Abstract
Despite the clinical importance of Helicobacter pylori in human gastric disorders, its exact route of transmission is still uncertain. Based on the contentious hypothesis and findings of previous investigations, water may play an important role in the transmission of H. pylori to humans. This study was carried out to investigate the vacA, cagA, oipA, iceA and babA2 genotype status and antimicrobial resistance properties of H. pylori strains isolated from the drinking water samples of four major provinces in Iran. A total of 400 drinking water samples were cultured and tested. H. pylori‐positive strains were analyzed for the presence of various genotypes and antimicrobial resistance. Twelve of 400 (3%) water samples were positive for H. pylori. Samples from Isfahan province had the highest, while those from Shiraz had the lowest prevalence of H. pylori. The seasonal distribution was also determined, with the highest prevalence of bacteria in the summer season (7.36%). H. pylori strains harbored the highest levels of resistance against ampicillin (100%), erythromycin (75%), clarithromycin (75%), and trimethoprim (58.3%). The most commonly detected genotypes were vacAs1a (83.3%), vacAm1a (66.6%), vacAs2 (50%) and cagA (50%). The presence of similar genotypes in the H. pylori strains of drinking water and those of human clinical samples suggest that contaminated water maybe the sources of bacteria. Spiramycin and furazolidone are suggested for the treatment of cases of H. pylori infection.
Keywords: antibiotic resistance properties, drinking water, genotypes, genotyping, Helicobacter pylori, Iran
Abbreviations
- AM
ampicillin
- AMX
amoxicillin
- babA
blood group antigen‐binding adhesion
- cag
cytotoxin‐associated gene
- Cef
cefsulodin
- CLR
clarithromycin
- CLSI
Clinical Laboratory Standards Institute
- ER
erythromycin
- FZL
furazolidone
- H. pylori
Helicobacter pylori
- iceA
induced by contact with the epithelium antigen
- Lev
levofloxacin
- Met
metronidazole
- oip
outer inflammatory protein
- RIF
rifampin
- Spi
spiramycin
- TRP
trimethoprim
- vacA
vacuolating cytotoxin
Helicobacter pylori is a gram‐negative, microaerophilic and spiral‐shaped bacterium that efficiently colonizes the human gastric mucosa 1. Bacterial colonization of the gastric mucosa is the main cause of ulcers in the stomach and duodenum 1. H. pylori is also known as a causative agent of peptic ulcer disease, type B gastritis, gastric adenocarcinoma, and mucosa‐associated lymphoid tissue lymphoma 1, 2. It has been estimated that 17–86% of hospitalized patients with peptic ulcers were infected with H. pylori 2, 3, 4. Documented data showed that the H. pylori colonizes more than 50% of the world's population 5. Despite the high incidence of bacteria in human populations, no reservoir outside of the human stomach has been identified 6. Transmission presumably occurs through fecal–oral and oral–oral routes. One of the most commonly reported reservoir of the H. pylori outside of human stomach is contaminated water 7, 8. An epidemiological association between water sources and the prevalence of H. pylori infection has been identified 8. The hypothesis of water being a route of transmission of H. pylori 8, 9, 10 is supported by epidemiologic studies that have observed a higher prevalence of H. pylori infection in developing countries which suffered from problems related to the sanitary distribution of water among the population 11.
To appraise the pathogenicity of H. pylori especially in possible sources of transmission like water, evaluation of latent virulence factors and genotypes is essential. The most commonly important virulence factors among H. pylori strains of different clinical outcomes of human and animal beings are the vacuolating cytotoxin (vacA), induced by contact with the epithelium antigen (iceA), cytotoxin‐associated gene (cag), blood group antigen‐binding adhesion (babA), and outer inflammatory protein (oip) 12, 13, 14, 15, 16. These genes are usually induced by adhesion and invasion to the gastric epithelial cells 12, 13, 14, 15, 16. Genotyping using these virulence markers is considered as one of the best approaches to determinate correlations between H. pylori isolates from different samples.
In order to appraise the pathogenicity of H. pylori, study the antimicrobial resistance properties is another important point. Treatment is a critical point in the epidemiology of H. pylori infection in humans, since therapeutic options have become somewhat limited because of the presence of multidrug‐resistant strains of this bacterium 14, 15, 16, 17. Moreover, to the best of our knowledge, we could not find any published data on the antibiotic resistance pattern of H. pylori strains isolated from drinking water samples.
Data on the epidemiology and transmission of H. pylori is extremely significant in order to prevent its distribution and to identify high‐risk populations, especially in areas that have high rates of gastritis, peptic ulcers, and gastric cancer such as Iran 13, 17, 18, 19. Considering the unclear epidemiological aspects of H. pylori in Iranian drinking water sources, the present investigation was carried out in order to study the exact status of vacA, cagA, iceA, oipA and babA2 genotypes and the antibiotic resistance patterns of H. pylori isolates from drinking water samples.
Materials and methods
Sample collection
From January 2014 to January 2015, overall 400 drinking water samples were collected from the various parts of Isfahan, Shiraz, Yazd, and Shahrekord province, Iran. All samples were collected from various seasons of the year including summer (n = 95), autumn (n = 100), winter (n = 110), and spring (n = 95). Samples (100 mL in 1000‐mL glass bottles containing 0.5 g of sodium thiosulphate for dechlorination) were transported to the lab on ice, and used within 2 h of collection. All samples were collected in aseptic conditions away from any cross‐contamination in separate glass bottles. Drinking water samples of these major cities of Iran were treated using chlorination.
