Abstract
The cell wall is a dynamic structure that is important for the pathogenicity of Candida albicans. Mannan, which is located in the outermost layer of the cell wall, has been shown to contribute to the pathogenesis of C. albicans, however, the molecular mechanism by which this occurs remains unclear. Here we identified a novel α-1,6-mannosyltransferase encoded by MNN10 in C. albicans. We found that Mnn10 is required for cell wall α-1,6-mannose backbone biosynthesis and polysaccharides organization. Deletion of MNN10 resulted in significant attenuation of the pathogenesis of C. albicans in a murine systemic candidiasis model. Inhibition of α-1,6-mannose backbone extension did not, however, impact the invasive ability of C. albicans in vitro. Notably, mnn10 mutant restored the invasive capacity in athymic nude mice, which further supports the notion of an enhanced host antifungal defense related to this backbone change. Mnn10 mutant induced enhanced Th1 and Th17 cell mediated antifungal immunity, and resulted in enhanced recruitment of neutrophils and monocytes for pathogen clearance in vivo. We also demonstrated that MNN10 could unmask the surface β-(1,3)-glucan, a crucial pathogen-associated molecular pattern (PAMP) of C. albicans recognized by host Dectin-1. Our results demonstrate that mnn10 mutant could stimulate an enhanced Dectin-1 dependent immune response of macrophages in vitro, including the activation of nuclear factor-κB, mitogen-activated protein kinase pathways, and secretion of specific cytokines such as TNF-α, IL-6, IL-1β and IL-12p40. In summary, our study indicated that α-1,6-mannose backbone is critical for the pathogenesis of C. albicans via shielding β-glucan from recognition by host Dectin-1 mediated immune recognition. Moreover, our work suggests that inhibition of α-1,6-mannose extension by Mnn10 may represent a novel modality to reduce the pathogenicity of C. albicans.
Author Summary
Mannan plays a crucial role in cell wall structure and virulence of the opportunistic pathogen Candida albicans. Both the invasive ability of the pathogen and the host defense against the pathogen contribute to the outcome of invasive infection. In the present study, we identified a novel α-1,6-mannosyltransferase, which was responsible for cell wall α-1,6-mannose backbone extension in C. albicans. We determined that α-1,6-mannose backbone is necessary for the pathogenesis of C. albicans due to its ability to shield β-(1,3)-glucan from the host Dectin-1 recognition and Th1/Th7 response. Our study highlights a novel strategy to enhance the host immune response towards C. albicans.
Introduction
Candida albicans is a common fungal microorganism that colonizes the oral, genital and gastrointestinal surfaces of most healthy individuals. The maintenance of colonization is the result of a complex balance between fungal proliferation and host immune recognition. Despite host immune defenses aimed at clearing pathogens, C. albicans has developed numerous strategies to evade host immune detection [1]. In immunocompromised patients, C. albicans may disseminate into bloodstream, causing life-threatening systemic candidiasis [2, 3]. The associated mortality rates of systemic infection are reported to be greater than 30%, highlighting the potential critical impact of C. albicans on global health burden [4–6].
The mature cell wall of C. albicans is a complex structure of cross-linked polysaccharides and glycosylated proteins. The cell wall is not only required for maintaining cell shape and stability, but also is critically related to immunogenicity and virulence of C. albicans. The outer layer of the cell wall is comprised of glycosylated mannoproteins that are post-translationally modified by N- and O-linked mannosides [7]. N-linked mannan chains are specifically required for cell morphology, phagocytosis, and immune recognition of C. albicans by host dendritic cells [8]. The core structure of N-mannan is a dolichol pyrophosphate anchored oligosaccharide comprised of three glucose, nine mannose and two N-acetylglucosamine residues (Glc3Man9GlcNAc2). The outer chain branched mannan is attached to the N-mannan core through an α-1,6-backbone. Addition of the first α-1,6-mannose is catalyzed by mannosyltransferase Och1. Notably, Och1 mutant strains of C. albicans demonstrate attenuated virulence in animal models with systemic infection [9]. Extension of α-1,6-mannose backbone by mannose residues is performed by the enzyme complexes mannan polymerase I (M-Pol I) and II (M-Pol II) [10]. The α-1,6-backbone is then further modified with additional α-1,2-mannose units by Mnn2 family and Mnn5, which similarly, are critical for C. albicans virulence in mice or Galleria mellonella [11, 12]. The outer side chains are further capped with either α-1,3-mannose or β-1,2-mannose units via Mnn1 family and β-1,2-mannosyltransferases (BMTs). The C. albicans MNN1 gene family contains six members, of which only MNN14 represent a critical factor for pathogenicity in vivo [13]. Bmt1 and Bmt3, which are required for the addition of the first and second β-1,2-mannose units respectively, are not associated with the virulence of C. albicans [14]. Although a variety of C. albicans mannosylation mutants have been found to be less pathogenic in vivo, the mechanisms of host clearance associated with abnormal mannan structures remains unclear.
The cell wall polysaccharides of C. albicans are mainly composed of multiple layers of carbohydrates, including mannans, β-glucans, and chitins [3]. These polysaccharides serve as pathogen-associated molecular patterns (PAMPs) that can be recognized by host-expressed pattern recognition receptors (PRRs) to initiate an innate immune response [1]. Several PRRs, such as toll-like receptors (TLRs), spleen tyrosine kinase (Syk)-coupled C-type lectin receptors (CLRs), and nucleotide binding oligomerization domain (Nod)-like receptors (NLRs), can recognize PAMPs on the surface of C. albicans [15–17]. The PRRs engagement by C. albicans PAMPs triggers innate immune cells to respond and renders antigen-presenting cells competent to prime T cells. A complex signaling cascades, including nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways, among others, lead to Th1 and Th17 activation and an adaptive immune response [18–21].
Dectin-1, a myeloid-expressed Syk-coupled receptor, can recognize β-(1,3)-glucan carbohydrates on the surface of various fungi [22–24]. Clinical studies have demonstrated that patients with Dectin-1 Y238X mutation are highly susceptible to mucosal C. albicans infection [25]. However, live C. albicans, including yeast and hyphae forms, binds to Dectin-1 in highly specialized pattern in vitro, except in the region between the parental yeast cell and the mature bud [26]. During infection, β-(1,3)-glucan of C. albicans is completely masked in earlier stages, while large percentages are exposed at later stages in a morphotype-independent fashion [27]. Shielding of β-(1,3)-glucan favors fungal survival and persistence by escaping Dectin-1 mediated immune recognition [28]. Previous studies have indicated that unmasking C. albicans β-(1,3)-glucan elicits a stronger host immune response towards C. albicans via several experimental manipulations such as drug treatment and several key genes deletion [29–31].
Mnn10, an important subunit of cis Golgi mannan polymerase, was identified as an α-1,6-mannosyltransferase which is responsible for mannan backbone extension in non-pathogenic fungal species such as Saccharomyces cerevisiae and Kluyveromyces lactis [32, 33]. In the present study, we first characterized the role of α-1,6-mannose backbone in C. albicans pathogenicity. We demonstrated that inhibition of α-1,6-mannose backbone extension can block the development of invasive C. albicans infection, and suggested α-1,6-mannose backbone extension is essential for the evasion of host Dectin-1 mediated immune response towards C. albicans.
Results
Mnn10 possesses α-1,6-mannosyltransferase activity, and is required for α-1,6-mannose backbone extension in C. albicans
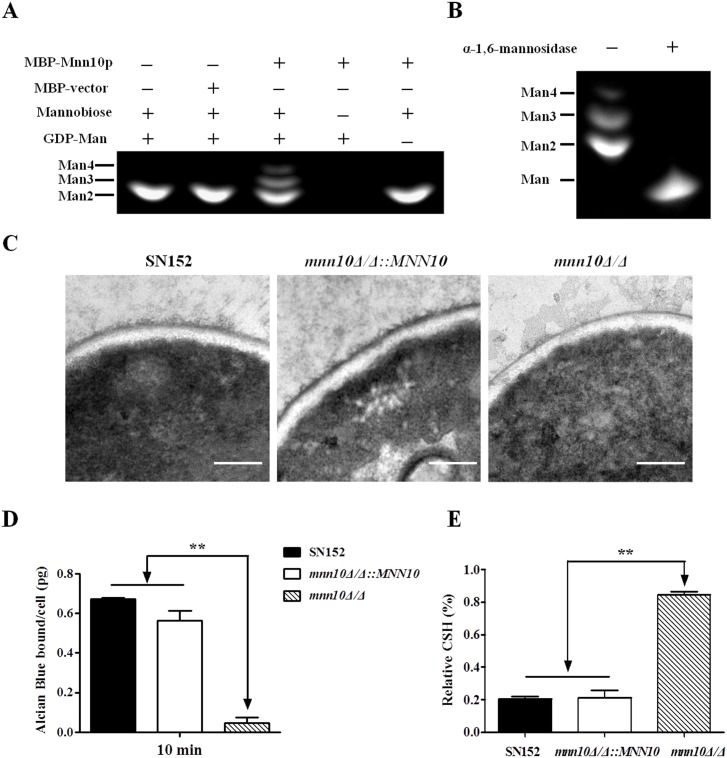
To analyze enzymatic activity, we used a bacterial expression system to produce MBP-fused Mnn10 protein from a pMAL-p5X vector and purified the protein by amylose magnetic beads (S1 Fig). The purified proteins were subjected to a mannosyltransferase assay system, which required α-1,6-linked mannobiose as an acceptor and GDP-mannose as a donor for proper mannosyltransferase activity [12, 34]. Our results indicated that Mnn10 catalyzes the transformation of GDP-mannose to α-1,6-mannobiose to form mannotriose and mannotetraose (Fig 1A). Consistently, the reaction products could be digested to mannose via α-1,6-mannosidase treatment (Fig 1B). Thus our results demonstrate that Mnn10 is able to transfer mannose from the donor onto the acceptor substrates to form α-1,6-linked oligomannose.
Fig 1. Mnn10 has mannosyltransferase activity and is required for α-1,6-mannose backbone length.
(A) Mannosyltransferase activity assay of Mnn10. The reaction of expressed MBP-fused Mnn10 protein or MBP protein incubated with α-1,6-mannobiose (Man 2), GDP-mannose (GDP-Man) or controls. The reaction products were labeled with ANTS and separated by fluorophore-assisted carbohydrate gel electrophoresis (FACE). Man 3, mannotriose; Man 4, mannotetraose. (B) α-1,6-mannose assay. The reaction products of (A) were treated with or without α-1,6-mannosidase treatment, and then subjected to FACE. (C) Representative cell wall ultrastructures of SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ strains were observed by transmission electron microscopy. The scale bar represents 0.2 μm. (D) Alcian Blue binding assay. The cells were incubated with Alcian Blue for 10 min and the amount of dye bound to the cell wall were calculated. Data represent the mean amount of dye bound per cell ± SD from triplicates of one representative experiment of three. (E) Cell surface hydrophobicity of the indicated C. albicans strains was measured by water-hydrocarbon two-phase assay. Data are means ± SD of triplicates of one representative experiment of three. **, P < 0.01 [One-way ANOVA with Bonferroni post-test (D, E)].
To further confirm the role of MNN10 in α-1,6-mannose backbone extension, we generated mnn10Δ/Δ null mutant strain and mnn10Δ/Δ::MNN10 revertant strain using the homologous recombination method. The genotype was confirmed by PCR and the expression of MNN10 was determined by quantitative RT-PCR (S2 Fig). When compared to the parental strain SN152, transmission electron microscopy (TEM) analysis noted a significantly shortened external layer of mannan fibril surrounding the cell wall in mnn10Δ/Δ. Notably, MNN10 gene rescue was sufficient to restore the expected length of mannan fibril (Fig 1C).
The phosphomannan of C. albicans cell wall, characterized by Alcian Blue dye binding, is attached to the branched mannan from the α-1,6-mannose backbone [35]. Therefore, the content of phosphomannan reveals the length of α-1,6-mannose backbone. The Alcian Blue assay demonstrated significantly decreased binding of the dye to mnn10Δ/Δ as compared to the parental strain, which could be rescued by reintegration of MNN10 into mnn10Δ/Δ, confirming the role of MNN10 in α-1,6-mannose backbone extension (Fig 1D). Inhibition of α-1,6-mannose backbone extension via deletion of MNN10 also affected the surface property of C. albicans such as hydrophobicity (Fig 1E). Furthermore, the change of cell wall structures impaired the resistance of mnn10Δ/Δ to various stresses including calcofluor white, fluconazole, miconazole, and caspofungin (S3 Fig).
Taken together, our results suggest that Mnn10 protein possesses α-1,6-mannosyltransferase activity and is crucial in α-1,6-mannose backbone extension in C. albicans.
