Abstract
Background
Conventional ECMO is bulky/non-ambulatory and requires multiple blood transfusions. We hypothesized that a percutaneous, paracorporeal artificial lung (PAL) could be established through a single venous cannulation to provide long-term ambulatory respiratory support.
Methods
Our PAL system was tested in 11 healthy sheep. Avalon Elite™ DLC, inserted through right jugular vein into SVC, RA and IVC, was connected to a CentriMag pump and compact hollow fiber gas exchanger, forming a short circuit PAL system. All sheep were moved to ICU and were ambulatory following anesthesia recovery. Hemodynamics and device performance were measured daily.
Results
The ambulatory PALs were successfully established in all 11 sheep. The sheep were awake, ate and moved freely in the metabolic cage with no need of artificial nutrition and blood transfusion. All sheep had stable hemodynamics with 2 L/min of average circuit flow, above 9.2 g/dl of Hgb levels throughout the experiment. A progressive decrease of O2 transfer and CO2 removal capacity was observed. The sheep were euthanized between 10–24 days for the reasons of bleeding (n=2), gas exchanger failure (n=6), and DLC issues (n=3).
Conclusions
We successfully established up to 24 days long-term ambulatory PAL in 11 animals using our patented DLC through a single-site percutaneous venous cannulation. However, critical bleeding/thrombosis formation and gas exchanger durability remain two major challenges for long term ambulatory PAL.
Keywords: extracorporeal membrane oxygenation, long-term ambulatory paracorporeal artificial lung, dual lumen cannula, sheep
Introduction
Chronic lung diseases are the third leading cause of death in America. As chronic lung disease reaches end stage, lung transplantation becomes an established choice for effective treatment. Although lung transplant has increased in number and waiting time has been reduced to approximately five months,1 the demand for a long-term respiratory support strategy persists to bridge these patients to transplantation. Low tidal volume ventilation and extracorporeal membrane oxygenation (ECMO) have been shown to improve patient outcome.2–4 However, mechanical ventilation requires intubation and sedation which limits patient mobility. Furthermore, it may result in ventilator-induced lung injury. Conventional ECMO, with a bulky/complicated circuit, is time limited, labor intensive, traumatic to blood, and non-ambulatory, requiring frequent blood transfusion, sedation, and enteral nutrition.5
Our hypothesis is that mechanical ventilation can be eliminated with efficient respiratory support allowing patient mobility, normal nutrition, and improved physical condition and promoting rapid patient recovery. To achieve patient mobility a compact ECMO system with simple blood vessel access is also required. Our goal is to develop a percutaneous, ambulatory paracorporeal artificial lung (PAL) system. This PAL system may also serve as a platform to test gas exchanger prototypes for long-term durability and gas exchange performance. This PAL system includes a low resistant, compact gas exchanger, and a compact centrifugal pump to form a simple/short paracorporeal circuit. The critical component is a highly efficient Avalon Elite™ Bicaval Dual Lumen Cannula (DLC), formerly known as the Wang-Zwische DLC, that connects the patient to the circuit via a single site venous cannulation, allowing effective respiratory support and patient mobility.6 In this study, we applied this ambulatory PAL system in healthy sheep to test different compact gas exchanger prototypes for up to four weeks.
Materials and Methods
1. The PAL system
Our PAL system consisted of a double lumen cannula (Avalon Elite™ DLC, Avalon Laboratories, LLC, Rancho Dominguez, CA), a less blood traumatic centrifugal blood pump (CentriMag, Levitronix LLC, Waltham, MA),7 and three types of gas exchanger made of different hollow fibers, including a standard polypropylene hollow fiber (Affinity, Medtronics, Minneapolis, MN); solid silicone membrane hollow fiber (SSMHF, MedArray, Ann Arbor, MI); and perfluorocopolymer coated polypropylene hollow fiber (PFCP, Compact Membrane Systems, Inc., Wilmington, DE). The pump and gas exchanger were connected by 3/8 inch Tygon tubing (Norton Performance Plastics, Wayne, NJ), and flushed and primed with heparinized 0.9% sodium chloride (3 IU/ml heparin).
2. Animal preparation and surgical procedure
This study was approved by the Institutional Animal Care and Use Committee at the University of Kentucky and was conducted in compliance with the “Guide for the Care and Use of Laboratory Animals”. The animals received 24-hour cageside care and adherence to the animal management protocol was monitored by a veterinarian. Blood samples were taken two days prior to surgery to measure complete blood cell count (CBC), blood chemistry panel, and platelet activation as baselines.
Eleven cross bred female sheep (35–45 kg) were used in the study. Under general anesthesia, the right femoral artery and vein were each cannulated with 16-gauge catheters (Intracath, Becton-Dickinson, Sandy, UT) via cut-down for hemodynamic monitoring, blood sampling and intravenous fluid/drug administration.
