Abstract
Purpose
‘Phenocopy’ frontotemporal dementia (phFTD) patients may clinically mimic the behavioral variant of FTD (bvFTD), but do not show functional decline or abnormalities upon visual inspection of routine neuroimaging. We aimed to identify abnormalities in gray matter (GM) volume and perfusion in phFTD and to assess whether phFTD belongs to the FTD spectrum. We compared phFTD patients with both healthy controls and bvFTD patients.
Materials & methods
Seven phFTD and 11 bvFTD patients, and 20 age-matched controls underwent structural T1-weighted magnetic resonance imaging (MRI) and 3D pseudo-continuous arterial spin labeling (pCASL) at 3T. Normalized GM (nGM) volumes and perfusion, corrected for partial volume effects, were quantified regionally as well as in the entire supratentorial cortex, and compared between groups taking into account potential confounding effects of gender and scanner.
Results
PhFTD patients showed cortical atrophy, most prominently in the right temporal lobe. Apart from this regional atrophy, GM volume was generally not different from either controls or from bvFTD. BvFTD however showed extensive frontotemporal atrophy. Perfusion was increased in the left prefrontal cortex compared to bvFTD and to a lesser extent to controls.
Conclusion
PhFTD and bvFTD show overlapping cortical structural abnormalities indicating a continuum of changes especially in the frontotemporal regions. Together with functional changes suggestive of a compensatory response to incipient pathology in the left prefrontal regions, these findings are the first to support a possible neuropathological etiology of phFTD and suggest that phFTD may be a neurodegenerative disease on the FTD spectrum.
Keywords: Phenocopy frontotemporal dementia, Behavioral variant frontotemporal dementia, Arterial spin labeling-MRI, Cerebral blood flow, Gray matter volume
Highlights
-
•
Both phFTD and bvFTD show frontotemporal cortical structural abnormalities.
-
•
PhFTD shows left frontal hyperperfusion, suggestive of functional compensation.
-
•
Overlapping findings in phFTD and bvFTD findings suggest a common disease spectrum.
1. Introduction
FTD is a presenile neurodegenerative disorder affecting the frontal and temporal lobes, with the behavioral variant (bvFTD) as its most common subtype. BvFTD is characterized by progressive deterioration in social and personal conduct (Neary et al., 1998). Core clinical features are behavioral disinhibition, apathy, loss of empathy, and perseverative, stereotypical or compulsive behavior. In addition to these symptoms, the diagnosis of probable bvFTD requires frontotemporal changes on neuroimaging and a gradual decline in functional abilities (Rascovsky et al., 2011). A subset (reports range from 7% up to 37% (Hornberger et al., 2009, Khan et al., 2012)) of predominantly male patients presents with behavioral changes characteristic of bvFTD, but without abnormalities on structural magnetic resonance imaging (MRI) or fluorodeoxyglucose-positron emission tomography (FDG-PET) (Davies et al., 2006, Kerklaan et al., 2014, Kipps et al., 2007, Kipps et al., 2009a). In addition, these patients have a more benign disease course (Davies et al., 2006) and do not show a decline in activities of daily living (Mioshi and Hodges, 2009). This clinical syndrome is referred to as ‘phenocopy’ FTD (phFTD) (Hornberger et al., 2008).
Because normal neuroimaging features and no cognitive decline over time are reported in these patients, a neurodegenerative etiology is disputed. Autopsy findings are sparse, but have not shown features of neurodegeneration (Diehl-Schmid et al., 2007, Kertesz et al., 2005). Very recently, repeat expansion in the C9ORF72 gene has been associated with very slowly progressive FTD, resembling phFTD. Some patients with this mutation have initially been diagnosed with phFTD (Gomez-Tortosa et al., 2014, Khan et al., 2012), but currently, phFTD is still defined as a clinical syndrome. An alternative notion is that phFTD patients might have a pre-existent psychiatric disorder and decompensate during mid-life (Kipps et al., 2010, Manes, 2012, Piguet et al., 2011).
In the present study we used advanced quantitative MRI techniques and analyses to investigate both structural and functional abnormalities in phFTD in more detail, as the typical behavioral changes in phFTD imply neurophysiological changes which may be detected with these advanced methods (Khan et al., 2012). We used arterial spin labeling (ASL)-MRI to quantify brain perfusion with higher spatial resolution than thus far achieved with PET (Wong et al., 1999). Focal atrophy can be detected by regional quantification of gray matter volume on structural imaging. Gray matter volume and perfusion in phFTD patients were compared with both healthy controls and bvFTD patients in order to assess whether phFTD belongs to the FTD spectrum.
2. Methods
2.1. Participant selection
PhFTD and bvFTD patients were recruited from the Alzheimer Center Southwest Netherlands at Erasmus MC, Rotterdam, The Netherlands, which is a tertiary referral center with special focus on FTD. Exclusion criteria for both phFTD and bvFTD patients were contraindications for MR imaging and lack of hetero-anamnestic information. In addition, phFTD patients were excluded when there was a diagnosis of dementia, or when other neurological or psychiatric disorders were suspected.
Of the fifteen patients that fulfilled the criteria for phFTD, i.e. behavioral features but no imaging findings consistent with bvFTD, and no progression for at least one year after initial diagnostic work-up, six patients declined to participate; one was excluded due to refusal of neuropsychological assessment; and one eventually showed progressive cognitive impairment at neuropsychological follow-up, resulting in the analysis of seven phFTD patients (Table 1). One patient showing an asymptomatic cortical infarct in the right parietal lobe on MRI was retained in the analyses, as no residual clinical symptoms were reported and the infarct was not in a region of interest for FTD. Image processing results were visually checked and did not show any effect of the infarct on segmentation or registration procedures. All phFTD patients were screened for the presence of the repeat expansion of the C9ORF72 gene. We chose to screen for this causative mutation only, and not other mutations associated with psychiatric symptoms in bvFTD, as it has been specifically associated with slowly progressive bvFTD (Gomez-Tortosa et al., 2014, Khan et al., 2012).
Table 1.
Clinical details of phFTD and bvFTD patients included in the analyses. Described are the behavioral and cognitive profiles of phFTD patient at the time of inclusion, and those of bvFTD patients at the time of diagnosis. Also listed are basis of diagnosis, number of visits and follow up, and (decline) of functional status as assessed by asking patients and/or their caregivers about the patient's ability to perform (instrumental) activities of daily living (as detailed in the Methods).
