Abstract
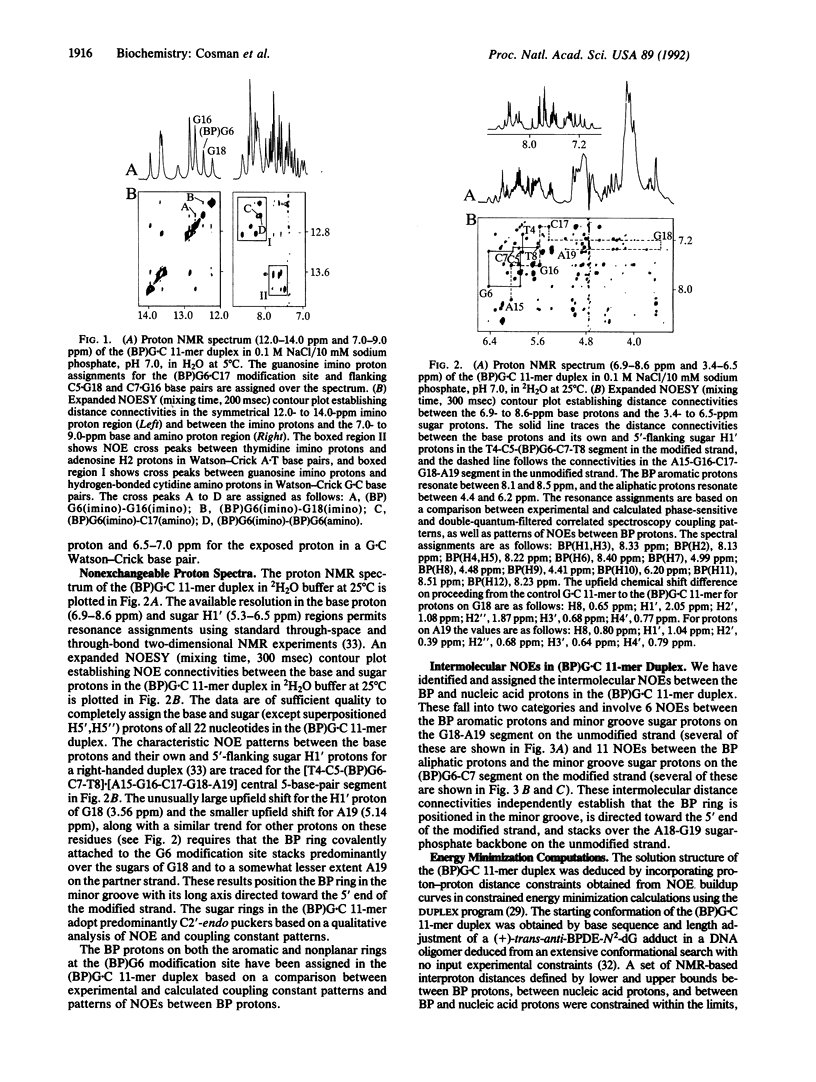
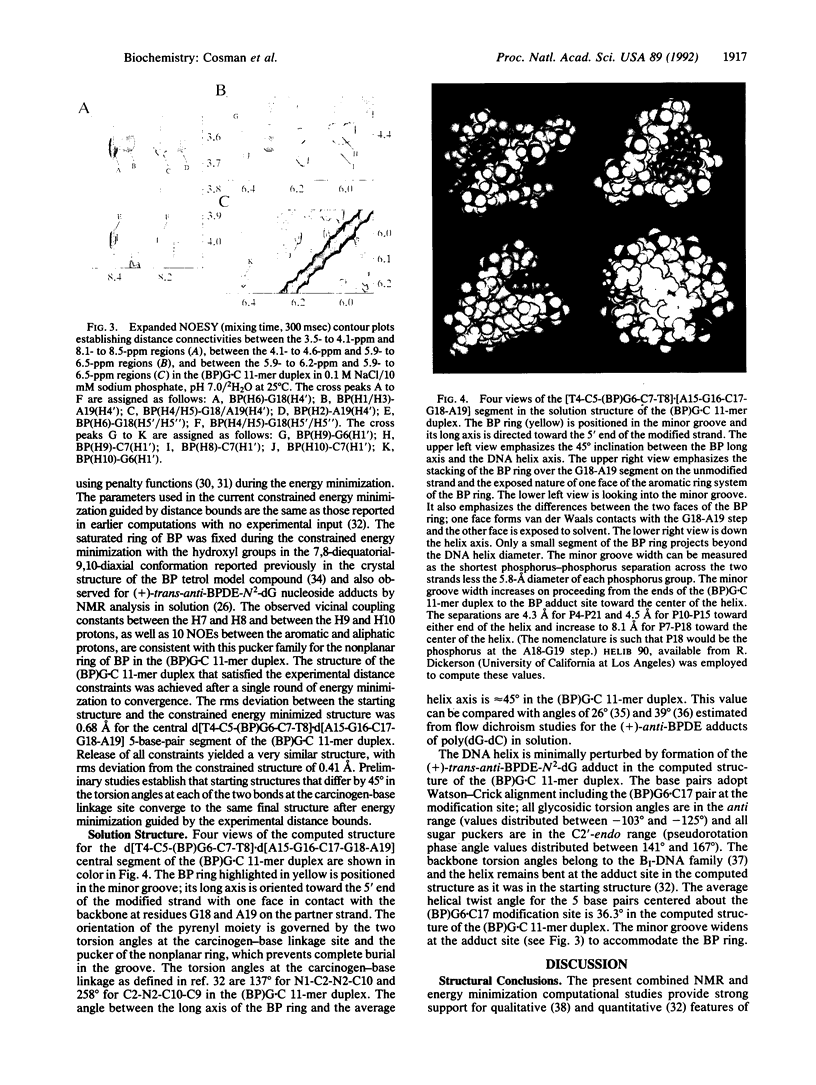
We have synthesized, separated, and purified approximately 10 mg of a deoxyundecanucleotide duplex containing a single centrally positioned covalent adduct between (+)-anti-benzo[a]pyrene (BP) diol epoxide and the exocyclic amino group of guanosine. Excellent proton NMR spectra are observed for the (+)-trans-anti-BP diol epoxide-N2-dG adduct positioned opposite dC and flanked by G.C pairs in the d[C1-C2-A3-T4-C5-(BP)G6-C7-T8-A9-C10-C11].d[12- G13-T14-A15-G16-C17-G18-A19-T20-G 21-G22] duplex +ADdesignated (BP)G.C 11-mer+BD. We have determined the solution structure centered about the BP covalent adduct site in the (BP)G.C 11-mer duplex by incorporating intramolecular and intermolecular proton-proton distance bounds deduced from the NMR data sets as constraints in energy minimization computations. The BP ring is positioned in the minor groove and directed toward the 5' end of the modified strand. One face of the BP ring of (BP)G6 is stacked over the G18 and A19 sugar-phosphate backbone on the partner strand and the other face is exposed to solvent. A minimally perturbed B-DNA helix is observed for the d[T4-C5-(BP)G6-C7-T8].d[A15-G16-C17-G18-A19] segment centered about the adduct site with Watson-Crick alignment for both the (BP)G6.C17 pair and flanking G.C pairs. A widening of the minor groove at the adduct site is detected that accommodates the BP ring whose long axis makes an angle of approximately 45 degrees with the average direction of the DNA helix axis. Our study holds future promise for the characterization of other steroisomerically pure adducts of BP diol epoxides with DNA to elucidate the molecular basis of structure-activity relationships associated with the stereoisomer-dependent spectrum of mutational and carcinogenic activities.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A. K., Islam S. A., Neidle S. Computer modelling studies of the covalent interactions between DNA and the enantiomers of anti-7,8-diol,9,10-epoxy-benzo[a]pyrene. J Biomol Struct Dyn. 1983 Dec;1(4):873–881. doi: 10.1080/07391102.1983.10507490. [DOI] [PubMed] [Google Scholar]