MNN10 is required for cell wall polysaccharides biosynthesis and organization in C. albicans
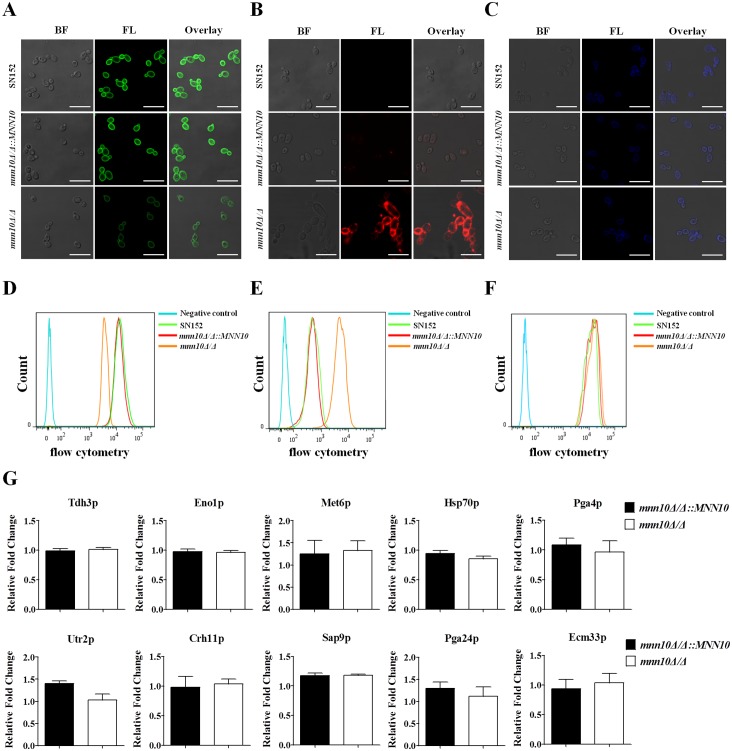
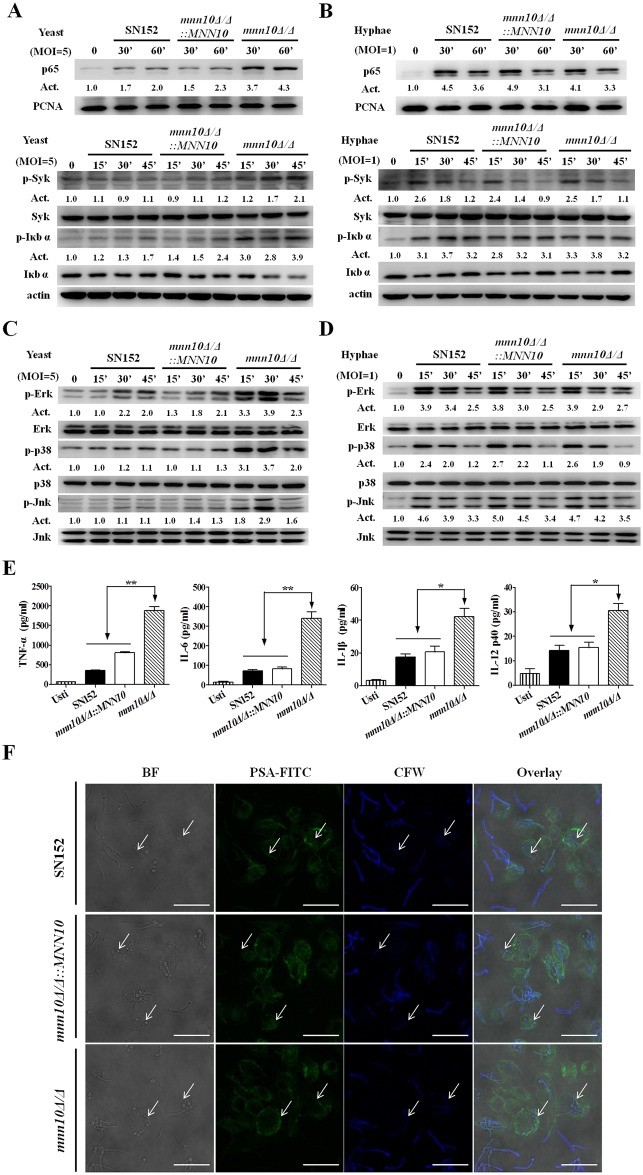
The cell wall polysaccharides of C. albicans consist of an inner layer of chitin and β-glucan, and an outer fibrillar layer of mannan. To examine the effects of MNN10 deletion on cell wall polysaccharides biosynthesis and organization, C. albicans yeasts or hyphae were stained with Concanavalin A (ConA), anti-β-glucan antibody and calcofluor white (CFW). We observed and quantified the polysaccharides by confocal laser scanning microscopy and flow cytometry, respectively. ConA staining indicated that mnn10 mutant, either in yeast cells or hyphae, had a markedly decreased fluorescence intensity which is suggested lower mannose content as compared to the parental or revertant strain (Figs 2A, 2D and S4A). β-(1,3)-glucan, a well-characterized PAMP of C. albicans, is buried underneath the outer layer of the cell wall. We found a remarkable exposure of β-(1,3)-glucan on the cell surface of mnn10 mutant (Figs 2B, 2E and S4B). However, no significant difference of CFW binding was visualized in mnn10 mutant strain, suggesting normal chitin content (Fig 2C and 2F).
Fig 2. Mnn10 is required for cell wall polysaccharides organization, but not virulence factors attachment.
Representative fluorescence micrographs of three cell wall carbohydrate layers from SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ, which were stained with ConA-FITC to visualise mannan (A), β-glucan antibody to visualize β-(1,3)-glucan (B) and calcofluor white to visualise chitin (C). Bright field (BF), fluorescence (FL), and overlay are shown individually. Scale bar represents 10 μm. The fluorescence intensity was quantified by flow cytometry [mannan (D), β-(1,3)-glucan (E), chitin (F)]. Data are representative of three independent experiments. (G) Relative fold change of representative virulence related cell wall proteins (CWPs) in mnn10 mutant compared to mnn10Δ/Δ::MNN10. The cell wall proteins of SN152, mnn10Δ/Δ::MNN10, and mnn10Δ/Δ strains were analyzed by LC-MS/MS on high-resolution instruments, respectively. Raw files were processed by Max Quant for peptide/protein identification and quantification. The relative data were changed to the ratio of mnn10Δ/Δ::MNN10 and mnn10Δ/Δ to SN152. Data are represented as means ± SEM of three independent experiments. P > 0.05 (Student’s t-test).
The highly glycosylated cell wall proteins (CWPs) of C. albicans often act as virulence factors and contribute to cell wall integrity, promote biofilm formation, mediate adherence to host cells, and promote invasion of epithelial layers [36–38]. To further evaluate the effect of α-1,6-mannose extension in C. albicans CWPs anchorage, we analyzed CWPs extraction by LC-MS/MS. Twenty representative CWPs were identified in the cell wall of the parental and mnn10 mutant strain (S1 Table). We found that MNN10 deletion had almost no effect on the anchorage of CWPs. Notably several of the proteins are considered to mediate specific roles in the development of invasion, such as cell-surface antigens involved in virulence (Tdh3p, Eno1p, Met6p, Hsp70p), proteins involved in cell wall biosynthesis and assembly (Pga4p, Utr2p, Crh11p), and proteins involved in adhesions and cell wall morphogenesis (Sap9p, Pga24p, Ecm33p) (Fig 2G).
Taken together, our results demonstrate that inhibition of α-1,6-mannose backbone extension by MNN10 deletion resulted in abnormal cell wall polysaccharides biosynthesis and organization, but had no effect on the anchorage of CWPs including virulence factors.
Inhibition of α-1,6-mannose backbone extension has no effect on the invasive capacity of C. albicans
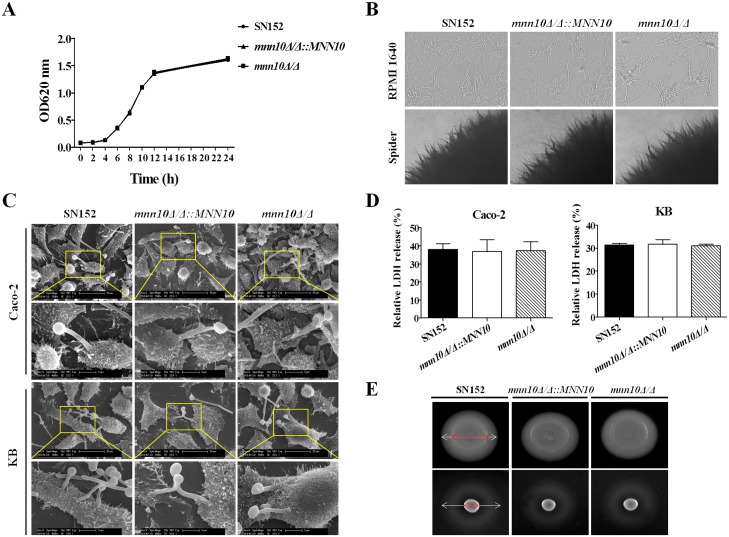
The composition and organization of the cell wall in C. albicans plays an important role in the initiation and maintenance of invasive infections. In the context of MNN10 deletion, we investigated the virulence of mnn10 mutant strain in vitro. Multiplication and yeast-to-hypha transition are the prerequisites of C. albicans invasive disease [39]. The growth curves obtained demonstrated that MNN10 did not impact the growth of C. albicans in vitro (Fig 3A). Moreover, mnn10 mutant also did not show defective filamentation in either liquid (RPMI1640, 10% serum liquid medium) or solid medium (Spider and Lee’s agar medium) favoring hyphal growth (Figs 3B and S5A).
Fig 3. Inhibition of α-1,6-mannose backbone extension in C. albicans does not affect its invasive capacity in vitro.
(A) Growth curves of SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ strains. (B) Representative photomicrographs of indicated C. albicans growing in liquid RPMI 1640 culture for 3 h at 37°C or on spider agar media for 5 days at 37°C to induce hyphal form. (C) Representative micrographs of scanning electron microscope of Caco-2 and KB cells invaded or penetrated by SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ after 2 h co-incubation (MOI = 1). (D) Epithelial cells damage was determined by assaying LDH release. Relative LDH release from Caco-2 or KB cells was measured after 12 h incubation with C. albicans (MOI = 0.1). Data are represented as means ± SD from triplicates of one representative experiment of three. P > 0.05 (One-way ANOVA with Bonferroni post-test). (E) Extracellular phospholipase and hemolytic activity assays. The phospholipase activity was examined by spotted C. albicans on egg yolk agar at 37°C for 3 days and observed the width of the zone of precipitation around each colony (top panel). The hemolytic activity was assessed by growing the indicated C. albicans strains on sugar-enriched sheep blood agar at 37°C for 3 days to observe the presence of a distinct translucent halo around the colonies (lower panel). Representative images are shown from one of three independent experiments.
Adhesion to the epithelium, such as oral and intestinal epithelial cells, and hyphal penetration are the first steps of C. albicans invasion [40]. Our results indicate that deletion of MNN10 did not affect the ability of C. albicans to adhere Caco-2 intestinal epithelial and KB buccal epithelial cells (P > 0.05, S5B Fig). Following adhesion, we used scanning electron microscopy (SEM) to evaluate the ability of hyphal penetration and invasion of mnn10 mutant strain. After a 2 h co-culture, we observed hyphal forms, of all the indicated strains, penetrated Caco-2 and KB cells at the apical face and microvillis attached to the hyphae at the point of penetration, indicating that MNN10 gene deletion had no effect on the invasion ability of C. albicans in vitro (Fig 3C). Moreover, no significant difference, in epithelial cell damage, was observed among strains (P > 0.05, Fig 3D).
Phospholipases and hemolysins secreted by C. albicans can also induce host cells damage [41, 42]. We screened extracellular phospholipase and hemolytic activity by growing C. albicans strains on either egg yolk agar or sheep blood agar. As compared to the parental and revertant strains, mnn10 mutant induced similar zones of precipitation or clearance around the colonies, indicating that MNN10 deletion was not significantly associated with phospholipases and hemolysins secretion (Fig 3E).
As such, our results suggest that MNN10 deletion did not affect the invasive capacity of C. albicans in vitro.