Isolation of H. pylori
Samples were filtered through 0.45‐μm filter membrane (Albet Co., Barcelona, Spain). Each membrane was then immersed into 2 mL of Tryptic Soy Broth (TSB, Merck, Darmstadt, Germany) for 1 h. After that, each 2 mL TSB was taken and cultured for H. pylori. Samples were cultured on Brucella agar (Merck) containing campylobacter‐selective supplement (5 mg·L−1, Merck), trimethoprim (0.25 mg·L−1), colistin methanesulfonate (30 mg·L−1), cycloheximide (100 mg·L−1), nalidixic acid (30 mg·L−1), trimethoprim (30 mg·L−1), vancomycin (10 mg·L−1) (Sigma, St Louis, MO, USA), amphotericin B (10 mg·L−1), sheep blood (5%), and 7% fetal calf serum (Sigma). After 72 h incubation at 37 °C in microaerophilic condition (85% N2, 10% CO2 and 5% O2,) using MART system (Anoxamat, Lichtenvoorde, The Netherlands), the bacterial growth was tested and confirmed as H. pylori using Gram staining, urease, and oxidase tests. For comparison, a reference strain of H. pylori (ATCC 43504) was employed.
Antimicrobial susceptibility testing
Pure cultures of H. pylori were applied for antibiotic susceptibility test. One strain from each H. pylori‐positive sample was selected for this aim. Antimicrobial susceptibility test was accomplished by the Kirby–Bauer disk diffusion method using Mueller–Hinton agar (Merck) supplemented with 5% defibrinated sheep blood and 7% fetal calf serum, according to the Clinical Laboratory Standards Institute (CLSI, 2012) 20. The antimicrobial resistance of H. pylori was measured against the widely used antibiotics in cases of H. pylori gastric ulcer. The following antimicrobial disks (HiMedia Laboratories, Mumbai, India) were used: ampicillin (10 μg), metronidazole (5 μg), erythromycin (5 μg), clarithromycin (2 μg), amoxicillin (10 μg), levofloxacin (5 μg), rifampin (30 μg), cefsulodin (30 μg), trimethoprim (25 μg), furazolidone (1 μg) and spiramycin (100 μg). After incubation at 37 °C for 48 h in a microaerophilic atmosphere (85% N2, 10% CO2, and 5% O2,), the susceptibility of the H. pylori was measured against each antimicrobial agents. Results were construed in accordance with interpretive criteria provided by CLSI 20. The H. pylori ATCC 43504 was used as quality control organisms in antimicrobial susceptibility determination.
DNA extraction and H. pylori 16S rRNA gene amplification
Suspected colonies were also identified as H. pylori based on the PCR technique. Genomic DNA was extracted from the colonies with typical characters of H. pylori using a DNA extraction kit for cells and tissues (Roche Applied Science, Mannheim, Germany, 11814770001) according to the manufacturer's instructions and its concentration was assessed by optic densitometry. Extracted DNA was amplified for the 16S rRNA gene (primers: HP‐F: 5′‐CTGGAGAGACTAAGCCCTCC‐3′ and HP‐R: 5′‐ATTACTGACGCTGATTGTGC‐3′) 21. PCR reactions were performed in a final volume of 50 μL containing 5 μL 10× buffer (Fermentas, Mannheim, Germany) + MgCl2, 2 mm dNTP (Fermentas), 2 units of Taq DNA polymerase (Fermentas), 100 ng genomic DNA as a template, and 25 picomole of each primer. PCR was performed using a thermal cycler (Eppendorf Co., Hamburg, Germany) under the following conditions: an initial denaturation for 2 min at 94 °C; 30 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s and a final extension at 72 °C for 8 min. H. pylori (ATCC 43504) was employed as a positive control in this part of the study.
Genotyping of vacA, cagA, iceA, babA2, and oipA genotypes in the H. pylori isolates of drinking water
The genotype data refer to pooled colonies (individual water samples) and not individual isolates of H. pylori. The presence of the iceA1, iceA2, oipA, cagA, babA2 genotypes and also various genotypes of vacA alleles (s1a, s1b, s1c, m1a, m1b and m2) were determined using PCR technique. List of primers and PCR program are shown in Table 1 22, 23, 24, 25, 26, 27, 28, 29, 30. PCR amplifications were performed in a programmable thermal cycler (Master Cycle Gradiant; Eppendorf) and all runs included one negative DNA control consisting of PCR grade water and two or more positive controls (26695, J99, SS1, Tx30, 88‐23 and 84–183).
Table 1.