- Andersen R. W., Whitlow M. D., Teeter M. M., Mohr S. C. A-DNA accommodates adducts derived from diol epoxides of polycyclic aromatic hydrocarbons bound in a "side-stacking" mode. J Biomol Struct Dyn. 1987 Oct;5(2):383–404. doi: 10.1080/07391102.1987.10506401. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Oncogenes and human cancer: cause or consequence? Carcinogenesis. 1986 Jul;7(7):1037–1042. doi: 10.1093/carcin/7.7.1037. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Brookes P., Osborne M. R. Mutation in mammalian cells by stereoisomers of anti-benzo[a] pyrene-diolepoxide in relation to the extent and nature of the DNA reaction products. Carcinogenesis. 1982;3(10):1223–1226. doi: 10.1093/carcin/3.10.1223. [DOI] [PubMed] [Google Scholar]
- Buening M. K., Wislocki P. G., Levin W., Yagi H., Thakker D. R., Akagi H., Koreeda M., Jerina D. M., Conney A. H. Tumorigenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides in newborn mice: exceptional activity of (+)-7beta,8alpha-dihydroxy-9alpha,10alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5358–5361. doi: 10.1073/pnas.75.11.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R. H., Maher V. M., McCormick J. J. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8680–8684. doi: 10.1073/pnas.87.21.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. C., Hilton B. D., Roman J. M., Dipple A. DNA adducts from carcinogenic and noncarcinogenic enantiomers of benzo[a]pyrene dihydrodiol epoxide. Chem Res Toxicol. 1989 Sep-Oct;2(5):334–340. doi: 10.1021/tx00011a011. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Cosman M., Ibanez V., Geacintov N. E., Harvey R. G. Preparation and isolation of adducts in high yield derived from the binding of two benzo[a]pyrene-7,8-dihydroxy-9,10-oxide stereoisomers to the oligonucleotide d(ATATGTATA). Carcinogenesis. 1990 Sep;11(9):1667–1672. doi: 10.1093/carcin/11.9.1667. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R., Miller J. A., Miller E. C., Yang N. C. Covalent intercalative binding to DNA in relation to the mutagenicity of hydrocarbon epoxides and N-acetoxy-2-acetylaminofluorene. Cancer Res. 1978 Oct;38(10):3247–3255. [PubMed] [Google Scholar]
