Summary
Protumorigenic PD-1high B cells, induced in hepatocellular carcinoma, suppress tumor-specific T cell response via IL-10-dependent pathways upon PD-1/PD-L1 interaction. Anti-PD-1 or anti-PD-L1 antibodies may function not only through blocking the PD-1 co-inhibitory pathway in T cells, but also via abolishing the suppressive function of regulatory B cells.
Tumor cells often induce an immunosuppressive microenvironment against the antitumor immunity. Besides regulatory T cells (Treg), myeloid derived suppressor cells (MDSC), and tumor-associated macrophages (TAM), regulatory B cells (Breg) have been reported recently to be the key immune suppressers that silence antitumor responses and promote tumor progression in some types of tumors. Breg cells were first discovered as an inhibitor in autoimmune diseases, since B-cell-deficient mice (μMT) failed to control experimental autoimmune encephalomyelitis (EAE) and could not undergo spontaneous remission (1). The exacerbated EAE in μMT mice was latterly attributed to a deficiency of in IL-10-producing B cells (2), or so called B10 cells. Emerging data indicate that tumor-infiltrating B cells are not irrelevant bystanders in tumor progression, but rather actively regulate antitumor immune responses (3-6). Tumor-infiltrating B cells with distinct phenotypes and functions may play specific roles in anti-tumor responses (5). Despite recent advances in understanding the role of tumor-infiltrating B cells, direct evidence supporting an immunosuppressive role for B cells in human cancers is still lacking, such as specific knowledge of the subset compositions, regulation, and functional relevance of B cells in cancer environments.
In this issue of Cancer Discovery, Xiao and colleagues present a comprehensive study on a novel PD-1high regulatory B cell subset in human hepatocellular carcinoma (7). This newly identified subset of PD-1high regulatory B cell is correlated with the tumor stage and early recurrence of patients. PD-1high Breg cells exhibit a unique CD5hi CD24−/+ CD27hi CD38dim phenotype that differs from conventional regulatory B cells. PD-1high B cells can be specifically induced by culture supernatant of primary HCC cells in vitro. Environmental hyaluronan (HA) fragments from hepatoma cells induce PD-1high Breg cells via TLR4 activation, and TLR4-mediated Bcl-6 upregulation is critical for induction of PD-1high Breg cells. Triggering PD-1 through PD-L1 induces production of IL-10 by PD-1high Breg cells, which suppresses tumor-specific immunity and promotes tumor progression.
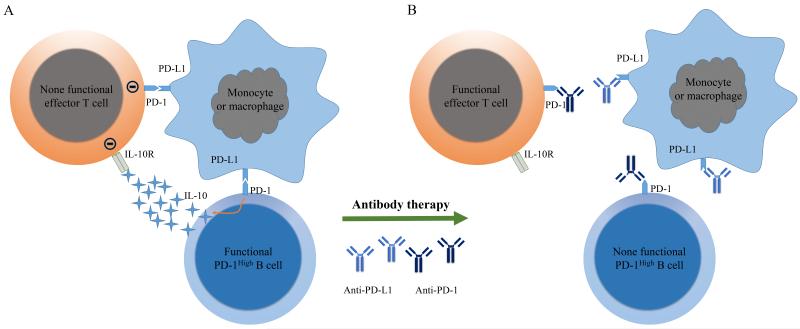
Immunosurveillance, as a primary defense against cancer, is a process for the immune system to recognize and destroy tumor cells. However, some tumor cells can escape elimination by the immune system via activation of immunosuppressive feedback loops, which result in inducing T-cell tolerance and the failure of tumor rejection. PD-1 signaling is one such inhibitory pathway, and its activation has been investigated in several cancer types (8). Binding of PD-1 on T cells with PD-L1 on antigen presenting cells (APCs) and other cells is a major mechanism trigging T cell exhaustion during tumor progression. Costimulatory receptors primarily function to modify TCR signaling. But PD-1 exhibits a more dominant function on inhibiting IFN-γ, TNF-α, and IL-2 production than promoting cellular proliferation (9). PD-1 can be expressed on T cells, B cells, natural killer T cells, activated monocytes, and dendritic cells (DCs; ref. 9). The role of PD-1 on T cells has been well studied, but not so well studied on B cells. Xiao and colleagues demonstrate that PD-1 expression on B cells endows them with the suppressive function. Upon interaction with PD-L1, PD-1high B cells start to produce IL-10. IL-10 serves as an immunosuppressive cytokine that regulates cytotoxic T cell activation. Prolonged exposure of immune cells to the inhibitory cytokines, such as IL-10, during tumor progression causes T cell dysfunction. These results identify a new mechanism for how PD-1/PD-L1 interactions between regulatory B cells and other cells induce the immune tolerance of T cells in the tumor. Anti-PD-1 and anti-PD-L1 antibodies have been broadly tested in several types of cancers in clinical trials (8). The main mechanism of tumor control by these antibodies is believed to be through blocking inhibitory signaling pathway in T cells. But anti-PD-1 and anti-PD-L1 antibodies can also work through