Oligonucleotide primers and PCR conditions used for genotyping of Helicobacter pylori strains isolated from Iranian drinking water samples 22, 23, 24, 25, 26, 27, 28, 29, 30
| Genes | Primer sequence (5′–3′) | Size of product (bp) | Volume of PCR reaction (50 μL) | PCR programs |
|---|---|---|---|---|
| vacA s 1 a |
F: CTCTCGCTTTAGTAGGAGC
R: CTGCTTGAATGCGCCAAAC |
213 | ||
| vacA s 1 b | F: AGCGCCATACCGCAAGAG CTGCTTGAATGCGCCAAAC | 187 | ||
| vacA s 1 c |
F: CTCTCGCTTTAGTGGGGYT
R: CTGCTTGAATGCGCCAAAC |
213 |
5 μL PCR buffer 10× (Fermentas) 1.5 mm MgCl2 200 μm dNTP (Fermentas) 0.5 μm of each primers F and R 1.25 U Taq DNA polymerase (Fermentas) 2.5 μL DNA template |
1 cycle: 95 °C for 1 min 32 cycle: 95 °C for 45 s 64 °C for 50 s 72 °C for 70 s 1 cycle: 72 °C for 5 min |
| vacA s 2 |
F: GCTAACACGCCAAATGATCC
R: CTGCTTGAATGCGCCAAAC |
199 | ||
| vacA m 1 A |
F: GGTCAAAATGCGGTCATGG
R: CCATTGGTACCTGTAGAAAC |
290 | ||
| vacA m 1 B |
F: GGCCCCAATGCAGTCATGGA
R: GCTGTTAGTGCCTAAAGAAGCAT |
291 | ||
| vacA m 2 |
F: GGAGCCCCAGGAAACATTG
R: CATAACTAGCGCCTTGCA |
352 | ||
| cag A |
F: GATAACAGCCAAGCTTTTGAGG
R: CTGCAAAAGATTGTTTGGCAGA |
300 |
5 μL PCR buffer 10× (Fermentas) 2 mm MgCl2 150 μm dNTP (Fermentas) 0.75 μm of each primers F and R 1.5 U Taq DNA polymerase (Fermentas) 3 μL DNA template |
1 cycle: 94 °C for 1 min 32 cycle: 95 °C for 60 s 56 °C for 60 s 72 °C for 60 s 1 cycle: 72 °C for 10 min |
| iceA 1 |
F: GTGTTTTTAACCAAAGTATC
R: CTATAGCCASTYTCTTTGCA |
247 |
5 μL PCR buffer 10× (Fermentas) 2 mm MgCl2 200 μm dNTP (Fermentas) 0.5 μm of each primers F and R 1.5 U Taq DNA polymerase (Fermentas) 5 μL DNA template |
1 cycle: 94 °C for 1 min 32 cycle: 94 °C for 60 s 56 °C for 60 s 72 °C for 60 s 1 cycle: 72 °C for 8 min |
| iceA 2 |
F: GTTGGGTATATCACAATTTAT
R: TTRCCCTATTTTCTAGTAGGT |
229/334 | ||
| oip A |
F: GTTTTTGATGCATGGGATTT
R: GTGCATCTCTTATGGCTTT |
401 |
5 μL PCR buffer 10× (Fermentas) 2.5 mm MgCl2 200 μm dNTP (Fermentas) 0.5 μm of each primers F and R 2 U Taq DNA polymerase (Fermentas) 3 μL DNA template |
1 cycle: 94 °C for 2 min 32 cycle: 94 °C for 60 s 56 °C for 60 s 72 °C for 60 s 1 cycle: 72 °C for 10 min |
| BabA2 |
F: CCAAACGAAACAAAAAGCGT
R: GCTTGTGTAAAAGCCGTCGT |
271 |
5 μL PCR buffer 10× (Fermentas) 1.5 mm MgCl2 200 μm dNTP (Fermentas) 0.5 μm of each primers F and R 1.25 U Taq DNA polymerase (Fermentas) 2.5 μL DNA template |
1 cycle: 95 °C for 1 min 30 cycle: 91 °C for 60 s 45 °C for 60 s 72 °C for 60 s 1 cycle: 72 °C for 8 min |
Gel electrophoresis
The PCR amplification products (10 μL) were subjected to electrophoresis in a 2% agarose gel in 1× TBE buffer (Fermentas) at 80 V for 30 min, stained with ethidium bromide, and images were obtained in a UVIdoc gel documentation systems (UK). The PCR products were identified by 100 bp DNA size marker (Fermentas).
Statistical analysis
Data were transferred to Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA, USA) for analysis. Using spss 16.0 statistical software (SPSS Inc., Chicago, IL, USA), Chi‐square test and Fisher's exact two‐tailed test were performed and differences were considered significant at values of P < 0.05. The distribution of H. pylori genotypes isolated from drinking water was statistically analyzed.
Results
A total of 400 drinking water samples were studied for the presence of H. pylori, its genotypes and antimicrobial resistance properties. Table 2 shows the total distribution of H. pylori in the drinking water samples of four major province of Iran. Of 400 drinking water samples collected, 12 samples (3%) were contaminated with H. pylori. The results of culture method were confirmed using the 16S rRNA‐based PCR technique. The water samples of Isfahan province were the most contaminated, while those of Shiraz province were less contaminated. Statistically significant difference was seen between the distributions of H. pylori and zone of sample collection (P < 0.05). Seasonal distribution of H. pylori in the drinking water samples of various parts of Iran is shown in Fig. 1. We found that the drinking water samples of summer seasons had the highest levels of H. pylori‐contamination (7.36%), followed by spring and autumn (2.1% and 2% respectively). Statistically significant difference was seen between the distributions of H. pylori and season of sample collection (P < 0.05).
Table 2.