| Behavioral and cognitive profile | Diagnosis based on | Follow up | Functional status | |
|---|---|---|---|---|
| phFTD patients | ||||
| 1 | Behavioral symptoms: Behavioral disinhibition, loss of empathy |
No progression for 4 years | Clinical 5 visits 5 year FU Neuropsychological 4 visits 4 year FU |
Stable as reported by both patient and caregiver. |
| Neuropsychological evaluation per domain (3rd visit): Orientation to person, time, place: unimpaired Memory: average Language: below average to average Attention: average to poor Executive functions: average to poor Information processing speed: below average Visuoconstructive ability: unimpaired Social cognition: poor Conclusion: although the impairments listed above are suspect for FTD, the absence of evident cognitive decline and the long disease course render this diagnosis less likely. | ||||
| 2 | Behavioral symptoms: Behavioral disinhibition, apathy, compulsive behavior, hyperorality |
No progression for 9 years | Clinical 9 visits 9 years Neuropsychological 3 visits 8.8 years |
Stable as reported by both patient and caregiver. |
| Neuropsychological evaluation per domain (3rd visit): Orientation to person, time, place: unimpaired Memory: unimpaired Language: average to poor Attention: average to poor Executive functions: average to below average Information processing speed: below average Visuoconstructive ability: unimpaired Social cognition: poor Conclusion: although the impairments listed above and the clinical presentation are suspect for FTD, the patient's intact insight into his/her own functioning, the absence of any evident cognitive decline and the very long disease course render this diagnosis less likely. | ||||
| 3 | Behavioral symptoms: Behavioral disinhibition, loss of empathy, loss of insight |
No progression for 1 year | Clinical 3 visits 1 year Neuropsychological 2 visits 1 year |
Stable as reported by both patient and caregiver. |
| Neuropsychological evaluation per domain (2nd visit): Orientation to person, time, place: unimpaired Memory: highly variable (unimpaired to poor) Language: average to poor Attention: below average to poor Executive functions: average to poor Information processing speed: average Visuoconstructive ability: unimpaired Social cognition: poor Conclusion: compared to the previous neuropsychological evaluation there is no evident cognitive decline. | ||||
| 4 | Behavioral symptoms: Behavioral disinhibition, loss of insight |
No progression for 5 years | Clinical 5 visits 5 year Neuropsychological 3 visits 4.8 years |
Patient reports minor difficulties at work, but performs activities of daily living independently and has no difficulties operating appliances according to caregiver. |
| Neuropsychological evaluation per domain (3rd visit): Orientation to person and place: unimpaired; to time: sufficient Memory: unimpaired to below average Language: below to above average Attention: average to poor Executive functions: below average to poor Information processing speed: average to below average Visuoconstructive ability: unimpaired Social cognition: average Conclusion: although the impairments listed above and the clinical presentation as well as the cognitive decline reported by patient and caregiver are suspect for FTD, the absence of evident cognitive decline renders this diagnosis less likely. | ||||
| 5 | Behavioral symptoms: Behavioral disinhibition, apathy, loss of empathy |
No progression for 6 years | Clinical 9 visits 6 years Neuropsychological 5 visits 5.9 years |
Minor difficulties reported by patient at first and second visits which had stabilized or improved at later visits, e.g. disorientation while driving, but not anymore when driving using GPS navigation, confirmed by caregiver. |
| Neuropsychological evaluation per domain (5th visit): Orientation to person, time, place: unimpaired Memory: average Language: average Attention: average to above average Executive functions: above average Information processing speed: above average Visuoconstructive ability: unimpaired Social cognition: below average Conclusion: the results are similar to previous neuropsychological examinations. There are no indications for cognitive impairment. | ||||
| 6 | Behavioral symptoms: Behavioral disinhibition, apathy, loss of empathy |
No progression for 1.2 years (slight functional decline but no clinical or cognitive deterioration). | Clinical 5 visits 1.2 years Neuropsychological 2 visits 1.2 years |
Very slow progression but performs activities of daily living independently according to caregiver. |
| Neuropsychological evaluation per domain (2nd visit): Orientation to person, time, place: unimpaired Memory: average to poor Language: average Attention: below to above average Executive functions: average to poor Information processing speed: average Visuoconstructive ability: unimpaired Social cognition: below average Conclusion: although the impairments listed above and the clinical presentation are suspect for FTD, the patient's intact insight into his/her own functioning and the absence of any cognitive decline render this diagnosis less likely. | ||||
| 7 | Behavioral symptoms: Behavioral disinhibition, apathy, loss of empathy, hyperorality |
No progression for 10 years | Clinical 9 visits 10.1 years Neuropsychological 4 visits 9.7 years |
Activities of daily living are scarcely performed due to the patient's severe apathy according to caregiver. |
| Neuropsychological evaluation per domain (4th visit): Orientation to person, time, place: unimpaired Memory: average to poor Language: average to poor Attention: below average to poor Executive functions: average to poor Information processing speed: average Visuoconstructive ability: unimpaired Social cognition: poor Conclusion: although the impairments listed above and the clinical presentation as well as the cognitive decline reported by the caregiver are suspect for FTD, the absence of evident cognitive decline renders this diagnosis less likely. | ||||
| bvFTD patients | ||||
| 1 | Behavioral symptoms: Behavioral disinhibition, loss of empathy |
Clinical presentation as well as functional and cognitive decline | Clinical 2 visits 1 year Neuropsychological evaluation 2 visits 1 years |
Functional decline reported by caregiver of several (instrumental) activities of daily living. |
| Neuropsychological evaluation per domain (2nd visit): Orientation to person and place: unimpaired; to time: impaired Memory: average to poor Language: below average Attention: poor Executive functions: average to poor Information processing speed: average Visuoconstructive ability: poor Conclusion: although previous neuropsychological evaluation did not provide any indication for a neurodegenerative disorder, the newly reported information by patient and caregiver and the cognitive decline substantiated by the current evaluation support the diagnosis of FTD. | ||||
| 2 | Behavioral symptoms: Apathy, loss of empathy |
Clinical presentation as well as functional and cognitive decline | Clinical 3 visits 1.8 years Neuropsychological evaluation 2 visits 1.3 years |
Functional decline reported by patient and caregiver of several (instrumental) activities of daily living. |
| Neuropsychological evaluation per domain (2nd visit): Orientation to person, time, place: unimpaired Memory: above average to average Language: unimpaired to below average Attention: average to poor Executive functions: average to below average Information processing speed: below average Visuoconstructive ability: below average Conclusion: compared to the previous neuropsychological evaluation, memory remained intact, but there is cognitive decline specifically in the domains of attention and executive functioning, indicative of a dementia syndrome, most likely FTD. | ||||
| 3 | Behavioral symptoms: Behavioral disinhibition, apathy, loss of empathy, loss of insight, hyperorality |
Clinical presentation, functional decline and cognitive impairment | Clinical 3 visits 2 months Neuropsychological evaluation 1 visit |
Functional decline reported by caregiver of several (instrumental) activities of daily living. |
| Neuropsychological evaluation per domain (1st visit): Orientation to person and time: unimpaired; to place: impaired Memory: below average to poor Language: poor Attention: average to poor Executive functions: poor Visuoconstructive ability: average Conclusion: the cognitive profile is indicative of dementia, clinically presenting as FTD. | ||||
| 4 | Behavioral symptoms: Apathy, loss of empathy, compulsive behavior |
Clinical presentation as well as and functional and cognitive decline | Clinical 3 visits 1.3 years Neuropsychological evaluation 2 visits 1.3 years |
Progressive functional decline as reported by caregiver. |
| Neuropsychological evaluation per domain (2nd visit): Orientation to person, time, place: unimpaired Memory: poor Language: below average to poor Attention: average to poor Executive functions: unimpaired Visuoconstructive ability: unimpaired Information processing speed: below average Conclusion: the decline in the cognitive domains of langue, attention and information processing speed compared to the previous neuropsychological evaluation, as well as the current overall cognitive profile and clinical presentation are indicative of FTD. | ||||
| 5 | Behavioral symptoms: Apathy, loss of insight, stereotyped and compulsive behavior, hyperorality |
Clinical presentation as well as functional and cognitive decline | Clinical 4 visits 2 years Neuropsychological evaluation 2 visits 1.3 years |
Progressive functional decline as reported by caregiver, eventual admission to nursing home. |
| Neuropsychological evaluation per domain (2nd visit): Orientation to person,: unimpaired; to time and place: impaired Memory: average to poor Language: average to poor Attention: below average to poor Executive functions: poor Information processing speed: average Visuoconstructive ability: poor (due to executive dysfunction) Conclusion: the cognitive decline in domains of attention and executive functioning and impairment on other domains, combined with the clinical presentation fit the profile of bvFTD. | ||||
| 6 | Behavioral symptoms: Behavioral disinhibition, compulsive behavior, hyperorality |
Clinical presentation as well as functional and cognitive impairment. | Clinical 6 visits 2.4 years Neuropsychological evaluation 1 visit |
Minor difficulties with operating appliances, grocery shopping, and laundry, as reported by caregiver. |
| Neuropsychological evaluation per domain (1st visit): Orientation to person, time, place: unimpaired Memory: unimpaired Language: below average to poor Attention: below average Executive functions: below average Visuoconstructive ability: unimpaired Conclusion: the mildly impaired attention and executive functions, combined with impaired language and intact memory and praxis, may be indicative of FTD | ||||