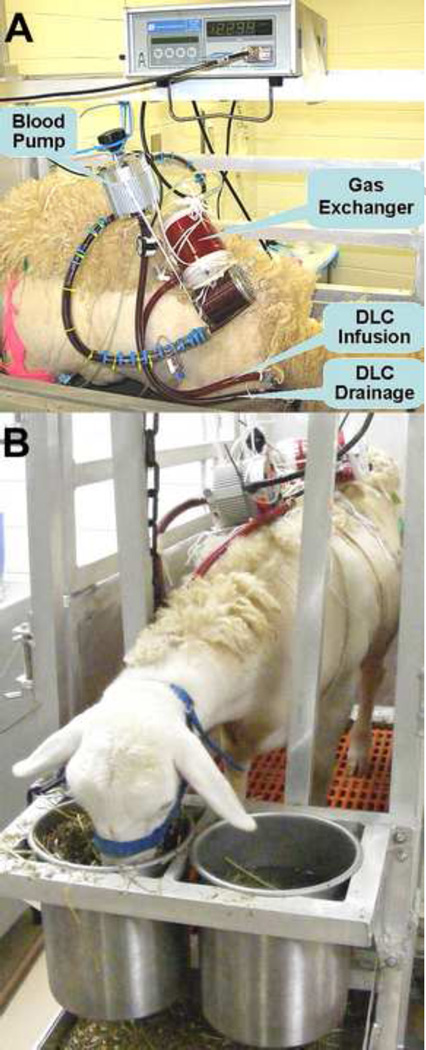
Through a cut-down in the neck, the right jugular vein was identified and exposed. After systemic anticoagulation with a bolus of heparin (120 IU/kg), a 27Fr DLC was inserted into the superior vena cava (SVC), traversing the right atrium with the tip positioned in the inferior vena cava (IVC). Correct positioning of the DLC was assured by anatomical data from our previous sheep autopsies. The CentriMag pump and a membrane gas exchanger were connected and primed with heparinized saline (3 IU/ml). Then, the DLC drainage outlet was connected to the pump inlet, and the DLC infusion lumen outlet was connected to the gas exchanger outlet, establishing the PAL system (Figure 1A). The pump was turned on, and O2 sweep gas was connected to the gas exchanger. The venous blood is withdrawn from both SVC and IVC through the DLC drainage lumen into the pump-gas exchanger. Oxygenated blood was returned to the right atrium toward the tricuspid valve through the DLC infusion lumen, avoiding recirculation. The incision on the neck was closed with continuous running suture and the DLC connector secured on the sheep’s neck by individual stitch. The sheep was moved into a metabolic cage and transferred to ICU.
Figure 1.
Configuration of our DLC based PAL system (A) and the animal after surgery (B). Sheep stayed awake and could eat and move freely inside cage.
3. Postsurgical care, monitoring and data collection
In the ICU, the gas exchanger was secured on the sheep’s back, and the CentriMag pump was hung on a weight-balanced pulley. This attachment allowed the sheep to move freely along with the PAL system, achieving in-cage ambulation and guaranteeing the circuit security (Figure 1B).
The sheep were extubated after recovery from anesthesia with spontaneous breathing and end tidal (ET) CO2 less than 50 mmHg. They were allowed free access to food and water throughout the study period.
Heparin was infused continuously and titrated to maintain activated clotting time (ACT) of 180–220 seconds. For the first 3 days post-surgery, buprenorphine (0.005–0.010 mg/kg iv; Bedford Laboratories, Bedford, OH) and enrofloxacin (2.5–5.0 mg/kg iv; Bayer Healthcare, Shawnee Mission, KS) were administered. Indications for gas exchanger replacement were: (1) gas exchange compromise/failure; (2) progressive plasma leakage; (3) blood leakage; (4) massive air into blood phase; and/or (5) severe clot formation within the gas exchanger.
Hemodynamic variables including heart rate (HR), mean arterial pressure (MAP), central venous pressure (CVP) were continuously monitored (MP50, Philips Medical Systems, Boblingen, Germany) and recorded every 6 hours. The circuit blood flow (Qb) was continuously monitored by a Transonic H9XL tubing flow sensor and HT 110 flowmeter (Transonic System Inc, Ithaca, NY). The CentriMag pump was adjusted to maintain circuit blood flow at 2 L/min. Blood was sampled from the femoral artery/vein and pre/post–gas exchanger for blood gas analysis (Cobas; Roche, Mannheim, Germany). Pre/post-gas exchanger blood pressures were measured for gas exchanger resistance calculation. The CO2 concentration in exhaust sweep gas was measured by an in-line CO2 sensor on the Philips monitor. Since the sweep gas was 100% O2, the CO2 removal equaled CO2 concentration in exhaust sweep gas, formulated as:
CO2 removal =sweep gas flow × CO2 concentration in exhaust sweep gas.