Total distribution of Helicobacter pylori in the drinking water samples in four major provinces of Iran
| Province | No. of samples collected | No. of H. pylori‐positive samples (%) | No. of H. pylori‐positive samples confirmed by PCR (%) |
|---|---|---|---|
| Isfahan | 120 | 5 (4.16) | 5 (4.16) |
| Shiraz | 110 | 2 (1.8) | 2 (1.8) |
| Yazd | 100 | 3 (3) | 3 (3) |
| Shahrekord | 70 | 2 (2.8) | 2 (2.8) |
| Total | 400 | 12 (3) | 12 (3) |
Figure 1.
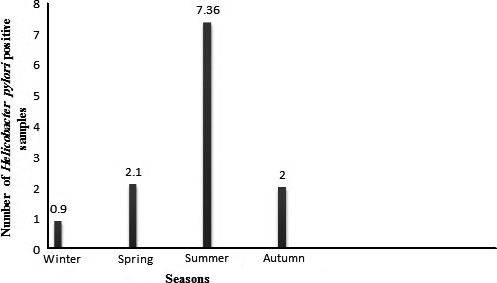
Seasonal distribution of Helicobacter pylori in Iranian drinking water samples. The number of samples collected in summer, autumn, winter, and spring seasons were 95, 100, 110 and 95, respectively. Number of positive strains obtained from the water samples collected from summer, autumn, winter, and spring seasons were seven, two, one, and two strains, respectively. Prevalence of H. pylori in each season is shown by percentage.
The results of antimicrobial resistance patterns of H. pylori isolates of Iranian drinking water samples is shown in Table 3. H. pylori strains of our investigation harbored the highest levels of resistance against ampicillin (100%), erythromycin (75%), clarithromycin (75%) and trimethoprim (58.3%) antimicrobial agents. There were statistically significant differences in the levels of antibiotic resistance between ampicillin and rifampin (P = 0.022), ampicillin and cefsulodin (P = 0.028), clarithromycin and furazolidone (P = 0.033), ampicillin and spiramycin (P = 0.034), and amoxicillin and furazolidone (P = 0.029).
Table 3.
Antimicrobial resistance pattern of Helicobacter pylori isolates from Iranian drinking water samples
| Types of Samples (no. positive results) | Pattern of antibiotic resistance (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AM10a | Met5 | ER5 | CLR2 | AMX10 | Lev5 | RIF30 | Cef30 | TRP25 | FZL1 | Spi100 | |
| Isfahan (5) | 5 (100) | 3 (60) | 4 (80) | 4 (80) | 3 (60) | 3 (60) | 2 (40) | 2 (40) | 3 (60) | 1 (20) | 1 (20) |
| Shiraz (2) | 2 (100) | 1 (50) | 2 (100) | 2 (100) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | – | – |
| Yazd (3) | 3 (100) | 1 (33.3) | 2 (66.6) | 2 (66.6) | 1 (33.3) | 1 (33.3) | – | – | 2 (66.6) | – | – |
| Shahrekord (2) | 2 (100) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | – | – | 1 (50) | – | 1 (50) |
| Total (12) | 12 (100) | 6 (50) | 9 (75) | 9 (75) | 6 (50) | 6 (50) | 3 (25) | 3 (25) | 7 (58.3) | 1 (8.3) | 2 (16.6) |
AM10: ampicillin (10 μg), Met5: metronidazole (5 μg), ER5: erythromycin (5 μg), CLR2: clarithromycin (2 μg), AMX10: amoxicillin (10 μg), Lev5: levofloxacin (5 μg), RIF30: rifampin (30 μg), Cef30: cefsulodin (30 μg), TRP25: trimethoprim (25 μg), FZL1: furazolidone (1 μg), and Spi100: spiramycin (100 μg).
Distribution of various genotypes of vacA alleles, cagA, iceA1, iceA2, oipA and babA2 is shown in table 4. The most commonly detected genotypes among the H. pylori isolates of drinking water were vacAs1a (83.3%), vacAm1a (66.6%), vacAs2 (50%) and cagA (50%). The prevalence of iceA1, iceA2, oipA and babA2 genotypes were 41.6%, 16.6%, 33.3% and 16.6%, respectively.
Table 4.
