Abstract
OBJECTIVE
To assess risk of stroke and MI after zoster in a U.S. community population of older adults.
DESIGN
A community cohort study (1986–2011) comparing risk for stroke and MI in adults ≥50 with and without zoster. Odds ratios are presented for MI and stroke at 3, 6, 12 and 36 months after index zoster plus hazard ratios for long-term risk (up to 28.6 years).
SETTING
Olmsted County, Minnesota.
PARTICIPANTS
All adult residents of Olmsted County, aged ≥50 at the time of medical record confirmed zoster (n = 4,862) and 19,433 sex and age matched individuals with no history of zoster.
EXPOSURE
Zoster.
MAIN OUTCOMES
Incident MI and stroke.
RESULTS
Overall, individuals with zoster had more risk or confounding factors for MI and stroke, suggesting that they had worse health status overall. When controlling for the multiple risk factors, those with zoster were at increased for stroke at 3 months post zoster compared to those without a history of zoster (OR 1.53 (95% confidence interval (CI95) 1.10–2.33, P = .04. The association between zoster and MI at 3 months was not robust across analytic methods. Zoster was not associated with an increased risk for either stroke or MI at any point beyond 3 months.
CONCLUSIONS AND RELEVANCE
Zoster was associated with only a short- term increased risk of stroke which may be preventable with prevention of zoster.
Introduction
Varicella zoster virus (VZV) causes varicella (chickenpox) after which virus becomes latent in neurons of ganglia along the entire neuraxis.1,2 As VZV-specific cell-mediated immunity declines in older and immunocompromised individuals, VZV reactivates to produce zoster (shingles). More than 95% of the world’s adult population is infected with VZV3,4 and up to one-third will develop zoster in their lifetime.5,6 Zoster can be complicated by myelitis, meningoencephalitis, vasculopathy, multiple ocular disorders7–19 and giant cell arteritis.10
Epidemiological studies outside the U.S. have suggested an increased risk of stroke and myocardial infarction (MI) after zoster.11–16 Those studies used national or regional administrative databases, assessing the association at time points ranging from weeks11,13,14,15 to years12 after zoster. None used medical record confirmation, most relying on zoster diagnostic codes,11–13,15, while one used antiviral prescriptions as a proxy for zoster.14 No similar studies of the U.S. population have been reported.
Herein, we assessed the risk of stroke and MI in a U.S. community-based population comparing outcomes among patients 50 years and older with medical record confirmed episodes of zoster and age and sex matched control patients without zoster. We assessed the risk of zoster-associated stroke or MI at 3, 6, 12 and 36 months as well as 20 years after zoster, adjusting for several known stroke and MI risk factors..
Methods
Study Design
This is a retrospective study of a population-based cohort of older adults with zoster comparing their rates of post zoster myocardial infarction (MI) and stroke to a cohort of age and sex matched individuals from the same community who had no medical record history of zoster. Patients were followed for a mean of 7.1 years (range 0–28.6 years). All adults aged 50 years and older with a confirmed zoster episode between January 1, 1986 and October 1, 2011 (N= 4,862) were included in the zoster cohort.. The no zoster cohort, (N= 19,433, included approximately 4 individuals matched by birthdate (+/− 1 year) and sex to the individuals in the zoster cohort. All patients had not refused medical record research authorization as required by Minnesota Statute.16,17 The study was approved by Institutional Review Boards of the Olmsted Medical Center and the Mayo Clinic. Risks for stroke and MI were assessed separately.
Participants, Data Collection and Adjudication of Cases of Zoster
Patients with zoster were identified using resources of the Rochester Epidemiology Project (REP), an electronic database that collects and links medical diagnoses for all patients receiving care in Olmsted County, MN.18–21 Diagnostic code and visit data are collected and linked by patient across two large systems (the Mayo Clinic and the Olmsted Medical Center) as well as three small clinics with one to three clinicians. Capture is estimated to be over 98% of each Olmsted County, MN community resident’s medical events.18
The zoster cohort was identified using a broad group of zoster related diagnostic codes (International Classification of Diseases version 9, ICD-9).6,9,22–24 The broad spectrum of codes increased sensitivity of electronic identification, and medical record review of each potential case assured specificity of the diagnosis. Confirmation required documentation of acute pain and dermatomal or rarely disseminated rash or organ damage consistent with zoster.25 Individuals with recurrent zoster were included since we have previously reported that recurrent zoster is not rare24 and our medical record review removed individuals with recurrent herpes simplex infection. Zoster patients included in the stroke analyses had no history of stroke before their index date and those included in the MI analyses had no history of MI before their index date.
The patients in the no zoster cohort were also selected using REP resources by matching each zoster patient with four patients whose birthdate was ± 1 year, who were of the same sex and had no zoster diagnoses in the five years prior to their inclusion in the cohort. All individuals in both cohorts lived in Olmsted County and most receive care from each of the two medical systems.18–20 Individuals included in the MI analyses had no history of prior MI and those in the stroke analyses had no history of prior stroke.