MNN10 gene is required for normal pathogenicity of C. albicans in a murine systemic candidiasis model
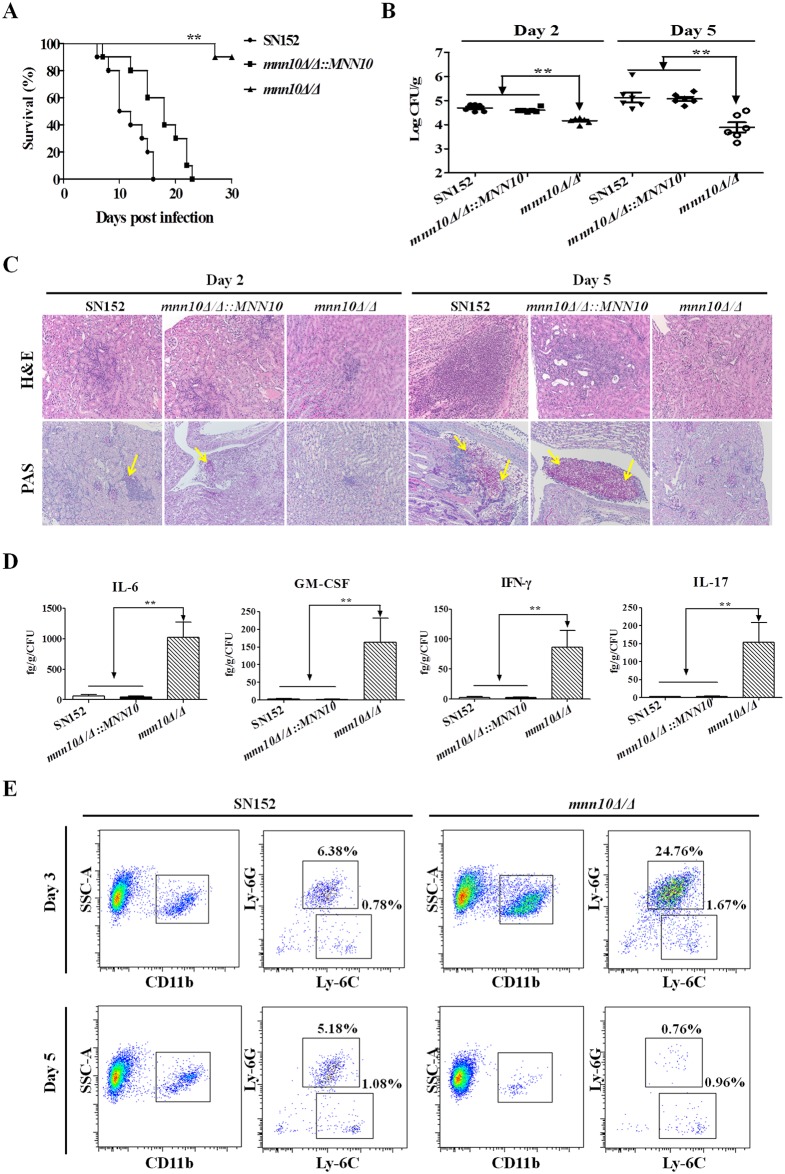
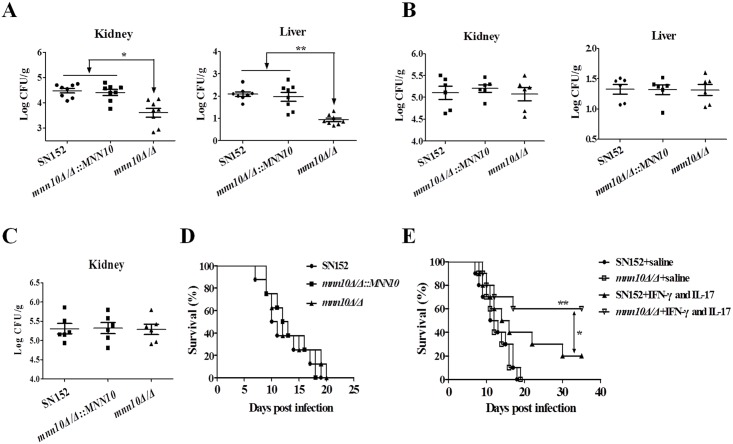
To examine the effects of α-1,6-mannose backbone of C. albicans on host infection in vivo, we compared the pathogenicity of SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ in a murine systemic candidiasis model. Mice infected with mnn10 mutant strain had a much higher survival rate than those infected with the parental or revertant strain at a lethal dose (5×105 CFU). Over a 30-day observation period, only one mouse infected with the mnn10 mutant strain died. By contrast, all of the mice infected with the parental or revertant strain died within 30 days (P < 0.01, Fig 4A). The median survival analysis demonstrated that the 50% survival limit was attained at 10 days for mice infected with the parental strain and 18 days for the revertant strain, respectively. At day 2 or day 5 post-infection, mice infected with mnn10 mutant had significantly lower fungal burdens in the kidneys and livers as compared to those infected with either the parental or revertant strain (P < 0.01 and P < 0.05, respectively; Figs 4B and S6A). Moreover, Hematoxylin and eosin (H&E) staining revealed that during prolonged infections, inflammatory influx and tissue necrosis of the kidneys were aggravated in mice infected with either parental or MNN10 revertant strains (Fig 4C, top panel). Periodic acid-Schiff (PAS) staining also identified more hyphae in the kidneys of parental or revertant strain infected mice as compared to the mnn10 mutant strain (Fig 4C, bottom panel). After normalized to organ CFU burden of infection, IL-6, GM-CSF, IFN-γ and IL-17 in the kidneys of mnn10 mutant infected mice were markedly higher than mice infected with parental or revertant strains (P < 0.01, Fig 4D) (the actual cytokine values were shown in S7 Fig).
Fig 4. Mnn10 is required for C. albicans systemic infection.
C57BL/6 mice were infected with 5×105 CFU SN152, mnn10Δ/Δ::MNN10 or mnn10Δ/Δ strain in 200 μl sterile saline via lateral tail vein. (A) Survival of C57BL/6 mice infected with the indicated strains was monitored for over 30 days (n = 10 per group). Data are representative of three independent experiments. (B) Quantification of the fungal burden in kidney tissues of C57BL/6 mice (n = 6 per group) infected with indicated C. albicans strains at day 2 and day 5. Data are representative of three independent experiments. (C) Representative H&E (for the inflammatory cells influx and the extent of tissue necrosis) and PAS (for C. albicans) staining of kidneys from C57BL/6 mice infected with indicated strains at day 2 and 5. Arrows indicate C. albicans filaments in the tissues. Magnification = 200 ×. (D) ELISA assays for IL-6, GM-CSF, IFN-γ and IL-17 in homogenized kidneys from C57BL/6 mice infected with indicated C. albicans at day 5 (n = 8 per group). The cytokine levels were normalized to burden of infection in each individually kidney as fg/g tissue/CFU. Data are means ± SD and are representative of three independent experiments. (E) The cellular inflammation in the kidneys of SN152 or mnn10Δ/Δ infected mice. SSChighCD11b+Ly-6C+Ly-6G+ neutrophils and SSChighCD11b+Ly-6C+Ly-6G- monocytes in the kidneys were detected at Day 3 and Day 5 by flow cytometry. Data are representative images of five mice. *, P < 0.05; **, P < 0.01 [Log-rank test (A) and Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test (B, D)].
Furthermore, we performed a flow cytometry analysis to detect cellular inflammation in the kidneys of infected mice. Time-course analysis revealed that mnn10Δ/Δ infected mice recruited more SSChighCD11b+Ly-6C+Ly-6G+ neutrophils and SSChighCD11b+Ly-6C+Ly-6G- monocytes in the kidney than mice infected with the SN152 strain at day 2 and day 3, respectively (Figs 4E and S8). The cellular inflammation in the kidneys of mnn10Δ/Δ infected mice reached a peak at day 3 post-infection, when compared to SN152 infected mice. With decreased fungal burden, neutrophils and monocytes were reduced in the kidney of mnn10Δ/Δ infected mice at day 5 post-infection as compared with SN152 infected mice (Fig 4E).
Taken together, the results suggest that inhibition of α-1,6-mannose extension by MNN10 deletion significantly impacted the pathogenesis of C. albicans by enhancing the host antifungal defense in vivo.
MNN10 deletion does not affect the pathogenicity of C. albicans in athymic nude mice
To further explore whether host antifungal defense was crucial for the clearance of mnn10 mutant in vivo, we investigated the pathogenicity of mnn10 mutant strain in BALB/c mice and athymic nude mice (BALB/c background). BALB/c mice infected with mnn10 mutant displayed significantly lower fungal burdens in the kidneys and livers as compared to those infected with either the parental or revertant strain (P < 0.05 and P < 0.01, respectively; Fig 5A). However, the results of kidneys and livers fungal burdens indicated mnn10 was not required for the C. albicans pathogenesis in athymic nude mice (Fig 5B). To expand upon these findings, longer time course and survival experiments were performed. At day 10 post-infection, no significant difference in the levels of fungal burden of kidneys was observed between mice infected with mnn10 mutant strain versus those infected with the parental or revertant strains (Fig 5C). Furthermore, there was no significant survival difference among these strains infected mice (Fig 5D).
Fig 5. Mnn10 is not required for C. albicans systemic infection in athymic nude mice.
BALB/c mice or athymic nude mice (BALB/c background) were infected with 3×105 CFU of SN152, mnn10Δ/Δ::MNN10 or mnn10Δ/Δ strain via lateral tail vein, respectively. The kidney and liver fungal burdens of BALB/c mice (n = 8 per group) (A) and athymic nude mice (n = 6 per group) (B) 5 days after infection. *, P < 0.05; **, P < 0.01 (Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test). (C) The kidney fungal burdens of athymic nude mice (n = 6 per group) 10 days after infection. (D) Survival of athymic nude mice infected with the indicated C. albicans (n = 8 per group). (E) Survival of SN152 and mnn10Δ/Δ infected athymic nude mice treated with combination of IFN-γ (100 ng) and IL-17 (100 ng) or the same volume of sterile saline. Data shown are representative of three independent experiments. **, P < 0.01 (mnn10Δ/Δ+IFN-γ and IL-17 versus mnn10Δ/Δ+saline); *, P < 0.05 (mnn10Δ/Δ+IFN-γ and IL-17 versus SN152+IFN-γ and IL-17) [Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test (A, B, C) and Log-rank test (D, E)].
To confirm the role of elevated IFN-γ and IL-17 in the infected mice (Fig 4D) in clearing mnn10 mutant strain, we performed an adoptive immunotherapy experiment. The combination of IFN-γ and IL-17 treatment significantly improved the survival of athymic nude mice infected with mnn10Δ/Δ (P < 0.01, Fig 5E). Moreover, mnn10 mutant infected mice treated with cytokines had a markedly higher survival rate than SN152 infected mice with the same treatment modality (P < 0.05, Fig 5E). Furthermore, we used neutralizing antibodies to IL-17 and/or IFN-γ to elucidate the importance of these cytokines in C57BL/6 mice infected with mnn10Δ/Δ. Compared with mice treated with isotype antibody rat IgG1, mice receiving anti-IFN-γ, anti-IL-17A, or both antibodies exhibited significantly higher fungal burdens in the kidneys and livers (S9 Fig) (P < 0.05). These results suggest that IFN-γ and IL-17 played an important role in the enhanced host defense against mnn10 mutant strain in vivo.
Inhibition of α-1,6-mannose backbone extension in C. albicans induces enhanced host innate immune response
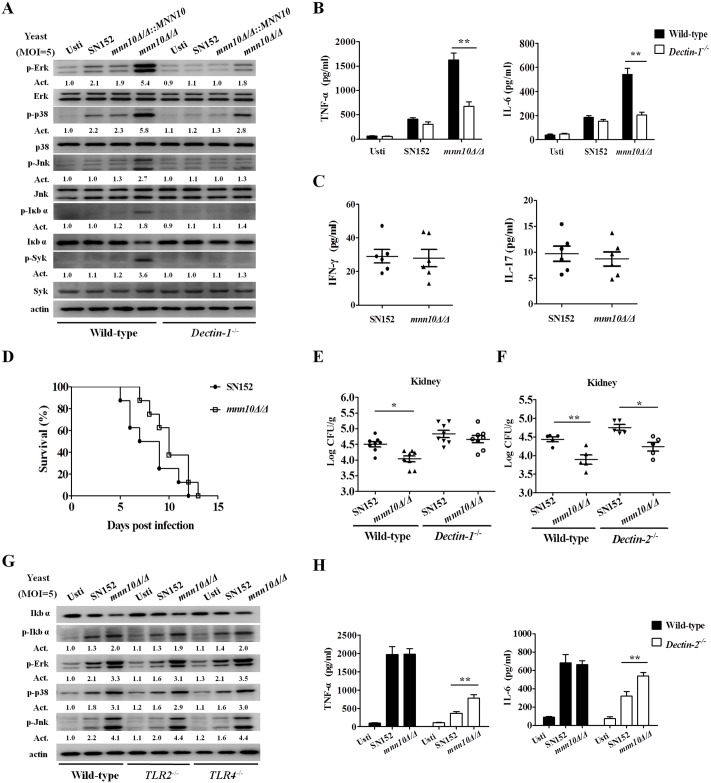
Our results suggested that the enhanced host antifungal immunity was the main factor that contributed to the diminished virulence of mnn10 mutant strain. Thus, we further investigated the myeloid cell recognition and response to mnn10 mutant using a macrophages-C. albicans interaction model. We found that mnn10 mutant yeast cells induced more nuclear translocation of NF-κB (p65), phosphorylation of Syk and IκBα, together with IκBα degradation in thioglycolate-elicited peritoneal macrophages (Fig 6A). Consistently, mnn10 mutant yeast cells also induced more ERK phosphorylation, p38 phosphorylation, and JNK phosphorylation in macrophages than the parent parental or revertant strains (Fig 6C). We also detected significantly higher levels of inflammatory cytokines including TNF-α, IL-6, IL-1β and IL-12p40 in macrophages induced by mnn10Δ/Δ yeast cells (Fig 6E). However, no differences in NF-κB and MAPK signaling activation were observed in macrophages stimulated by hyphal forms of the parental, mnn10 mutant and revertant strains, the same as the production of proinflammatory cytokines (Figs 6B, 6D and S10).
Fig 6. α-1,6-mannose backbone inhibition in C. albicans yeasts, but not hyphae, could induce an increased immune responses.
(A and B) Thioglycollate-elicited peritoneal macrophages were stimulated with UV-inactivated SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ yeasts (MOI = 5) (A) or hyphae (MOI = 1) (B) for the indicated times. The nuclear extracts (top panel) and total cell lysates (lower panel) were subjected to immunoblotting analysis with the indicated antibodies of NF-κB signaling. (C and D) Thioglycollate-elicited peritoneal macrophages were challenged with UV-inactivated SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ yeasts (MOI = 5) (C) or hyphae (MOI = 1) (D) for the indicated times. The total cell lysates were subjected to immunoblotting with the indicated antibodies of MAPK signaling. Numbers between blots indicate activity (Act) of phosphorylation of MAPK or NF-κB pathways, as measured by densitometry. (E) ELISA results for cytokines TNF-α, IL-6, IL-1β and IL-12p40 in cell supernatants of thioglycollate-elicited peritoneal macrophages, which were stimulated with the indicated C. albicans yeasts (MOI = 5) for 6 h. Usti, unstimulated. Data are means ± SD of triplicates from one representative experiment of three. Usti, unstimulated. *, P < 0.05; **, P < 0.01 (One-way ANOVA with Bonferroni post-test). (F) Phagocytosis of C. albicans by thioglycollate-elicited peritoneal macrophages. Live C. albicans was co-cultured with the macrophages grown on coverslips in multiwell plates for 90 min. After staining with CFW (1 μg/ml) and PSA-FITC (20 μg/ml) for 10 min, the samples were viewed by confocal laser scanning microscope directly. Scale bar represents 10 μm. Arrows indicate the internalized C. albicans cells inaccessible to staining with CFW. Bright field (BF), fluorescein isothiocyanate-conjugated pisum sativum agglutinin (PSA-FITC), calcofluor white (CFW) and overlay are shown individually.