Total distribution of various genotypes in Helicobacter pylori strains of Iranian drinking water samples
| Types of samples (no. of positive results) | Distribution of various genotypes (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| s1a | s1b | s1c | s2 | m1a | m1b | m2 | cagA | iceA1 | iceA2 | oipA | babA2 | |
| Isfahan (5) | 4 (80) | 2 (40) | 1 (20) | 3 (60) | 4 (80) | 2 (40) | 2 (40) | 3 (60) | 2 (40) | 1 (20) | 2 (40) | 1 (20) |
| Shiraz (2) | 2 (100) | 1 (50) | – | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | – | – | – |
| Yazd (3) | 2 (66.6) | 1 (33.3) | – | 1 (33.3) | 2 (66.6) | – | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (33.3) | 1 (33.3) |
| Shahrekord (2) | 2 (100) | 1 (50) | – | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | 1 (50) | – | 1 (50) | – |
| Total (12) | 10 (83.3) | 5 (41.6) | 1 (8.3) | 6 (50) | 8 (66.6) | 4 (33.3) | 5 (41.6) | 6 (50) | 5 (41.6) | 2 (16.6) | 4 (33.3) | 2 (16.6) |
Discussion
The present investigation was carried out to study the prevalence of H. pylori in the drinking water samples of four major provinces in Iran as well as to determine the vacA, cagA, iceA, oipA and babA2 genotype status and antibiotic resistance properties of bacterial isolates. Our results showed that 3% of drinking water samples were contaminated with H. pylori. Although the prevalence of bacterium is low, according to daily and high consumption of water, it is very impressive. Despite the results of a previous study which revealed that viable helicobacters were not detected in any of the 151 samples from the United Kingdom 31, the results of our investigation showed that all of the H. pylori isolates of drinking water were viable in culture media. Bahrami et al. 32 tried to detect H. pylori in city water, dental units’ water, and bottled mineral water of Iran. Their results showed that the prevalence of H. pylori in 2 of 50 tap water samples (4%), 2 of 35 dental units’ water (5.8%) samples, and 1 of 40 water coolers in public places (3%) were contaminated with H. pylori. Some possible reasons for the high prevalence of H. pylori in drinking water samples in Iran are (a) the lack of efficient methods for water purification, (b) using river water for drinking in some areas of Iran like Isfahan and Yazd province, (c) the possibility of the presence of bacterial colonies as a biofilm in the pipes used for water transfer, (d) application of open water accumulation sources in some areas like Shahrekord and Shiraz, (e) the possibility of leakage of household, industrial, and agricultural wastewater to the sources of drinking water, and finally (f) lack of personal hygiene of refinery rooms’ staff. One possible explanation for the higher prevalence of H. pylori in the drinking water of Isfahan province is due to primary contamination of the Zayande‐rood River. The Zayande‐rood River is the main source of drinking water supply of the Isfahan province. This river comes from the Zagros Mountains. After passing through several towns, agricultural lands, and industrial areas, the river reaches the Isfahan steel company and then enters the Isfahan purification facility. Several sources of pollution including Isfahan steel company, towns, villages, industrial factories, and agriculture exist along the path of this river. Entrance of industrial, agricultural, urban and rural waste, and waste waters into the Zayande‐rood River are the main source of water contamination. In addition to Isfahan, the Zayande‐rood River are the main source of water supply for Yazd province. Therefore, primary contamination of Zayande‐rood River and weak performance of refinery rooms are two important factors which support the significant presence of H. pylori in the drinking water of Yazd province. Despite our finding, no H. pylori was found in the water sources of several studies 11, 33, 34. This could be attributed to the fact that H. pylori can survive for a short period of time in water 33. Moreover, the method employed for H. pylori isolation may lack sufficient sensitivity to recover very low numbers of H. pylori 33, 35, 36.
Marked seasonality with the high prevalence in summer season was seen in the H. pylori strains of drinking water of our study. The main reason for the highest prevalence of H. pylori strains in summer in Iran is the fact that during this time climatic events, heat, rain, and thunderstorms, as well as variation in barometric pressure may have influence on the prevalence of bacteria. After analyzing the average temperatures of these four seasons in Isfahan province (17 °C for spring, 32 °C for summer, 16 °C for autumn and 5 °C for winter), it was determined that the prevalence rate of H. pylori strains in each season is related with their average temperatures. Yahaghi et al. 15 reported the similar seasonal distribution of the H. pylori strains in vegetable and salad samples. They showed statistically significant differences in the incidence of H. pylori between hot and cold seasons of the year.
The results of our study revealed that the presence of H. pylori in drinking water could be associated with clinical infections. It is because of the high presence of resistant and virulent strains of H. pylori in Iranian drinking water samples. We found that the bacterial strains of our investigation harbored the high levels of resistance against ampicillin (100%), erythromycin (75%), clarithromycin (75%), trimethoprim (58.3%), metronidazole (50%), amoxicillin (50%) and levofloxacin (50%) antimicrobial agents. Similar findings have been reported previously by Thyagarajan et al. 37, Yahaghi et al. 15, Bang et al. 38, and Secka et al. 39. Bang et al. 38 reported that the H. pylori isolates of human clinical samples were highly resistant to metronidazole (34.7%), clarithromycin (16.7%), and amoxicillin (11.8%). Mirzaei et al. 40 reported that the prevalence of resistance of H. pylori isolates of Iranian clinical samples against metronidazole, clarithromycin, and amoxicillin were 56.3%, 14.6% and 4.2%, respectively. Previous study which was conducted on drinking water showed that the H. pylori isolates were resistant against metronidazole (36.4%), clarithromycin (0.9%), amoxicillin (0%), tetracycline (1.8%) and furazolidone (4.5%) 41. Epidemiological investigations of Iran, Nigeria, India, Senegal, China, Saudi Arabia, Taiwan, Colombia, Thailand, Brazil, Egypt and Argentina showed that the H. pylori isolates of human clinical specimens had the highest levels of resistance against metronidazole, amoxicillin, quinolones, and tetracycline (WGO 42) which was similar to our results.
The prevalence of resistance against human‐based antimicrobial agents in the H. pylori strains of drinking water samples could indirectly confirm the human‐based routes of water infections. One possible explanation for the high prevalence of resistance against ampicillin (100%), erythromycin (75%), clarithromycin (75%), trimethoprim (58.3%), metronidazole (50%), amoxicillin (50%) and levofloxacin (50%) antimicrobial agents in our study maybe the excessive and indiscriminate prescription of these antibiotics in the treatment of cases of H. pylori infections in Iran. The possibility of considering spiramycin and furazolidone antibiotics as an alternative for treatment of H. pylori could be suggested in Iranian cases of infections. We found impressive high percentage of resistances to clarithromycin (75%), ampicillin (100%), and amoxycillin (50%), which were much higher than those reported by other investigations worldwide 40, 41, 42. These antibiotics are one of the first‐choice treatment agents for H. pylori infection and the high prevalence of resistance against these antibiotics are due to the irregular, intense, and illegal prescription of clarithromycin not only for the cases of H. pylori infections but also for all types of infectious diseases of the digestive tract. This matter has serious country‐based concern.