Stroke and MI
Our primary outcome was incident (first event) stroke or MI after the index date which was the date of zoster in the zoster cohort members and for the “matched” zoster individuals in community cohort patients. Transient ischemic attacks (TIA) were not included as an outcome since TIA is often not a definitive event.12,14 Conversely, a previous study in the Olmsted County population reported that diagnoses of stroke and MI have high clinical accuracy when the diagnostic code in the REP is taken from hospital discharge data or death certificates.26
The occurrence of a stroke or MI was assessed using the codes listed in Supplemental eTable 1. For each case and control, all stroke or MI events were identified using the date associated with the earliest available stroke or MI code as the event date. All strokes and MIs were included as events of interest if they occurred 30 days or less before the index date of zoster through the follow-up period since replication and spread of VZV begins before zoster rash.27
Covariates
Diagnostic codes used to adjust analyses for multiple other morbidities were obtained for each case using the REP diagnostic index which records all diagnoses for each medical encounter. Overall, 92% of cases and all control patients had at least 5 years of health care data to search for covariates before their index date. Only diagnoses before the individual’s index date were included as risk or confounding factors for that patient. Data on current and former smoking status and obesity were poorly recorded in the diagnostic coding data and were therefore not included in analyses.
To enhance the list of diagnostic codes used to identify potential risk factors for stroke and MI, we used the list of factors published by the US Department of Health and Human Services Taskforce (US-DHHS) in 2010.28–30 The list includes diagnostic codes for the major risk factors for MI and stroke such as hypertension, dyslipidemia, coronary artery disease (including MI for stroke), cardiac arrhythmias, congestive heart failure, diabetes, vasculopathies and stroke (for MI), depression and chronic obstructive pulmonary disease. (See Supplemental eTable 2.)
The REP diagnostic code data for all visits for each patient were searched electronically to identify all visits with any ICD-9 codes for the risk and confounding factors from their first visit to any Olmsted County health care facility until the last visit 30 days before the index date. The ICD-9 codes were then pooled into the risk factor domains: hypertension, dyslipidemia, coronary artery disease (includes MI), arrhythmias, congestive heart failure, diabetes, depression, chronic obstructive pulmonary disease, vasculopathies and stroke as proposed by the US DHHS.28–30 We added a domain of “anxiety”31 based on recent work suggesting that it is a risk factor for MI.32 To decrease the risk of false-positive diagnoses for any condition, we required that a patient received two codes for a given condition separated by more than 30 days for that condition to be considered present.33,34
Statistical Analyses
Only individuals with no MI before the zoster index date were included in zoster and MI analyses. Similarly, only individuals with no stroke before the index date were included in zoster and stroke analyses.
The zoster and no zoster cohorts were compared for demographic and co-morbidity frequency using Chi-squared tests. We examined the effect of HZ on risk for stroke using 3 methods: 1) univariate and multi-variate logistic regression analysis of the unpaired subjects, including age and sex as predictors with a step-down method, deleting non-significant variables to reduce the number of co-morbidity predictors, 2) conditional logistic regression for matched pairs, and 3) survival analyses (time to stroke or MI) presented using Kaplan-Meier curves, and Cox proportional hazards models for the full follow-up period, with case status, age, sex, and other co-morbidities, as predictors.35 The S-Plus statistics package v. 7.0.6 (Tibco Software) was used for all computations.
Results
The 4,862 patients with zoster and 19,433 patients with no zoster patients had substantial rates of morbidity and multi-morbidity diagnosed before the index date (Table 1). The rates of chronic conditions were higher among the zoster cohort than among the no zoster cohort patients when stratifying by 1–3 or more chronic conditions: 1 condition, 24.7% vs. 23.1%; 2 conditions, 21.3% vs 17.5%; and for 3 or more conditions 27.7% vs 21.7% for cases and controls respectively; P < .001. The sex ratio of cases and controls did not differ. The average age of the patients for the stroke analyses differed by 0.7 years (P=<.001) and by 0.5 years for the MI analyses (P=.01). Neither difference was considered clinically significant. Since we included patients with fatal stroke, no minimum follow-up period was required.
Table 1.