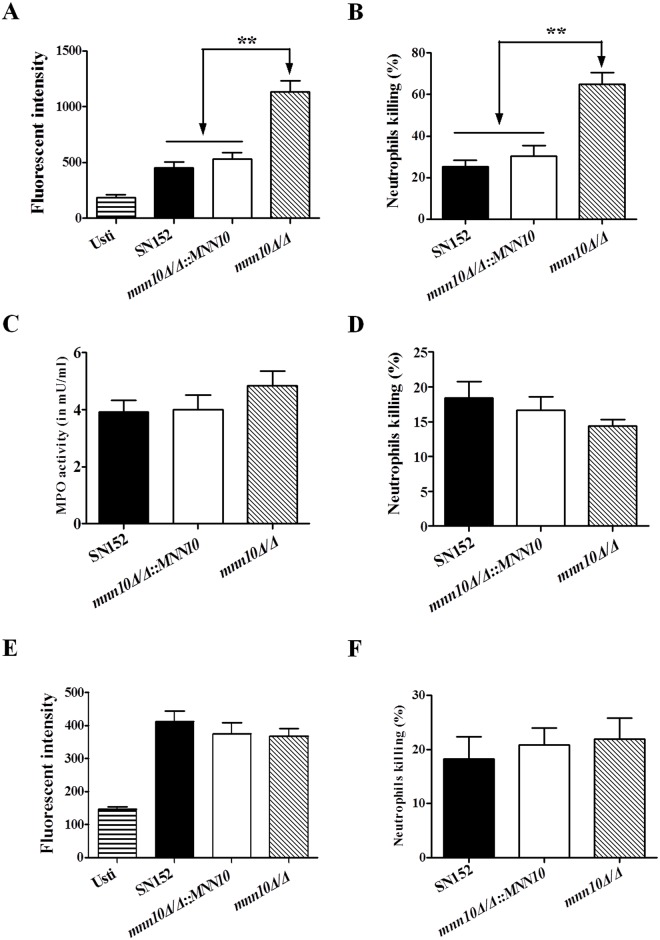
We performed a macrophage phagocytosis assay to investigate whether mnn10 mutant was differentially taken up from the parental or revertant strain. Our results suggest that no significant difference of the macrophage phagocytosis was visually appreciated between mnn10 mutant, and the parental or revertant strains in the initial infection stage (Figs 6F and S11). However, we found that thioglycollate-elicited peritoneal neutrophils could produce significantly more ROS and thus destroy mnn10Δ/Δ more efficiently (Fig 7A and 7B). While neutrophils can target pathogens in modalities such as myeloperoxidase (MPO), no significant difference in the intracellular MPO activity of neutrophils stimulated by mnn10 mutant versus parental or revertant strain was observed (Fig 7C). To determine whether the enhanced killing was dependent on ROS production, we scavenged ROS by 2 mM L-ascorbic acid in the co-culture medium, and found that the ability of neutrophils to eliminate mnn10Δ/Δ was diminished with decreased ROS (Fig 7D and 7E). However, we found that removal of mnn10 mutant hyphae by neutrophils was similar in parental or revertant strains (Fig 7F).
Fig 7. Neutrophils killed mnn10 mutant more efficiently with an augmented respiratory burst.
(A, E) Neutrophils respiratory burst assay. The cellular reactive oxygen species production of thioglycollate-elicited peritoneal neutrophils were measured after incubation with C. albicans for 1 h (MOI = 1) with (E) or without (A) 2 mM ascorbic acid. (B, D) Neutrophils killing assay. Neutrophils (6×105 cells) were incubated with 3×104 CFU viable C. albicans for 1 h with (D) or without (B) 2 mM ascorbic acid. Then the suspension was plated on SDA agar for 48 h to count live C. albicans colonies. (C) Neutrophils were incubated with the indicated C. albicans for 1 h (MOI = 1). The cellular level of MPO in neutrophils was measured after incubation with C. albicans. (F) Neutrophils (6×105 cells) were incubated with 3×104 CFU C. albicans hyphae for 1 h. Then the suspension was plated on SDA agar to count live C. albicans colonies. Data shown are means ± SD of triplicates from one representative experiment of three. Usti, unstimulated. **, P < 0.01 (One-way ANOVA with Bonferroni post-test).
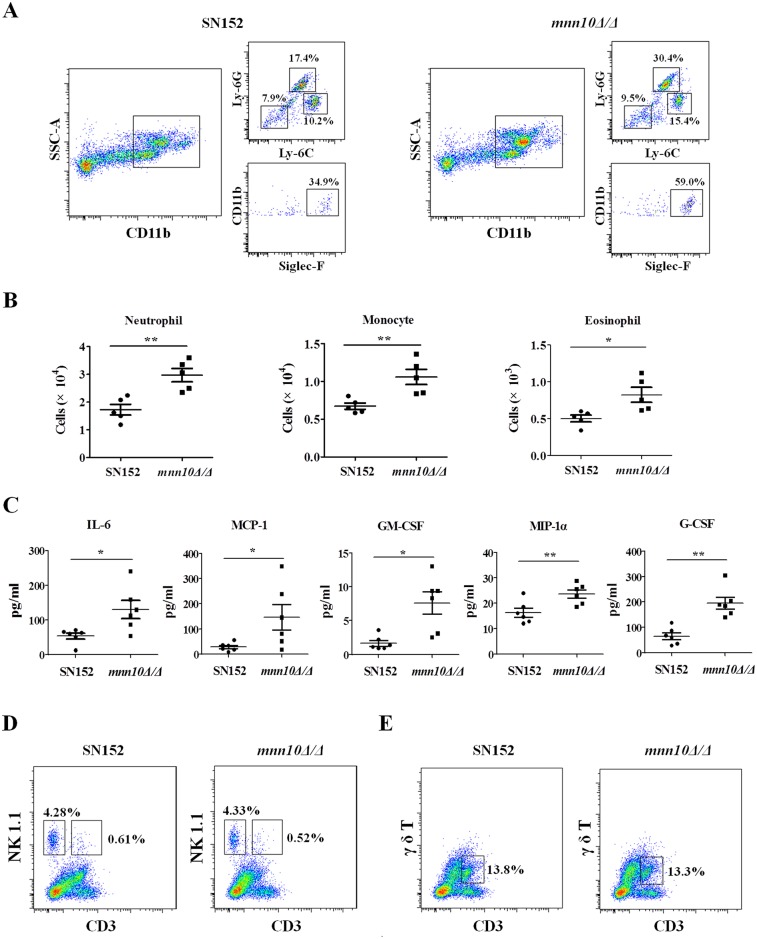
We further explored how enhanced leukocytes recognition of mnn10 mutant strain affected inflammation response in vivo using a peritoneal infection model. Mice were injected intraperitoneally with C. albicans, and flow cytometry performed 4 h later revealed that mnn10Δ/Δ infected mice recruited more inflammatory cells in the peritoneum than SN152 infected mice, including SSChighCD11b+Ly-6C+Ly-6G+ neutrophils, SSChighCD11b+Ly-6C+Ly-6G- monocytes and SSChighCD11b+Siglec-F+ eosinophils (Fig 8A and 8B). The enhanced inflammatory cell recruitment was also associated with increased production of specific cytokines and growth factors such as IL-6, MCP-1, MIP-1α, G-CSF and GM-CSF (Fig 8C). However, this analysis failed to reveal significant difference in the inflammatory cells, including CD3-NK1.1+ NK cells, CD3+NK1.1+ NKT cells, and CD3+γ/δ T+ cells, between mnn10Δ/Δ and SN152 infected mice (Fig 8D and 8E).
Fig 8. Mnn10 mutant stimulated enhanced inflammatory responses in vivo.
(A, B, C) C57BL/6 mice were intraperitoneal infected with 5×105 UV-inactivated C. albicans of SN152 or mnn10Δ/Δ strain for 4 h. (A) Flow cytometry for SSChighCD11b+Ly-6C+Ly-6G+ neutrophils, SSChighCD11b+Ly-6C+Ly-6G- monocytes and SSChighCD11b+Siglec-F+ eosinophils in the peritoneum. Data are representative images of five mice. (B) Scatter plots of myeloid cell subsets in the peritoneum cavities (n = 5 per group). (C) ELISA assays for cytokines, chemokines and growth factors in lavage fluid from the inflamed peritoneal cavities (n = 6 per group). Data are representative of three independent experiments. (D, E) C57BL/6 mice were intraperitoneal infected with 5×105 live SN152 or mnn10Δ/Δ strain for 2 days. CD3-NK1.1+ NK cells (D), CD3+NK1.1+ NKT cells (D), and CD3+γ/δ T+ cells (E) in the peritoneum were detected by flow cytometry. Data are representative images of five mice. MCP-1, chemokine CCL2; MIP-1α, chemokine CCL3; GM-CSF, granulocyte-monocyte colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor. *, P < 0.05; **, P < 0.01 [Mann-Whitney nonparametric t-test (B, C)].
Enhanced host antifungal immunity induced by mnn10 mutant is Dectin-1 dependent
Several PRRs, such as TLRs and CLRs, are involved in host defense during C. albicans infection. We hypothesized that the enhanced host immune response induced by mnn10 mutant may be attributed to the cell wall β-(1,3)-glucan exposure. We stimulated thioglycollate-elicited peritoneal macrophages from wild-type or Dectin-1-deficient mice with mnn10 mutant yeasts, and found that activation of NF-κB and MAPK signaling was defective in Dectin-1-deficient macrophages (Fig 9A). Consequently, the mnn10 mutant yeasts could not significantly increase the production of inflammatory cytokines such as TNF-α and IL-6 in Dectin-1-deficient macrophage cells (Fig 9B). The removal of mnn10 mutant by neutrophils from Dectin-1-deficient mice was similar in parental or revertant strains (S12A Fig). Moreover, no significant difference in the inflammatory cytokines, including IL-6, GM-CSF, IFN-γ and IL-17, were detected between the kidneys of mnn10Δ/Δ and SN152 infected Dectin-1-deficient mice (Figs 9C and S12B). The survival curves indicated that mnn10 mutant strain presented similar pathogenicity with the parental strain SN152 in Dectin-1-deficient mice (Fig 9D). Dectin-1-deficient mice had similar kidney or liver fungal burdens when infected with the parental SN152 or mnn10Δ/Δ strain (P > 0.05, Figs 9E and S6B).
Fig 9. Inflammatory responses, stimulated by C. albicans mnn10 mutant, were Dectin-1 dependent.
(A) Thioglycollate-elicited peritoneal macrophages from wild-type or Dectin-1-deficient mice were stimulated with UV-inactivated C. albicans yeast SN152, mnn10Δ/Δ::MNN10 or mnn10Δ/Δ (MOI = 5) for 30 minutes. The total cell lysates were then analyzed by immunoblotting with the indicated antibodies. Numbers between blots indicate activity (Act) of phosphorylation of MAPK or NF-κB pathways, as measured by densitometry. (B) Thioglycollate-elicited peritoneal macrophages from wild-type or Dectin-1-deficient mice were stimulated with the UV-inactivated C. albicans yeasts (MOI = 5) for 6 h. The amount of TNF-α and IL-6 in supernatants was determined by ELISA. Data are means ± SD of triplicates. (C) ELISA assays for IFN-γ and IL-17 in homogenized kidney from infected Dectin-1-deficient mice at day 5 (n = 6 per group). (D) Survival of Dectin-1-deficient mice infected with 5×105 CFU C. albicans of SN152 or mnn10Δ/Δ strain (n = 8 per group). (E and F) The kidneys fungal burden of Dectin-1-deficient mice (E) or Dectin-2-deficient mice (F) infected with 3×105 C. albicans at day 5. (G) Wild-type, TLR2-, and TLR4-deficient thioglycollate-elicited peritoneal macrophages were stimulated with UV-inactivated SN152 or mnn10Δ/Δ strain of C. albicans yeast cells (MOI = 5) for 30 min for preparing cell lysates. Samples were subjected to immunoblotting analysis using indicated antibodies. (H) Thioglycollate-elicited peritoneal macrophages from wild-type or Dectin-2-deficient mice were stimulated with the UV-inactivated C. albicans hyphae (MOI = 1) for 6 h. The amount of TNF-α and IL-6 in supernatants was determined using ELISA. Data shown are representative of three independent experiments. Usti, unstimulated. *, P < 0.05; **, P < 0.01 [Two-way ANOVA with Bonferroni post-test (B, H); Mann-Whitney nonparametric t-test (C); Log-rank test (D); Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test (E, F)].