Genotyping of H. pylori isolates showed that in the water samples of all studied areas of Iran, vacAs1a (83.3%), vacAm1a (66.6%), vacAs2 (50%) and cagA (50%) genotypes were high. As far as we know, only one study tried to study the genotype status of H. pylori strains of untreated municipal waste water samples 43. The results of this study showed the high presence of vacAs1a, vacAm1a, and vacAs1am1a. High presence of vacAs1a, vacAm1a, vacAs2, cagA alleles and also s1am1a, s2m1a, s1bm1a, s1bm1b, s1a m2, s2m2 and m1am2 genotypes in the H. pylori strains of human clinical samples such as gastric biopsy, feces, and saliva have been reported previously 44, 45, 46.
Total prevalence of iceA1, iceA2, oipA and babA2 genotypes in the H. pylori strains of our survey were 41.6%, 16.6%, 33.3% and 16.6%, respectively. In a study which was conducted in Iran on human clinical samples 47, the prevalence of cagA, iceA1, iceA2, oipA and babA2 genotypes were 62.2%, 48.6%, 16.2%, 81.1% and 94.6%, respectively. Similarity in the genotyping pattern of H. pylori in all provinces of our study and its close proximity with those of human clinical samples of other investigations have indirectly shown the human‐based contamination of drinking water samples in these areas of Iran.
Helicobacter pylori strains carrying the s1m1 mosaic combination of the gene vacA exhibit higher levels of cytotoxic activity than s1m2 strains, while s2m2 strains secrete the toxin with low or no vacuolating activity in vitro and is rarely associated with gastric disease 13, 14, 15, 48. The severity of diseases caused by strains which express babA is greater than diseases by strains that do not express the gene 49. The expression of iceA1 is upregulated on contact between H. pylori and human epithelial cells, and may be related with peptic ulcer disease. The expression of oipA is associated with IL‐8 induction and is related with severe clinical outcomes 13, 14, 15, 48.
With respect to the high levels of similarities in the H. pylori strains of drinking water of our study and those of other investigations which were conducted on clinical samples, it could be concluded that contaminated water may be the sources of H. pylori infection for humans. However, there are significant differences between the drinking water isolates and those from patients: resistance to clarithromycin and amoxicillin was much higher in the drinking water isolates than those found in Iranian clinical samples, and the presence of oipA‐ and babA2‐positive strains was much higher in clinical isolates than those from the drinking water in this study. The main reasons for the above findings are higher resistance of H. pylori strains of water samples than those of clinical isolates.
Conclusion
In conclusion, drinking water samples in Iran harbor H. pylori similar in genotypes of vacA, cagA, iceA, oipA and babA2 alleles with isolates recovered from various types of human clinical samples. High prevalence of H. pylori in our samples suggest that contaminated drinking water in these area of Iran maybe the sources of the bacteria and that it entered the human population after a period of time. There was no high diversity in the genotyping pattern of H. pylori between the different areas of Iran which may have shown that there was one source of contamination for drinking water. Prescription of spiramycin and furazolidone antibiotics as an alternative approach for treatment of H. pylori could be suggested.
Author contributions
All authors contributed equally to this work. All authors read and approved the final manuscript.
References
- 1. Suzuki R, Shiota S and Yamaoka Y (2012) Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori . Infect Genet Evol 12, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shrestha S, Paudel P, Pradhan GB, Shrestha L and Bhattachan CL (2012) Prevalence study of H. pylori infection in dyspeptic patients coming to Nepal Medical College Teaching Hospital, Jorpati, Kathmandu. Nepal Med Coll J 14, 229–233. [PubMed] [Google Scholar]
- 3. Vu C and Ng YY (2000) Prevalence of Helicobacter pylori in peptic ulcer disease in a Singapore hospital. Singapore Med J 41, 478–481. [PubMed] [Google Scholar]
- 4. Mastromarino P, Conti C, Donato K, Strappini PM, Cattaruzza MS and Orsi GB (2005) Does hospital work constitute a risk factor for Helicobacter pylori infection. J Hosp Infect 60, 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown LM (2000) Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev 22, 283–297. [DOI] [PubMed] [Google Scholar]
- 6. Allaker RP, Young KA, Hardie JM, Domizio P and Meadows NJ (2002) Prevalence of Helicobacter pylori at oral and gastrointestinal sites in children: evidence for possible oral‐tooral transmission. J Med Microbiol 51, 312–317. [DOI] [PubMed] [Google Scholar]
- 7. Baker KH and Hegarty JP (2001) Presence of Helicobacter pylori in drinking water is associated with clinical infection. Scand J Infect Dis 33, 744–746. [DOI] [PubMed] [Google Scholar]