Demographic and Risk Factor Data
| Patients for Zoster and Stroke Analyses |
Patients for the Zoster and MI Analyses |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) with zoster 4478 Total |
N (%) without zoster/ 16800 Total |
Chi- square P-value |
N (%) with zoster 4454 Total |
N (%) without zoster 16740 Total |
Chi- square P-value |
||||
| Sex = female | 2782 (62.1) | 10513 (62.6) | .59 | 2826 (63.4) | 10831 (64.7) | .12 | |||
| Age mean (range) | 68.5 (50,106) | 67.8 (49,106) | < .001 | 68.6 (50,106) | 68.1 (49,106) | .01 | |||
| Age 50–64 | 1964 (43.9) | 7717 (45.9) | .01 | 1944 (43.6) | 7604 (45.4) | .04 | |||
| Age 65–79 | 1662 (37.1) | 6163 (36.7) | .61 | 1647 (37.0) | 6053 (36.2) | .32 | |||
| Age 80+ | 852 (19) | 2920 (17.4) | .01 | 863 (19.4) | 3083 (18.4) | .02 | |||
| Morbidities | 2065 (46.4) | 6526 (39.0) | < .001 | ||||||
| Hypertension | 2068 (46.2) | 6473 (38.5) | < .001 | 2065 (46.4) | 6526 (39.0) | < .001 | |||
| CAD | 861 (19.2) | 2380 (14.2) | < .001 | 668 (15.0) | 1818 (10.9) | < .001 | |||
| MI before index | 312 (7.0) | 900 (5.4) | < .001 | NA | NA | NA | |||
| Arrhythmias | 953 (21.3) | 2589 (15.4) | < .001 | 917 (20.6) | 2543 (15.2) | < .001 | |||
| Dyslipidemia | 1941 (43.3) | 5955 (35.4) | < .001 | 1873 (42.1) | 5808 (34.7) | < .001 | |||
| Depression | 850 (19) | 2640 (15.7) | < .001 | 862 (19.4) | 2718 (16.2) | < .001 | |||
| Diabetes | 873 (19.5) | 2769 (16.5) | < .001 | 866 (19.4) | 2741 (16.4) | < .001 | |||
| Substance abuse | 183 (4.1) | 649 (3.9) | .52 | 188 (4.2) | 621 (3.7) | .12 | |||
| Anxiety | 662 (14.8) | 2020 (12) | < .001 | 663 (14.9) | 2053 (12.3) | < .001 | |||
| Vasculopathy | 118 (2.6) | 316 (1.9) | .002 | 174 (3.9) | 509 (3.0) | < .001 | |||
| Number of events for each analysis (Strokes) | Number of events for each analysis (MI) | 35 (0.8) | 81 (0.5) | .02 | |||||
| within 3 months | 33 (0.7) | 73 (0.4) | .02 | 24 (0.5) | 48 (0.3) | .02 | |||
| in 6 months | 46 (1) | 123 (0.7) | .06 | 35 (0.8) | 81 (0.5) | .02 | |||
| in 1 year | 71 (1.6) | 235 (1.4) | .39 | 61 (1.4) | 155 (0.9) | .01 | |||
| in 3 years | 176 (3.9) | 591 (3.5) | .20 | 154 (3.5) | 450 (2.7) | .01 | |||
| ever | 562 (12.6) | 1844 (11) | .003 | 443 (9.9) | 1430 (8.5) | < .001 | |||
| Follow-up Period | 1272 (28.6) | 4637 (27.7) | .26 | ||||||
| 90 daus | 4425 (98.8) | 16716 (99.5) | .03 | 4405 (98.9) | 16673 (99.6) | .03 | |||
| 3 years | 4151 (92.7) | 15758 (93.8) | .06 | 4102 (92.1) | 15652 (93.5) | .05 | |||
| > 5 years | 2508 (56) | 9155 (54.5) | .07 | 2481 (55.7) | 9034 (54.0) | .04 | |||
| > 10 years | 1281 (28.6) | 4659 (27.7) | .25 | 1272 (28.6) | 4637 (27.7) | .26 | |||
| > 15 years | 506 (11.3) | 1904 (11.3) | .97 | 519 (11.7) | 1915 (11.4) | .71 | |||
For analyses of zoster and stroke, we removed all patients with stroke before the index date which yielded 4,478 individuals with zoster and 16,800 individuals with no zoster. Stroke was associated with zoster in the first 3 months [odds ratio (OR) = 1.7, CI95 1.13–2.57], and the first 6 months after zoster (OR=1.41, CI95 1.0–1.98) in univariate logistic regression that included only zoster and stroke. In stepwise multivariate logistic regression the association of zoster and stroke remained significant only at 3 months after zoster (OR 1.53, CI95 1.01–2.33). When considered individually, many factors in addition to zoster were significantly associated with stroke. However, in stepwise logistic regression which accounted for multiple risk factors and zoster simultaneously, most risk factors became non-significant, leaving only cardiac arrhythmias, a history of vasculopathy, age and zoster as significantly associated with stroke. (Table 2). The results were robust across different analysis methods, specifically conditional logistic regression and assessment of hazard ratios. (Supplementary Table e3).
Table 2.