The O2 transfer was calculated based on blood gas analysis (Hgb level and its oxygen saturation percentage) and blood flow8:
O2 transfer =1.34 × hemoglobin ×10 × ΔO2 sat × Qb + 0.003 × ΔPO2 ×Q.
ΔO2 sat and ΔPO2 presented change in blood O2 saturation and in oxygen pressure across the gas exchange device, respectively.
Post surgical CBC, blood chemistry panel and platelet activation were measured daily the 1st week, twice a week the 2nd week, and weekly thereafter. Platelet activation was determined using flow cytometry (FACSCalibur flow cytometer and Cell Quest software Version 3.4, Becton Dickinson; San Jose, CA) to detect CD62 expression.9
4. Termination of the experiment and post experimental examination
Sheep experiments were terminated if the DLC kinked, broke, or dislocated, or if sheep suffered from massive bleeding (Hgb<5g/dl). The experiment duration was designed for 1–4 weeks according to different purposes of experimental design. For the perfluorocopolymer coated Affinity gas exchanger, experiment was terminated when gas exchanger failed. For the silicone fiber gas exchanger, the experiment was terminated after gas exchanger was replaced twice. For the uncoated Affinity gas exchanger, the experiment lasted up to four weeks allowing multiple device replacements.
A bolus of heparin (120 IU/kg) was injected intravenously immediately before euthanasia with beuthanasia-D (Schering-Plough Animal Health, Union, NJ). Necropsy was conducted to visualize DLC position in vivo and to examine cannulation related injury and thrombus formation in blood vessel and the heart. Also, a post experimental examination on gas exchanger and the pump head was performed.
5 Data Analysis
All values were expressed as mean ± standard deviation. For physiological and device functional data, significant difference at various time points was determined with one-way ANOVA (analysis of variance) with multiple comparisons. Using Student t-test, changes in hematology, platelet activation, and blood chemistry were compared between pre-surgical baselines and post-surgical measurements. A p value of < 0.05 was considered statistically significant.
Results
A total of 15 gas exchangers have been tested in the 11 animals. All eleven sheep survived the surgery and recovered from anesthesia. Our DLC allowed PAL connection to the sheep with single-site venous cannulation. Therefore, after surgery, the sheep stayed awake and alert, standing and sitting freely inside the metabolic cage. The sheep freely ate hay/feed pellets and drank water with no need of artificial feeding (Figure 1B). This is in contrast to a traditional ECMO patient who requires mechanical ventilation, sedation, and enteral nutrition with gastric tube, and consequently lacks ambulation.
The experimental length was 10–24 days. The individual lengths and causes of termination are listed in Table 1. Two animals were terminated because of DLC kinking at day 11 and one due to DLC dislocation at day 18. Two sheep were sacrificed for bleeding-caused low hemoglobin level (<5g/dl) at day16 (groin hematoma) and day 25 (back hematoma). All the others were terminated for gas exchanger issues such as blood leakage at day 18 (n=1) and gas exchange failure from day 18 to 25 (n=5).
Table 1.
Experimental length and termination of individual animal.
| Animal No. |
Experimental Length (day) |
Gas Exchanger Ffiber Type* |
Gas Exchanger Replacement |
Cause of termination |
|---|---|---|---|---|
| 1 | 24 | Affinity | D12, Failure; plasma leakage D21, Failure |
Bleeding |
| 2 | 10 | Affinity | DLC kinking | |
| 3 | 17 | SSMHF | D4, Clot formation | gas exchanger leakage (blood and gas) |
| 4 | 21 | SSMHF | D15, Failure | Gas exchange failure |
| 5 | 20 | PFCP | Gas exchange failure | |
| 6 | 10 | PFCP | DLC kinking | |
| 7 | 15 | PFCP | Bleeding | |
| 8 | 20 | PFCP | Gas exchange failure | |
| 9 | 17 | PFCP | DLC dislocation | |
| 10 | 17 | PFCP | Gas exchanger failure |
|
| 11 | 24 | PFCP | Gas exchanger failure |
Affinty: standard polypropylene hollow fiber.
SSMHF: solid silicone hollow fiber.
PFCP: perfluorocopolymer coated polypropylene hollow fiber.
