwelling medical devices and ever increasing use of immunosuppressive therapies, broad spectrum antibacterial agents and total parenteral nutrition (TPN), all of which are major risk factors for the development of invasive candidiasis.
Several laboratory animal studies have demonstrated the efficacy of LAmB treatment for invasive infections caused by Candida species. Dosages of 2.5-10 mg/kg are comparable to the efficacy of DAmB (0.75 mg/kg/day) for disseminated Candida albicans infection [81]. In a study of mice infected with Candida glabrata, a dose-dependent reduction of kidney fungal burden was observed for dosages up to 20 mg/kg/day however complete clearance was only achieved in combination with caspofungin or micafungin [46].
The majority of early clinical studies for invasive candidiasis and candidemia were performed using DAmB as the “gold standard”. A clinically effective dosage of LAmB was not demonstrated until relatively recently. Two clinical trials performed in adults [82] and in children [83] compared the response to LAmB 3 mg/kg with micafungin, an echinocandin. Both studies demonstrated comparable clinical outcomes for both study arms. LAmB was associated with more infusion reactions and nephrotoxicity compared with micafungin.
6.5. Cryptococcal meningitis
Cryptococcal meningitis is a neglected infection that is a leading cause of global infectious morbidity and mortality. The majority of cases occur in resource poor settings and in patients with HIV/AIDS. In high resource settings, cryptococcal meningitis is also seen in the context of solid organ transplantation.
There are limited pre-clinical data for LAmB that specifically relate to cryptococcal meningitis. LAmB was comparable in efficacy to DAmB in an early murine study of systemic cryptococcosis [81]. More recently, a murine model of cryptococcal meningitis was used to investigate the pharmacodynamics of LAmB and flucytosine. Mice were treated with 3, 10 and 20 mg/kg of LAmB. A dose dependent reduction in organism burden in the brain was observed. A regimen of 3 mg/kg/day was submaximal, while the highest dose (20mg/kg/day) resulted in a decline in fungal cerebral burden without achieving sterilization [50].
In the clinical setting, DAmB at a dose of 1 mg/kg/day in combination with flucytosine (5FC) has shown a mortality benefit over DAmB monotherapy and considered the standard of care for induction therapy of cryptococcal meningitis [84]. In many cryptococcal meningitis patients, in particular solid organ transplant recipients, renal impairment is frequently present, which makes the administration of a 2-week induction course of DAmB a challenge. Consequently, LAmB is increasingly used for this purpose, and there is evidence that it is clinically effective for CNS cryptococcosis [85]. However, there is still considerable uncertainty regarding the optimal dosage of LAmB for cryptococcal meningitis. A randomized trial comparing LAmB 3, LAmB 6 mg/kg with DAmB 0.7 mg/kg did not reveal any significant differences in clinical outcomes or mortality, although this study was not powered to distinguish these regimens on the basis of efficacy [86]. A dose of LAmB of 4mg/kg was comparable to DAmB 7mg/kg in one small study [87]. A small phase II study showed a 74% clinical response rate with a dose of 3mg/kg [88].
The potential benefits of combining LAmB with flucytosine are not yet known. A previously mentioned murine study, when bridged to humans, suggests that LAmB 3 mg/kg is associated with a submaximal antifungal effect, while the use of LAmB 6 mg/kg alone or LAmB 3 mg/kg plus flucytosine is associated with a near maximal antifungal effect [50]. Current clinical treatment guidelines currently recommend a dose of 3-4mg/kg/day LAmB in combination with flucytosine during the induction phase[89], whilst FDA approval is for a dose of 6mg/kg/day initially. Further investigation into optimal dosing is clearly required.
6.6. Leishmaniasis
Leishmaniasis is caused by the protozoan parasite Leishmania spp., and is transmitted by the sandfly. Its most severe form, visceral leishmaniasis (VL), can be fatal and is most common in resource-limited settings such as India and East Africa. A study using a murine model of visceral leishmaniasis studied exposure-response relationships of LAmB [90]. A dose of 0.8 mg/kg reduced the parasite load by log10 4-6 parasites/g tissue in the liver and spleen compared with controls. Dosages of LAmB 5 mg/kg and 50 mg/kg, given alternate days for 6 doses, resulted in complete sterilization of the liver, spleen and lungs.
Several clinical studies have demonstrated excellent outcomes with the use of LAmB in visceral leishmaniasis, with the WHO recommending a cumulative dose of 20 mg/kg [91]. However, the minimum effective dose remains unknown. This is especially important because most cases occur in resource-limited settings, where the cost of LAmB is prohibitive.
6.7. Mucorales
Mucormycosis, or zygmoycosis, is a rare yet devastating infection, most commonly caused by Rhizopus spp., which primarily affects profoundly immunosuppressed patients and patients with diabetic ketoacidosis.
The polyenes are first-line agents for the treatment of infections caused by the mucorales, and LAmB is frequently used for this indication. A regimen of 10 mg/kg/day is required to induce a decline in the fungal burden in the lung in a neutropenic pulmonary model of Rhizopus oryzae infection [92]. Comparable dosages are required to induce prolongation of survival in a diabetic murine model of disseminated infection [48].
Clinical data with the use of LAmB in invasive mucormycosis are extremely limited and are hampered by the low incidence and very high mortality regardless of the choice of antifungal therapy. Dosages of at least 5mg/kg/day are generally used but there is little data to guide optimum therapy in this setting. Early surgical intervention may be just as important as the use of an effective antifungal agent [93]. One prospective, non-randomized study using 10 mg/kg/day LAmB (with surgery in two-thirds of cases) demonstrated a 45% response rate [94]. Given the high mortality rates of mucormycosis, combinations of LAmB with an azole (posaconazole or isavuconazole) and/or an echinocandin warrant study in clinical trials.
6.8. Histoplasmosis
Histoplasmosis is often HIV associated and carries a high mortality in this setting unless treated aggressively.
An early murine study of disseminated histoplasmosis demonstrated comparable efficacy of a regimen of LAmB of 3 mg/kg/day to 1 mg/kg/day of DAmB [49]. A prophylaxis study in a murine model of disseminated histoplasmosis demonstrated that a single dose of LAmB 10mg/kg given 7 days prior to challenge completely prevented the development of infection in both immunocompetent or immunosuppressed mice, with no detectable fungal growth in the spleen. Two weeks later, there was still no growth detected in the spleens of immunocompetent mice; however, 40% of immunosuppressed mice had succumbed to infection by this stage [16].
A phase II clinical trial comparing LAmB at 3 mg/kg daily for 14 days with DAmB 0.7 mg/kg in patients with HIV-associated disseminated histoplasmosis demonstrated increased survival with LAmB with significantly less toxicity [95]. A retrospective analysis of patients treated with LAmB or itraconazole for histoplasmosis demonstrated a significantly higher rate of blood culture sterility at two weeks (85% with LAmB, compared to 53% with itraconazole [96]).