- 8. Aziz RK, Khalifa MM and Sharaf RR (2015) Contaminated water as a source of Helicobacter pylori infection: a review. J Adv Res 6, 539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gião MS, Azevedo NF, Wilks SA, Vieira MJ and Keevil CW (2010) Effect of chlorine on incorporation of Helicobacter pylori into drinking water biofilms. Appl Environ Microbiol 76, 1669–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellack NR, Koehoorn MW, MacNab YC and Morshed MG (2006) A conceptual model of water's role as a reservoir in Helicobacter pylori transmission: a review of the evidence. Epidemiol Infect 134, 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Percival SL and Thomas JG (2009) Transmission of Helicobacter pylori and the role of water and biofilms. J Water Health 7, 469–477. [DOI] [PubMed] [Google Scholar]
- 12. Arévalo‐Galvis A, Trespalacios‐Rangell AA, Otero W, Mercado‐Reyes MM and Poutou‐Piñales RA (2012) Prevalence of cagA, vacA, babA2 and iceA genes in H. pylori strains isolated from Colombian patients with functional dyspepsia. Pol J Microbiol 61, 33–40. [PubMed] [Google Scholar]
- 13. Momtaz H, Dabiri H, Souod N and Gholami M (2014) Study of Helicobacter pylori genotype status in cows, sheep, goats and human beings. BMC Gastroenterol 14, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mousavi S, Safarpoor Dehkordi F and Rahimi E (2014) Virulence factors and antibiotic resistance of Helicobacter pylori isolated from raw milk and unpasteurized dairy products in Iran. J Venom Anim Toxins Incl Trop Dis 20, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yahaghi E, Khamesipour F, Mashayekhi F, Safarpoor Dehkordi F, Sakhaei MH, Masoudimanesh M and Khayyat Khameneie M (2014) Helicobacter pylori in vegetables and salads: genotyping and antimicrobial resistance properties. Biomed Res Int 2014, 757941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ranjbar R, Khamesipour F, Jonaidi‐Jafari N and Rahimi E (2016) Helicobacter pylori in bottled mineral water: genotyping and antimicrobial resistance properties. BMC Microbiol 16, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shokrzadeh L, Alebouyeh M, Mirzaei T, Farzi N and Zali MR (2015) Prevalence of multiple drug‐resistant Helicobacter pylori strains among patients with different gastric disorders in Iran. Microb Drug Resist 21, 105–110. [DOI] [PubMed] [Google Scholar]
- 18. Siavoshi F, Malekzadeh R, Daneshmand M and Ashktorab H (2005) Helicobacter pylori endemic and gastric disease. Dig Dis Sci 50, 2075–2080. [DOI] [PubMed] [Google Scholar]
- 19. Eshraghian A (2014) Epidemiology of Helicobacter pylori infection among the healthy population in Iran and countries of the Eastern Mediterranean Region: a systematic review of prevalence and risk factors. World J Gastroenterol 20, 17618–17625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clinical and Laboratory Standards Institute (CLSI) (2012). Performance Standards for Antimicrobial Susceptibility Testing. Twenty‐Second Informational Supplement M100‐S21. CLSI, Wayne, PA. [Google Scholar]
- 21. Ho SA, Hoyle JA and Lewis FA (1991) Direct polymerase chain reaction test for detection of Helicobacter pylori in humans and animals. J Clin Microbiol 29, 2543–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Versalovic J, Koeuth T and Lupski JR (1991) Distribution of repetitive DNA sequences in Eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 19, 6823–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tumurru MK, Cover TL and Blaser MJ (1993) Cloning and expression of a highmolecular mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun 61, 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ and Cover TL (1995) Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem 270, 17771–17777. [DOI] [PubMed] [Google Scholar]
- 25. Peek RM, Thompson SA, Donahue JP, Tham KT, Atherton JC, Blaser MJ and Miller GG (1998) Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians 110, 531–544. [PubMed] [Google Scholar]
- 26. Wang J, Chi DS, Laffan JJ, Li C, Ferguson DA Jr, Litchfield P and Thomas E (2002) Comparison of cytotoxin genotypes of Helicobacter pylori in stomach and saliva. Dig Dis Sci 47, 1850–1856. [DOI] [PubMed] [Google Scholar]
- 27. Kauser F, Hussain MA, Ahmed I, Habeeb A, Khan AA and Ahmed N (2005) Comparing genomes of Helicobacter pylori strains from the high‐altitude desert of Ladakh, India. J Clin Microbiol 43, 1538–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamazaki S, Yamakawa A, Okuda T, Ohtani M, Suto H, Ito Y, Yamazaki Y, Keida Y, Higashi H, Hatakeyama M et al (2005) Distinct diversity of vacA, cagA, and cagE genes of Helicobacter pylori associated with peptic ulcer in Japan. J Clin Microbiol 43, 3906–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chomvarin C, Namwat W, Chaicumpar K, Mairiang P, Sangchan A, Sripa B, Tor‐Udom S and Vilaichone RK (2008) Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis 12, 30–36. [DOI] [PubMed] [Google Scholar]
- 30. Thirumurthi S and Graham DY (2012) Helicobacter pylori infection in India from a western perspective. Indian J Med Res 136, 549–562. [PMC free article] [PubMed] [Google Scholar]