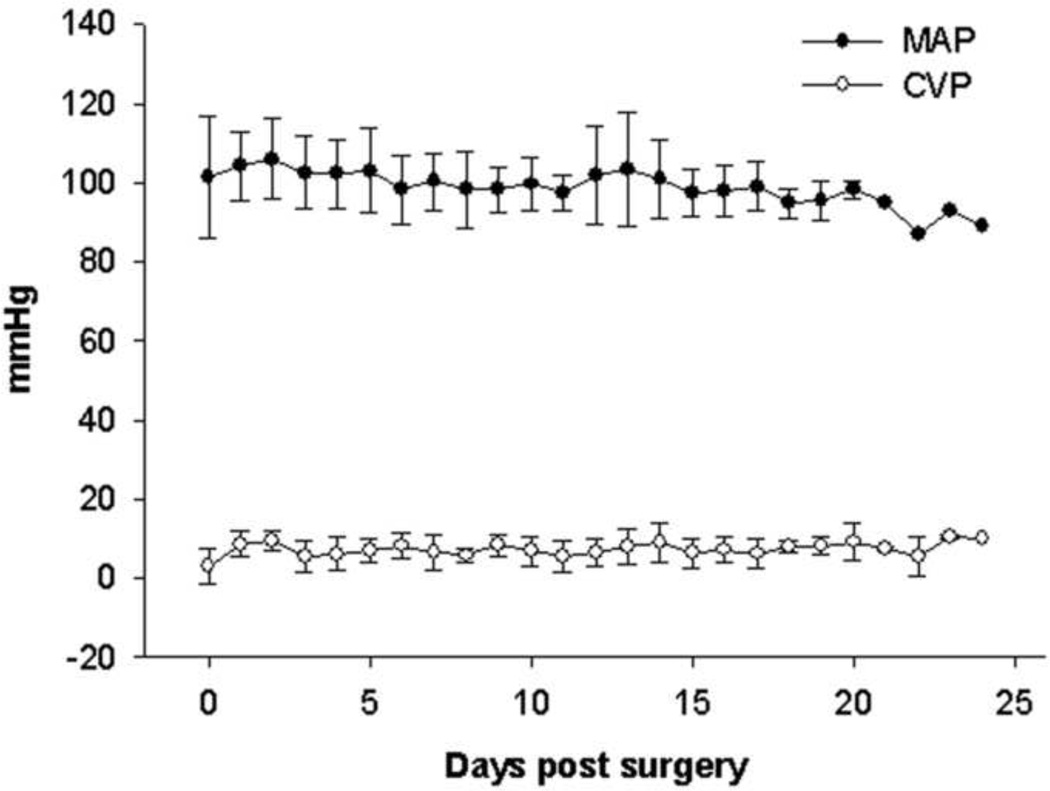
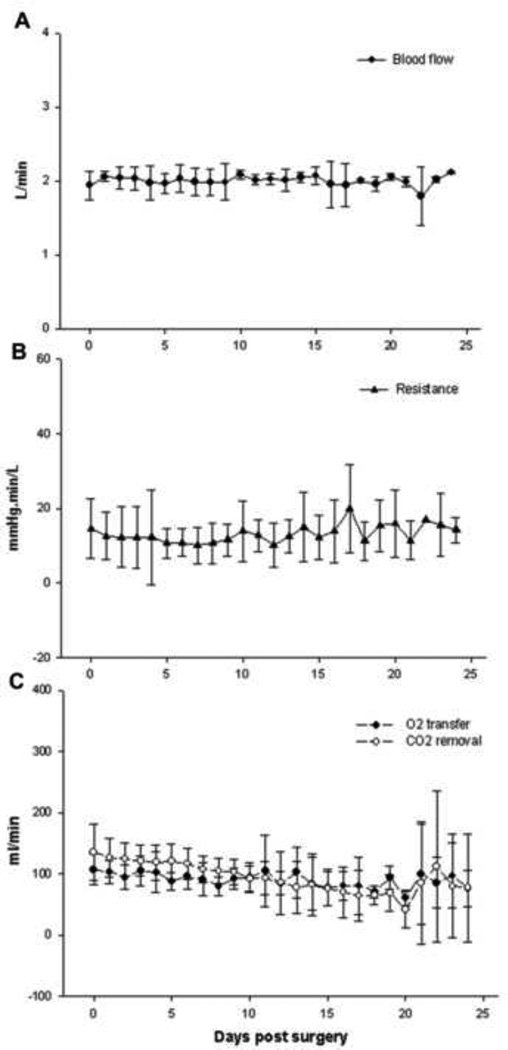
All sheep had stable hemodynamics throughout the experimental period (Figure 2) with no need of inotropic medicine. Two animals experienced low circuit blood flow leading to experimental termination. All the other sheep had very stable circuit blood flow throughout the study, averaging 2 L/min, which is sufficient for gas exchange in the present study and was the same as the level in our previous study.6 (Figure 3A). The average device resistance ranged from 10.0 ± 1.6 to 20.0 ± 4.2 mm Hg·min/L and did not alter significantly over the experiment (P>0.05, Figure 3B).
Figure 2.
Stable hemodynamics during the experimental period. MAP, mean arterial pressure; CVP, central venous pressure. P>0.05, respectively.
Figure 3.
Stable circuit blood flow (A) and resistance (B) over time, P>0.05, respectively. Gas exchange performance over time (C) showing a gradual decrease in capacity of O2 transfer (P<0.05) and in CO2 removal (P<0.01).