7. New Strategies for LAmB
The lack of new antifungal agents on the market necessitates optimizing the use of currently available drugs. Until recently, LAmB essentially was used as a straight substitute for DAmB while retaining the same intravenous dosing schedules, however greater recognition of its PK/PD have led to efforts to investigate shortened or intermittent dosing schedules and investigate novel routes of administration.
7.1. Alternative Routes of Administration - Aerosolised Therapy
Given the lung is the primary site of infection for many invasive fungal diseases, aerosolized LAmB has been investigated for its potential to deliver amphotericin B directly to the site of infection. Amphotericin B deoxycholate can be nebulised [97], but is associated with a higher incidence of bronchospasm compared with LAmB. LAmB can also be nebulised without disrupting liposomes. One experimental model reported that lung tissue of mice exposed to aerosolized LAmB for three × 20 minute periods accumulated a maximum concentration of 43 μg/g at one hour after the third exposure [98]. Even after 336 hours, the lung concentration of amphotericin B remained high enough (24 μg/g lung tissue) to prevent subsequent pulmonary infection with Cryptococcus neoformans. Similarly, mice treated with aerosolized LAmB for three one hour intervals and had > 200 μg/g in the lungs 24h after the last dose. A small amount of amphotericin B is deposited in the upper airway, but there was no systemic drug exposure. These intrapulmonary concentrations prevented the establishment of invasive infection following intranasal challenge with A. fumigatus.
7.1.1. Clinical experience with aerosolized LAmB
Patients receiving lung transplants are a high-risk group for invasive fungal pulmonary disease so pulmonary-focused prophylaxis is of particular clinical interest though clinical data remains limited. A pharmacokinetic study measured amphotericin B concentrations in bronchioalveoar lavage fluid (BAL) in 27 lung transplant patients receiving nebulised LAmB at a dose of 25mg, 3 times per week [99]. Mean concentrations were 11.1 mg/L after 2 days and 3 mg/L after 14 days, with the final concentration adjusted based on the assumption that approximately 1% of recovered fluid represents epithelial lining fluid in the lung. No significant systemic exposure was observed and there was no apparent effect on respiratory function. The same research group published their clinical experience of 104 patients receiving this same regime of aerosolized LAmB [100]. The breakthrough fungal infection rate was 7.7%, lower than the 10% failure rate in a historical control group of 49 patients at their centre receiving nebulised DAmB but without reaching statistical significance. The regime was found to be minimally toxic and acceptable to patients.
A randomized, placebo controlled study of 271 patients with prolonged neutropenia investigated twice weekly inhalation of 12.5 mg LAmB for the prevention of invasive pulmonary aspergillosis (IPA) [101]. There was a statistically significant reduction in the incidence of IPA in the LAmB group, (4% vs. 13% in the control arm, p <0.05), with minimal toxicity observed.
Clinical experience of treatment of established infection with nebulised LAmB remains limited. One successful outcome was reported in a patient who received 50 mg nebulised LAmB twice daily for an ABPA-associated empyema, having had to switch from aerosolised DAmB because of bronchospasm.[102]
7.2. Catheter Lock therapy
Fungal biofilms in catheters complicate the treatment of fungal infection. The biofilm shields fungal cells from otherwise effective concentrations of drug and limits immunological responses. Although conventional DAmB appears to be inhibited by Candida biofilms [103], LAmB appears to retain activity in this setting. An indwelling catheter model in rabbits suggests that 3-day old C. albicans biofilms can be effectively treated with LAmB as a lock therapy. The drug lock was administered at 10 mg/mL for 8 hours each day. After 7 days of AmBisome lock therapy, scanning EMs showed that AmBisome-treated catheters were free of biofilm and all catheter cultures were negative [104]. While echinocandins are currently favored for catheter lock therapy in patients, individual case reports have reported successful catheter salvage [105]. LAmB may be a useful agent in this setting however at present this remains strictly an investigational approach and requires significant further clinical study before it is likely to be adopted in routine clinical practice.
8. Novel dosing regimens for LAmB
8.1. Intermittent or Single Dose Therapy
Because of the prolonged mean tissue residence time of LAmB, which ranges from several days to weeks [16, 20] the use of a number of innovative regimens may be possible. Intermittent regimens reduce the cost and side-effects of LAmB therapy, and can potentially extend the use of this antifungal agent to ambulatory settings, such as outpatient antimicrobial therapy (OPAT) and haemato-oncology day units, and to the developing world. Intermittent therapy with LAmB has been investigated in several preclinical studies that have demonstrated the efficacy for candidiasis [20] coccidiodomycosis [106] cryptocococcosis [107], histoplasmosis [49]and visceral leishmaniasis [90], These preclinical results have formed the experimental basis to further investigate the utility of intermittent therapy in clinical settings, for the treatment of cryptococcal meningitis and visceral leishmaniasis [108]
A new multicentre clinical trial for cryptococcal meningitis in Africa is currently in progress comparing a single dose of 10 mg/kg, intermittent dosing of LAmB (10mg/kg on day one, two dosages of 5mg/kg on days 3 and 7), compared with 3 mg/kg/day, all administered on a fluconazole backbone [109].
Given its long residual time in the liver and spleen, short course dosing or a single dose of LAmB is especially attractive for the treatment of visceral leishmaniasis and is being actively investigated in a series of clinical trials, largely supported by The Drugs for Neglected Diseases Initiative (DNDI). A single dose of 7.5 mg/kg therapy for 203 patients in India resulted in a 90% cure rate at 6 months [108]. Another trial of 304 patients found non-inferiority of LAmB (10 mg/kg) compared to DAmB (15 mg/kg total dose; 1 mg/kg alternate days for 30 days) [110]. This high single dose however carries the risk of significant infusion reactions. A lower single dose (5mg/kg) in combination with miltefosine is a promising strategy [111].
Interestingly, single dosing does not appear to be effective in treating VL in East Africa, for reasons that remain unclear [112]. A clinical trial is underway to investigate combination therapies of LAmB at 10mg/kg single dose with miltefosine or sodium stibogluconate.
8.2. Intermittent dosing for prophylaxis of IFI
Intermittent dosing is being increasingly investigated for the prophylaxis of invasive fungal infection.
