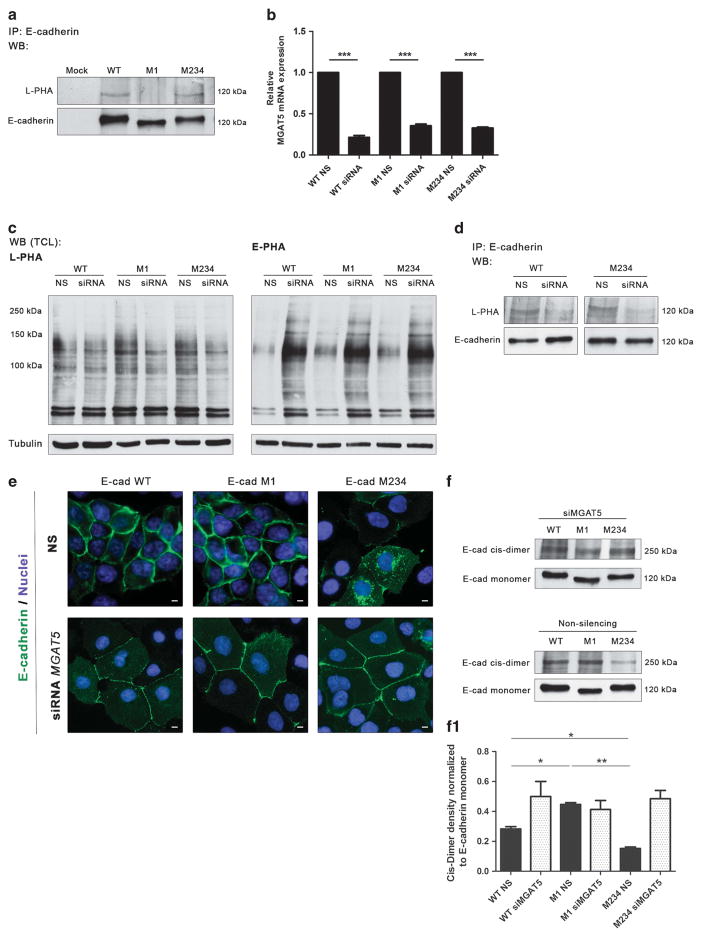
Figure 5.
Knockdown of GnT-V induces the recovery of E-cadherin expression and functions. (a) Immunoprecipitation of E-cadherin M234 showed an increased L-PHA reactivity compared with the M1 mutant, revealing a significant increase in β1,6 GlcNAc-branched N-glycan structures modification in comparison with E-cadherin M1. (b) MGAT5 mRNA expression of AGS cells expressing E-cadherin WT, M1 and M234 after MGAT5 silencing. Around 70–80% of MGAT5 silencing was observed in WT, M1 and M234 mutants. NS, non-silencing. The relative mRNA expression of siRNA cells expressing E-cadherin WT, M1 and M234 are expressed as the fold increase, compared with the respective non-silencing cells, which was taken as 1 (Student’s t-test: *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). (c) Lectin blot analysis of GnT-V and GnT-III products on total cell lysate from AGS cells expressing E-cadherin WT, M1 and M234 after MGAT5 knockdown. A decreased L-PHA reactivity after MGAT5 knockdown and a competitive increase of E-PHA reactivity were observed. (d) MGAT5 silencing resulted in a remarkable decreased L-PHA reactivity for the E-cadherin band from the E-cadherin WT and E-cadherin M234, suggesting that Asn-554 is occupied with β1,6 GlcNAc-branched N-glycans. (e) Immunofluorescence analysis of AGS cells expressing E-cadherin WT, M1 and M234 after MGAT5 knockdown. After MGAT5 silencing, E-cadherin M234 showed an increased localization at the cell–cell membrane. White size bars ~ 5 μm. (f) Evaluation of the impact of MGAT5 silencing on cis-dimer formation of E-cadherin. Knockdown of MGAT5 leads to a similar ratio of E-cadherin cis-dimer formation in AGS cells expressing E-cadherin M1 and M234. (f1) Bar graphs. Amounts of E-cadherin cis-dimer were determined from the ratio of densities of E-cadherin cis-dimer/E-cadherin monomer. Results are reported as the mean ± s.d. of two independent experiment. See also Supplementary Figure S6.