- 31. Watson CL, Owen RJ, Said B, Lai S, Lee JV, Surman‐Lee S and Nichols G (2004) Detection of Helicobacter pylori by PCR but not culture in water and biofilm samples from drinking water distribution systems in England. J Appl Microbiol 97, 690–698. [DOI] [PubMed] [Google Scholar]
- 32. Bahrami AR, Rahimi E and Ghasemian Safaei H (2013) Detection of Helicobacter pylori in city water, dental units’ water, and bottled mineral water in Isfahan, Iran. ScientificWorldJournal 2013; Article ID 280510, 5 pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gomes BC and De Martinis ECP (2004) The significance of Helicobacter pylori in water, food and environmental samples. Food Control 15, 397–403. [Google Scholar]
- 34. Janzon A, Sjöling A, Lothigius A, Ahmed D, Qadri F and Svennerholm AM (2009) Failure to detect Helicobacter pylori DNA in drinking and environmental water in Dhaka, Bangladesh, using highly sensitive real‐time PCR assays. Appl Environ Microbiol 75, 3039–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Queralt N and Araujo R (2007) Analysis of the survival of H. pylori within a laboratory‐based aquatic model system using molecular and classical techniques. Microb Ecol 54, 771–777. [DOI] [PubMed] [Google Scholar]
- 36. Angelidis AS, Tirodimos I and Bobos M (2011) Detection of Helicobacter pylori in raw bovine milk by fluorescence in situ hybridization (FISH). Int J Food Microbiol 151, 252–256. [DOI] [PubMed] [Google Scholar]
- 37. Thyagarajan SP, Ray P, Das BK, Ayyagari A, Khan AA, Dharmalingam S, Rao UA, Rajasambandam P, Ramathilagam B, Bhasin D et al (2003) Geographical difference in antimicrobial resistance pattern of Helicobacter pylori clinical isolates from Indian patients: multicentric study. J Gastroenterol Hepatol 18, 1373–1378. [DOI] [PubMed] [Google Scholar]
- 38. Bang SY, Han DS, Eun CS, Kim JE, Ahn SB, Sohn JH, Jeon YC and Kang JO (2007) Changing patterns of antibiotic resistance of Helicobacter pylori in patients with peptic ulcer disease. Korean J Gastroenterol 50, 356–362. [PubMed] [Google Scholar]
- 39. Secka O, Berg DE, Antonio M, Corrah T, Tapgun M, Walton R, Thomas V, Galano JJ, Sancho J, Adegbola RA et al (2013) Antimicrobial susceptibility and resistance patterns among Helicobacter pylori strains from The Gambia, West Africa. Antimicrob Agents Chemother 57, 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mirzaei N, Poursina F, Faghri J, Talebi M, Khataminezhad MR, Hasanzadeh A and Ghasemian Safaei H (2013) Prevalence of resistance to Helicobacter pylori strains to selected antibiotics in Isfahan, Iran. Jundishapur J Microbiol 6, e6342. [Google Scholar]
- 41. Bahrami AR, Aminipour Harandi MR, Kazemi Kheirabadi E, Sharifian B, Ghasemian Safaei H and Rahimi E (2011) Antimicrobial resistance of Helicobacter pylori isolates from cow feces, raw milk and drinking water in Iran. Middle East J Sci Res 10, 698–701. [Google Scholar]
- 42. Hunt RH, Xiao SD, Megraud F, Leon‐Barua R, Bazzoli F, van der Merwe S, Vaz Coelho LG, Fock M, Fedail S, Cohen H et al (2011) Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis 20, 299–304. [PubMed] [Google Scholar]
- 43. Yingzhi Lu, Redlinger TE, Avitia R, Galindo A and Goodman K (2002) Isolation and genotyping of Helicobacter pylori from untreated municipal wastewater. Appl Environ Microbiol 68, 1436–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saribasak H, Salih BA, Yamaoka Y and Sander E (2004) Analysis of Helicobacter pylori genotypes and correlation with clinical outcome in Turkey. J Clin Microbiol 42, 1648–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miernyk K, Morris J, Bruden D, McMahon B, Hurlburt D, Sacco F, Parkinson A, Hennessy T and Bruce M (2011) Characterization of Helicobacter pylori cagA and vacA genotypes among Alaskans and their correlation with clinical disease. J Clin Microbiol 49, 3114–3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Havaei SA, Mohajeri P, Khashei R, Salehi R and Tavakoli H (2014) Prevalence of Helicobacter pylori vacA different genotypes in Isfahan, Iran. Adv Biomed Res 3, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sedaghat H, Moniri R, Jamali R, Arj A, Razavi Zadeh M, Moosavi SGA, Rezaei M and Pourbabaee M (2014) Prevalence of Helicobacter pylori vacA, cagA, cagE, iceA, babA2, and oipA genotypes in patients with upper gastrointestinal diseases. Iran J Microbiol 6, 14–21. [PMC free article] [PubMed] [Google Scholar]
- 48. Torres LE, Melián K, Moreno A, Alonso J, Sabatier CA, Hernández M, Bermúdez L and Rodríguez BL (2009) Prevalence of vacA, cagA and babA2 genes in Cuban Helicobacter pylori isolates. World J Gastroenterol 15, 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ben Mansour K, Fendri C, Zribi M, Masmoudi A, Labbene M, Fillali A, Mami NB, Najjar T, Meherzi A, Sfar T et al (2010) Prevalence of Helicobacter pylori vacA, cagA, iceA and oipA genotypes in Tunisian patients. Ann Clin Microbiol Antimicrob 9, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]