In one study of prophylaxis against invasive fungal infection, a regimen of LAmB 7.5 mg/kg weekly was given to 21 patients with graft-versus host diseases [113]. Approximately one-third of patients developed infusion-related symptoms and one-third required treatment cessation because of a serious adverse reaction (creatinine increase, chest pain or hypotension). The regimen appeared to be effective although the study was not a comparative study or sufficiently powered to detect clinically meaningful antifungal efficacy. Another study compared a regimen of 2 mg/kg, 3 times per week to placebo and found no difference in fungal outcomes in neutropenic patients and bone marrow transplant recipients [114].
The efficacy of a single weekly dose of LAmB 10 mg/kg for 4 or 8 weeks for patients with acute leukemia undergoing induction or consolidation chemotherapy, or allogeneic stem cell transplantation, respectively, was investigated [115]. No other systemic antifungal therapy was used. The primary endpoint was tolerability and safety of the antifungal regimen. Progressive enrollment of the HSCT recipients was abandoned because of a greater than 10% incidence of serious adverse events (e.g. dyspnoea, chest pain, tubule - interstitial nephritis, renal insufficiency, anuria and anaphylactic shock), suggesting higher dosages of liposomal amphotericin B may be problematic for this vulnerable subgroup. This study was underpowered to detect clinically meaningful antifungal efficacy of a high-dose intermittently administered dosage regimen.
A recent trial of 5mg/kg LAmB twice weekly for the prevention of IFI in patients with acute lymphoblastic leukemia showed a reduction of IFI compared to the placebo group (RR 0.33) but this did not reach statistical significance (p=0.24)[116]. The optimal regimen (i.e. both dose and schedule of administration) therefore remains undefined. Pharmacokinetic-pharmacodynamic studies with bridging to the clinical setting are a potential tool with which this question could be further addressed.
9. Expert Opinion
The lipid composition of AmBisome has resulted in a drug delivery formulation for amphotericin B that has reduced toxicity, and is active against a broad spectrum of pathogenic fungi as well as Leishmania spp. LAmB distributes to many tissues and achieves effective concentrations that enable treatment of many invasive fungal diseases.
Extensive preclinical studies have provided a reasonable understanding of drug distribution, elimination and antifungal effect. There is expanding knowledge related to the pharmacodynamics of LAmB for invasive candidiasis, invasive aspergillosis and cryptococcal meningitis. There are, however, many remaining questions that are related to the pharmacology and optimal clinical use of LAmB.
Perhaps one of the most interesting and underexploited properties of LAmB is its prolonged mean residence time in tissues. This property suggests that for some indications, LAmB could be given intermittently, as a short course or even as a single dose without compromising efficacy. This has the potential to significantly reduce both the cost and possible adverse events, and extend the use of LAmB to ambulatory settings. Moreover, these shortened regimens could have a major impact particularly in infections such as cryptococcosis and leishmaniasis which overwhelmingly are seen in parts of the world where the cost of LAmB is otherwise prohibitive.
There is a striking paucity of clinical data in special populations such as neonates, young children, pregnant women and morbidly obese patients. This needs to be urgently addressed.
AmBisome is now in its second decade of clinical use. There are still opportunities to better utilize this agent for the treatment of life-threatening invasive fungal diseases. This is especially important given the rising threat of antifungal drug resistance and the relative paucity of new antifungal agents.
KEY POINTS.
Liposomal amphotericin B is a safe and effective agent for treatment of a wide range of medically important opportunistic fungal pathogens including aspergillosis, cryptococcal meningitis, invasive candidiasis and mucormycosis.
Liposomal amphotericin B has been consistently demonstrated to have less nephrotoxicity and infusional toxicity than other formulations of amphotericin B.
The molecular pharmacology of liposomal amphotericin B is increasingly understood. Amphotericin B is preferentially retained within the liposome until contact between the liposome and the fungus, which then enables amphotericin B to disengage from the liposome and bind ergosterol in the fungal membrane.
Relatively little is known about the tissue pharmacokinetics of liposomal amphotericin B. The polyenes exhibit prolonged mean residence times in tissues making innovative regimens such as abbreviated and intermittent therapy possible
In addition to its antifungal activity, liposomal amphotericin B is also active against the protozoal parasite Leishmania spp. Its potential for use in single dose or intermittent treatment regimens in the treatment of visceral leishmaniasis is of major clinical interest and is being actively investigated in clinical trials.
ACKNOWLEDGEMENTS AND DECLARATION OF INTEREST
Neil Stone is supported through a Clinical Fellowship via the Wellcome Trust Strategic Award in Medical Mycology and Fungal Immunology (Aberdeen University)
William Hope has acted as a consultant or received research support from Astellas Pharma, Gilead, Pfizer Inc., F2G, Pulmocide, and Basilea and is supported by a National Institutes of Health Research (NIHR) Clinician Scientist Award.
Tihana Bicanic has attended Advisory Boards for Gilead Sciences Inc and Basilea, has received sponsorship for conference attendance from Gilead Sciences Ltd and Astellas Pharma Inc and is the co-recipient of a 2015 Gilead UK and Ireland Fellowship on Invasive fungal disease.