Univariate and Mulivariate Stepwise Logistic Regression for Stroke at All Time Periods after Zoster
| Univariate analyses: Stroke and Zoster | ||
| Time After Index | OR (95% CI) | P-value |
| 3 months | 1.70 (1.13–2.57) | .01 |
| 6 months | 1.41 (1–1.98) | .05 |
| 1 year | 1.14 (0.87–1.48) | .35 |
| 3 years | 1.12 (0.95–1.33) | .19 |
| Multiviariate Stepwise Logistic Regression Models: | ||
| Variable | OR (95% CI) | P-value |
| Model at 3 months | ||
| Zoster | 1.53 (1.01–2.33) | .04 |
| Age vs. age 50–64: age 65–79 | 3.32 (1.84–5.99) | <.001 |
| age 80+ | 6.37 (3.49–11.62) | <.001 |
| Arrhythmias | 1.74 (1.15–2.65) | .009 |
| Vasculopathy | 2.52 (1.24–5.11) | 0.01 |
| Model at 6 months | ||
| Zoster | 1.28 (0.91–1.8) | .16 |
| Age vs age 50–64: age 65–79 | 1.77 (1.41–2.23) | <.001 |
| age 80+ | 1.47 (1.31–1.64) | <.001 |
| Hypertension | 1.81 (1.31–2.51) | <.001 |
| Vasculopathy | 2.58 (1.47–4.54) | .001 |
| Model at 1 year | ||
| Zoster | 1.04 (0.79–1.36) | .79 |
| Age vs age 50–64: age 65–79 | 1.53 (1.3–1.81) | <.001 |
| age 80+ | 1.45 (1.33–1.57) | <.001 |
| Hypertension | 1.73 (1.34–2.23) | <.001 |
| Coronary artery disease | 1.44 (1.09–1.89) | .01 |
| Dyslipidemia | 0.66 (0.5– 0.86) | .002 |
| Vasculopathy | 3.07 (2–4.7) | <.001 |
| Model at 3 years | ||
| Zoster | 1.02 (0.86–1.22) | .81 |
| Age vs age 50–64: age 65–79 | 1.88 (1.68–2.11) | <.001 |
| age 80+ | 1.56 (1.47–1.65) | <.001 |
| Sex = Female | 0.90 (0.83–0.93) | .008 |
| Hypertension | 1.67 (1.41–1.96) | <.001 |
| Coronary artery disease | 1.41 (1.17–1.68) | <.001 |
| Dyslipidemia | 0.74 (0.62–0.87) | <.001 |
| Depression | 1.29 (1.07–1.55) | .009 |
| Vasculopathy | 1.68 (1.20–2.36) | .002 |
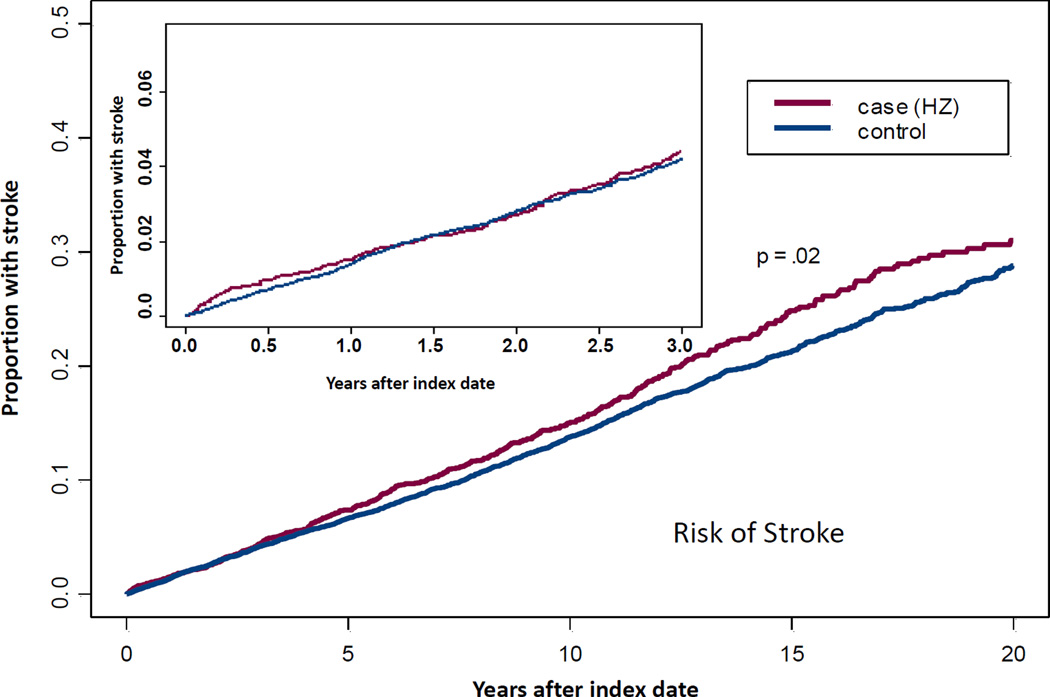
While simple survival analysis demonstrated a difference in time to first stroke after zoster for case versus control patients over 20 years [HR = 1.11, CI 95 1.01–1.23, P = .02), the association was no longer significant when adjusting for comorbidities in a Cox proportional hazards model (P = .14). The top portion of the Figure shows the Kaplan Meier plot for 20 year follow- up with an insert highlighting the first 3 years after zoster.