- Eriksson M., Nordén B., Jernström B., Gräslund A. Binding geometries of benzo[a]pyrene diol epoxide isomers covalently bound to DNA. Orientational distribution. Biochemistry. 1988 Feb 23;27(4):1213–1221. doi: 10.1021/bi00404a022. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E., Gagliano A., Ivanovic V., Weinstein I. B. Electric linear dichroism study on the orientation of benzo[alpha]pyrene-7,8-dihydrodiol 9,10-oxide covalently bound to DNA. Biochemistry. 1978 Nov 28;17(24):5256–5262. doi: 10.1021/bi00617a027. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E., Zinger D., Ibanez V., Santella R., Grunberger D., Harvey R. G. Properties of covalent benzo[a]pyrene diol epoxide-DNA adducts investigated by fluorescence techniques. Carcinogenesis. 1987 Jul;8(7):925–935. doi: 10.1093/carcin/8.7.925. [DOI] [PubMed] [Google Scholar]
- Gräslund A., Jernström B. DNA-carcinogen interaction: covalent DNA-adducts of benzo(a)pyrene 7,8-dihydrodiol 9,10-epoxides studied by biochemical and biophysical techniques. Q Rev Biophys. 1989 Feb;22(1):1–37. [PubMed] [Google Scholar]
- Hare D. R., Wemmer D. E., Chou S. H., Drobny G., Reid B. R. Assignment of the non-exchangeable proton resonances of d(C-G-C-G-A-A-T-T-C-G-C-G) using two-dimensional nuclear magnetic resonance methods. J Mol Biol. 1983 Dec 15;171(3):319–336. doi: 10.1016/0022-2836(83)90096-7. [DOI] [PubMed] [Google Scholar]
- Hingerty B. E., Figueroa S., Hayden T. L., Broyde S. Prediction of DNA structure from sequence: a build-up technique. Biopolymers. 1989 Jul;28(7):1195–1222. doi: 10.1002/bip.360280703. [DOI] [PubMed] [Google Scholar]
- Hogan M. E., Dattagupta N., Whitlock J. P., Jr Carcinogen-induced alteration of DNA structure. J Biol Chem. 1981 May 10;256(9):4504–4513. [PubMed] [Google Scholar]
- Jeffrey A. M., Jennette K. W., Blobstein S. H., Weinstein I. B., Beland F. A., Harvey R. G., Kasal H., Miura I., Nakanishi K. Letter: Benzo[a]pyrene-nucleic acid derivative found in vivo: structure of a benzo[a]pyrenetetrahydrodiol epoxide-guanosine adduct. J Am Chem Soc. 1976 Sep 1;98(18):5714–5715. doi: 10.1021/ja00434a060. [DOI] [PubMed] [Google Scholar]
- Koreeda M., Moore P. D., Yagi H., Yeh H. J., Jerina D. M. Alkylation of polyguanylic acid at the 2-amino group and phosphate by the potent mutagen (+/-)-7beta,8alpha-dihydroxy-9beta,10beta-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene. J Am Chem Soc. 1976 Oct 13;98(21):6720–6722. doi: 10.1021/ja00437a061. [DOI] [PubMed] [Google Scholar]