| 7 | Behavioral symptoms: Behavioral disinhibition, loss of empathy, compulsive behavior |
Clinical presentation, functional decline and cognitive impairment. C9ORF72 mutation present. | Clinical 5 visits 1.5 years Neuropsychological evaluation 1 visit |
Progressive functional decline as reported by caregiver, eventual admission to day care (5 days a week). |
| Neuropsychological evaluation per domain (1st visit): Orientation to person, time, place: unimpaired Memory: poor Language: below average to poor Attention: average to poor Executive functions: average Visuoconstructive ability: average Conclusion: the focal impairment in the language domain could possibly be attributed to logopenic progressive aphasia (LPA) but the absence of memory impairment and the prominent behavioral symptoms are not typical for LPA. | ||||
| 8 | Behavioral symptoms: Behavioral disinhibition, loss of empathy, loss of insight, stereotyped and compulsive behavior |
Screened for MAPT mutation before the onset of symptoms because of positive family history. Clinical conversion to FTD confirmed 1.3 years later (based on clinical presentation, cognitive and functional decline). | Clinical 5 visits 1.3 year Neuropsychological evaluation 2 visits 1.4 year |
Functional decline reported initially by patient and later by caregiver of several (instrumental) activities of daily living. |
| Neuropsychological evaluation per domain (2nd visit): Orientation to person, time, place: unimpaired Memory: average to below average Language: below average to poor Attention: unimpaired Executive functions: unimpaired to below average Information processing speed: unimpaired Visuoconstructive ability: unimpaired Social cognition: poor Conclusion: the cognitive profile of impairment in language, memory and social cognition, combined with the cognitive decline compared to the previous neuropsychological evaluation and clinical presentation, are compatible with (conversion to) bvFTD. | ||||
| 9 | Behavioral symptoms: Behavioral disinhibition, apathy, loss of empathy, compulsive behavior |
Clinical presentation as well as functional and cognitive decline. | Clinical 2 visits 1 week Neuropsychological 1 visit. Test results of neuropsychological evaluation conducted 6 months earlier elsewhere were also available. |
Functional decline of several (instrumental) activities of daily living decline reported by caregiver. |
| Neuropsychological evaluation per domain (1st visit, compared to neuropsychological exam performed elsewhere): Orientation to person and place: unimpaired; to time: sufficient Memory: highly variable (unimpaired to poor) average Language: highly variable (unimpaired to poor) Attention: unimpaired Executive functions: average to poor Information processing speed: below average Visuoconstructive ability: unimpaired Social cognition: poor Conclusion: the cognitive profile of impairment in language, memory and social cognition, combined with the cognitive decline compared to the previous neuropsychological evaluation [conducted elsewhere] and clinical presentation, are compatible with bvFTD. | ||||
| 10 | Behavioral symptoms: Loss of empathy, loss of insight |
Clinical presentation, cognitive impairment and functional decline. | Clinical 4 visits 0.8 year Neuropsychological Test results of neuropsychological evaluation conducted earlier elsewhere were available. |
Performs activities of daily living independently but has progressive difficulties operating appliances and managing finances, as reported by caregiver. |
| Neuropsychological exam conducted elsewhere showed poor performance on multiple domains, particularly executive functioning and language | ||||
| 11 | Behavioral symptoms: Apathy, loss of empathy |
Screened for MAPT mutation when symptoms first manifested because of positive family history. Diagnosis based on mutation, clinical presentation, cognitive and functional decline. | Clinical 2 visits 1 month Neuropsychological Test results of two neuropsychological evaluations with 1.2 year interval conducted elsewhere were available. |
Increasing interference with daily functioning, as reported by caregiver. |
| Neuropsychological evaluation per domain (2nd neuropsychological exam performed elsewhere): Orientation to person and place: unimpaired; to time: impaired Memory: poor Language: highly variable (unimpaired to poor) Attention: unimpaired Executive functions: below average to poor Information processing speed: below average Visuoconstructive ability: unimpaired Social cognition: poor Conclusion: compared to the previous evaluation there is a decline in orientation and language | ||||
Twelve bvFTD patients with possible bvFTD (Rascovsky et al., 2011) with an onset before 65 years and a Mini Mental State Examination (MMSE) score ≥ 20 were prospectively recruited as part of a larger ongoing study on advanced MR neuroimaging in the early stage of presenile dementia. If the diagnosis at the initial visit was uncertain, patients were followed up on until a definitive diagnosis was established based on the criteria by (Rascovsky et al., 2011). One patient was excluded from analysis due to poor perfusion data quality as a result of severe motion artifacts, resulting in the analysis of 11 bvFTD patients (Table 1).
Healthy age-matched controls were recruited through advertisement and from the patients' peers. They were matched for gender with the phFTD patients. Exclusion criteria were history of neurological or psychiatric disorders and contraindications for MRI. Of the twenty-three controls, two were excluded due to missing data and one because of below-average scores on neuropsychological assessment, resulting in the analysis of twenty healthy controls.
The study was approved by the medical ethics committee of Erasmus MC. All participants gave written informed consent.
2.2. Neuropsychological and psychiatric assessment
All participants underwent extensive neuropsychological examination as part of routine diagnostic work-up, assessing language and speech, attention and mental processing speed, executive functions, memory, and social cognition. PhFTD patients had an additional assessment to verify whether they fulfilled the criterion of no cognitive decline for at least one year. Additionally, functional status and possible decline thereof was determined by asking both phFTD and bvFTD patients and their caregivers about the patient's ability to perform (instrumental) activities of daily living, such as cooking, transportation, financial management, grooming, bathing, dressing, and eating. Functional decline excluded the diagnosis of phFTD.
PhFTD patients were assessed by an experienced psychiatrist to rule out major psychiatric disorders other than dementia. Clinical assessment by the expert psychiatrist was based on interviews with the patients and their caregivers, the Brief Psychiatric Rating Scale (BPRS (Overall and Gorham, 1962); Dutch translation (Dingemans, 1986)), and the psychiatrist's observations. The assessment served to determine whether pre-existent psychiatric disorders were absent, such as personality disorders and autism spectrum disorders, that would serve as an alternative explanation of the current behavioral symptoms. Specifically, late-onset psychotic disorders, manic episodes, and depressive or anxiety disorders were ruled out as these are more likely to mimic bvFTD (and thus phFTD).
2.3. Image acquisition
Patients underwent MR imaging on two identical 3T scanners (Discovery MR750 system GE Healthcare, USA) with identical protocols. Seven healthy controls and all phFTD patients were scanned on one, and 13 healthy controls and all bvFTD patients on the other scanner.
2.3.1. Structural imaging
For gray matter volumetric assessment and anatomical reference, a high-resolution three-dimensional (3D) inversion recovery (IR) fast spoiled gradient-echo (FSPGR) T1-weighted (T1w) scan was acquired (inversion time (TI) 450 ms, echo time (TE) 3.06 ms, repetition time (TR) 7.904 s, flip angle 12°, ASSET factor 2, isotropic resolution 1 mm3 in a 240 mm field of view (FOV), 176 sagittal slices, total acquisition time 4 min and 41 s).
2.3.2. Perfusion imaging
Perfusion images were acquired using whole brain 3D pseudo-continuous ASL (p-CASL), currently the recommended sequence for clinical use (Alsop et al., 2015). With exception of the post labeling delay (1525 ms in the current study), perfusion scans were acquired using the recommended parameters (interleaved fast spin-echo stack-of-spiral readout of 512 sampling points on 8 spirals, background suppressed, labeling duration 1450 ms, TE 10.5 ms, TR 4632 ms, isotropic resolution 3.3 mm3 in a 240 mm FOV, 36 axial slices, number of excitations (NEX) 3, total acquisition time 4 min and 29 s). The labeling plane was positioned 9 cm below the anterior commissure–posterior commissure line.
2.4. Image data processing
We processed imaging data according to the methods described in detail by Bron et al., 2014, as briefly outlined below. In summary, cerebral blood flow (CBF) values from gray matter (GM) corrected for partial volume effects were obtained using the following methods.
2.4.1. Tissue segmentation
Using the unified tissue segmentation method in SPM8 (Statistical Parametric Mapping, London, UK), we segmented T1w images into GM, white matter and cerebrospinal fluid maps. The GM maps were subsequently used to derive GM volumes and CBF.
2.4.2. ASL post-processing
The ASL data consisted of a difference image and a control image. Quality of all images were visually assessed by checking for motion, susceptibility and watershed artifacts. GM maps were rigidly registered with the difference image (Elastix registration software (Klein et al., 2010)) and registrations were checked visually. Tissue maps were transformed to ASL image space to perform partial volume (PV) correction, and PV effects in ASL difference and control images were subsequently corrected using local linear regression within a 3D kernel based on tissue maps (Asllani et al., 2008). We quantified PV-corrected ASL images as CBF maps using the single-compartment model (Alsop et al., 2015). CBF maps were transformed to T1w image space for further analysis.
2.4.3. ROI labeling
We defined regions of interest (ROIs) for each participant using a multi-atlas approach. This involved registration of 30 labeled T1w images, each containing 83 cortical and subcortical ROIs (Gousias et al., 2008, Hammers et al., 2003), to the participants' T1w images. The labels of the 30 atlas images were fused by means of majority voting to obtain a final ROI labeling (Heckemann et al., 2006). Rigid, affine, and non-rigid B-spline transformation models were applied successively for registration to the participants' nonuniformity-corrected T1w images (Tustison et al., 2010). Both the participants' and the labeled T1w images were masked for this registration using the Brain Extraction Tool (Smith, 2002).