Figure.
Stroke: Time to First Stroke Over 20 Years after Zoster Compared with Time to First Stroke in Control Subjects Having No Zoster.
All subjects had no stroke prior to index date of zoster using that date also as index date for age and sex matched controls with no history of zoster. Time to stroke was estimated with Kaplan-Meier curves with Cox proportional hazards models for the full follow-up period, with case status, age, sex, and other co-morbidities, as predictors. The sample included 4,478 cases and 16,800 controls. Using a Cox proportional hazards model, controlling for multiple co-morbidities zoster was not associated with long term risk of stroke (P = .14). The insert highlights the shorter term risk over 0 to 3 years. Using step wise logistic regression accounting for multiple co-morbidities, the association of zoster and stroke remained significant at only 3 months after zoster, OR 1.53 (CI 95 1.01, 2.33), P = .044.
Myocardial Infarction: Time to First Myocardial Infarction (MI) Over 20 Years after Zoster Compared with Time to First MI in Control Subjects Having No Zoster.
All subjects had no MI prior to index date of zoster using that date also as index date for age and sex matched controls with no history of zoster. Time to MI was estimated with Kaplan-Meier curves with Cox proportional hazards models for the full follow-up period, with case status, age, sex, and other co-morbidities, as predictors. The sample included 4,454 case and 16,740 control patients. Using a Cox proportional hazards model, controlling for multiple co-morbidities zoster was not associated with long term risk of stroke (P = .13). The insert highlights the shorter term evaluations of MI and zoster. Using step wise logistic regression accounting for multiple co-morbidities, the association of zoster and MI remained significant at only 3 months after zoster 1.68 (CI 95 1.03, 2.75), P = .04.
Zoster and MI analyses included 4,454 individuals with zoster and 16,740 individuals with no zoster, all with no history of prior MI before their index date. The mean age was 68.2 (range 49.1–106 years) with an average of 7.0 years of follow-up (range 0–27.8 years). When considering zoster and each risk factor alone (univariate analyses), MI was associated with zoster at 3 months, 6 months and 1 and 3 years with a declining odds ratio over longer times after zoster from 1.88 (CI95 1.15–3.08) at 3 months to 1.29 (CI95 1.08–1.56) at 3 years (Table 3). However, when controlling for other risk and confounding factors in stepwise logistic regression, zoster remained significantly associated with MI at only 3 months with non-MI coronary artery disease diagnosed before their index date and age as the only other significant factors (Table 3). MI and zoster are not strongly associated at any point in time using any type of analyses and the association of MI and zoster identified at 3 month by logistic regression is not robust across differing analytic approaches.(See Supplemental Table) The associations between MI and zoster are non-significant at 6, 12 and 36 months time with all methods.
Table 3.
Univariate and Mutivairate Stepwise Logistic Regression for Myocardial Infarction at All Time Periods after Zoster
| Univariate logistic regression: MI and Zoster | ||
| Time after index: | OR (95% CI) | p-value |
| 3 months | 1.88 (1.15–3.08) | .01 |
| 6 months | 1.63 (1.09–2.42) | .02 |
| 1 year | 1.49 (1.10–2.00) | .009 |
| 3 years | 1.29 (1.08–1.56) | .006 |
| Multivariate Stepwise Logistic Regression Models | ||
| Model for 3 months | ||
| Variable | OR (95% CI) | P-value |
| Zoster | 1.68 (1.03–2.75) | .04 |
| Age vs age<65: age 65–79 | 2.31 (1.19–4.47) | .01 |
| age 80+ | 3.51 (1.77–6.95) | <.001 |
| Coronary artery disease | 4.17 (2.56–6.78) | <.001 |
| Model for 6 months | ||
| Zoster | 1.44 (0.97–2.15) | .07 |
| Age vs age<65: age 65–79 | 2.3 (1.35–3.92) | .002 |
| age 80+ | 4.02 (2.34–6.9) | <.001 |
| Coronary artery disease | 3.55 (2.4–5.25) | <.001 |
| Diabetes | 1.62 (1.08–2.43) | .02 |
| Model for 1 year | ||
| Zoster | 1.33 (0.99–1.8) | .06 |
| Age vs age<65: age 65–79 | 2.61 (1.77–3.85) | <.001 |
| age 80+ | 4.93 (3.31–7.34) | <.001 |
| Sex = Male | 1.64 (1.24–2.17) | <.001 |
| Coronary artery disease | 2.14 (1.58–2.91) | <.001 |
| Depression | 1.43 (1.03–1.98) | .03 |
| Diabetes | 1.73 (1.29–2.33) | <.001 |
| Model for 3 years | ||
| Zoster | 1.17 (0.97–1.41) | .11 |
| Age vs age<65: age 65–79 | 2.26 (1.8–2.85) | <.001 |
| age 80+ | 4.51 (3.56–5.73) | <.001 |
| Sex = Female | 1.49 (1.25–1.76) | <.001 |
| Hypertension | 1.35 (1.13–1.62) | .001 |
| Coronary artery disease | 2.01 (1.66–2.43) | <.001 |
| Diabetes | 1.47 (1.21–1.78) | <.001 |
Simple survival analysis demonstrated a difference in time to first MI for patients with zoster compared to control patients (HR = 1.13, CI95 1.01–1.25, P = .03) but that association became non-significant after adjusting for co-morbidities (P=.13). The lower portion of the Figure shows both 20-year follow-up results as well as the insert for results from the first 3 years.