References
- 1.Wingard JR, White MH, Anaissie E, Raffalli J, Goodman J, Arrieta A, et al. A randomized, double-blind comparative trial evaluating the safety of liposomal amphotericin B versus amphotericin B lipid complex in the empirical treatment of febrile neutropenia. L Amph/ABLC Collaborative Study Group. Clin Infect Dis. 2000 Nov;31(5):1155–63. doi: 10.1086/317451. [DOI] [PubMed] [Google Scholar]
- 2.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965 Aug;13(1):238–52. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 3.Adler-Moore JP, PR DEVELOPMENT, CHARACTERIZATION, EFFICACY AND MODE OF ACTION OF AMBISOME,' A UNILAMELLAR LIPOSOMAL FORMULATION OF AMPHOTERICIN B. Journal of Liposome Research. 1993:429–50. [Google Scholar]
- 4.Papahadjopoulos D, Jacobson K, Nir S, Isac T. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim Biophys Acta. 1973 Jul;311(3):330–48. doi: 10.1016/0005-2736(73)90314-3. [DOI] [PubMed] [Google Scholar]
- 5.Fujii G, Chang JE, Coley T, Steere B. The formation of amphotericin B ion channels in lipid bilayers. Biochemistry. 1997 Apr;36(16):4959–68. doi: 10.1021/bi962894z. [DOI] [PubMed] [Google Scholar]
- 6.Adler-Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother. 2002 Feb;49(Suppl 1):21–30. doi: 10.1093/jac/49.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- 7.Wasan EK, Gershkovich P, Zhao J, Zhu X, Werbovetz K, Tidwell RR, et al. A novel tropically stable oral amphotericin B formulation (iCo-010) exhibits efficacy against visceral Leishmaniasis in a murine model. PLoS Negl Trop Dis. 2010;4(12):e913. doi: 10.1371/journal.pntd.0000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamill RJ. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs. 2013 Jun;73(9):919–34. doi: 10.1007/s40265-013-0069-4. [DOI] [PubMed] [Google Scholar]
- 9.Adler-Moore JP, Proffitt RT. Amphotericin B lipid preparations: what are the differences? Clin Microbiol Infect. 2008 May;14(Suppl 4):25–36. doi: 10.1111/j.1469-0691.2008.01979.x. [DOI] [PubMed] [Google Scholar]
- 10.Adler-Moore J. AmBisome targeting to fungal infections. Bone Marrow Transplant. 1994;14(Suppl 5):S3–7. [PubMed] [Google Scholar]
- 11.Lestner JM, Howard SJ, Goodwin J, Gregson L, Majithiya J, Walsh TJ, et al. Pharmacokinetics and pharmacodynamics of amphotericin B deoxycholate, liposomal amphotericin B, and amphotericin B lipid complex in an in vitro model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2010 Aug;54(8):3432–41. doi: 10.1128/AAC.01586-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemoto K, Yamamoto Y, Ueda Y. Evaluation of antifungal pharmacodynamic characteristics of AmBisome against Candida albicans. Microbiol Immunol. 2006;50(8):579–86. doi: 10.1111/j.1348-0421.2006.tb03832.x. [DOI] [PubMed] [Google Scholar]
- 13.Readio JD, Bittman R. Equilibrium binding of amphotericin B and its methyl ester and borate complex to sterols. Biochim Biophys Acta. 1982 Feb;685(2):219–24. doi: 10.1016/0005-2736(82)90103-1. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K, Osada M, Takemoto K, Yamamoto Y, Asai T, Oku N. Temperature-dependent transfer of amphotericin B from liposomal membrane of AmBisome to fungal cell membrane. J Control Release. 2010 Jan;141(2):208–15. doi: 10.1016/j.jconrel.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008 Feb;61(2):235–7. doi: 10.1093/jac/dkm476. [DOI] [PubMed] [Google Scholar]
- 16.Garcia A, Adler-Moore JP, Proffitt RT. Single-dose AmBisome (Liposomal amphotericin B) as prophylaxis for murine systemic candidiasis and histoplasmosis. Antimicrob Agents Chemother. 2000 Sep;44(9):2327–32. doi: 10.1128/aac.44.9.2327-2332.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson JA, Adler-Moore JP, Schwartz J, Jensen GM, Proffitt RT. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob Agents Chemother. 2006 Jun;50(6):2122–31. doi: 10.1128/AAC.00315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boswell GW, Bekersky I, Buell D, Hiles R, Walsh TJ. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob Agents Chemother. 1998 Feb;42(2):263–8. doi: 10.1128/aac.42.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Etten EW, ten Kate MT, Stearne LE, Bakker-Woudenberg IA. Amphotericin B liposomes with prolonged circulation in blood: in vitro antifungal activity, toxicity, and efficacy in systemic candidiasis in leukopenic mice. Antimicrob Agents Chemother. 1995 Sep;39(9):1954–8. doi: 10.1128/aac.39.9.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith PJ, Olson JA, Constable D, Schwartz J, Proffitt RT, Adler-Moore JP. Effects of dosing regimen on accumulation, retention and prophylactic efficacy of liposomal amphotericin B. J Antimicrob Chemother. 2007 May;59(5):941–51. doi: 10.1093/jac/dkm077. [DOI] [PubMed] [Google Scholar]
- 21.Bekersky I, Boswell GW, Hiles R, Fielding RM, Buell D, Walsh TJ. Safety, toxicokinetics and tissue distribution of long-term intravenous liposomal amphotericin B (AmBisome): a 91-day study in rats. Pharm Res. 2000 Dec;17(12):1494–502. doi: 10.1023/a:1007605024942. [DOI] [PubMed] [Google Scholar]
- 22.Lee JW, Amantea MA, Francis PA, Navarro EE, Bacher J, Pizzo PA, et al. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob Agents Chemother. 1994 Apr;38(4):713–8. doi: 10.1128/aac.38.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bekersky I, Boswell GW, Hiles R, Fielding RM, Buell D, Walsh TJ. Safety and toxicokinetics of intravenous liposomal amphotericin B (AmBisome) in beagle dogs. Pharm Res. 1999 Nov;16(11):1694–701. doi: 10.1023/a:1018997730462. [DOI] [PubMed] [Google Scholar]
- 24.Gregoriadis G. Overview of liposomes. J Antimicrob Chemother. 1991 Oct;28(Suppl B):39–48. doi: 10.1093/jac/28.suppl_b.39. [DOI] [PubMed] [Google Scholar]
- 25.Gershkovich P, Wasan EK, Lin M, Sivak O, Leon CG, Clement JG, et al. Pharmacokinetics and biodistribution of amphotericin B in rats following oral administration in a novel lipid-based formulation. J Antimicrob Chemother. 2009 Jul;64(1):101–8. doi: 10.1093/jac/dkp140. [DOI] [PubMed] [Google Scholar]
- 26.Groll AH, Giri N, Petraitis V, Petraitiene R, Candelario M, Bacher JS, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis. 2000 Jul;182(1):274–82. doi: 10.1086/315643. [DOI] [PubMed] [Google Scholar]