2.4.4. ROI analysis
For all ROIs, we derived GM volumes and mean GM CBF values which were checked for outliers due to previously unnoticed artifacts or registration errors. The subcortical ROIs, cerebellum, brainstem, ventricles and white matter were excluded from analysis. ROIs that parcellated gyri in multiple sections were combined to constitute entire gyri (supplementary Table 1). GM volumes and mean GM CBF values were subsequently obtained for the left and right hemisphere separately. Regional GM volumes were divided by the total intracranial volume to correct for head size and are referred to as normalized GM (nGM) volumes.
2.5. Data analysis
Using SPSS Statistics, version 20.0 (New York, USA) we first analyzed differences in gender and scanner across groups with Fisher's exact test. As these were significantly different between groups (p < 0.05), we then used hierarchical regression to sequentially assess the effects of scanner, gender, and group on nGM and CBF. Only the nGM and regional CBF ROIs that showed a significant effect of group but did not show significant effects of scanner and/or gender were further tested for differences between groups. This was done using a nonparametric Kruskal-Wallis test with Dunn-Bonferroni correction for multiple comparisons as nGM, CBF, age and MMSE were not normally distributed across groups (Shapiro-Wilk test p < 0.05). The findings were visually represented in boxplots of nGM and CBF for each of the brain lobes. Statistical thresholds were set at p < 0.05. Results were visualized by overlaying the ROIs as defined by Gousias et al., 2008 and Hammers et al., 2003 that showed group differences on a volume render of a skull-stripped T1w template in MRIcron NIfTI viewer (Chris Rorden, Version 1, April 2010).
3. Results
3.1. Participant characteristics
Age was not different between groups (H(2) = 1.129, p > 0.05, Kruskal-Wallis test) (Table 2). MMSE was significantly different between groups (F(2) = 10.182, p < 0.05, Kruskal-Wallis test): both phFTD and bvFTD patients had significantly lower MMSE scores than controls.
Table 2.
Participant characteristics.
| Controls | phFTD | bvFTD | |
|---|---|---|---|
| N (male) | 20 (20) | 7 (7) | 11 (5) |
| Median age in years (25th–75th percentile) | 64 (62–66) | 61 (60–70) | 63 (57–66) |
| Median MMSE (25th–75th percentile) | 28 (28–30) | 27 (26–28) | 27 (24–28) |
bvFTD = behavioral variant frontotemporal dementia; IQR = interquartile range; MMSE = Mini Mental State Examination; phFTD = phenocopy frontotemporal dementia; SD = standard deviation.
None of the phFTD patients had a C9ORF72 mutation, nor could their behavioral disturbances be attributed to an underlying psychiatric disorder. Neuropsychological assessment was normal in one and suggestive of FTD in six phFTD patients, but did not demonstrate progressive decline.
Median follow-up to establish definitive diagnosis of bvFTD was 1.4 years (range 1.7 months–2.4 years).
3.2. Gray matter volumetric changes
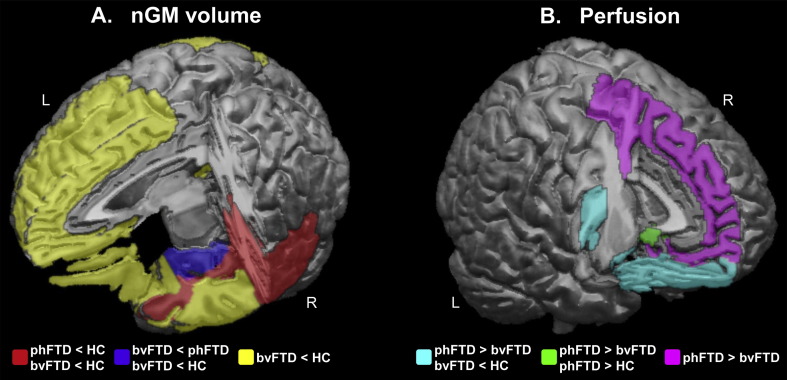
There were significant differences in nGM volume between groups mostly in frontal and temporal regions (Fig. 1A, Table 3). PhFTD patients had lower supratentorial nGM volume than controls which was most pronounced in the right posterior temporal lobe, right superior temporal gyrus and bilateral fusiform gyrus. BvFTD showed extensive bilateral frontotemporal nGM volume loss compared to controls. Compared to phFTD, bvFTD showed lower nGM volume in the right hippocampal formation and the right amygdala. Other nGM volumes were not significantly different between bvFTD and phFTD. This spectrum of findings, with mean nGM volumes being highest in controls, lowest in bvFTD and in-between in phFTD, was particularly apparent in the frontal and temporal lobes (Fig. 2A).
Fig. 1.
Schematic overview of cortical regions showing (A) normalized GM volume and (B) perfusion abnormalities. Panel 1A shows in red regional nGM atrophy present in both phFTD and bvFTD; in blue regional nGM volume loss in bvFTD compared to both phFTD and controls; and in yellow regional nGM volume loss in in bvFTD when compared to controls, but not compared to phFTD. Panel 1B shows in cyan hyperperfusion in phFTD compared to bvFTD in regions that show hypoperfusion in bvFTD compared to controls; in green regional hyperperfusion in phFTD compared to both bvFTD and controls; and in violet regional hyperperfusion in phFTD compared to bvFTD.
HC = healthy controls; phFTD = phenocopy frontotemporal dementia; bvFTD = behavioral variant frontotemporal dementia; nGM = normalized gray matter. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Median nGM volume [% ICV] and 25th and 75th percentile (in parentheses) for healthy controls (HC), phFTD and bvFTD patients.
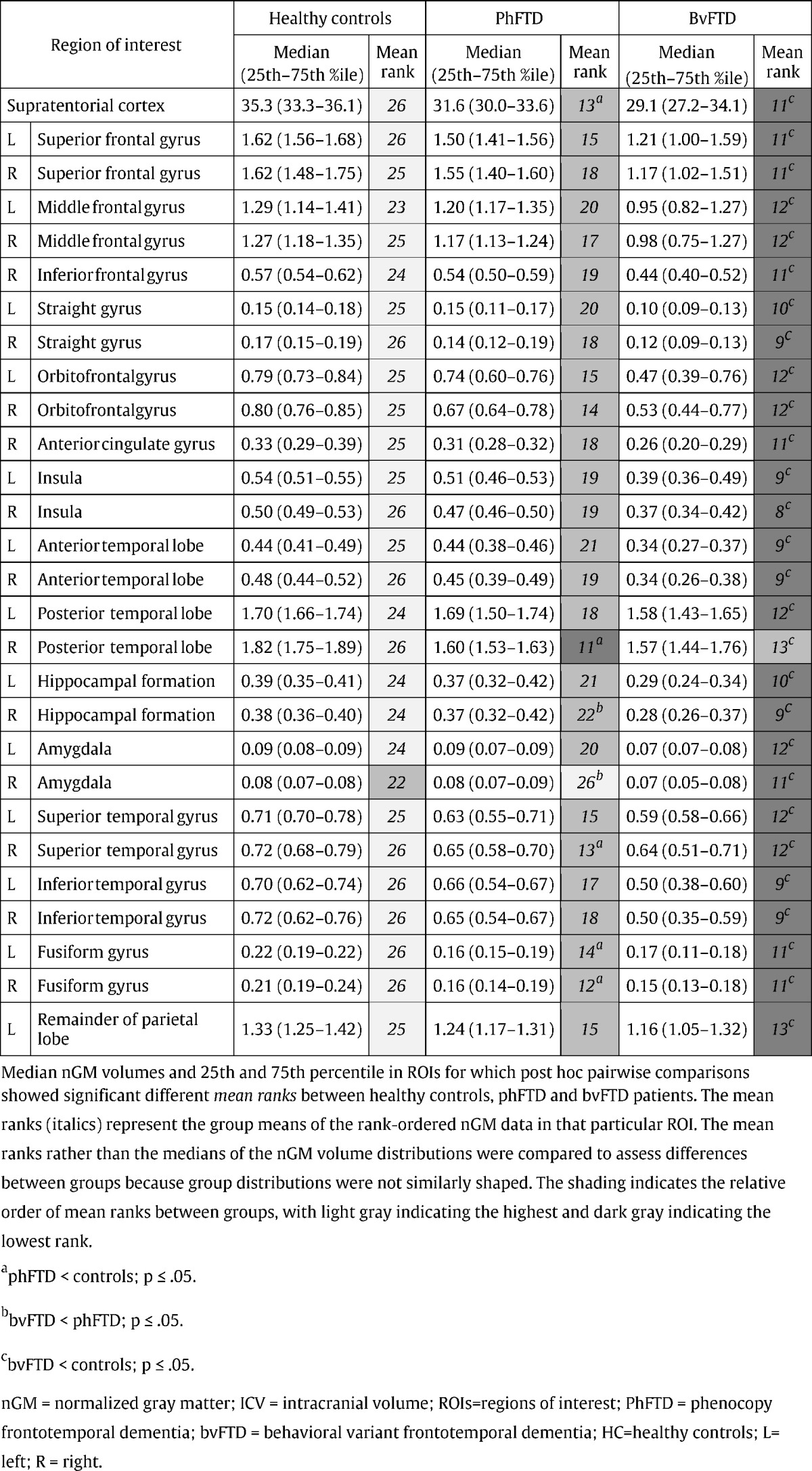
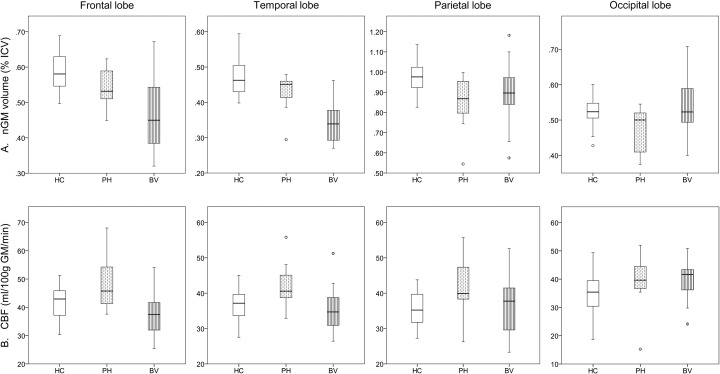
Fig. 2.