Discussion
In our geographically defined U.S. population of older adults, zoster was associated with an increased risk of stroke for three months after zoster even when controlling for multiple risk and confounding factors. The association was robust across multiple analytic strategies. The association between MI and zoster was less strong and not robust across different analytic methods suggesting that this association requires additional evaluation in larger data sets. No increased risk for either stroke or MI continued beyond 3 months including from survival analysis for up to 20 years post zoster when adjusting for cardiovascular and cerebral vascular risk factors and confounding factors such as diabetes. Patients with zoster had significantly higher rates of other chronic diseases including the presence of multiple chronic conditions compared to those without zoster.
Table 4 summarizes the epidemiological studies assessing stroke, MI and TIA risk after zoster. All studies other than ours used administrative data to identify zoster which can result in 5–15% false-positive “cases”.25 Most studies focused only on stroke or stroke and TIA, accounting for multiple cardiovascular risk factors, but used differing study designs. Kang et al.11 and Breuer et al.12 compared zoster cases to age and sex matched control patients with no history of zoster before the date of zoster in the matched case. Langan et al.13 used patients as their own controls comparing stroke rates in the year before zoster with stroke rates in the year after zoster. This may improve “matching” but limits the assessment period to one year. Lin et al.15 included only cases with herpes zoster ophthalmicus (HZO) which we were unable to report over our entire period. Sreenivasan et al.14 used prescribed antiviral medications to identify zoster, a proxy that may be problematic since antiviral agents are often used to treat other herpes virus infections.
Table 4.
Summary of Studies Assessing risk of stroke or MI after zoster
| Author, year, country |
Design | Population | Sample size | Stroke/TIA— short-term results |
Stroke/TIA— long-term results |
MI—short- term results |
MI—long-term results |
|---|---|---|---|---|---|---|---|
| Breuer et al., 2014, UK | Matched cohort—age, sex and practice site Zoster from administrative data Follow-up Mean, 6.8 years (range 1 – 24 years) |
All ages, attending GP offices Zoster from administrative data 2002–2010 |
106,601 cases 213,202 controls Mean age, 57.8 years |
NA | Cox proportional hazard ratios Older (>40 years) TIA---HR = 1.15 (1.09–1.21) Stroke—not significant Younger (<40 years) Stroke---HR 2.42 (1.34–4.36) TIA--- HR 1.49 (1.04–2.15) |
NA | Cox proportional hazard ratios Older (>40 years) MI---HR = 1.10 (1.05–1.16) Younger (≤40 years) MI----HR 1.74 (1.13–2.66) |
| Kang et al., 2009, Taiwan | Case and control cohorts, age- and sex-matched Zoster from administrative data |
Adults 18+ Zoster from administrative data 1997–2001 |
7,760 cases 23,800 controls Mean age, 46.7 years |
Kaplan-Meier and log rank test Stroke and TIA combined All ages: Crude--I year HR 1.31 (1.07–1.61) Adjusted--1 year HR 1.31 (1.06–1.60) |
NA | NA | NA |
| Langan et al., 2014, UK | Cohort comparing individual in year before zoster with year after. Zoster from adminis-trative data |
Adults 18+ Zoster from administrative data 1987–2012 |
6,584 eligible cases who served as their own controls Mean age at stroke, 77 years |
Conditional Poisson regression, incidence rate ratios (IRR) All ages: 1–4 weeks, IRR 1.63 (1.32–2.02) 5–12 weeks, IRR 1.42 (1.21–1.68) 13–26 weeks, IRR 1.23 (1.07–1.42) 27–52 weeks, not significant |
NA | NA | NA |
| Lin et al, 2010, Taiwan | Case and control cohorts, age by decade and sex-matched HZO from medical record review |
Adults 18+ Zoster from administrative data 2003–2004 |
658 cases with HZO 1,974 controls without zoster Mean age 56.9 years |
Kaplan-Meier log rank test Stroke All ages: Crude HR 5.15 (3.31–8.33) Adjusted HR 4.52 (2.40–11.10) |
NA | NA | NA |
| Sreenivasan et al, 2013, Denmark | Cohort “Exposed” presumed zoster—prescribed antiviral therapy | Adults 18+ Zoster from antiviral proxy 1995– 2008 |
4.6 million cohort 117, 926 zoster cases Mean age – not reported |
Poisson regression incident rate ratios (IRR) All ages: 2 weeks IRR, 2.27 (1.83–2.82) [no difference in age groups] 1 year IRR, 1.17 (1.09–1.24) [risk in younger than 40 years—p .0002] |
Poisson regression incident rate ratios (IRR): Stroke and TIA combined All ages: >1 year IRR, 1.05 (1.02–1.09) [not significant in >60 years] |
NA | NA |
| Yawn et al., 2015, US | Case/control, age-and sex-matched in same geographic area Zoster confirmed by medical records Mean follow-up, 7.1 years (range 0 – 28 years) |
Adults 50+ Zoster confirmed by medical records 1986–2010 |
4,862 zoster cases 19,433 control patients Mean age, 68.1 years |