However, our results demonstrated that other PRRs involved in antifungal immunity did not contribute to the enhanced immune responses elicited by mnn10 mutant. By example, mnn10 mutant strain presented similar pathogenicity in Dectin-2 deficient mice when compared to SN152 in the wild type mice (P < 0.05, Figs 9F and S6C). Dectin-2 deficiency had no effect on the inflammatory cytokines production such as TNF-α and IL-6 in macrophages, and TLR2 or TLR4 deficiency had no effect on the activation of NF-κB and MAPK signaling in macrophages when challenged with mnn10 mutant (Fig 9G and 9H).
Taken together, these data suggest that the enhanced immune response induced by α-1,6-mannose backbone inhibition in C. albicans was Dectin-1 dependent.
Discussion
During C. albicans infection, both yeast cells and hyphae can be found in infected organs or tissues, and innate immune cells discriminate them using different PRRs to elicit a protective immune response [43]. Previous studies have shown that the mannan structure of C. albicans plays an important role in the development of invasive infection. Here we first determined that the cell wall α-1,6-mannose backbone maintained the pathogenicity of C. albicans by preventing host, Dectin-1 mediated, recognition of β-(1,3)-glucan. These results highlight a previously unappreciated relationship between cell wall mannan structure and pathogenicity of C. albicans.
Several genes are involved in the biosynthesis of the cell wall mannan in C. albicans. Cell wall mannan structure mutant strains, induced by deletion of several genes including OCH1, MNN2 and MNN5, often represent a less pathogenic strain in vivo [9, 11, 12]. Herein, we determined that Mnn10 has α-1,6-mannosyltransferase activity, and is responsible for α-1,6-mannose backbone biosynthesis in C. albicans (Fig 1). We also highlighted the role of MNN10 in pathogenicity of C. albicans. Our studies using mnn10 null mutant strain demonstrated that MNN10 is required for C. albicans pathogenicity during a systemic candidiasis model in mice (Fig 4).
Infection is mediated by the interplay between a pathogen’s ability to invade host, versus the host attempts to recognize and destroy the pathogen. Several cell wall proteins on the surface of C. albicans act as virulence factors to invade host [39, 44]. Cell wall proteomic analysis indicated that MNN10 deletion had no effect on virulence factors of C. albicans (Fig 2G). Several steps, including adhesion to the epithelium, epithelium penetration and invasion by hyphae, vascular dissemination, and endothelial colonization, are involved in the development of invasive candidiasis [2]. However, the data from our study in vitro indicated that the mnn10 mutant was not defective in its invasive capacity (Fig 3). Therefore, we hypothesize enhanced immune recognition of the mutant strain by the host, rather than decreased virulence, contributed to the attenuated pathogenicity. The normal pathogenicity of mnn10 mutant in athymic nude mice (BALB/c background) further confirmed our hypothesis (Fig 5B, 5C and 5D). The difference of mnn10 mutant clearance in BALB/c mice versus athymic nude mice may be attributed to thymus, which is an important organ for the differentiation and maturation of T lymphocytes. Both Th1 and Th17 cells mediate host protection against C. albicans infection [19, 45]. IFN-γ and IL-17 are the key cytokines produced by Th1 and Th17 cells, which recruit neutrophils and macrophages to destroy the pathogen. IFN-γ and IL-17 elevation, and corresponding neutrophil responses were observed in the kidneys of mnn10 mutant infected mice, indicating that the mnn10 mutant could stimulate stronger antifungal response (Fig 4D and 4E). Although the source of IFN-γ and IL-17 can be from innate lymphocytes, our results suggest that elevated levels of IFN-γ and IL-17 elicited by mnn10 mutant in vivo are likely not derived from innate lymphocytes such as NK cells, NKT cells and γ/δ T cells (Fig 8D and 8E). Intracellular cytokine staining analysis revealed that mnn10Δ/Δ infected mice induced more IFN-γ-producing and IL-17A-producing α/β T cells than SN152 infected mice (S13 Fig). Therefore, our study suggested that inhibition of α-1,6-mannose backbone extension in C. albicans induced enhanced T lymphocyte mediated immune response in vivo.
The host innate immune cells involved in invading pathogens recognition are predominantly monocytes and neutrophils in circulation and macrophages in infected tissues. Inflammatory cytokines and chemokines can recruit innate immune cells to infected tissues. In a peritoneal infection model, we demonstrated that mnn10 mutant strain could recruit more neutrophils and monocytes by inducing cytokines and chemokines including IL-6, MCP-1, GM-CSF, MIP-1α and G-CSF in the peritoneal cavity (Fig 8A, 8B and 8C). IL-6 and G-CSF can promote neutrophil production and activation against C. albicans infection [46]. GM-CSF and MIP-1α are involved in potentiating neutrophil functions and maturation [47, 48]. MCP-1 is a crucial mediator to recruit monocytes in inflammation in vivo [49]. Neutrophils contribute to the initial step to kill fungi, and are especially important in neutropenic and immunosuppressed individuals [50]. Our data also determined that neutrophils mainly eliminated mnn10 mutant strain in a ROS-dependent manner (Fig 7). These results suggest that inhibition of C. albicans α-1,6-mannose backbone extension by MNN10 deletion could enhance host innate immune recognition.
Immune recognition could render antigen presenting cells competent to prime T cells, and thereby drive the adaptive Th1 and Th17 immune response. After encountering pathogens, the host macrophages secrete several cytokines, leading to the induction of Th cell differentiation [3]. Our study suggests that C. albicans mnn10 did not play an important role in the initial phagocytosis stage of macrophages (Figs 6F and S11). By contrast, mnn10 mutant yeast of S. cerevisiae was poorly taken up by primary macrophages, as compared to the parental strain [51]. We hypothesize that the differential macrophage phagocytosis of mnn10 mutant might be attributed to the pathogenicity and immunogenicity differences between C. albicans and S. cerevisiae. However, we demonstrated that mnn10 mutant strain elicited enhanced recognition by macrophages. The increased cellular responses of macrophages were associated with NF-κB and MAPK pathway activation, and inflammatory cytokine productions including TNF-α, IL-6, IL-1β and IL-12p40 (Fig 6A, 6C and 6E). TNF-α was involved in the innate immune response against Candida infection through promotion of neutrophil production and activation [52]. IL-6 and IL-23 (consisting of IL-12p40 and p19) contributed to Th17 differentiation induced by C. albicans and Staphylococcus aureus, and IL-1β was essential pro-inflammatory regulators of Th17 cells both at priming and effect phase [53]. Therefore, we suggest that the enhanced recognition by innate immune cells could promote Th cell response and thus contributes to host clearance of mnn10 mutant strain in vivo.
The special PAMPs on the surface of C. albicans could be recognized by PRRs of innate immune cells to initiate the host immunity. The skeletal component of C. albicans cell wall is based on a core structure of β-(1,3)-glucan that is covalently linked to β-(1,6)-glucan, chitin, and an outer layer of mannoproteins [3]. Recognition of β-(1,3)-glucan by Dectin-1 has been reported to be important in host antifungal defense [22]. However, β-(1,3)-glucan on the surface of C. albicans was normally shielded by the outer mannan layer from being recognized by Dectin-1 on innate immune cells [2, 27]. Deletion of certain C. albicans genes, such as the phospholipids phosphatidylserine synthase gene CHO1 and the histidine kinase gene CHK1, could unmask β-glucans of C. albicans, specifically recognized by Dectin-1 and leading to more host immune responses [30, 31]. Antifungal compounds, such as caspofungin and gepinacin, can also cause the exposure of β-(1,3)-glucan in C. albicans and elicit a stronger host immune response [29, 54]. Our results demonstrate that inhibition of α-1,6-mannose backbone extension by MNN10 deletion could unmask the concealed β-(1,3)-glucan in either yeast or hyphal form (Figs 2B, 2E and S4B). The exposure of β-(1,3)-glucan may be due to the fact that the outer structure of cell surface do not adequately conceal the inner layer. The enhanced inflammatory responses stimulated by mnn10 mutant, including inflammatory signaling activation and cytokine secretion, were markedly down-regulated in Dectin-1-deficient macrophages, suggesting that they were Dectin-1 dependent (Fig 9A and 9B). Moreover, our study indicates that mnn10 mutant restored its pathogenicity in Dectin-1-deficient mice, further confirming our hypothesis (Fig 9D and 9E). While mnn10 mutant strain also stimulated enhanced response of macrophage cells from TLR-2, TLR-4 deficient mice or showed less pathogenicity in Dectin-2 deficient mice, indicating the enhanced inflammatory responses induced by mnn10 mutant strain had little associations with other PRRs involved in host antifungal defense (Fig 9F, 9G and 9H). We concluded that enhanced Dectin-1 dependent immune recognition of mnn10 mutant strain could induce increased inflammatory cytokines secretion in antigen presenting cells such as macrophages, which further regulated Th1 and Th17 cell differentiation. IFN-γ secreted from Th1 lymphocytes and IL-17 secreted from Th17 lymphocytes ultimately mediated the clearance of mnn10 mutant strain in vivo.
Previous studies have reported that deficiency of Och1 or Mnn2 family involved in mannan biosynthesis of C. albicans could also lead to β-glucan exposure and decreased mannan [9, 11]. However, Och1 and Mnn2 mutant elicited a host reduced immune response. As we know, PRRs can bind to short oligosaccharides and the precise carbohydrate epitopes to elicit antifungal immunity. We believe these discrepancies may be attributed to the differential effect of these genes on the mannan structure and the precise exposed carbohydrate epitopes of β-glucan. In addition, these previous studies concluded that decreased mannan, rather than β-glucan exposure, was the major PAMP recognized by host immune system in Och1 and Mnn2 mutant [9, 11]. The present study not only indicates that deletion of MNN10 in C. albicans results in decreased mannose content and a more remarkable exposure of β-(1,3)-glucan on the cell surface, but also suggests that β-(1,3)-glucan of mnn10 mutant strain is the major PAMP and induced enhanced Dectin-1 dependent immune response.
In conclusion, we first identified that Mnn10 as an α-1,6-mannosyltransferase, which is involved in the cell wall α-1,6-mannose backbone extension and maintained pathogenicity of C. albicans by evading host Dectin-1 mediated antifungal immunity. In addition, there are no mammalian homologs of Mnn10 protein, thus our results provide a new potential antifungal therapeutic strategy for modulating the host immune response to C. albicans.
Materials and Methods
Ethics statement
All mouse experimental procedures were performed in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals approved by the State Council of People’s Republic of China. The protocol was approved by the Institutional Animal Care and Use Committee of Tongji University (Permit Number: TJLAC-014-013).
Mice
Female C57BL/6 mice, BALB/c nude mice and athymic nude mice (BALB/c background) were obtained from Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. TLR2 deficient and TLR4 deficient mice (C57BL/6 background) were purchased from Shanghai Biomodel Organism Science & Technology Development Company. Dectin-1-deficient (Clec7a -/-) mice were kindly provided by Dr. Gordon D. Brown (the mice were backcrossed for nine generations on the C57BL/6 background) and Dectin-2-deficient (Clec4n -/-) mice were kindly provided by Dr. Yoichiro Iwakura (C57BL/6 background) [55, 56].
Cell lines, reagents and antibodies
KB buccal epithelial carcinoma cell line and Caco-2 intestinal epithelial cell line were purchased from BIOK&KM. Alcian Blue, ANTS, HF-pyridine, percoll, calcofluor white (CFW), fluorescein isothiocyanate-conjugated pisum sativum agglutinin (PSA-FITC) and dihydrorhodamine 123 were purchased from Sigma-Aldrich. Antibodies against phospho-ERK, p38, phospho-p38, JNK, phospho-JNK, phospho-IκBα, Syk, phospho-Syk, p65, and PCNA were purchased from Cell Signaling Technologies. Antibodies against ERK, IκBα and β-actin were from Santa Cruz Biotechnology. Alexa-488-labeled and Cy3-labeled goat anti-mouse antibodies were purchased from Life Technologies. Antibody against β-(1,3)-glucan was purchased from Biosupplies Inc. The following antibodies, along with the appropriate isotype controls were used in flow cytometry: peridinin-chlorophyll-protein-complex anti-Ly-6C (clone HK1.4, Biolegend), Alexa Fluor 647 Siglec-F (clone E50-2440, BD Pharmingen), phycoerythrin-Cy7-conjugated anti-CD11b (clone M1/70, Biolegend), phycoerythrin-conjugated anti-Ly-6G (clone 1A8, Biolegend), fluorescein isothiocyanate-conjugated anti-CD3ε (clone 145-2C11, Biolegend), allophycocyanin anti-NK-1.1 (clone PK136, Biolegend) and allophycocyanin anti-TCR γ/δ (clone GL3, Biolegend).
Strain growth conditions and construction
All strains were maintained on SDA agar plates (1% peptone, 4% dextrose, and 1.8% agar) and grown in YPD broth (1% yeast extract, 2% peptone, and 2% dextrose) at 30°C. For hyphal growth, C. albicans yeast cells were cultured in RPMI 1640 medium [10.4 g RPMI 1640 (Gibco BRL), 34.5 g morpholinepropanesulfonic acid (Sigma), and 2.0 g NaHCO3, pH 7.0, in 1 liter double-distilled water sterilized by filtration] at 37°C for 3 h.
To construct MNN10 null mutant strain (mnn10Δ/Δ), the entire open reading frame of MNN10 was deleted from the parental strain SN152 by homologous recombination of auxotrophic markers HIS1 and LEU2 using a fusion-PCR-based strategy as previously described [57, 58]. To construct MNN10 revertant strain (mnn10Δ/Δ::MNN10), the fusion fragment containing MNN10 ORF and C. albicans SAT1-flipper cassette was transformed into mnn10Δ/Δ and the SAT marker was subsequently looped out as described previously [59]. All of the strains and the primers used in this study were listed in S2 and S3 Tables.