A) normalized GM (% ICV) and B) CBF (ml/100 g GM/min) in the different lobes for healthy controls (HC), phFTD (PH) and bvFTD (BV) patients. The central box represents values from lower to upper quartile (25-75th percentile), the middle line represents the median, and vertical bars extend from minimum to maximum value. Spheres outside the bars indicate extreme values (value ≥ 1.5 × interquartile range). Note that GM volumes in phFTD are generally in-between those of HC and bvFTD, and that perfusion in phFTD is generally higher than in bvFTD and controls.
HC = healthy controls; phFTD = phenocopy frontotemporal dementia; bvFTD = behavioral variant frontotemporal dementia; nGM = normalized gray matter; ICV = intracranial volume; CBF = cerebral blood flow.
3.3. Perfusion changes in the gray matter
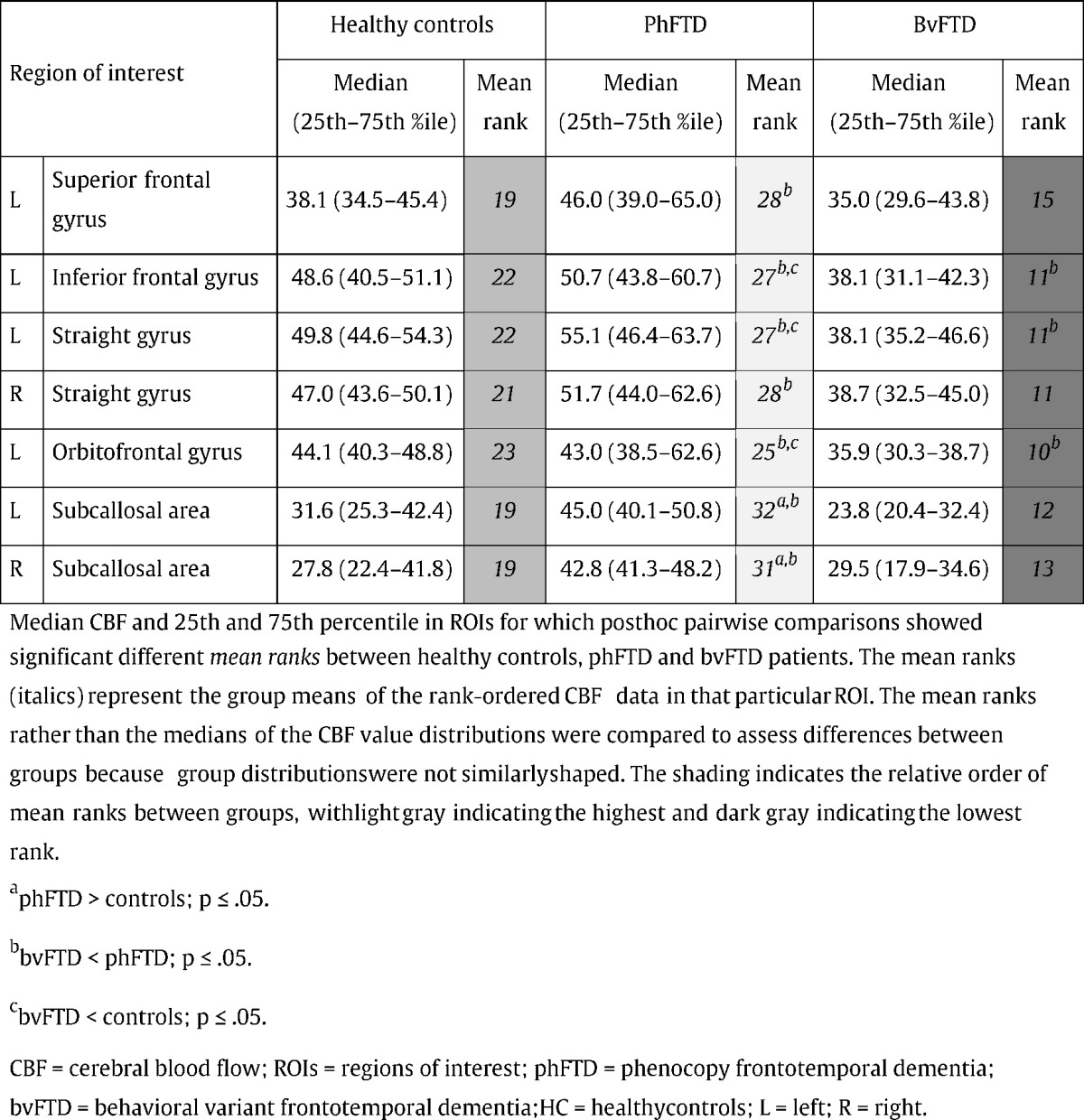
There were significant differences in CBF between groups in frontal regions (Fig. 1B, Table 4). CBF in the bilateral subcallosal area was higher in phFTD than both in bvFTD and controls, as illustrated in Fig. 2B. CBF in bvFTD was lower than in phFTD in the left superior and inferior frontal gyrus, the left orbitofrontal gyrus, and in the bilateral straight gyrus. bvFTD showed lower CBF than controls in the left inferior frontal and straight gyrus, and the left orbitofrontal gyrus. Note that differences between groups were not located in watershed regions and can therefore not be attributed to watershed artifacts.
Table 4.
Median CBF and 25th and 75th percentile (in parentheses) for healthy controls (HC), phFTD and bvFTD patients.
4. Discussion
To the best of our knowledge, our study is the first to show cortical brain abnormalities in phFTD. We found cortical atrophy in phFTD, most prominently in the right superior and posterior temporal lobe, and the fusiform gyrus bilaterally. Furthermore, we found left frontal hyperperfusion in phFTD compared to bvFTD and to a lesser extent to controls, which may reflect functional compensation for incipient pathology.
Regional right temporal atrophy was not only seen in phFTD but also present in bvFTD, suggesting similar underlying pathophysiology. Atrophy in right temporal regions has been linked to impaired emotion recognition and empathy in neurodegenerative disease (Rankin et al., 2006, Rosen et al., 2006), and more specifically to emotional blunting in bvFTD (Lee et al., 2014). In addition, frontotemporal atrophy lateralized to the right hemisphere is more often associated with socially undesirable behavior in FTD than when lateralized to the left (Mychack et al., 2001). The fact that we found atrophy in this specific region may explain why symptoms in phFTD patients are mostly isolated to the behavioral domain, in contrast to bvFTD patients who show a more widespread frontotemporal atrophy and additional cognitive and functional decline.