- Krugh T. R., Graves D. E., Stone M. P. Two-dimensional NMR studies on the anthramycin-d(ATGCAT)2 adduct. Biochemistry. 1989 Dec 26;28(26):9988–9994. doi: 10.1021/bi00452a017. [DOI] [PubMed] [Google Scholar]
- Lawley P. D. Mutagens as carcinogens: development of current concepts. Mutat Res. 1989 Jul;213(1):3–25. doi: 10.1016/0027-5107(89)90028-6. [DOI] [PubMed] [Google Scholar]
- Loeb L. A. Endogenous carcinogenesis: molecular oncology into the twenty-first century--presidential address. Cancer Res. 1989 Oct 15;49(20):5489–5496. [PubMed] [Google Scholar]
- Loechler E. L. Adduct-induced base-shifts: a mechanism by which the adducts of bulky carcinogens might induce mutations. Biopolymers. 1989 May;28(5):909–927. doi: 10.1002/bip.360280502. [DOI] [PubMed] [Google Scholar]
- Marshall C. J., Vousden K. H., Phillips D. H. Activation of c-Ha-ras-1 proto-oncogene by in vitro modification with a chemical carcinogen, benzo(a)pyrene diol-epoxide. Nature. 1984 Aug 16;310(5978):586–589. doi: 10.1038/310586a0. [DOI] [PubMed] [Google Scholar]
- Meehan T., Straub K., Calvin M. Benzo[alpha]pyrene diol epoxide covalently binds to deoxyguanosine and deoxyadenosine in DNA. Nature. 1977 Oct 20;269(5630):725–727. doi: 10.1038/269725a0. [DOI] [PubMed] [Google Scholar]
- Neidle S., Subbiah A., Kuroda R., Cooper C. S. Molecular structure of (+/-)-7,8,9,10-tetrahydroxy-7,8,9,10-tetrahydrobenzo(a)pyrene determined by x-ray crystallography. Cancer Res. 1982 Sep;42(9):3766–3768. [PubMed] [Google Scholar]
- Norman D., Abuaf P., Hingerty B. E., Live D., Grunberger D., Broyde S., Patel D. J. NMR and computational characterization of the N-(deoxyguanosin-8-yl)aminofluorene adduct [(AF)G] opposite adenosine in DNA: (AF)G[syn].A[anti] pair formation and its pH dependence. Biochemistry. 1989 Sep 5;28(18):7462–7476. doi: 10.1021/bi00444a046. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Hare D. DNA and RNA: NMR studies of conformations and dynamics in solution. Q Rev Biophys. 1987 Aug;20(1-2):35–112. doi: 10.1017/s0033583500004224. [DOI] [PubMed] [Google Scholar]
- Rao S. N., Lybrand T., Michaud D., Jerina D. M., Kollman P. A. Molecular mechanics simulations on covalent complexes between polycyclic carcinogens and B-DNA. Carcinogenesis. 1989 Jan;10(1):27–38. doi: 10.1093/carcin/10.1.27. [DOI] [PubMed] [Google Scholar]