- 27.Groll AH, Lyman CA, Petraitis V, Petraitiene R, Armstrong D, Mickiene D, et al. Compartmentalized intrapulmonary pharmacokinetics of amphotericin B and its lipid formulations. Antimicrob Agents Chemother. 2006 Oct;50(10):3418–23. doi: 10.1128/AAC.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002 Mar;46(3):828–33. doi: 10.1128/AAC.46.3.828-833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Würthwein G, Young C, Lanvers-Kaminsky C, Hempel G, Trame MN, Schwerdtfeger R, et al. Population pharmacokinetics of liposomal amphotericin B and caspofungin in allogeneic hematopoietic stem cell recipients. Antimicrob Agents Chemother. 2012 Jan;56(1):536–43. doi: 10.1128/AAC.00265-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong Y, Shaw PJ, Nath CE, Yadav SP, Stephen KR, Earl JW, et al. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrob Agents Chemother. 2006 Mar;50(3):935–42. doi: 10.1128/AAC.50.3.935-942.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hope WW, Goodwin J, Felton TW, Ellis M, Stevens DA. Population pharmacokinetics of conventional and intermittent dosing of liposomal amphotericin B in adults: a first critical step for rational design of innovative regimens. Antimicrob Agents Chemother. 2012 Oct;56(10):5303–8. doi: 10.1128/AAC.00933-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother. 2002 Mar;46(3):834–40. doi: 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh TJ, Yeldandi V, McEvoy M, Gonzalez C, Chanock S, Freifeld A, et al. Safety, tolerance, and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob Agents Chemother. 1998 Sep;42(9):2391–8. doi: 10.1128/aac.42.9.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriyama B, Torabi-Parizi P, Pratt AK, Henning SA, Pennick G, Shea YR, et al. Pharmacokinetics of liposomal amphotericin B in pleural fluid. Antimicrob Agents Chemother. 2010 Apr;54(4):1633–5. doi: 10.1128/AAC.01438-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogelsinger H, Weiler S, Djanani A, Kountchev J, Bellmann-Weiler R, Wiedermann CJ, et al. Amphotericin B tissue distribution in autopsy material after treatment with liposomal amphotericin B and amphotericin B colloidal dispersion. J Antimicrob Chemother. 2006 Jun;57(6):1153–60. doi: 10.1093/jac/dkl141. [DOI] [PubMed] [Google Scholar]
- 36.Strenger V, Meinitzer A, Donnerer J, Hofer N, Dornbusch HJ, Wanz U, et al. Amphotericin B transfer to CSF following intravenous administration of liposomal amphotericin B. J Antimicrob Chemother. 2014 Sep;69(9):2522–6. doi: 10.1093/jac/dku148. [DOI] [PubMed] [Google Scholar]
- 37.Heinemann V, Bosse D, Jehn U, Kähny B, Wachholz K, Debus A, et al. Pharmacokinetics of liposomal amphotericin B (Ambisome) in critically ill patients. Antimicrob Agents Chemother. 1997 Jun;41(6):1275–80. doi: 10.1128/aac.41.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurtz R, Itokazu G, Rodvold K. Antimicrobial dosing in obese patients. Clin Infect Dis. 1997 Jul;25(1):112–8. doi: 10.1086/514505. [DOI] [PubMed] [Google Scholar]
- 39.Polso AK, Lassiter JL, Nagel JL. Impact of hospital guideline for weight-based antimicrobial dosing in morbidly obese adults and comprehensive literature review. J Clin Pharm Ther. 2014 Dec;39(6):584–608. doi: 10.1111/jcpt.12200. [DOI] [PubMed] [Google Scholar]
- 40.Nath CE, McLachlan AJ, Shaw PJ, Coakley JC, Earl JW. Amphotericin B dose optimization in children with malignant diseases. Chemotherapy. 2007;53(2):142–7. doi: 10.1159/000100013. [DOI] [PubMed] [Google Scholar]
- 41.Pagliano P, Carannante N, Rossi M, Gramiccia M, Gradoni L, Faella FS, et al. Visceral leishmaniasis in pregnancy: a case series and a systematic review of the literature. J Antimicrob Chemother. 2005 Feb;55(2):229–33. doi: 10.1093/jac/dkh538. [DOI] [PubMed] [Google Scholar]
- 42.Takemoto K, Yamamoto Y, Ueda Y, Sumita Y, Yoshida K, Niki Y. Comparative study on the efficacy of AmBisome and Fungizone in a mouse model of pulmonary aspergillosis. J Antimicrob Chemother. 2006 Apr;57(4):724–31. doi: 10.1093/jac/dkl005. [DOI] [PubMed] [Google Scholar]
- 43.Al Nakeeb Z, Petraitis V, Goodwin J, Petraitiene R, Walsh TJ, Hope WW. Pharmacodynamics of Amphotericin B Deoxycholate, Amphotericin B Lipid Complex and Liposomal Amphotericin B against Aspergillus fumigatus. Antimicrob Agents Chemother. 2015 Feb; doi: 10.1128/AAC.04723-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clemons KV, Schwartz JA, Stevens DA. Experimental central nervous system aspergillosis therapy: efficacy, drug levels and localization, immunohistopathology, and toxicity. Antimicrob Agents Chemother. 2012 Aug;56(8):4439–49. doi: 10.1128/AAC.06015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Etten EW, van den Heuvel-de Groot C, Bakker-Woudenberg IA. Efficacies of amphotericin B-desoxycholate (Fungizone), liposomal amphotericin B (AmBisome) and fluconazole in the treatment of systemic candidosis in immunocompetent and leucopenic mice. J Antimicrob Chemother. 1993 Nov;32(5):723–39. doi: 10.1093/jac/32.5.723. [DOI] [PubMed] [Google Scholar]
- 46.Olson JA, Adler-Moore JP, Smith PJ, Proffitt RT. Treatment of Candida glabrata infection in immunosuppressed mice by using a combination of liposomal amphotericin B with caspofungin or micafungin. Antimicrob Agents Chemother. 2005 Dec;49(12):4895–902. doi: 10.1128/AAC.49.12.4895-4902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clemons KV, Stevens DA. Therapeutic efficacy of a liposomal formulation of amphotericin B (AmBisome) against murine blastomycosis. J Antimicrob Chemother. 1993 Sep;32(3):465–72. doi: 10.1093/jac/32.3.465. [DOI] [PubMed] [Google Scholar]
- 48.Ibrahim AS, Gebremariam T, Husseiny MI, Stevens DA, Fu Y, Edwards JE, et al. Comparison of lipid amphotericin B preparations in treating murine zygomycosis. Antimicrob Agents Chemother. 2008 Apr;52(4):1573–6. doi: 10.1128/AAC.01488-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Graybill JR, Bocanegra R. Liposomal amphotericin B therapy of murine histoplasmosis. Antimicrob Agents Chemother. 1995 Aug;39(8):1885–7. doi: 10.1128/aac.39.8.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Connor L, Livermore J, Sharp AD, Goodwin J, Gregson L, Howard SJ, et al. Pharmacodynamics of liposomal amphotericin B and flucytosine for cryptococcal meningoencephalitis: safe and effective regimens for immunocompromised patients. J Infect Dis. 2013 Jul;208(2):351–61. doi: 10.1093/infdis/jit164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mistro S, Maciel IeM, de Menezes RG, Maia ZP, Schooley RT, Badaró R. Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Clin Infect Dis. 2012 Jun;54(12):1774–7. doi: 10.1093/cid/cis290. [DOI] [PubMed] [Google Scholar]