Expression and purification of Mnn10 protein
The non-transmembrane region of C. albicans MNN10 encoding amino acid residues 70 to 335 was cloned into pMAL-p5X (NEB) including MBP tag. And then the plasmid was transformed into BL21 (DE3) pLysS cells for expressing MBP-fused Mnn10 protein. The transformants were cultured overnight at 37°C and diluted 1:100 in fresh LB culture. When the medium OD600 was up to 0.6 at 37°C, IPTG at a final concentration of 0.1 mM was added and the cells were grown overnight at 16°C. MBP-fused Mnn10 protein was purified by amylose resin (NEB) according to the protocols as previously described [60]. The supernatant was passed through a 0.45 μm filter and bound to amylose resin by gravity flow. Unspecific proteins were washed off by applying 10 column volumes (CVs) of column buffer. The protein of interest was then eluted by elute buffer (column buffer added with 10 mM maltose). The eluate was finally dialyzed against buffer (25 mM Tris-HCl, pH7.5) for 2 h at room temperature.
α-1,6-Mannosyltransferase activity assay in vitro
The mannosyltransferase activity assay was performed according to the method as described previously [61, 62]. The purified MBP-fused Mnn10 protein (8 μg) was subjected to the buffer (10 mM MnCl2, 50 mM HEPES, 10 mM α-1,6-D-mannobiose and 5 mM GDP-D-Mannose, pH 7.2). The α-1,6-mannose extended reaction was incubated at 30°C for 1 h. After labeled with 0.75 μmol 8-Aminonaphthalene-1,3,6-trisulfonic acid (ANTS), the products were separated by electrophoresis on 40% polyacrylamide gels and detected by UV light on a transilluminator (3500 R, Tanon). The reaction products were then digested with α-1,6-mannosidase (NEB) overnight at 37°C according to manufacturer’s instructions, and analyzed by FACE.
Alcian Blue binding assay
The Alcian Blue binding assay was carried out as described previously [35]. 1.5×107 exponentially growing C. albicans cells were washed with 0.02 M HCl, and then incubated with 30 μg/ml Alcian Blue for 10 min at room temperature. The supernatant was measured at OD600 and the concentration of Alcian Blue was determined by reference to a standard curve. The amount of dye bound to C. albicans cells were calculated by subtracting the amount of dye in the supernatant.
Cell surface hydrophobicity
Exponentially growing C. albicans cells were washed and resuspended in PBS buffer (OD600 = 1.0), and 0.75 ml cyclohexane was then added to the above 3 ml cell suspension. The mixtures were vortexed for 3 min and settled for 20 min at room temperature, the OD600 of the aqueous phase was measured. The relative hydrophobicity was measured as [(OD600 of the control minus OD600 after octane overlay)/OD600 of the control] × 100% [63].
Isolation and analysis of cell wall proteins (CWPs)
The covalent and non-covalent CWPs were isolated as previously described [64, 65]. The major types of covalently linked CWPs are glycosylphosphatidylinositol anchored proteins (GPI-APs) and proteins with internal repeats (Pir proteins). GPI-CWPs were released by resuspending the cell wall debris in undiluted HF-pyridine and incubated at 0°C for 3 h. Pir proteins were specifically released by incubating cell wall debris with 30 mM NaOH at 4°C for 16 h. The non-covalent CWPs of C. albicans were extracted by SDS buffer (50 mM Tris-HCl, 2% SDS, 100 mM EDTA, and 10 mM DTT, pH 8.0). The whole CWPs were then mixed and further digested by trypsin for the analysis of LC-MS/MS on high-resolution instruments (LTQ-Orbitrap XL and Velos, Thermo Fisher). Raw files were processed by MaxQuant soft for peptide/protein identification and quantification.
Confocal laser scanning microscopy
To stain β-(1,3)-glucan of the cell wall, exponentially growing C. albicans yeast cells were washed in PBS (for hyphal form assays, 1×106 C. albicans cells were cultured in RPMI 1640 medium at 37°C for 3 h on a microscope slide in a six-well plate), and then incubated with anti-β-(1,3)-glucan antibody overnight at 4°C and then stained by Cy3-labeled antibody for 1 h at 30°C. To stain mannan and chitin of the cell wall, C. albicans yeast cells or hyphae were washed in PBS and incubated in the dark with 50 μg/ml ConA to stain for α-mannopyranosyl or 30 μg/ml CFW for chitin for 30 min. The above stained cells were washed and scanned at 63 × magnification with confocal laser scanning microscope (TCS SP5; Leica). Micrograph pictures were then acquired and analyzed by LAS AF Lite program.
Transmission electron microscopy
5×107 exponentially growing C. albicans cells were washed in PBS and then fixed in 4 ml fixative solution (3% paraformaldehyde, 3.6% glutaraldehyde, pH 7.2) for 24 h at 4°C. After post-fixation of samples with 1% phosphotungstic acid for 2 h, they were washed by distilled water, block-stained with uranyl acetate, dehydrated in alcohol, immersed in propylenoxide, and embedded in glycide-ether. Ultrathin sections were observed under a transmission electron microscope (Hitachi H-800, Japan) at 120 kV.
Assessment of virulence in vitro
To measure the growth curve of C. albicans, exponentially growing cells were washed and resuspended in fresh YPD broth (OD600 = 0.1), and then the optical density was determined at the indicated time point. To observe the hyphal growth, C. albicans cells were sub-cultured at 37°C in either RPMI 1640 medium plus 10% (vol/vol) heat-inactivated fetal calf serum (FCS) or spider solid medium.
Phospholipase activity and hemolytic activity of C. albicans strains were screened as described previously [66, 67]. Briefly, the suspension of yeast cells were spotted on egg yolk agar or sugar-enriched sheep blood agar and incubated at 37°C for 3 days. The phospholipase activity of each strain was observed by measuring the width of zone of precipitation around the colony. The presence of a distinct translucent halo around the colony indicated positive hemolytic activity.
C. albicans invasion and epithelial cell damage assays
The Caco-2 or KB cells were grown as approximate 80%-90% confluent monolayer in MEM medium with 20% (vol/vol) heat-inactivated FCS. For SEM, the cells were grown on 8 mm diameter glass coverslips. Each coverslip was infected with 1×106 live C. albicans yeast cells. After 2 h of infection, the cells were gently washed with PBS prior to 1% OsO4 and then examined using a XL-30 scanning electron microscope (Philips, Holland) as described previously [68].
For the cell damage assay, 80%-90% confluent monolayer of Caco-2 or KB cells was infected with 1×105 live C. albicans yeast cells for 12 h, respectively. Lactate dehydrogenase (LDH) in the medium released from control or infected epithelial cells was determined by LDH Assay kit (Beyotime, China) according to the manufacturer’s instructions. Maximal LDH release was obtained by adding 0.1 ml of 1% Triton X-100 to each well and vigorously disrupting the epithelial layers 1 h before the end of incubation period. The relative LDH activity was measured as [(OD490 of infected cells minus OD490 of the control)/(OD490 of maximal LDH release minus OD490 of the control)] × 100%.
Thioglycollate-elicited peritoneal macrophages and neutrophils preparation
Thioglycollate-elicited peritoneal macrophages and neutrophils were isolated as previously described [69]. Briefly, C57BL/6 mice were injected intraperitoneally with 2 ml 3% (wt/vol) thioglycollate (Merck). Peritoneal cells were collected by washing with PBS containing 0.5 mM EDTA 14 h later and 72 h later, for neutrophils and macrophages isolation, respectively. The cells were cultured in RPMI1640 plus 10% (vol/vol) heat-inactivated FCS.
Neutrophils killing assay in vitro
The killing assay was carried out as described previously [70]. Thioglycollate-elicited peritoneal neutrophils were mixed with live C. albicans [multiplicity of infection (MOI) = 1:20] in a 24-well plate, and were kept for 1 h at 4°C to settle the cells before being transferred to 37°C for another 1 h. Control plates were kept in parallel at 4°C during the incubation. Then the cells were mixed and plated on SDA agar for counting live C. albicans colonies for 48 h at 30°C.
For analysis of reactive oxygen species (ROS), the inflammatory cells were co-cultured with C. albicans (MOI = 1) in RPMI medium containing 10 μM dihydrorhodamine 123 for 1 h at 37°C. After incubation the fluorescent intensity of the oxidized dihydrorhodamine 123 was measured by a multi-mode microplate reader (excitation wavelength, 485 nm; emission wavelength, 538 nm). Cells loaded with dihydrorhodamine 123 but not treated with C. albicans were used to assess background of ROS production. For analysis of myeloperoxidase (MPO), the neutrophils were lysed by 1% Triton X-100 for 10 min and the MPO activity in neutrophil lysates was measured using an enzyme assay as described previously [71].
Macrophage-C. albicans interaction
C. albicans yeast cells or hyphae were exposed to four doses of 100,000 μjoules/cm2 in a CL-1000 Ultraviolet Crosslinker (UVP), with agitation between each dose to treat cells evenly [29]. The thioglycollate-elicited macrophages were stimulated with the UV-inactivated C. albicans yeasts (MOI = 5) or hyphae (MOI = 1) for the indicated time. Macrophage phagocytosis assay was performed as previously described, exponentially growing C. albicans cells were washed in PBS buffer and added to the monolayer macrophages (MOI = 5) at the indicated time (30 min, 60 min, 90 min and 120 min). CFW staining was performed for C. albicans and PSA-FITC staining was performed for macrophages. CFW/PSA-FITC stained samples were scanned immediately at 63 × magnification with confocal laser scanning microscope. Micrograph pictures were acquired and analyzed by LAS AF Lite program.
Western blotting
The cells were lysed in lysis buffer (250 mM NaCl, 50 mM HEPES, 1 mM EDTA, 1% NP-40, protease inhibitors, pH 7.4) for total cell lysates. For nuclear extracts, cells were lysed in lysis buffer (10 mM KCl, 10 mM HEPES, 0.1 mM EDTA, 0.4% NP-40, protease inhibitors, pH 7.9). The nuclear pellets were harvested, washed with the lysis buffer and resuspended in the extraction buffer (20 mM HEPES, 400 mM NaCl, 1 mM EDTA, protease inhibitors, pH 7.9), and then incubated with vortexing at 4°C for 30 minutes. The cell lysates were subjected to SDS-PAGE, blotted with the indicated primary antibodies and secondary antibodies, and then developed with the chemiluminescence method according to the manufacturer’s instructions (Millipore) using the ECL detection system (GE Healthcare). The densitometry of indicated blot was quantified using Image J software (National Institutes of Health, USA).
Cytokine production measurement
Concentrations of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-1β and IL-12p40 in cell culture supernatant, gamma interferon (IFN-γ) and IL-17 in tissue homogenates, IL-6, chemokines monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), and granulocyte and granulocyte-monocyte colony-stimulating factors (G-CSF/GM-CSF) in peritoneal lavage fluid, were measured with commercially available Ready-Set-Go cytokine kits (eBioscience) or cytokine multiplex kits (R&D systems) in triplicate times according to the manufacturer’s instructions.
Murine systemic candidiasis model
For the C. albicans infection in vivo, groups of C57BL/6 female mice or BALB/c female mice (6–8 weeks) were injected via lateral tail vein with 200 μl of a suspension containing indicated live C. albicans (5×105 cells for C57BL/6 mice and 3×105 cells for BALB/c mice) in sterile saline. Mice were monitored daily and were killed after 2 days or 5 days of infection. The kidneys and livers were removed, and then homogenized in 0.5 ml PBS for fungal burdens measurement or fixed in 10% neutral formalin for H&E and PAS staining. Supernatants of kidney and livers homogenates were harvested and stored at -80°C for the measurement of cytokine production.
Cellular inflammation assay in the kidney
C57BL/6 mice were injected with 5×105 CFU C. albicans and sacrificed at 12 h, 1 day, 2 days, 3 days, 4 days or 5 days post-infection and the kidneys were removed. The kidneys were minced into tissue pieces and digested for 1 h at 37°C. Then the digested tissues were passed through a 70-mm filter, washed and centrifuged in a 40%/70% percoll gradient for leukocytes isolation [72]. The leukocytes at the interphase were then analyzed by flow cytometry (BD FACS).
Murine peritoneal infection model
C57BL/6 mice were injected intraperitoneally with 5×105 CFU C. albicans and were killed after 4 h or 2 days. The peritoneal infiltrate was collected by lavage with ice-cold PBS containing 0.5 mM EDTA, and then the red blood cells were lysed. The inflammatory cells were counted and blocked with PBS containing 5% heat-inactivated FCS and 1 mM sodium azide at 4°C. The populations of the cells were analyzed by flow cytometry to determine the leukocyte composition as described before [22].
Statistical analysis
At least three biological replicates were performed for all experiments unless otherwise indicated. Log-rank test was used to evaluate the equality of survival curves. The two-tailed Student’s t-test was used for analysis of two groups and multiple groups were analyzed by one-way analysis of variance with Bonferroni post-tests. For analysis of nonparametrically distributed data, the Mann-Whitney test or Kruskal-Wallis test was used. Statistical significance was set at a p-value in the figures as: *, P < 0.05; **, P < 0.01.