removing the suppressive function of Breg cells. As shown in Fig. 1A, PD-1/PD-L1 interactions could induce T cell dysfunction through two different pathways in the tumor: 1) PD-L1 on monocytes or macrophages directly inducing effector T-cell dysfunction by triggering PD-1 on T cells; 2) PD-L1 triggers PD-1 on regulatory B cells for producing IL-10, inducing effector T-cell dysfunction. Thus anti-PD-1 or anti-PD-L1 antibodies may also function in two different ways: 1) directly interrupting the interaction of T cell PD-1 with PD-L1 on monocytes or macrophages; 2) interrupting interaction of PD-1 on regulatory B cells with PD-L1 on monocytes or macrophages, removing the suppressive effect of regulatory B cells on effector T cells (Fig. 1B). These two different pathways can exist separately or co-exist in the tumor microenvironment. This mechanism explains why anti-PD-1 or anti-PD-L1 can also control some tumors with PD-1 negative tumor infiltrating T lymphocytes (8).
Figure 1.
The role of PD-1/PD-L1 interaction in suppressing tumor-specific T cell and the potential mechanism of how anti-PD-1/anti-PD-L1 works in tumor treatment. A. By interacting directly with PD-L1+ monocytes or macrophages, the PD-1high B cells induce T cell dysfunction via IL-10-dependent pathways and PD-L1+ monocytes or macrophages can also directly induce the T cell dysfunction by PD-L1/PD-1 interaction. B. Anti-PD-1 or anti-PD-L1 antibody can block the PD-1/PD-L1 interactions of T cell or PD-1high B cells with monocytes or macrophage, which rescues the function of effector T cells.
To overcome tumor immune tolerance, identification of the dominant immunosuppressive cells and pathways in the tumor will be an alternative way to re-design future treatment. Each individual tumor microenvironment is possibly controlled by a distinct profile of suppressive molecules or cellular population. This study has demonstrated that PD-1high B cell is one sort of the immunosuppressive cells that is specifically induced in the hepatocellular carcinoma microenvironment. If PD-1high B cells are the dominant immunosuppressive cells in HCC, or even other types of cancer, anti-CD20 antibody can be used to transiently deplete these cells and remove the suppression (10). The authors propose that soluble factors derived from cancer cell promote PD-1 expression. Determining the nature of such factor(s) in the future will bring great benefit to clinical treatment. In addition to the proposed monocytes, other sources of PD-L1 interacting with PD-1 on B cells cannot be ruled out yet. It would be interesting to know whether B and/or T cell dysfunction can be corrected by PD-1 blockade on B cells, and whether IL-10 production from those Breg can be suppressed after treatment in clinic. The requirement of PD-1 or PD-L1 signaling for generation of those Bregs should be investigated in the following-up study. Together, this study has opened a new avenue to study B cell-mediated suppression and provided new insights into the possible manipulation by B cell-mediated suppression through PD-1 overexpression.
Acknowledgments
Grant Support
This work is supported by National Nature and Science Foundation of China grant (No. 81172814) to H. Peng; and supported by the U.S. National Institutes of Health through National Cancer Institute grants CA141975 to Y.-X. Fu.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest
REFERENCES
- 1.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–8. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 3.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 4.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer Res. 2011;71:3505–15. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Gallastegui N, Rosenblatt JD. Regulatory B cells in anti-tumor immunity. Int Immunol. 2015;27:521–30. doi: 10.1093/intimm/dxv034. [DOI] [PubMed] [Google Scholar]
- 6.Roghanian A, Fraser C, Kleyman M, Chen J. B Cells Promote Pancreatic Tumorigenesis. Cancer Discov. 2016;6:230–2. doi: 10.1158/2159-8290.CD-16-0100. [DOI] [PubMed] [Google Scholar]
- 7.Xiao X, Lao XM, Chen MM, Liu RX, Wei Y, Ouyang FZ, et al. PD-1High Identifies a Novel Regulatory B Cell Population in Human Hepatoma that Promotes Disease Progression. Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-15-1408. [DOI] [PubMed] [Google Scholar]
- 8.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8:328rv4. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–21. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]