General gas exchange performance throughout experiment is shown in Figure 3C. Both O2 transfer and CO2 removal capacity gradually decreased over time (P<0.05 and P<0.01, respectively). O2 transfer decreased from 107 ± 8 to 76 ± 21 ml/min. CO2 removal significantly reduced from 139 ± 12 ml/min to 78 ± 63 ml/min. After day 21, one of the two remaining sheep underwent Affinity gas exchanger replacement, which might account for the high error bars.
Among the total of 15 gas exchangers tested in the 11 sheep, four gas exchangers were Affinity in 2 animals, 4 were SSMHF in 2 animals, and 7 were PFCP in 7 animals. Four gas exchangers were replaced for thrombosis formation inside (in 1 SSMHF device) and gas exchange performance deterioration (in 1 SSMHF and 2 Affinity devices). In the 24-day survival sheep on Affinity gas exchanger, there were two replacements, one at day 9 due to gas exchange failure and the other at day 21 due to gas exchange failure plus plasma leakage. In the 21-day surviving sheep with SSMHF device, the replacement happened at day 15, also due to gas exchange dysfunction. In the 17-day surviving animal with SSMHF gas exchanger, the device was changed at day 4 because of severe thrombus formation.
Plasma leakage occurred in two Affinity devices at day 10 and 12, and in three PFCP devices at day 10, 16 and 21, respectively. Blood leakage took place in two SSMHF devices at 9 hours after running and at day 14. Also, mild air leakage happened to two SSMHF at day 10 and 14, as well as to two PFCP devices at day 10 and 13, respectively.
Hematology and platelet activation data are summarized in Table 2. The Hgb levels were above 9.2 g/dl throughout the experiment with no need for blood transfusion, attributed to daily feeding similar to a healthy animal. Compared with pre-surgery baseline of 12.7 ± 1.8g/dl, hemoglobin was decreased immediately after surgery but remained at stable and satisfactory levels. WBC counts increased significantly at day 1 and 2 after surgery but were still within normal range. Throughout the rest of the study period, WBC counts returned to pre-surgery values and were stable. Platelet counts were within physiological range throughout experiment although they were decreased after surgery (Table 2). Platelet activation as measured by flow cytometry analysis did not increase significantly from pre-surgery baseline throughout the experimental study.
Table 2.
Hematology and platelet activation
| Days post surgery |
pre | 0 | 1 | 2 | 3 | 7 | 14 | 18–21 | >21 |
|---|---|---|---|---|---|---|---|---|---|
| WBC (×103/µl) |
5.5 ± 2.8 |
4.4 ± 1.5 | 9.9 ± 2.6** |
8.3 ± 2.9* |
6.7 ± 2.7 |
7.9 ± 3.4 |
6.1 ± 2.1 |
6.1 ± 3.0 |
4.7 ± 1.2 |
| Hgb (g/dl) |
12.7 ± 1.8 |
10.2 ± 1.4* |
9.5 ± 1.6** |
9.2 ± 1.3** |
9.2 ± 1.2** |
9.6 ± 1.2** |
10.5 ± 1.7* |
10.5 ± 1.6* |
10.8 ± 2.9 |
| Platelet (×103/µl) |
436 ± 135 |
331 ± 121 |
228 ± 94** |
208 ± 71** |
222 ± 89** |
314 ± 67 |
313 ± 65* |
272 ± 65* |
321 ± 114 |
| Platelet activation (%) |
5.8 ± 2.5 |
7.4 ± 3.1 | 7.1 ± 2.3 |
7.0 ± 2.9 |
5.6 ± 3.3 |
4.6 ± 2.0 |
5.9 ± 2.5 |
8.4 ± 2.4 |
5.2 ± 1.7 |
Comparing with pre-surgical baselines,
P<0.05;
P<0.01
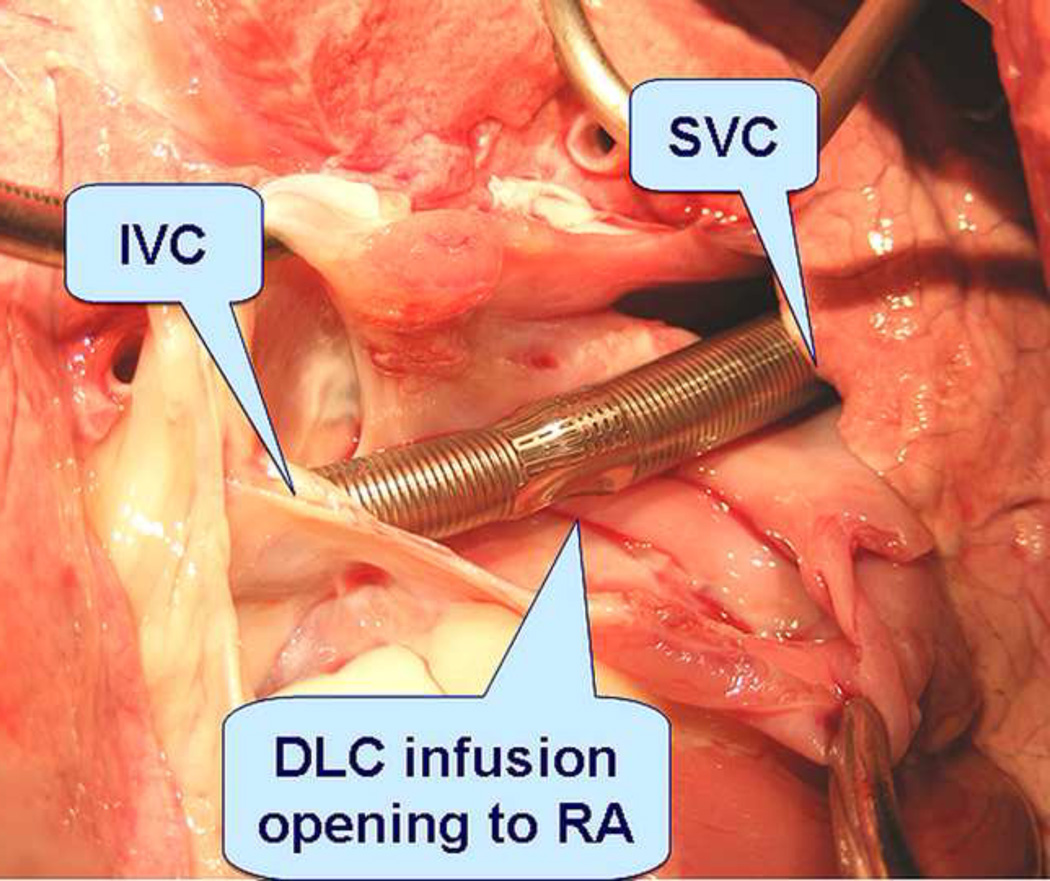