- 52.Wasan KM, Morton RE, Rosenblum MG, Lopez-Berestein G. Decreased toxicity of liposomal amphotericin B due to association of amphotericin B with high-density lipoproteins: role of lipid transfer protein. J Pharm Sci. 1994 Jul;83(7):1006–10. doi: 10.1002/jps.2600830716. [DOI] [PubMed] [Google Scholar]
- 53.Loo AS, Muhsin SA, Walsh TJ. Toxicokinetic and mechanistic basis for the safety and tolerability of liposomal amphotericin B. Expert Opin Drug Saf. 2013 Nov;12(6):881–95. doi: 10.1517/14740338.2013.827168. [DOI] [PubMed] [Google Scholar]
- 54.Sau K, Mambula SS, Latz E, Henneke P, Golenbock DT, Levitz SM. The antifungal drug amphotericin B promotes inflammatory cytokine release by a Toll-like receptor- and CD14-dependent mechanism. J Biol Chem. 2003 Sep;278(39):37561–8. doi: 10.1074/jbc.M306137200. [DOI] [PubMed] [Google Scholar]
- 55.Roden MM, Nelson LD, Knudsen TA, Jarosinski PF, Starling JM, Shiflett SE, et al. Triad of acute infusion-related reactions associated with liposomal amphotericin B: analysis of clinical and epidemiological characteristics. Clin Infect Dis. 2003 May;36(10):1213–20. doi: 10.1086/374553. [DOI] [PubMed] [Google Scholar]
- 56.Szebeni J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M, et al. Liposome-induced pulmonary hypertension: properties and mechanism of a complement-mediated pseudoallergic reaction. Am J Physiol Heart Circ Physiol. 2000 Sep;279(3):H1319–28. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- 57.Wade RL, Chaudhari P, Natoli JL, Taylor RJ, Nathanson BH, Horn DL. Nephrotoxicity and other adverse events among inpatients receiving liposomal amphotericin B or amphotericin B lipid complex. Diagn Microbiol Infect Dis. 2013 Jul;76(3):361–7. doi: 10.1016/j.diagmicrobio.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Patel GP, Crank CW, Leikin JB. An evaluation of hepatotoxicity and nephrotoxicity of liposomal amphotericin B (L-AMB) J Med Toxicol. 2011 Mar;7(1):12–5. doi: 10.1007/s13181-010-0120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer MA, Winkelmayer WC, Rubin RH, Avorn J. The hepatotoxicity of antifungal medications in bone marrow transplant recipients. Clin Infect Dis. 2005 Aug;41(3):301–7. doi: 10.1086/431586. [DOI] [PubMed] [Google Scholar]
- 60.Kolve H, Ahlke E, Fegeler W, Ritter J, Jürgens H, Groll AH. Safety, tolerance and outcome of treatment with liposomal amphotericin B in paediatric patients with cancer or undergoing haematopoietic stem cell transplantation. J Antimicrob Chemother. 2009 Aug;64(2):383–7. doi: 10.1093/jac/dkp196. [DOI] [PubMed] [Google Scholar]
- 61.Chamilos G, Luna M, Lewis RE, Chemaly R, Raad II, Kontoyiannis DP. Effects of liposomal amphotericin B versus an amphotericin B lipid complex on liver histopathology in patients with hematologic malignancies and invasive fungal infections: a retrospective, nonrandomized autopsy study. Clin Ther. 2007 Sep;29(9):1980–6. doi: 10.1016/j.clinthera.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 62.Administration FD, editor. FDA. AmBisome® Package Insert. Mar, 2012. [Google Scholar]
- 63.Lewis RE, Albert ND, Kontoyiannis DP. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008 Nov;52(11):4178–80. doi: 10.1128/AAC.00715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Penack O, Schwartz S, Martus P, Reinwald M, Schmidt-Hieber M, Thiel E, et al. Low-dose liposomal amphotericin B in the prevention of invasive fungal infections in patients with prolonged neutropenia: results from a randomized, single-center trial. Ann Oncol. 2006 Aug;17(8):1306–12. doi: 10.1093/annonc/mdl128. [DOI] [PubMed] [Google Scholar]
- 65.Uhlenbrock S, Zimmermann M, Fegeler W, Jürgens H, Ritter J. Liposomal amphotericin B for prophylaxis of invasive fungal infections in high-risk paediatric patients with chemotherapy-related neutropenia: interim analysis of a prospective study. Mycoses. 2001 Dec;44(11–12):455–63. doi: 10.1046/j.1439-0507.2001.00706.x. [DOI] [PubMed] [Google Scholar]
- 66.Singh N, Paterson DL, Gayowski T, Wagener MM, Marino IR. Preemptive prophylaxis with a lipid preparation of amphotericin B for invasive fungal infections in liver transplant recipients requiring renal replacement therapy. Transplantation. 2001 Apr;71(7):910–3. doi: 10.1097/00007890-200104150-00016. [DOI] [PubMed] [Google Scholar]
- 67.Shah T, Lai WK, Gow P, Leeming J, Mutimer D. Low-dose amphotericin for prevention of serious fungal infection following liver transplantation. Transpl Infect Dis. 2005 Sep-Dec;7(3–4):126–32. doi: 10.1111/j.1399-3062.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 68.Fortun J, Martin-Davila P, Moreno S, Barcena R, de Vicente E, Honrubia A, et al. Prevention of invasive fungal infections in liver transplant recipients: the role of prophylaxis with lipid formulations of amphotericin B in high-risk patients. J Antimicrob Chemother. 2003 Nov;52(5):813–9. doi: 10.1093/jac/dkg450. [DOI] [PubMed] [Google Scholar]
- 69.Prentice HG, Hann IM, Herbrecht R, Aoun M, Kvaloy S, Catovsky D, et al. A randomized comparison of liposomal versus conventional amphotericin B for the treatment of pyrexia of unknown origin in neutropenic patients. Br J Haematol. 1997 Sep;98(3):711–8. doi: 10.1046/j.1365-2141.1997.2473063.x. [DOI] [PubMed] [Google Scholar]
- 70.Walsh TJ, Finberg RW, Arndt C, Hiemenz J, Schwartz C, Bodensteiner D, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. National Institute of Allergy and Infectious Diseases Mycoses Study Group. N Engl J Med. 1999 Mar;340(10):764–71. doi: 10.1056/NEJM199903113401004. [DOI] [PubMed] [Google Scholar]
- 71.Walsh TJ, Teppler H, Donowitz GR, Maertens JA, Baden LR, Dmoszynska A, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004 Sep;351(14):1391–402. doi: 10.1056/NEJMoa040446. [DOI] [PubMed] [Google Scholar]