Our findings are in contrast to previous studies, in which no atrophy in phFTD was found using semi-quantitative ratings (Davies et al., 2006, Pennington et al., 2011). One possible explanation might be that such semiquantitative rating was not sufficiently sensitive. However, other studies using the potentially more sensitive VBM method did not show any abnormalities either (Kipps et al., 2009a, Kipps et al., 2009b), except for one case study reporting non-specific parieto-occipital, thalamic and subtle frontoinsular atrophy (Khan et al., 2012). The discrepancy with the present study may lie in the fact that we used highly specific patient selection criteria, i.e. behavioral features consistent with bvFTD, without progression for at least one year, without psychiatric disorders and without C9ORF72 mutations. It may also be due to methodological differences between voxel-wise and ROI analyses. ROI analysis circumvents the problem of inter-individual anatomical variability, as well as subsequent corrections for such variability that may compromise resolution (such as smoothing). Additionally, statistical power of ROI analysis is hampered less by corrections for multiple comparisons than voxel-wise testing.
Apart from the focal right temporal atrophy, nGM volumes in phFTD were generally not different from neither bvFTD nor from controls. Only the right hippocampal formation and amygdala showed more atrophy in bvFTD compared with phFTD, suggesting preservation of those regions in phFTD, whereas these were severely affected in bvFTD (Barnes et al., 2006). Of note is that otherwise, nGM volumes were similar between phFTD and bvFTD, despite widespread GM loss in bvFTD compared to controls. These findings suggest that there is a continuum in nGM volumes ranging from normal on the one end to clearly abnormal in bvFTD on the other, with phFTD in-between. Together with the overlapping finding in both phFTD and bvFTD of right temporal lobe atrophy, this suggests that phFTD may be a disease on the FTD spectrum.
Our study was the first to use ASL-MRI in phFTD to assess perfusion. ASL is tightly coupled to brain metabolism and function as measured with FDG-PET, but previous PET studies failed to find any abnormalities in phFTD (Kerklaan et al., 2014, Kipps et al., 2009a). We found higher perfusion in phFTD in the bilateral straight gyrus and left superior, inferior and orbital frontal gyrus compared to bvFTD, and to a lesser extent compared to controls. Some of these regions, i.e. in the left inferior frontal gyrus, correspond to those showing hypoperfusion in bvFTD compared to controls. Such hyperperfusion in phFTD relative to bvFTD may reflect a compensatory process of increased activity to compensate for incipient pathology in regions affected in bvFTD (Hu et al., 2010). Such functional compensation is not commonly seen in clinical cases of bvFTD patients, particularly not on FDG-PET. As ASL and FDG-PET correlate generally well (Cha et al., 2013, Chen et al., 2011), such occasionally reported hyperperfusion with ASL may in fact be the result of a commonly applied normalization procedure, in which CBF is divided by gray matter volume. Because in bvFTD atrophy often exceeds hypoperfusion (Zhang et al., 2011), division of relatively intact CBF by relatively extensive volume loss may lead to an overestimation of (corrected) CBF and thus hyperperfusion. Similarly, hypermetabolism has been observed after global but not cerebellar normalization of FDG PET data in frontotemporal dementia (e.g. Dukart et al., 2010). In the present study, we did not divide CBF by gray matter volume (only by intracranial volume to correct for head size) and therefore did not artificially ‘induce’ hyperperfusion. Our findings in bvFTD patients are thus in line with the extensive FDG-PET literature (e.g. Diehl-Schmid et al., 2007), and the fact that we did not find hyperperfusion in bvFTD, but only in phFTD patients leads us to speculate that such hyperperfusion may be unique to phFTD pathophysiology.
A similar pattern of hyperperfusion could be observed in the right straight gyrus, where perfusion was increased in phFTD compared to bvFTD, while there was a trend (p = 0.06) towards hypoperfusion in bvFTD compared to controls. The other hyperperfused regions in phFTD relative to bvFTD, namely the superior frontal gyrus and subcallosal region, did not show hypoperfusion in bvFTD. Although not observed in our bvFTD sample, left superior frontal hypoperfusion has been found in FTD in previous ASL studies (Du et al., 2006, Tosun et al., 2012, Zhang et al., 2011). Similarly, PET studies have reported subcallosal hypometabolism in FTD (Kanda et al., 2008, Salmon et al., 2003, Schroeter et al., 2007). Therefore, a compensatory process may still be hypothesized. Possible functional compensation would be expected to occur prior to volume loss, and in fact the regions that showed hyperperfusion did not show any clear volume loss, in line with this hypothesis. Even in the presence of volume loss, though, this does not exclude an ongoing process of functional compensation with an upregulation of remaining functional tissue. On the other hand, it can be postulated that in the context of functional compensation volume loss is expected to occur at some point, which would not be consistent with the observations in phFTD to date. However, this is not necessarily the case if neuronal dysfunctioning is largely non-progressive. Because of the cross-sectional design of the study, any interpretation in this context remains speculative.
Taken together, our findings in phFTD suggest functional compensation as well as focal structural abnormalities overlapping with those found in bvFTD. Overlapping focal cortical atrophy was limited to the right temporal lobe, consistent with the disease-specific prominent behavioral changes of phFTD, while cortical volumes in the remaining frontotemporal regions were in-between normal and those in bvFTD. These findings support the idea that phFTD is a disease of the FTD spectrum. One could even wonder whether phFTD is not simply an early manifestation of bvFTD. The notion of phFTD as a neurodegenerative disease is however still disputed due to the absence of disease progression in these patients. Psychiatric disorders have been proposed as an alternative or contributory etiology (Gossink et al., 2016, Kipps et al., 2010, Manes, 2012, Piguet et al., 2011). In support of this view, imaging findings show substantial overlap between FTD and disorders such as schizophrenia (Andreasen et al., 1997, Olabi et al., 2011) and depression (Dotson et al., 2009, Drevets et al., 1997). In addition, phFTD patients may carry a C9ORF72 mutation (Gomez-Tortosa et al., 2014, Khan et al., 2012) which is not only associated with bvFTD, but also with psychotic symptoms (Snowden et al., 2012). Hence, there is substantial overlap between phenotype and possibly genotype of psychiatric syndromes and the FTD spectrum. Yet in our phFTD patients, alternative psychiatric diagnoses were ruled out, which renders interpretation in the context of neurodegenerative disease more likely. In addition, none of the phFTD patients had a mutation of the C9ORF72 gene. Therefore, as of yet, phFTD still seems to be described best as a clinical syndrome. As such, our phFTD population comprised patients with a distinct clinical profile: behavioral features consistent with bvFTD, without progression for at least one year, without psychiatric disorders and without the C9ORF72 mutation. This well-defined clinical profile may have enabled a first link between the typical behavioral changes in phFTD and potential neurophysiological changes as detected with imaging. In support of this, the imaging abnormalities observed in our phFTD sample showed almost full spatial overlap with those observed in the bvFTD sample. Nevertheless, future studies defining the spectrum of both neurodegenerative and psychiatric etiologies that cause behavioral changes later in life (Krudop et al. 2014) are necessary to resolve this debate.