In post experimental necropsy, massive subcutaneous hematoma was confirmed in the two sheep (day16 at groin; day 25 at right back, respectively), and no other bleeding was found. Necropsy also revealed correct positioning of the DLC inside the blood vessel in all but one of the animals, in whom the cannula dislocated at day 18. DLC kinking was visualized in two more sheep that survived 20 days and 24 days respectively without decrease in circuit blood flow. No visible thrombus was found in the cannulae or around the tip and ports (Figure 4). No visible injury, infarct or emboli were found in the hearts and blood vessels. The DLC kinking was observed in 2 sheep, and in both cases the kinking occurred at the part of the cannula that turned around at the sheep neck in the angle to facilitate device attachment onto sheep’s back. Thrombi/clots were not found in the pump heads but visualized in 11 out of 15 gas exchangers (2 of 4 Affinity; 2 of 4 SSMHF and all 7 PFCP devices). Thrombosis in 2 SSMHF and 2 PFCP devices appeared more remarkable.
Figure 4.
Necropsy confirmed correct DLC position. No visible injury and clot was found in the vessel and heart.
Discussion
Our patented DLC, coupled with a small centrifugal pump and a compact hollow fiber gas exchanger, established a simple PAL system. This system has a very short circuit, enabling ambulation and low blood resistance, minimizing blood trauma. It achieved long-term respiratory support through only a one-site percutaneous venous cannulation.9 In this PAL study, the ambulatory sheep obtained adequate nutrition from normal eating and maintained a satisfactory hemoglobin level with no need of additional artificial nutritional support or blood transfusion.
A long-term ambulatory PAL has been developed by another group; however, that PAL system requires an open chest surgery with direct cannulation to the heart (atrium) and anastomosis to the main PA.10 This major invasive surgery will have a significant and negative impact on the really sick patient. The major difference distinguishing our PAL system from others is the percutaneous, one-site venous PAL installation for efficient respiratory support, eliminating the major invasive surgery.
The DLC is the key component enabling our ambulatory PAL. However, the previous commercial ECMO DLC is only available for pediatric patients and has poor performance due to recirculation.11 This DLC is susceptible to kinking and collapse due to its weak construction. The drainage lumen may collapse from excessive negative pressure generated by the centrifugal pump. Therefore a bulky roller pump with a long gravity drainage circuit is required, restricting patient mobility. By contrast, our DLC has higher efficiency, stronger construction, and broader applicability for not only pediatric but also adult patients.9 It is designed to withdraw total venous blood from both IVC and SVC through one drainage lumen for oxygenation and to infuse the blood back to the RA to achieve total respiratory support. The two drainage lumen openings in the SVC and IVC are spatially separated from the infusion lumen opening in the RA, which maximally reduces recirculation and enhances blood transfer efficiency. The stainless steel wire reinforcement construction allows a stronger and larger DLC for adult patient application. The round-shaped membrane infusion lumen has geometric stability to tolerate high negative pressure generated by a compact centrifugal pump, eliminating the requirement for a bulky roller pump with a long gravity drainage circuit.6 Therefore, our DLC-based PAL is characterized by high performance, low resistance, short circuit, and one-site jugular vein cannulation, providing up to total respiratory support, eliminating the requirement of intubation/mechanical ventilation/ related sedation, and allowing our PAL sheep to be ambulatory.