- 72.Walsh TJ, Pappas P, Winston DJ, Lazarus HM, Petersen F, Raffalli J, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002 Jan;346(4):225–34. doi: 10.1056/NEJM200201243460403. [DOI] [PubMed] [Google Scholar]
- 73.Francis P, Lee JW, Hoffman A, Peter J, Francesconi A, Bacher J, et al. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar D-mannitol and serum galactomannan as markers of infection. J Infect Dis. 1994 Feb;169(2):356–68. doi: 10.1093/infdis/169.2.356. [DOI] [PubMed] [Google Scholar]
- 74.Kirkpatrick WR, Coco BJ, Patterson TF. Sequential or combination antifungal therapy with voriconazole and liposomal amphotericin B in a Guinea pig model of invasive aspergillosis. Antimicrob Agents Chemother. 2006 Apr;50(4):1567–9. doi: 10.1128/AAC.50.4.1567-1569.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olson JA, George A, Constable D, Smith P, Proffitt RT, Adler-Moore JP. Liposomal amphotericin B and echinocandins as monotherapy or sequential or concomitant therapy in murine disseminated and pulmonary Aspergillus fumigatus infections. Antimicrob Agents Chemother. 2010 Sep;54(9):3884–94. doi: 10.1128/AAC.01554-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leenders AC, Daenen S, Jansen RL, Hop WC, Lowenberg B, Wijermans PW, et al. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol. 1998 Oct;103(1):205–12. doi: 10.1046/j.1365-2141.1998.00944.x. [DOI] [PubMed] [Google Scholar]
- 77.Krüger W, Stockschläder M, Sobottka I, Betker R, De Wit M, Kröger N, et al. Antimycotic therapy with liposomal amphotericin-B for patients undergoing bone marrow or peripheral blood stem cell transplantation. Leuk Lymphoma. 1997 Feb;24(5–6):491–9. doi: 10.3109/10428199709055586. [DOI] [PubMed] [Google Scholar]
- 78.Ellis M, Spence D, de Pauw B, Meunier F, Marinus A, Collette L, et al. An EORTC international multicenter randomized trial (EORTC number 19923) comparing two dosages of liposomal amphotericin B for treatment of invasive aspergillosis. Clin Infect Dis. 1998 Dec;27(6):1406–12. doi: 10.1086/515033. [DOI] [PubMed] [Google Scholar]
- 79.Cornely OA, Maertens J, Bresnik M, Ebrahimi R, Ullmann AJ, Bouza E, et al. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial) Clin Infect Dis. 2007 May;44(10):1289–97. doi: 10.1086/514341. [DOI] [PubMed] [Google Scholar]
- 80.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008 Feb;46(3):327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 81.Adler-Moore JP, Chiang SM, Satorius A, Guerra D, McAndrews B, McManus EJ, et al. Treatment of murine candidosis and cryptococcosis with a unilamellar liposomal amphotericin B formulation (AmBisome) J Antimicrob Chemother. 1991 Oct;28(Suppl B):63–71. doi: 10.1093/jac/28.suppl_b.63. [DOI] [PubMed] [Google Scholar]
- 82.Kuse ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet. 2007 May;369(9572):1519–27. doi: 10.1016/S0140-6736(07)60605-9. [DOI] [PubMed] [Google Scholar]
- 83.Queiroz-Telles F, Berezin E, Leverger G, Freire A, van der Vyver A, Chotpitayasunondh T, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J. 2008 Sep;27(9):820–6. doi: 10.1097/INF.0b013e31817275e6. [DOI] [PubMed] [Google Scholar]
- 84.Day JN, Chau TT, Lalloo DG. Combination antifungal therapy for cryptococcal meningitis. N Engl J Med. 2013 Jun;368(26):2522–3. doi: 10.1056/NEJMc1305981. [DOI] [PubMed] [Google Scholar]
- 85.Sun HY, Alexander BD, Lortholary O, Dromer F, Forrest GN, Lyon GM, et al. Lipid formulations of amphotericin B significantly improve outcome in solid organ transplant recipients with central nervous system cryptococcosis. Clin Infect Dis. 2009 Dec;49(11):1721–8. doi: 10.1086/647948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hamill RJ, Sobel JD, El-Sadr W, Johnson PC, Graybill JR, Javaly K, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS-associated acute cryptococcal meningitis: a randomized, double-blind clinical trial of efficacy and safety. Clin Infect Dis. 2010 Jul;51(2):225–32. doi: 10.1086/653606. [DOI] [PubMed] [Google Scholar]
- 87.Leenders AC, Reiss P, Portegies P, Clezy K, Hop WC, Hoy J, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS. 1997 Oct;11(12):1463–71. doi: 10.1097/00002030-199712000-00010. [DOI] [PubMed] [Google Scholar]
- 88.Coker RJ, Viviani M, Gazzard BG, Du Pont B, Pohle HD, Murphy SM, et al. Treatment of cryptococcosis with liposomal amphotericin B (AmBisome) in 23 patients with AIDS. AIDS. 1993 Jun;7(6):829–35. doi: 10.1097/00002030-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 89.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2010 Feb;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gangneux JP, Sulahian A, Garin YJ, Farinotti R, Derouin F. Therapy of visceral leishmaniasis due to Leishmania infantum: experimental assessment of efficacy of AmBisome. Antimicrob Agents Chemother. 1996 May;40(5):1214–8. doi: 10.1128/aac.40.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bern C, Adler-Moore J, Berenguer J, Boelaert M, den Boer M, Davidson RN, et al. Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clin Infect Dis. 2006 Oct;43(7):917–24. doi: 10.1086/507530. [DOI] [PubMed] [Google Scholar]
- 92.Lewis RE, Albert ND, Liao G, Hou J, Prince RA, Kontoyiannis DP. Comparative pharmacodynamics of amphotericin B lipid complex and liposomal amphotericin B in a murine model of pulmonary mucormycosis. Antimicrob Agents Chemother. 2010 Mar;54(3):1298–304. doi: 10.1128/AAC.01222-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spellberg B, Ibrahim AS, Chin-Hong PV, Kontoyiannis DP, Morris MI, Perfect JR, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012 Mar;67(3):715–22. doi: 10.1093/jac/dkr375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lanternier F, Lortholary O. [AMBIZYGO: phase II study of high dose liposomal amphotericin B (AmBisome) [10 mg/kg/j] efficacy against zygomycosis] Med Mal Infect. 2008 Jun;38(Suppl 2):S90–1. doi: 10.1016/S0399-077X(08)73003-8. [DOI] [PubMed] [Google Scholar]
- 95.Johnson PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002 Jul;137(2):105–9. doi: 10.7326/0003-4819-137-2-200207160-00008. [DOI] [PubMed] [Google Scholar]