- Roche C. J., Jeffrey A. M., Mao B., Alfano A., Kim S. K., Ibanez V., Geacintov N. E. Dependence of conformations of benzo[a]pyrene diol epoxide-DNA adducts derived from stereoisomers of different tumorigenicities on base sequence. Chem Res Toxicol. 1991 May-Jun;4(3):311–317. doi: 10.1021/tx00021a009. [DOI] [PubMed] [Google Scholar]
- Singh S. B., Hingerty B. E., Singh U. C., Greenberg J. P., Geacintov N. E., Broyde S. Structures of the (+)- and (-)-trans-7,8-dihydroxy-anti-9,10-epoxy-7,8,9,10-tetrahydrobenzo(a)pyre ne adducts to guanine-N2 in a duplex dodecamer. Cancer Res. 1991 Jul 1;51(13):3482–3492. [PubMed] [Google Scholar]
- Slaga T. J., Bracken W. J., Gleason G., Levin W., Yagi H., Jerina D. M., Conney A. H. Marked differences in the skin tumor-initiating activities of the optical enantiomers of the diastereomeric benzo(a)pyrene 7,8-diol-9,10-epoxides. Cancer Res. 1979 Jan;39(1):67–71. [PubMed] [Google Scholar]
- Stevens C. W., Bouck N., Burgess J. A., Fahl W. E. Benzo[a]pyrene diol-epoxides: different mutagenic efficiency in human and bacterial cells. Mutat Res. 1985 Oct;152(1):5–14. doi: 10.1016/0027-5107(85)90040-5. [DOI] [PubMed] [Google Scholar]
- Van de Ven F. J., Hilbers C. W. Nucleic acids and nuclear magnetic resonance. Eur J Biochem. 1988 Dec 1;178(1):1–38. doi: 10.1111/j.1432-1033.1988.tb14425.x. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Oncogenes, antioncogenes, and the molecular bases of multistep carcinogenesis. Cancer Res. 1989 Jul 15;49(14):3713–3721. [PubMed] [Google Scholar]
- Weinstein I. B. The origins of human cancer: molecular mechanisms of carcinogenesis and their implications for cancer prevention and treatment--twenty-seventh G.H.A. Clowes memorial award lecture. Cancer Res. 1988 Aug 1;48(15):4135–4143. [PubMed] [Google Scholar]
- Weston A., Newman M. J., Mann D. L., Brooks B. R. Molecular mechanics and antibody binding in the structural analysis of polycyclic aromatic hydrocarbon-diol-epoxide--DNA adducts. Carcinogenesis. 1990 May;11(5):859–864. doi: 10.1093/carcin/11.5.859. [DOI] [PubMed] [Google Scholar]
- Wood A. W., Chang R. L., Levin W., Yagi H., Thakker D. R., Jerina D. M., Conney A. H. Differences in mutagenicity of the optical enantiomers of the diastereomeric benzo[a]pyrene 7,8-diol-9,10-epoxides. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1389–1396. doi: 10.1016/s0006-291x(77)80133-2. [DOI] [PubMed] [Google Scholar]