Supporting Information
SDS-PAGE analysis of Mnn10 expressed in Escherichia coli. Lane 1, molecular weight markers; lane 2, the culture supernatant of total protein of E. coli strain; lane 3 and 4, the expressed and purified supernatant of E. coli strain transformed with an empty vector pMAL-p5X; lane 5 and 6, expression and purification of MBP-fused Mnn10 protein.
(TIF)
(A) Fusion-PCR-based cassette method for disruption of MNN10 in two-step homologous recombination and SAT1 flipper cassette for construction of the reconstituted strain. (B, D) PCR confirmation of MNN10 gene deletion and its revertant strain by genomic DNA. Genomic DNA from mnn10 mutant and revertant strains were PCR amplified with the oligonucleotides indicated at the bottom of the figure. (C) Quantitative real-time RT-PCR analysis of MNN10 in C. albicans paretal strain SN152 and revertant strain mnn10Δ/Δ::MNN10. Gene expression is indicated as a fold change relative to SN152. Data are means ± SD of triplicates from one representative experiment of three.
(TIF)
The parental strain SN152, mnn10 mutant and revertant strains were spotted in 10-fold dilutions onto YPD agar plates supplemented with the indicated stresses. Plates were incubated for 48 h at 30°C.
(TIF)
Fluorescence micrographs of the cell wall carbohydrate layers from hyphal form of SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ, which were stained with ConA-FITC to visualise mannan (A), β-glucan antibody to visualise β-glucan (B). Bright field (BF), fluorescence (FL), and overlay are shown individually. Scale bar represents 10 μm.
(TIF)
(A) Exponentially growing C. albicans cells were incubated in RPMI 1640 medium plus 10% (vol/vol) heat-inactivated fetal calf serum for 3 h, or grew on Lee’s agar media for 5 days at 37°C. Representative photographs were shown. (B) The adherence of C. albicans to Caco-2 or KB cells was evaluated by co-incubating for 1 h in six-well tissue culture plates, after which the adherent colonies were counted. Data represent mean (± SD) of triplicates from one representative experiment of three.
(TIF)
(A) Liver fungal burden of wild-type mice infected with 5×105 CFU of the indicated C. albicans strains at day 2 and day 5. (B) The liver fungal burden of wild-type or Dectin-1 deficient mice infected with 3×105 CFU of the indicated C. albicans strains at day 5. (C) The liver fungal burden of wild-type or Dectin-2 deficient mice infected with 3×105 CFU of the indicated C. albicans strains at day 5. Data shown are representative of three independent experiments. **, P < 0.01; *, P < 0.05 (Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test).
(TIF)
C57BL/6 mice were infected with 5×105 CFU of SN152, mnn10Δ/Δ::MNN10 or mnn10Δ/Δ strain via lateral tail vein at day 2 and day 5 (A top panel, and B) (n = 8 per group). The cytokine levels were normalized to burden of infection in each individually kidney as fg/g tissue/CFU (A, bottom panel). Data are means ± SD and are representative of three independent experiments. *, P < 0.05; **, P < 0.01 (Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test).
(TIF)
C57BL/6 mice were infected with 5×105 CFU of parental strain SN152 or mnn10 mutant strain via lateral tail vein. (A) SSChighCD11b+Ly-6C+Ly-6G+ neutrophils and SSChighCD11b+Ly-6C+Ly-6G- monocytes in the kidneys were detected at the indicated time by flow cytometry. Data are representative images of five mice. (B) The absolute number of neutrophils and monocytes cells in the kidneys of SN152 or mnn10 mutant strain infected mice (n = 5 per group). *, P < 0.05; **, P < 0.01 (Two-way ANOVA with Bonferroni post-test).
(TIF)
Mice (n = 6 per group) were treated with 500 μg of anti-IFN-γ (clone XMG1.2, BioLegend), 100 μg of anti-IL-17A (clone TC11-18H10.1, BioLegend), mixture of anti-IFN-γ and anti-IL-17A, or rat IgG1 per mouse 1 day before and at day 1 and 3 after injection of mnn10 mutant strain. *, P < 0.05 (Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test).
(TIF)
Thioglycollate-elicited peritoneal macrophages were stimulated with the indicated C. albicans hyphae (MOI = 1) for 6 h. Usti, unstimulated. Data are means ± SD of triplicates from one representative experiment of three.
(TIF)
Live C. albicans was added to the macrophages grown on coverslips in multiwell plates at the indicated time. After adding CFW (1 μg/ml) and PSA-FITC (20 μg/ml) to the culture medium for 10 min, the samples were viewed by confocal laser scanning microscope directly. Scale bar represents 10 μm. Arrows indicate the internalized C. albicans cells inaccessible to staining with CFW. Bright field (BF) and fluorescence (FL) are shown individually.
(TIF)
(A) The thioglycollate-elicited peritoneal neutrophils from Dectin-1 deficient mice (6×105 cells) were incubated with 3×104 CFU C. albicans for 1 h. Then the suspension was plated on SDA agar to count live C. albicans colonies. Data are means ± SD of triplicates. (B) ELISA assays for IL-6, GM-CSF in homogenized kidney from infected Dectin-1 deficient mice at day 5 (n = 6 per group). Data are representative of three independent experiments.
(TIF)
C57BL/6 mice were infected with 5×105 CFU of parental strain SN152 or mnn10 mutant strain via lateral tail vein (n = 5 per group). Intracellular cytokine IFN-γ and IL-17 from α/β or γ/δ T cells were analyzed after gated on CD3+ T cells, and intracellular cytokine signals from NK cells were analyzed after gated on CD3- T cells.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Dr. Gordon D. Brown (University of Aberdeen) for providing Dectin-1 KO mice and Dr. Yoichiro Iwakura (Tokyo University of Science) for Dectin-2 KO mice. We also thank Dr. Suzanne M. Noble (University of California-San Francisco) for providing C. albicans SN152, plasmid pSN40, pSN52 and Dr. Joachim Morschhauser (University of Wurzburg) for plasmid pSFS2A.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financially supported by the National Natural Science Foundation of China (No 81330083, www.nsfc.gov.cn/, YYJ); the National Natural Science Foundation of China (No 81471924, 81202563, www.nsfc.gov.cn/, MMA); the National Key Basic Research Program of China (No 2013CB531602, www.973.gov.cn/, YYJ); the Natural Science Foundation of Science and Technology Commission of Shanghai Municipality (No 13ZR1437900, www.stcsm.gov.cn/, XH); the Fundamental Research Funds for the Central Universities, MMA; the Shanghai Science and Technology Support Program (No 14401902200, www.stcsm.gov.cn/, MMA); the Shanghai Science and Technology Support Program (No 14431902200, www.stcsm.gov.cn/, YYJ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011; 11: 275–288. 10.1038/nri2939 [DOI] [PubMed] [Google Scholar]
- 2. Gow NA, van de Veerdonk FL, Brown AJ, and Netea MG. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011; 10: 112–122. 10.1038/nrmicro2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Netea MG, Brown GD, Kullberg BJ, Gow NA. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol. 2008; 6: 67–78. [DOI] [PubMed] [Google Scholar]
- 4. Leroy O, Gangneux JP, Montravers P, Mira JP, Gouin F, Sollet JP, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med. 2009; 37: 1612–1618. 10.1097/CCM.0b013e31819efac0 [DOI] [PubMed] [Google Scholar]
- 5. Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, et al. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents. 2014; 43: 78–81. 10.1016/j.ijantimicag.2013.09.005 [DOI] [PubMed] [Google Scholar]
- 6. Sganga G, Bianco G, Fiori B, Nure E, Spanu T, Lirosi MC, et al. Surveillance of bacterial and fungal infections in the postoperative period following liver transplantation: a series from 2005–2011. Transplant Proc. 2013; 45: 2718–2721. 10.1016/j.transproceed.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 7. Hall RA, Gow NA. Mannosylation in Candida albicans: role in cell wall function and immune recognition. Mol Microbiol. 2013; 90: 1147–1161. 10.1111/mmi.12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cambi A, Netea MG, Mora-Montes HM, Gow NA, Hato SV, Lowman DW, et al. Dendritic cell interaction with Candida albicans critically depends on N-linked mannan. J Biol Chem. 2008; 283: 20590–20599. 10.1074/jbc.M709334200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bates S, Hughes HB, Munro CA, Thomas WP, MacCallum DM, Bertram G, et al. Outer chain N-glycans are required for cell wall integrity and virulence of Candida albicans . J Biol Chem. 2006; 281: 90–98. [DOI] [PubMed] [Google Scholar]
- 10. Southard SB, Specht CA, Mishra C, Chen-Weiner J, Robbins PW. Molecular analysis of the Candida albicans homolog of Saccharomyces cerevisiae MNN9, required for glycosylation of cell wall mannoproteins. J Bacteriol. 1999; 181: 7439–7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hall RA, Bates S, Lenardon MD, Maccallum DM, Wagener J, Lowman DW, et al. The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans . PLoS Pathog. 2013; 9: e1003276 10.1371/journal.ppat.1003276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bai C, Xu XL, Chan FY, Lee RT, Wang Y. MNN5 encodes an iron-regulated alpha-1,2-Mannosyltransferase important for protein glycosylation, cell wall integrity, morphogenesis, and virulence in Candida albicans . Eukaryot Cell. 2006; 5: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bates S, Hall RA, Cheetham J, Netea MG, MacCallum DM, Brown AJ, et al. Role of the Candida albicans MNN1 gene family in cell wall structure and virulence. BMC Res Notes. 2013; 6: 294 10.1186/1756-0500-6-294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mille C, Bobrowicz P, Trinel PA, Li H, Maes E, Guerardel Y, et al. Identification of a new family of genes involved in beta-1,2-mannosylation of glycans in Pichia pastoris and Candida albicans . J Biol Chem. 2008; 283: 9724–9736. 10.1074/jbc.M708825200 [DOI] [PubMed] [Google Scholar]
- 15. Netea MG, Van Der Graaf CA, Vonk AG, Verschueren I, Van Der Meer JW, Kullberg BJ. The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis. 2002; 185: 1483–1489. [DOI] [PubMed] [Google Scholar]
- 16. Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol. 2011; 23: 467–472. 10.1093/intimm/dxr046 [DOI] [PubMed] [Google Scholar]
- 17. Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009; 459: 433–436. 10.1038/nature07965 [DOI] [PubMed] [Google Scholar]
- 18. Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011; 29: 1–21. 10.1146/annurev-immunol-030409-101229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans . Cell Host Microbe. 2012; 11: 425–435. 10.1016/j.chom.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moyes DL, Runglall M, Murciano C, Shen C, Nayar D, Thavaraj S, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe. 2010; 8: 225–235. 10.1016/j.chom.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol. 2009; 10: 203–213. 10.1038/ni.1692 [DOI] [PubMed] [Google Scholar]
- 22. Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007; 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002; 196: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lowman DW, Greene RR, Bearden DW, Kruppa MD, Pottier M, Monteiro MA, et al. Novel structural features in Candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae versus yeast. J Biol Chem. 2014; 289: 3432–3443. 10.1074/jbc.M113.529131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009; 361: 1760–1767. 10.1056/NEJMoa0901053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005; 24: 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wheeler RT, Kombe D, Agarwala SD, Fink GR. Dynamic, morphotype-specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 2008; 4: e1000227 10.1371/journal.ppat.1000227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gow NA, Netea MG, Munro CA, Ferwerda G, Bates S, Mora-Montes HM, et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J Infect Dis. 2007; 196: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wheeler RT, Fink GR. A drug-sensitive genetic network masks fungi from the immune system. PLoS Pathog. 2006; 2: e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis SE, Hopke A, Minkin SC Jr, Montedonico AE, Wheeler RT, Reynolds TB. Masking of β-(1,3)-glucan in the cell wall of Candida albicans from detection by innate immune cells depends on phosphatidylserine. Infect Immun. 2014; 82: 4405–4413. 10.1128/IAI.01612-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klippel N, Cui S, Groebe L, Bilitewski U. Deletion of the Candida albicans histidine kinase gene CHK1 improves recognition by phagocytes through an increased exposure of cell wall beta-1,3-glucans. Microbiology. 2010; 156: 3432–3444. 10.1099/mic.0.040006-0 [DOI] [PubMed] [Google Scholar]
- 32. Jungmann J, Rayner JC, Munro S. The Saccharomyces cerevisiae Protein Mnn10p/Bed1p Is a Subunit of a Golgi Mannosyltransferase Complex. J Biol Chem. 1999; 274: 6579–6585. [DOI] [PubMed] [Google Scholar]
- 33. Bartkeviciūte D, Sasnauskas K. Disruption of the MNN10 gene enhances protein secretion in Kluyveromyces lactis and Saccharomyces cerevisiae . FEMS Yeast Res. 2004; 4: 833–840. [DOI] [PubMed] [Google Scholar]
- 34. Wiggins CA, Munro S. Activity of the yeast MNN1 alpha-1,3-mannosyltransferase requires a motif conserved in many other families of glycosyltransferases. Proc Natl Acad Sci U S A. 1998; 95: 7945–7950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hobson RP, Munro CA, Bates S, MacCallum DM, Cutler JE, Heinsbroek SE, et al. Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J Biol Chem. 2004; 279: 39628–39635. [DOI] [PubMed] [Google Scholar]