- 96.Wheat LJ, Cloud G, Johnson PC, Connolly P, Goldman M, Le Monte A, et al. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrob Agents Chemother. 2001 Aug;45(8):2354–7. doi: 10.1128/AAC.45.8.2354-2357.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamalaporn H, Leung K, Nagel M, Kittanakom S, Calvieri B, Reithmeier RA, et al. Aerosolized liposomal Amphotericin B: a potential prophylaxis of invasive pulmonary aspergillosis in immunocompromised patients. Pediatr Pulmonol. 2014 Jun;49(6):574–80. doi: 10.1002/ppul.22856. [DOI] [PubMed] [Google Scholar]
- 98.GILBERT BE. Liposomal aerosols in the management of pulmonary infections. Journal of aerosol medicine. 1996;9(1):111–22. doi: 10.1089/jam.1996.9.111. [DOI] [PubMed] [Google Scholar]
- 99.Monforte V, Ussetti P, López R, Gavaldà J, Bravo C, de Pablo A, et al. Nebulized liposomal amphotericin B prophylaxis for Aspergillus infection in lung transplantation: pharmacokinetics and safety. J Heart Lung Transplant. 2009 Feb;28(2):170–5. doi: 10.1016/j.healun.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Monforte V, Ussetti P, Gavaldà J, Bravo C, Laporta R, Len O, et al. Feasibility, tolerability, and outcomes of nebulized liposomal amphotericin B for Aspergillus infection prevention in lung transplantation. J Heart Lung Transplant. 2010 May;29(5):523–30. doi: 10.1016/j.healun.2009.11.603. [DOI] [PubMed] [Google Scholar]
- 101.Rijnders BJ, Cornelissen JJ, Slobbe L, Becker MJ, Doorduijn JK, Hop WC, et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: a randomized, placebo-controlled trial. Clin Infect Dis. 2008 May;46(9):1401–8. doi: 10.1086/586739. [DOI] [PubMed] [Google Scholar]
- 102.Purcell IF, Corris PA. Use of nebulised liposomal amphotericin B in the treatment of Aspergillus fumigatus empyema. Thorax. 1995 Dec;50(12):1321–3. doi: 10.1136/thx.50.12.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramage G, VandeWalle K, Bachmann SP, Wickes BL, López-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002 Nov;46(11):3634–6. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schinabeck MK, Long LA, Hossain MA, Chandra J, Mukherjee PK, Mohamed S, et al. Rabbit model of Candida albicans biofilm infection: liposomal amphotericin B antifungal lock therapy. Antimicrob Agents Chemother. 2004 May;48(5):1727–32. doi: 10.1128/AAC.48.5.1727-1732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buckler BS, Sams RN, Goei VL, Krishnan KR, Bemis MJ, Parker DP, et al. Treatment of central venous catheter fungal infection using liposomal amphotericin-B lock therapy. Pediatr Infect Dis J. 2008 Aug;27(8):762–4. doi: 10.1097/INF.0b013e318170b68b. [DOI] [PubMed] [Google Scholar]
- 106.Albert MM, Adams K, Luther MJ, Sun SH, Graybill JR. Efficacy of AmBisome in murine coccidioidomycosis. J Med Vet Mycol. 1994 Dec;32(6):467–71. doi: 10.1080/02681219480000621. [DOI] [PubMed] [Google Scholar]
- 107.Clemons KV, Stevens DA. Comparison of fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob Agents Chemother. 1998 Apr;42(4):899–902. doi: 10.1128/aac.42.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sundar S, Jha TK, Thakur CP, Mishra M, Singh VP, Buffels R. Single-dose liposomal amphotericin B in the treatment of visceral leishmaniasis in India: a multicenter study. Clin Infect Dis. 2003 Sep;37(6):800–4. doi: 10.1086/377542. [DOI] [PubMed] [Google Scholar]
- 109.AMBITION trial. http://wwwisrctncom/ISRCTN10248064
- 110.Sundar S, Chakravarty J, Agarwal D, Rai M, Murray HW. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010 Feb;362(6):504–12. doi: 10.1056/NEJMoa0903627. [DOI] [PubMed] [Google Scholar]
- 111.Sundar S, Sinha PK, Rai M, Verma DK, Nawin K, Alam S, et al. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011 Feb;377(9764):477–86. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- 112.Khalil EA, Weldegebreal T, Younis BM, Omollo R, Musa AM, Hailu W, et al. Safety and efficacy of single dose versus multiple doses of AmBisome for treatment of visceral leishmaniasis in eastern Africa: a randomised trial. PLoS Negl Trop Dis. 2014;8(1):e2613. doi: 10.1371/journal.pntd.0002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El-Cheikh J, Faucher C, Fürst S, Duran S, Berger P, Vey N, et al. High-dose weekly liposomal amphotericin B antifungal prophylaxis following reduced-intensity conditioning allogeneic stem cell transplantation. Bone Marrow Transplant. 2007 Mar;39(5):301–6. doi: 10.1038/sj.bmt.1705592. [DOI] [PubMed] [Google Scholar]
- 114.Kelsey SM, Goldman JM, McCann S, Newland AC, Scarffe JH, Oppenheim BA, et al. Liposomal amphotericin (AmBisome) in the prophylaxis of fungal infections in neutropenic patients: a randomised, double-blind, placebo-controlled study. Bone Marrow Transplant. 1999 Jan;23(2):163–8. doi: 10.1038/sj.bmt.1701543. [DOI] [PubMed] [Google Scholar]
- 115.Cordonnier C, Mohty M, Faucher C, Pautas C, Robin M, Vey N, et al. Safety of a weekly high dose of liposomal amphotericin B for prophylaxis of invasive fungal infection in immunocompromised patients: PROPHYSOME Study. Int J Antimicrob Agents. 2008 Feb;31(2):135–41. doi: 10.1016/j.ijantimicag.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 116.Cornely OLT, Maertens J, Castagnola C, Anagnostopoulos A, Allione B, Heussel C, Donnelly J, Agrawal S, Garner W, Simcock C, Hawkins M, et al. A Double-Blind, Multicentre, Randomised, Placebo-Controlled Study to Assess the Efficacy, Safety and Tolerability of Prophylactic Liposomal Amphotericin B (AmBisome®) for the Prevention of Invasive Fungal Infections in Subjects Receiving Remission-Induction Chemotherapy for Acute Lymphoblastic Leukaemia (AmBiGuard trial) ICAAC. 2014:2014. [Google Scholar]
- 117.Bellmann R, Egger P, Wiedermann CJ. Differences in pharmacokinetics of amphotericin B lipid formulations despite clinical equivalence. Clin Infect Dis. 2003 Jun;36(11):1500–1. doi: 10.1086/374876. [DOI] [PubMed] [Google Scholar]
- 118.Bellmann R. Clinical pharmacokinetics of systemically administered antimycotics. Curr Clin Pharmacol. 2007 Jan;2(1):37–58. doi: 10.2174/157488407779422311. [DOI] [PubMed] [Google Scholar]
- 119.Felton T, Troke PF, Hope WW. Tissue penetration of antifungal agents. Clin Microbiol Rev. 2014 Jan;27(1):68–88. doi: 10.1128/CMR.00046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Formulary BN. BNF. 2015 Sep; [Google Scholar]