ARDS patients have the problem of severe hypoxia and hypercapnia, which requires high ECMO circuit blood flow for high volume O2 transfer/CO2 removal. However, the main purpose of this study was to develop an ambulatory PAL for end stage chronic lung diseases, instead of ARDS. CO2 retention/hypercapnia is the critical problem of end stage chronic lung diseases and appropriate CO2 removal can reverse its pathophysiology. Total CO2 removal requires much less blood flow.12 Therefore, we choose 2 l/min blood flow in this study. However, if the patient has severe lung injury requiring high blood flow for high volume O2 transfer/CO2 removal, this 27 Fr DLC can run up to 4.4 l/min blood flow.13 There is also a 31 Fr version available.
The advantage of ambulation is obvious.9,14 First, it decreases the likelihood of lung infection because of spontaneous breathing with coughing ability. In our long term PAL, no antibiotic was applied except the prophylactic administration for the first 3 postoperative days. Secondly, avoidance of intubation/mechanical ventilation/sedation allows sheep to obtain adequate nutrition by natural eating, maintaining normal hemoglobin level, and avoiding blood transfusion with this minimal blood trauma PAL circuit.
Nevertheless, DLC related complications were observed in this study. The most serious complication was cannula kinking, resulting in decreased circuit blood flow in two sheep. The DLC kinking in our sheep study was caused by the vigorous sheep movement and the sharp angle for positioning the circuit onto the sheep’s back. This DLC requires precise alignment and orientation of infusion opening toward tricuspid valve. Displacement and misorientation of the DLC will compromise the PAL gas exchange performance. In the later stage of this study, smooth curving of the tubing coupled with multiple points security to the animal helped to prevent DLC kinking, displacement, and misorientation.
As always, the bleeding/thrombosis and gas exchanger failure were major challenges in long-term PAL.15,16 Bleeding/thrombosis were leading complications contributing to mortality in this PAL study, like ECMO and LVAD clinical application. Blood contacting foreign surface area activates the blood coagulation cascade and causes thrombosis. PAL/ECMO has the largest foreign surface area among artificial organs, requiring a high level of anticoagulation (systemic heparinization) to prevent thrombosis and embolization. However, the anticoagulation level is very hard to balance between thrombosis and bleeding.17 On one hand, current heparin based anticoagulation causes serious bleeding complication, contributing to significant ECMO morbidity and mortality.18 On the other hand, circuit thrombosis leads to systemic embolization and gas exchange impairment. In our present long-term PAL study, two sheep were euthanized for large hematoma formation and progressive decrease of blood hemoglobin, while thrombosis was found inside all the gas exchangers contributing to gas exchange failure.
The second major challenge in long-term PAL application is the limited durability of the gas exchanger. The microporous hollow fiber gas exchanger fails within 7–10 days and requires replacement.19,20 The gas exchanger failure is caused by: 1) plasma leakage from blood phase into sweep gas phase through the micropores on hollow fiber, diminishing gas transfer efficiency; 2) protein deposition on the gas exchange surface, increasing the distance between blood and sweep gas phases; and 3) thrombosis formation in the gas exchanger, reducing effective gas exchange surface area. Both SSMHF and PFCP are new true membrane hollow fiber prototypes developed to eliminate plasma leakage and increase gas exchange durability. However, plasma leakage still occurred in SSMHF and caused gas exchanger failure in our sheep study, demonstrating the inadequate coating durability. Significant thrombosis was observed in PFCP gas exchangers, which is related to non-commercial quality of the fibers. Therefore, the comparison study was stopped due to above prototype quality issues.
In summary, we successfully established up to 24 day, long-term ambulatory PAL in 11 animals, using our patented DLC through a single-site percutaneous venous cannulation. However, critical bleeding/thrombosis formation and gas exchanger durability remain two major challenges for long term ambulatory PAL.
Acknowledgments
Dr. Zwischenberger currently receives grant monies from the National Institutes of Health. Dr. Zwischenberger also receives royalties ($13.22/cannula) from Avalon for his patent on the double lumen cannula (AvalonElite™). Dr. Wang receives royalties ($39.66/cannula) from Avalon for his patent on the double lumen cannula (AvalonElite™). Dr. Wang also receives grant monies from the National Institutes of Health.
It should be noted that complimentary (no cost) AvalonElite™ double lumen cannulas were provided for this study by Avalon.
Funding Sources
This study was supported in part by grants from the National Institutes of Health (HL 064508 and HL 068375).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No other authors have any conflicts of interest to disclose.