Multivariate logistic regression ≥ 50 years: 3 months OR 1.53 (1.10–2.33) 6, 9, 12 and 36 months—not significant |
Kaplan Meier ≥ 50 years: HR 1.11 (1.10–1.22) Not significant when accounting for multiple risk factors |
Multivariate logistic regression ≥ 50 years: 3 months 1.68 (1.03–2.75) 6, 9, 12 and 36 months--not significant |
Kaplan Meier ≥ 50 years: HR 1.13 (1.01–1.27) Not significant when accounting for multiple risk factors |
Previous studies also differed in the time period of risk assessed. Both Langan et al.13 and Sreenivasan et al.14 reported results as early as two weeks after zoster which our sample size did not allow. However, our 53% increased risk for stroke at three months is similar to the 42% increase reported by Langan at 5–12 weeks.14 Like Langan et al, we also found no increased risk of MI or stroke at 1 year after zoster in older adults13, although both Kang et al.11 and Sreenivasan et al.14 reported increased risk of stroke at 1 year. Unfortunately, Kang et al.11 did not stratify stroke risk by age group reporting only in all adults >18 years of age, making comparison to our results difficult. Although Sreenivasan et al.14 reported a 17% increased risk of stroke in older adults at one year after zoster, the use of antiviral prescriptions as a proxy for zoster requires further confirmation.
Our results agreed with those from the only two other studies that assessed risk of stroke beyond 1 year, showing no long-term increased risk of stroke after zoster in adults older than 40 to 50 years of age when adjusted for multiple risk factors.12,14 The other two studies do report increased risk for stroke in patients younger than 40 years for whom we have no data. We agree with the authors of those studies that results for younger patients must be interpreted with caution due to difficulty in controlling for risk factors which are often not assessed or reported.12,14
Our study is the first large U.S. epidemiological study to report on risk of MI in the immediate post zoster period. We found that the association between zoster and MI at 3 months is not strong and not robust across different analytic methods. However, our cohorts had very few MIs in that brief period suggesting that the results should be further evaluated using a larger dataset. Breuer et al.12 reported a small 10% increased risk of MI in older adults in their long-term follow-up, similar to the unadjusted 13% increased risk we found long-term for MI. Considering the modestly increased risk and the large burden of co-morbid conditions in the zoster patients, these results deserve further scrutiny. Recent studies found that morbidity and multiple morbidities at younger ages strongly predict increasing morbidities with aging.36,37 In all reported studies, patients with zoster have greater numbers of chronic conditions at the time of zoster compared to age- and sex-matched controls. Thus, it is possible that an increased long-term risk of MI and stroke is due at least in part to the steeper multi-morbidity trajectory in zoster patients.
Mechanisms for Increased Short-term Risks Following Zoster
During the first 3 months after zoster, the increased risk of stroke is most likely due to productive VZV infection in intracerebral arteries after transaxonal spread of virus upon reactivation from cranial nerve ganglia. VZV has been found in intracerebral arteries as late as 10 months after zoster38 with pathological changes that include loss of smooth muscle cells which may contribute to aneurysm formation and hemorrhagic stroke.7 Similarly, any increased risk of MI within 3 months of zoster may be caused by virus infection of coronary arteries after transaxonal spread of VZV that reactivated from autonomic and dorsal root ganglia. A recent report described a patient on long-term steroids who developed thoracic zoster and died suddenly 5 months later; post-mortem examination revealed VZV in multiple coronary arteries, as well as in the posterior cerebral artery.39
Inflammatory cells that secrete soluble factors which contribute to vascular remodeling and can potentially disrupt pre-existing atherosclerotic plaques have also been detected in VZV-infected arteries40 and are noted as potentially important in the increased risk of MI after respiratory and urinary tract infections and sepsis.41,42
Limitations and Strengths
The Olmsted County older adult population is primarily Caucasian prohibiting assessment of the impact of race or ethnicity on outcomes. Only the Taiwan studies report race or ethnicity.11,15 Loss to follow up, primarily due to death in these elderly patients was almost 45% by year 5 but was less than 1% at 3 months (all due to non-stroke or MI related deaths) increasing to only 7% by 36 months. Therefore, it is unlikely that loss to follow up affected our 3 month to 3 year assessments and our long term assessment used Kaplan Meier calculations which account for death and other losses to follow up.