The present study has some limitations. Firstly, the sample size was small. This is inherent to phFTD being a rare disease, with only 15 documented cases in a large tertiary referral center as ours. Of note is that the current literature on phFTD has the same limitation, with a median sample size of only 9 patients. Many studies report findings based on cases or case series (N = 1–4; Diehl-Schmid et al., 2007, Gomez-Tortosa et al., 2014, Khan et al., 2012, Kipps et al., 2007). Studies investigating larger samples (N = 8–26; Davies et al., 2006, Hornberger et al., 2008, Hornberger et al., 2009, Kerklaan et al., 2014, Kipps et al., 2009a, Kipps et al., 2009b, Mioshi and Hodges, 2009) generally did not apply such stringent in- and inclusion criteria as we did, i.e. not adhering to all but only some of the criteria we used such as 1) clinical presentation with bvFTD symptomatology, 2) cognitive decline as assessed with repeated comprehensive neuropsychological testing, 3) repeated self-reported functional status by patient and caregiver, 4) explicit exclusion of other psychiatric disorders as assessed by an experienced psychiatrist, and 5) controlling for the presence of the C9ORF72 mutation associated with slowly progressive bvFTD. This variation in patient selection indicates the trade-off between sample size and potential patient heterogeneity in the context of rare disease. We chose to study a very well-defined phFTD sample, by strictly controlling for disease progression and alternative psychiatric etiology, at the cost of sample size and thus statistical power and generalizability. One patient did have an asymptomatic cortical infarct in the right parietal lobe, but as this did not affect image processing results we expect it did not influence our findings. Secondly, groups were not fully gender-matched and were scanned on two-albeit identical-scanners. We used hierarchical regression analysis to account for potential confounding effects of gender and scanner. Although this stringent analysis limited the number of regions that were eventually analyzed between groups and carries the risk of false negative results, it decreased the probability of false positives, and as such strengthens the validity of our findings. Finally, despite a one year follow-up to ensure the absence of progression, longer follow-up in a longitudinal study will be even better suited to assess whether patients show no or very slow progression. Therefore, follow-up of our phFTD sample is currently ongoing. In addition, the findings in the current study are based on group analysis which may not necessarily generalize to the individual patient level. In our follow-up study we intend to describe patients also on an individual basis, taking longitudinal findings into account. Ultimately, post-mortem examination is essential to determine whether neuropathology is present and if so, what type. Hence, studies investigating both neurodegenerative etiology and neuropsychiatric presentation of behavioral changes later in life (Krudop et al., 2014) may further elucidate the relationship between behavior and neurophysiology.
In conclusion, in addition to overlapping focal right temporal lobe atrophy in phFTD and bvFTD, we found a continuum of frontotemporal cortical volumes ranging from normal on the one end to clearly abnormal in bvFTD on the other, with phFTD in-between. Furthermore, we observed left frontal hyperperfusion in phFTD, suggestive of a compensatory process in response to incipient pathology in regions affected in FTD. To the best of our knowledge, our findings are the first evidence of a neuropathological substrate of phFTD and to possibly place it in an FTD spectrum. This may serve as the basis for further assessment in larger patient samples with longitudinal clinical and pathological follow-up.
The following is the supplementary data related to this article.
Regions 1 of interest (ROIs).
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
We would like to thank T.H. Wong and H.H. Meeter for conducting the genetic screening, and J.M. Papma for critical review of this manuscript. We would also like to thank the COST-AID (European Cooperation in Science and Technology—Arterial spin labeling Initiative in Dementia) Action BM1103 for facilitating meetings for researchers to discuss the development and application of ASL as a diagnostic tool for dementia.
This project was financially supported by a personal fellowship granted by the Erasmus University Rotterdam.
References
- Alsop D.C., Detre J.A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L., Lu H., MacIntosh B.J., Parkes L.M., Smits M. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the european consortium for ASL in dementia. Magn. Reson. Med. 2015;73(1):102–116. doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., O'Leary D.S., Flaum M., Nopoulos P., Watkins G.L., Boles Ponto L.L., Hichwa R.D. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349(9067):1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Asllani I., Borogovac A., Brown T.R. Regression algorithm correcting for partial volume effects in arterial spin labeling MRI. Magn. Reson. Med.: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2008;60(6):1362–1371. doi: 10.1002/mrm.21670. [DOI] [PubMed] [Google Scholar]
- Barnes J., Whitwell J.L., Frost C., Josephs K.A., Rossor M., Fox N.C. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch. Neurol. 2006;63(10):1434–1439. doi: 10.1001/archneur.63.10.1434. [DOI] [PubMed] [Google Scholar]
- Bron E.E., Steketee R.M.E., Houston G.C., Oliver R.A., Achterberg H.C., Loog M., van Swieten J.C., Hammers A., Niessen W.J., Smits M. Diagnostic classification of arterial spin labeling and structural MRI in presenile early stage dementia. Hum. Brain Mapp. 2014;35(9):4916–4931. doi: 10.1002/hbm.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y.H., Jog M.A., Kim Y.C., Chakrapani S., Kraman S.M., Wang D.J. Regional correlation between resting state FDG PET and pCASL perfusion MRI. J. Cereb. Blood Flow Metab. 2013;33(12):1909–1914. doi: 10.1038/jcbfm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Wolk D.A., Reddin J.S., Korczykowski M., Martinez P.M., Musiek E.S., Newberg A.B., Julin P., Arnold S.E., Greenberg J.H., Detre J.A. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77(22):1977–1985. doi: 10.1212/WNL.0b013e31823a0ef7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R.R., Kipps C.M., Mitchell J., Kril J.J., Halliday G.M., Hodges J.R. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch. Neurol. 2006;63(11):1627–1631. doi: 10.1001/archneur.63.11.1627. [DOI] [PubMed] [Google Scholar]
- Diehl-Schmid J., Grimmer T., Drzezga A., Bornschein S., Riemenschneider M., Forstl H., Schwaiger M., Kurz A. Decline of cerebral glucose metabolism in frontotemporal dementia: a longitudinal 18F-FDG-PET-study. Neurobiol. Aging. 2007;28(1):42–50. doi: 10.1016/j.neurobiolaging.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Dingemans P. Psychiatrisch Centrum AZUA; Amsterdam: 1986. Vertaling en Bewerking: Uitgebreide BPRS Handleiding, Los Angeles Project. [Google Scholar]
- Dotson V.M., Davatzikos C., Kraut M.A., Resnick S.M. Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J. Psychiatry Neurosci.: JPN. 2009;34(5):367–375. [PMC free article] [PubMed] [Google Scholar]
- Drevets W.C., Price J.L., Simpson J.R., Jr., Todd R.D., Reich T., Vannier M., Raichle M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Du A.T., Jahng G.H., Hayasaka S., Kramer J.H., Rosen H.J., Gorno-Tempini M.L., Rankin K.P., Miller B.L., Weiner M.W., Schuff N. Hypoperfusion in frontotemporal dementia and alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67(7):1215–1220. doi: 10.1212/01.wnl.0000238163.71349.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J., Mueller K., Horstmann A., Vogt B., Frisch S., Barthel H., Becker G., Moller H.E., Villringer A., Sabri O., Schroeter M.L. Differential effects of global and cerebellar normalization on detection and differentiation of dementia in FDG-PET studies. NeuroImage. 2010;49(2):1490–1495. doi: 10.1016/j.neuroimage.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Gomez-Tortosa E., Serrano S., de Toledo M., Perez-Perez J., Sainz M.J. Familial benign frontotemporal deterioration with C9ORF72 hexanucleotide expansion. Alzheimers Dement. 2014;10(5 Suppl.):S284–S289. doi: 10.1016/j.jalz.2013.09.013. [DOI] [PubMed] [Google Scholar]
- Gossink F.T., Dols A., Kerssens C.J., Krudop W.A., Kerklaan B.J., Scheltens P., Stek M.L., Pijnenburg Y.A. Psychiatric diagnoses underlying the phenocopy syndrome of behavioural variant frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry. 2016;87(1):64–68. doi: 10.1136/jnnp-2014-308284. [DOI] [PubMed] [Google Scholar]