References
- 1.U.S. Department of Health and Human Services. 2009 OPTN/SRTR Annual Report: Transplant Data 1999–2008. [Accessed October 20, 2011]; http://www.ustransplant.org/annual_reports/current/ [Google Scholar]
- 2.Presenti A, Zanella A, Patroniti N. Extracorporeal gas exchange. Curr Opin Crit Care. 2009;15:52–58. doi: 10.1097/MCC.0b013e3283220e1f. [DOI] [PubMed] [Google Scholar]
- 3.Hämmäinen P, Schersten H, Lemström K, et al. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: A descriptive study. The Journal of Heart and Lung Transplantation. 2011;30:103–107. doi: 10.1016/j.healun.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 4.ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Strueber M. Extracorporeal support as a bridge to lung transplantation. Curr Opin Crit Care. 2010;16:69–73. doi: 10.1097/MCC.0b013e3283353ce0. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Zhou X, Liu X, Sidor B, Lynch J, Zwischenberger JB. Wang-Zwische double lumen cannula-toward a percutaneous and ambulatory paracorporeal artificial lung. ASAIO J. 2008:54606–54611. doi: 10.1097/MAT.0b013e31818c69ab. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Zhou X, Lick SD, Liu X, Qian K, Zwischenberger JB. An ambulatory pulmonary and right heart assist device (OxyRVAD) in an ovine survival model. J Heart Lung Transplant. 2007;26:974–979. doi: 10.1016/j.healun.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Koziara J, Oh J, Akers W, Ferraris S, Mumper R. Blood compatibility of cetyl alcohol/polysorbate-based nanoparticles. Pharm Res. 2005;22:1821–1828. doi: 10.1007/s11095-005-7547-7. [DOI] [PubMed] [Google Scholar]
- 9.Javidfar J, Brodie D, Wang D, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2011;91:1763–1769. doi: 10.1016/j.athoracsur.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Wu ZJ, Zhang T, Bianchi G, et al. Thirty-day in-vivo performance of a wearable artificial pump-lung for ambulatory respiratory support. Ann Thorac Surg. 2012;93:274–281. doi: 10.1016/j.athoracsur.2011.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews A, Zwischenberger J, Cilley R, Drake K. Venovenous extracorporeal membrane oxygenation (ECMO) using a double-lumen cannula. Artif Organs. 1987;11:265–268. doi: 10.1111/j.1525-1594.1987.tb02669.x. [DOI] [PubMed] [Google Scholar]
- 12.Brunston RL, Jr, Zwischenberger JB, Tao W, Cardenas VJ, Jr, Traber DL, Bidani A. Total arteriovenous CO2 removal: simplifying extracorporeal support for respiratory failure. Ann Thorac Surg. 1997;64:1599–1605. doi: 10.1016/s0003-4975(97)01113-2. [DOI] [PubMed] [Google Scholar]
- 13.Bermudez CA, Rocha RV, Toyoda Y, et al. Extracorporeal membrane oxygenation for advanced refractory shock in acute and chronic cardiomyopathy. Ann Thorac Surg. 2011;92:2125–2131. doi: 10.1016/j.athoracsur.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 14.Garcia JP, Iacono A, Kon ZN, Griffith BP. Ambulatory extracorporeal membrane oxygenation: A new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg. 2010;139:e137–e139. doi: 10.1016/j.jtcvs.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Garcia JP, Kon ZN, Evans C, et al. Ambulatory veno-venous extracorporeal membrane oxygenation: Innovation and pitfalls. J Thorac Cardiovasc Surg. 2011;142:755–761. doi: 10.1016/j.jtcvs.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 16.Gaffney AM, Wildhirt SM, Griffin MJ, Annich GM, Radomski MW. Extracorporeal life support. BMJ. 2010;341:c5317. doi: 10.1136/bmj.c5317. [DOI] [PubMed] [Google Scholar]
- 17.Baird CW, Zurakowski D, Robinson B, et al. Anticoagulation and pediatric extracorporeal membrane oxygenation: impact of activated clotting time and heparin dose on survival. Ann Thorac Surg. 2007;83:912–920. doi: 10.1016/j.athoracsur.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Oliver WC. Anticoagulation and coagulation management for ECMO. Semin Cardiothorac Vasc Anesth. 2009;13:154–175. doi: 10.1177/1089253209347384. [DOI] [PubMed] [Google Scholar]
- 19.Sato H, Hall CM, Lafayette NG, et al. Thirty-day in-parallel artificial lung testing in sheep. Ann Thorac Surg. 2007;84:1136–1143. doi: 10.1016/j.athoracsur.2007.05.051. [DOI] [PubMed] [Google Scholar]
- 20.Haneya A, Philipp A, Mueller T, et al. Extracorporeal circulatory systems as a bridge to lung transplantation at remote transplant centers. Ann Thorac Surg. 2011;91:250–255. doi: 10.1016/j.athoracsur.2010.09.005. [DOI] [PubMed] [Google Scholar]