- 36. Nobile CJ, Nett JE, Andes DR, and Mitchell AP. Function of Candida albicans adhesin Hwp1 in biofilm formation. Eukaryot Cell. 2006; 5: 1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almeida RS, Brunke S, Albrecht A, Thewes S, Laue M, Edwards JE, et al. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog. 2008; 4: e1000217 10.1371/journal.ppat.1000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun JN, Solis NV, Phan QT, Bajwa JS, Kashleva H, Thompson A, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog. 2010; 6: e1001181 10.1371/journal.ppat.1001181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011; 9: 737–748. 10.1038/nrmicro2636 [DOI] [PubMed] [Google Scholar]
- 40. Filler SG, Swerdloff JN, Hobbs C, Luckett PM. Penetration and damage of endothelial cells by Candida albicans . Infect Immun. 1995; 63: 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ibrahim AS, Mirbod F, Filler SG, Banno Y, Cole GT, Kitajima Y, et al. Evidence implicating phospholipase as a virulence factor of Candida albicans . Infect Immun. 1995; 63: 1993–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rajendran R, Robertson DP, Hodge PJ, Lappin DF, Ramage G. Hydrolytic enzyme production is associated with Candida albicans biofilm formation from patients with type 1 diabetes. Mycopathologia. 2010; 170: 229–235. 10.1007/s11046-010-9319-0 [DOI] [PubMed] [Google Scholar]
- 43. Jia XM, Tang B, Zhu LL, Liu YH, Zhao XQ, Gorjestani S, et al. CARD9 mediates Dectin-1-induced ERK activation by linking Ras-GRF1 to H-Ras for antifungal immunity. J Exp Med. 2014; 211: 2307–2321. 10.1084/jem.20132349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Howlett JA, Squier CA. Candida albicans ultrastructure: colonization and invasion of oral epithelium. Infect Immun. 1980; 29: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wüthrich M, Deepe GS Jr, Klein B. Adaptive Immunity to Fungi. Annu Rev Immunol. 2012; 30: 115–148. 10.1146/annurev-immunol-020711-074958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Basu S, Quilici C, Zhang HH, Grail D, Dunn AR. Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans . Growth Factors. 2008; 26: 23–34. 10.1080/08977190801987513 [DOI] [PubMed] [Google Scholar]
- 47. Weisbart RH, Kwan L, Golde DW, Gasson JC. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987; 69: 18–21. [PubMed] [Google Scholar]
- 48. Hachicha M, Rathanaswami P, Naccache PH, McColl SR. Regulation of chemokine gene expression in human peripheral blood neutrophils phagocytosing microbial pathogens. J Immunol. 1998; 160: 449–454. [PubMed] [Google Scholar]
- 49. Wenzel U, Schneider A, Valente AJ, Abboud HE, Thaiss F, Helmchen UM, et al. Monocyte chemoattractant protein-1 mediates monocyte/macrophage influx in anti-thymocyte antibody-induced glomerulonephritis. Kidney Int. 1997; 51: 770–776. [DOI] [PubMed] [Google Scholar]
- 50. Gazendam RP, van Hamme JL, Tool AT, van Houdt M, Verkuijlen PJ, Herbst M, et al. Two independent killing mechanisms of Candida albicans by human neutrophils: evidence from innate immunity defects. Blood. 2014; 124: 590–597. 10.1182/blood-2014-01-551473 [DOI] [PubMed] [Google Scholar]
- 51. Keppler-Ross S, Douglas L, Konopka JB, Dean N. Recognition of yeast by murine macrophages requires mannan but not glucan. Eukaryot Cell. 2010; 9: 1776–1787. 10.1128/EC.00156-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Netea MG, van Tits LJ, Curfs JH, Amiot F, Meis JF, van der Meer JW, et al. Increased susceptibility of TNF-alpha lymphotoxin-alpha double knockout mice to systemic candidiasis through impaired recruitment of neutrophils and phagocytosis of Candida albicans . J Immunol. 1999; 163: 1498–1505. [PubMed] [Google Scholar]
- 53. Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012; 484: 514–518. 10.1038/nature10957 [DOI] [PubMed] [Google Scholar]
- 54. McLellan CA, Whitesell L, King OD, Lancaster AK, Mazitschek R, Lindquist S. Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem Biol. 2012; 7: 1520–1528. [DOI] [PubMed] [Google Scholar]
- 55. Vautier S, Drummond RA, Redelinghuys P, Murray GI, MacCallum DM, Brown GD. Dectin-1 is not required for controlling Candida albicans colonization of the gastrointestinal tract. Infect Immun. 2012; 80: 4216–4222. 10.1128/IAI.00559-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, et al. Dectin-2 Recognition of a-Mannans and Induction of Th17 Cell Differentiation Is Essential for Host Defense against Candida albicans . Immunity. 2010; 32: 681–691. 10.1016/j.immuni.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 57. Yan L, Côte P, Li XX, Jiang YY, Whiteway M. PalI domain proteins of Saccharomyces cerevisiae and Candida albicans . Microbiol Res. 2012; 167: 422–432. 10.1016/j.micres.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 58. Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans . Eukaryot Cell. 2005; 4: 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reuss O, Vik A, Kolter R, Morschhäuser J. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans . Gene. 2004; 341: 119–127. [DOI] [PubMed] [Google Scholar]
- 60. Kellermann OK, Ferenci T. Maltose-binding protein from Escherichia coli. Methods Enzymol. 1982; 90 Pt E: 459–463. [DOI] [PubMed] [Google Scholar]
- 61. Jungmann J, Munro S. Multi-protein complexes in the cis Golgi of Saccharomyces cerevisiae with a-1,6-mannosyltransferase activity. EMBO J. 1998; 17: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Striebeck A, Robinson DA, Schüttelkopf AW, van Aalten DM. Yeast Mnn9 is both a priming glycosyltransferase and an allosteric activator of mannan biosynthesis. Open Biol. 2013; 3: 130022 10.1098/rsob.130022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Klotz SA, Drutz DJ, Zajic JE. Factors governing adherence of Candida species to plastic surfaces. Infect Immun. 1985; 50: 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Groot PW, de Boer AD, Cunningham J, Dekker HL, de Jong L, Hellingwerf KJ, et al. Proteomic analysis of Candida albicans cell walls reveals covalently bound carbohydrate-active enzymes and adhesins. Eukaryot Cell. 2004; 3: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Castillo L, Calvo E, Martínez AI, Ruiz-Herrera J, Valentín E, Lopez JA, et al. A study of the Candida albicans cell wall proteome. Proteomics. 2008; 8: 3871–3881. 10.1002/pmic.200800110 [DOI] [PubMed] [Google Scholar]
- 66. Samaranayake LP, Raeside JM, MacFarlane TW. Factors affecting the phospholipase activity of Candida species in vitro . Sabouraudia. 1984; 22: 201–207. [PubMed] [Google Scholar]
- 67. Luo G, Samaranayake LP, Yau JY. Candida species exhibit differential in vitro hemolytic activities. J Clin Microbiol. 2001; 39: 2971–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dalle F, Wächtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, et al. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol. 2010; 12: 248–271. 10.1111/j.1462-5822.2009.01394.x [DOI] [PubMed] [Google Scholar]
- 69. Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans . Nat Immunol. 2007; 8: 39–46. [DOI] [PubMed] [Google Scholar]
- 70. Svobodová E, Staib P, Losse J, Hennicke F, Barz D, Józsi M. Differential interaction of the two related fungal species Candida albicans and Candida dubliniensis with human neutrophils. J Immunol. 2012; 189: 2502–2511. 10.4049/jimmunol.1200185 [DOI] [PubMed] [Google Scholar]
- 71. Ermert D, Niemiec MJ, Röhm M, Glenthøj A, Borregaard N, Urban CF. Candida albicans escapes from mouse neutrophils. J Leukoc Biol. 2013; 94: 223–236. 10.1189/jlb.0213063 [DOI] [PubMed] [Google Scholar]
- 72. Huang J, Meng S, Hong S, Lin X, Jin W, Dong C. IL-17C is required for lethal inflammation during systemic fungal infection. Cell Mol Immunol. 2015; 13: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SDS-PAGE analysis of Mnn10 expressed in Escherichia coli. Lane 1, molecular weight markers; lane 2, the culture supernatant of total protein of E. coli strain; lane 3 and 4, the expressed and purified supernatant of E. coli strain transformed with an empty vector pMAL-p5X; lane 5 and 6, expression and purification of MBP-fused Mnn10 protein.
(TIF)
(A) Fusion-PCR-based cassette method for disruption of MNN10 in two-step homologous recombination and SAT1 flipper cassette for construction of the reconstituted strain. (B, D) PCR confirmation of MNN10 gene deletion and its revertant strain by genomic DNA. Genomic DNA from mnn10 mutant and revertant strains were PCR amplified with the oligonucleotides indicated at the bottom of the figure. (C) Quantitative real-time RT-PCR analysis of MNN10 in C. albicans paretal strain SN152 and revertant strain mnn10Δ/Δ::MNN10. Gene expression is indicated as a fold change relative to SN152. Data are means ± SD of triplicates from one representative experiment of three.
(TIF)
The parental strain SN152, mnn10 mutant and revertant strains were spotted in 10-fold dilutions onto YPD agar plates supplemented with the indicated stresses. Plates were incubated for 48 h at 30°C.
(TIF)
Fluorescence micrographs of the cell wall carbohydrate layers from hyphal form of SN152, mnn10Δ/Δ::MNN10 and mnn10Δ/Δ, which were stained with ConA-FITC to visualise mannan (A), β-glucan antibody to visualise β-glucan (B). Bright field (BF), fluorescence (FL), and overlay are shown individually. Scale bar represents 10 μm.
(TIF)
(A) Exponentially growing C. albicans cells were incubated in RPMI 1640 medium plus 10% (vol/vol) heat-inactivated fetal calf serum for 3 h, or grew on Lee’s agar media for 5 days at 37°C. Representative photographs were shown. (B) The adherence of C. albicans to Caco-2 or KB cells was evaluated by co-incubating for 1 h in six-well tissue culture plates, after which the adherent colonies were counted. Data represent mean (± SD) of triplicates from one representative experiment of three.
(TIF)
(A) Liver fungal burden of wild-type mice infected with 5×105 CFU of the indicated C. albicans strains at day 2 and day 5. (B) The liver fungal burden of wild-type or Dectin-1 deficient mice infected with 3×105 CFU of the indicated C. albicans strains at day 5. (C) The liver fungal burden of wild-type or Dectin-2 deficient mice infected with 3×105 CFU of the indicated C. albicans strains at day 5. Data shown are representative of three independent experiments. **, P < 0.01; *, P < 0.05 (Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test).
(TIF)
C57BL/6 mice were infected with 5×105 CFU of SN152, mnn10Δ/Δ::MNN10 or mnn10Δ/Δ strain via lateral tail vein at day 2 and day 5 (A top panel, and B) (n = 8 per group). The cytokine levels were normalized to burden of infection in each individually kidney as fg/g tissue/CFU (A, bottom panel). Data are means ± SD and are representative of three independent experiments. *, P < 0.05; **, P < 0.01 (Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test).
(TIF)
C57BL/6 mice were infected with 5×105 CFU of parental strain SN152 or mnn10 mutant strain via lateral tail vein. (A) SSChighCD11b+Ly-6C+Ly-6G+ neutrophils and SSChighCD11b+Ly-6C+Ly-6G- monocytes in the kidneys were detected at the indicated time by flow cytometry. Data are representative images of five mice. (B) The absolute number of neutrophils and monocytes cells in the kidneys of SN152 or mnn10 mutant strain infected mice (n = 5 per group). *, P < 0.05; **, P < 0.01 (Two-way ANOVA with Bonferroni post-test).
(TIF)
Mice (n = 6 per group) were treated with 500 μg of anti-IFN-γ (clone XMG1.2, BioLegend), 100 μg of anti-IL-17A (clone TC11-18H10.1, BioLegend), mixture of anti-IFN-γ and anti-IL-17A, or rat IgG1 per mouse 1 day before and at day 1 and 3 after injection of mnn10 mutant strain. *, P < 0.05 (Kruskal-Wallis nonparametric One-way ANOVA with Dunns post-test).
(TIF)
Thioglycollate-elicited peritoneal macrophages were stimulated with the indicated C. albicans hyphae (MOI = 1) for 6 h. Usti, unstimulated. Data are means ± SD of triplicates from one representative experiment of three.
(TIF)
Live C. albicans was added to the macrophages grown on coverslips in multiwell plates at the indicated time. After adding CFW (1 μg/ml) and PSA-FITC (20 μg/ml) to the culture medium for 10 min, the samples were viewed by confocal laser scanning microscope directly. Scale bar represents 10 μm. Arrows indicate the internalized C. albicans cells inaccessible to staining with CFW. Bright field (BF) and fluorescence (FL) are shown individually.
(TIF)
(A) The thioglycollate-elicited peritoneal neutrophils from Dectin-1 deficient mice (6×105 cells) were incubated with 3×104 CFU C. albicans for 1 h. Then the suspension was plated on SDA agar to count live C. albicans colonies. Data are means ± SD of triplicates. (B) ELISA assays for IL-6, GM-CSF in homogenized kidney from infected Dectin-1 deficient mice at day 5 (n = 6 per group). Data are representative of three independent experiments.
(TIF)
C57BL/6 mice were infected with 5×105 CFU of parental strain SN152 or mnn10 mutant strain via lateral tail vein (n = 5 per group). Intracellular cytokine IFN-γ and IL-17 from α/β or γ/δ T cells were analyzed after gated on CD3+ T cells, and intracellular cytokine signals from NK cells were analyzed after gated on CD3- T cells.
(TIF)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.