- Gousias I.S., Rueckert D., Heckemann R.A., Dyet L.E., Boardman J.P., Edwards A.D., Hammers A. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. NeuroImage. 2008;40(2):672–684. doi: 10.1016/j.neuroimage.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Hammers A., Allom R., Koepp M.J., Free S.L., Myers R., Lemieux L., Mitchell T.N., Brooks D.J., Duncan J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003;19(4):224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckemann R.A., Hajnal J.V., Aljabar P., Rueckert D., Hammers A. Automatic anatomical brain MRI segmentation combining label propagation and decision fusion. NeuroImage. 2006;33(1):115–126. doi: 10.1016/j.neuroimage.2006.05.061. [DOI] [PubMed] [Google Scholar]
- Hornberger M., Piguet O., Kipps C., Hodges J.R. Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology. 2008;71(19):1481–1488. doi: 10.1212/01.wnl.0000334299.72023.c8. [DOI] [PubMed] [Google Scholar]
- Hornberger M., Shelley B.P., Kipps C.M., Piguet O., Hodges J.R. Can progressive and non-progressive behavioural variant frontotemporal dementia be distinguished at presentation? J. Neurol. Neurosurg. Psychiatry. 2009;80(6):591–593. doi: 10.1136/jnnp.2008.163873. [DOI] [PubMed] [Google Scholar]
- Hu W.T., Wang Z., Lee V.M., Trojanowski J.Q., Detre J.A., Grossman M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology. 2010;75(10):881–888. doi: 10.1212/WNL.0b013e3181f11e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T., Ishii K., Uemura T., Miyamoto N., Yoshikawa T., Kono A.K., Mori E. Comparison of grey matter and metabolic reductions in frontotemporal dementia using FDG-PET and voxel-based morphometric MR studies. Eur. J. Nucl. Med. Mol. Imaging. 2008;35(12):2227–2234. doi: 10.1007/s00259-008-0871-5. [DOI] [PubMed] [Google Scholar]
- Kerklaan B.J., Berckel B.N., Herholz K., Dols A., Flier W.M., Scheltens P., Pijnenburg Y.A. The added value of 18-fluorodeoxyglucose-positron emission tomography in the diagnosis of the behavioral variant of frontotemporal dementia. Am. J. Alzheimers Dis. Other Demen. 2014;29(7):607–613. doi: 10.1177/1533317514524811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A., McMonagle P., Blair M., Davidson W., Munoz D.G. The evolution and pathology of frontotemporal dementia. Brain J. Neurol. 2005;128(Pt 9):1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Khan B.K., Yokoyama J.S., Takada L.T., Sha S.J., Rutherford N.J., Fong J.C., Karydas A.M., Wu T., Ketelle R.S., Baker M.C. Atypical, slowly progressive behavioural variant frontotemporal dementia associated with C9ORF72 hexanucleotide expansion. J. Neurol. Neurosurg. Psychiatry. 2012;83(4):358–364. doi: 10.1136/jnnp-2011-301883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipps C.M., Davies R.R., Mitchell J., Kril J.J., Halliday G.M., Hodges J.R. Clinical significance of lobar atrophy in frontotemporal dementia: application of an MRI visual rating scale. Dement. Geriatr. Cogn. Disord. 2007;23(5):334–342. doi: 10.1159/000100973. [DOI] [PubMed] [Google Scholar]
- Kipps C.M., Hodges J.R., Fryer T.D., Nestor P.J. Combined magnetic resonance imaging and positron emission tomography brain imaging in behavioural variant frontotemporal degeneration: refining the clinical phenotype. Brain. 2009;132:2566–2578. doi: 10.1093/brain/awp077. [DOI] [PubMed] [Google Scholar]
- Kipps C.M., Nestor P.J., Acosta-Cabronero J., Arnold R., Hodges J.R. Understanding social dysfunction in the behavioural variant of frontotemporal dementia: the role of emotion and sarcasm processing. Brain. 2009;132:592–603. doi: 10.1093/brain/awn314. [DOI] [PubMed] [Google Scholar]
- Kipps C.M., Hodges J.R., Hornberger M. Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the ‘bvFTD phenocopy syndrome’. Curr. Opin. Neurol. 2010;23(6):628–632. doi: 10.1097/WCO.0b013e3283404309. [DOI] [PubMed] [Google Scholar]
- Klein S., Staring M., Murphy K., Viergever M.A., Pluim J.P. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging. 2010;29(1):196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- Krudop W.A., Kerssens C.J., Dols A., Prins N.D., Moller C., Schouws S., Barkhof F., van Berckel B.N., Teunissen C.E., van der Flier W.M. Building a new paradigm for the early recognition of behavioral variant frontotemporal dementia: late onset frontal lobe syndrome study. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2014;22(7):735–740. doi: 10.1016/j.jagp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Lee G.J., Lu P.H., Mather M.J., Shapira J., Jimenez E., Leow A.D., Thompson P.M., Mendez M.F. Neuroanatomical correlates of emotional blunting in behavioral variant frontotemporal dementia and early-onset alzheimer's disease. J. Alzheimers Dis.: JAD. 2014;41(3):793–800. doi: 10.3233/JAD-132219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F. Psychiatric conditions that can mimic early behavioral variant frontotemporal dementia: the importance of the new diagnostic criteria. Curr. Psychiatry Rep. 2012;14(5):450–452. doi: 10.1007/s11920-012-0294-7. [DOI] [PubMed] [Google Scholar]
- Mioshi E., Hodges J.R. Rate of change of functional abilities in frontotemporal dementia. Dement. Geriatr. Cogn. Disord. 2009;28(5):419–426. doi: 10.1159/000255652. [DOI] [PubMed] [Google Scholar]
- Mychack P., Kramer J.H., Boone K.B., Miller B.L. The influence of right frontotemporal dysfunction on social behavior in frontotemporal dementia. Neurology. 2001;56(11 Suppl. 4):S11–S15. doi: 10.1212/wnl.56.suppl_4.s11. [DOI] [PubMed] [Google Scholar]
- Neary D., Snowden J.S., Gustafson L., Passant U., Stuss D., Black S., Freedman M., Kertesz A., Robert P.H., Albert M. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Olabi B., Ellison-Wright I., McIntosh A.M., Wood S.J., Bullmore E., Lawrie S.M. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol. Psychiatry. 2011;70(1):88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Overall J.E., Gorham D.R. The brief psychiatric rating scale. Psychol. Rep. 1962;10(3):799–812. [Google Scholar]
- Pennington C., Hodges J.R., Hornberger M. Neural correlates of episodic memory in behavioral variant frontotemporal dementia. J. Alzheimers Dis.: JAD. 2011;24(2):261–268. doi: 10.3233/JAD-2011-101668. [DOI] [PubMed] [Google Scholar]
- Piguet O., Hornberger M., Mioshi E., Hodges J.R. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10(2):162–172. doi: 10.1016/S1474-4422(10)70299-4. [DOI] [PubMed] [Google Scholar]
- Rankin K.P., Gorno-Tempini M.L., Allison S.C., Stanley C.M., Glenn S., Weiner M.W., Miller B.L. Structural anatomy of empathy in neurodegenerative disease. Brain J. Neurol. 2006;129(Pt 11):2945–2956. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D., Mendez M.F., Kramer J.H., Neuhaus J., van Swieten J.C., Seelaar H., Dopper E.G., Onyike C.U. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J. Neurol. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H.J., Wilson M.R., Schauer G.F., Allison S., Gorno-Tempini M.L., Pace-Savitsky C., Kramer J.H., Levenson R.W., Weiner M., Miller B.L. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. 2006;44(3):365–373. doi: 10.1016/j.neuropsychologia.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Salmon E., Garraux G., Delbeuck X., Collette F., Kalbe E., Zuendorf G., Perani D., Fazio F., Herholz K. Predominant ventromedial frontopolar metabolic impairment in frontotemporal dementia. NeuroImage. 2003;20(1):435–440. doi: 10.1016/s1053-8119(03)00346-x. [DOI] [PubMed] [Google Scholar]
- Schroeter M.L., Raczka K., Neumann J., Yves von Cramon D. Towards a nosology for frontotemporal lobar degenerations-a meta-analysis involving 267 subjects. NeuroImage. 2007;36(3):497–510. doi: 10.1016/j.neuroimage.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden J.S., Rollinson S., Thompson J.C., Harris J.M., Stopford C.L., Richardson A.M., Jones M., Gerhard A., Davidson Y.S., Robinson A. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain J. Neurol. 2012;135(Pt 3):693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun D., Rosen H., Miller B.L., Weiner M.W., Schuff N. MRI patterns of atrophy and hypoperfusion associations across brain regions in frontotemporal dementia. NeuroImage. 2012;59(3):2098–2109. doi: 10.1016/j.neuroimage.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison N.J., Avants B.B., Cook P.A., Zheng Y., Egan A., Yushkevich P.A., Gee J.C. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging. 2010;29(6):1310–1320. doi: 10.1109/TMI.2010.2046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.C., Buxton R.B., Frank L.R. Quantitative perfusion imaging using arterial spin labeling. Neuroimaging Clin. N. Am. 1999;9(2):333–342. [PubMed] [Google Scholar]
- Zhang Y., Schuff N., Ching C., Tosun D., Zhan W., Nezamzadeh M., Rosen H.J., Kramer J.H., Gorno-Tempini M.L., Miller B.L. Joint assessment of structural, perfusion, and diffusion MRI in alzheimer's disease and frontotemporal dementia. Int. J. Alzheimers Dis. 2011;2011:546871. doi: 10.4061/2011/546871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regions 1 of interest (ROIs).