Although we included data from all hospital admissions and death records allowing us to capture patients who die from MI or stroke before hospital admission, we did not include “silent” strokes or MIs identified only by imaging. Timing of the initial “silent” event is difficult to ascertain and therefore not easily linked to a zoster or index date. We were unable to control for obesity or smoking status but know that the rate of current smokers in the Olmsted community population is less than one-third of the smoking rate reported in the largest UK study.12 Therefore, it is possible that differences could be due to residual confounding from the smoking, obesity or factors which we were unable to assess.
Strengths of our study include medical record confirmation of all zoster cases, eliminating the 10–15% overestimate of zoster when only administrative data are used for diagnosis.25 Moreover, risk factors for stroke and MI were identified using a broad set of diagnostic codes developed by an expert panel to facilitate assessing many important chronic conditions such as hypertension, depression and dyslipidemia.32 Codes included those used by other studies that provided their list of ICD-9 codes for risk factor identification as well as additional codes that may represent less commonly diagnosed but important representations of those chronic conditions. Finally, this is the first study to combine analytic strategies to assess both short term risk of stroke and MI at 3 months, 6 months, 1 year and 3 years. Our study also has the longest post zoster follow up period ever reported.
Conclusions
In adults aged 50 years and older, zoster is associated with an increased risk of stroke and possibly MI in the first 90 days, but not thereafter. Use of zoster vaccine may prevent zoster and therefore, the associated acute increased risk.
Supplementary Material
ACKNOWLEDGMENTS
Funding/Support: This study was supported by an investigator-initiated grant from Merck & Company (Yawn), NIA grant R01 AG034676 (Yawn) and NIH grant AG032958 (Gilden, Nagel).
Role of the Funder/Sponsor: None of the funders or sponsors had any role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: The authors thank Marina Hoffman for editorial assistance, and Cathy Allen for word processing and formatting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
All authors report no conflict of interest.
References
- 1.Cohen JI. Clinical practice: Herpes zoster. N Engl J Med. 2013;369(3):255–263. doi: 10.1056/NEJMcp1302674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel M, Gilden D. Editorial commentary: varicella zoster virus infection: generally benign in kids, bad in grown-ups. Clin Infect Dis. 2014;58(11):1504–1506. doi: 10.1093/cid/ciu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai K, Gebremeskel BG, Acosta CJ. Systematic review of incidence and complications of herpes zoster: towards a global perspective. BMJ Open. 2014;4(6):e004833. doi: 10.1136/bmjopen-2014-004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yawn BP, Gilden D. The global epidemiology of herpes zoster. Neurology. 2013;81(10):928–930. doi: 10.1212/WNL.0b013e3182a3516e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30. [PubMed] [Google Scholar]
- 6.Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82(11):1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- 7.Nagel MA, Traktinskiy I, Azarkh Y, Kleinschmidt-DeMasters B, Hedley-Whyte T, Russman A, VanEgmond EM, Stenmark K, Frid M, Mahalingam R, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011 Jul 26;77(4):364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert GJ. Herpes zoster ophthalmicus and delayed contralateral hemiparesis. Relationship of the syndrome to central nervous system granulomatous angiitis. JAMA. 1974;229(3):302–304. [PubMed] [Google Scholar]
- 9.Yawn BP, Wollan PC, St Sauver JL, Butterfield LC. Herpes zoster eye complications: rates and trends. Mayo Clin Proc. 2013;88(6):562–570. doi: 10.1016/j.mayocp.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilden D. Association of varicella zoster virus with giant cell arteritis. Monoclon Antib Immunodiagn Immunother. 2014 Jun;33(3):168–172. doi: 10.1089/mab.2014.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JH, Ho JD, Chen YH, Lin HC. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40(11):3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 12.Breuer J, Pacou M, Gautier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;83(2):e27–e33. doi: 10.1212/WNL.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 13.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58(11):1497–1503. doi: 10.1093/cid/ciu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sreenivasan N, Basit S, Wohlfahrt J, et al. The short- and long-term risk of stroke after herpes zoster - a nationwide population-based cohort study. PLoS ONE. 2013;8(7):e69156. doi: 10.1371/journal.pone.0069156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74(10):792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- 16.Yawn BP, Yawn RA, Geier GR, Xia Z, Jacobsen SJ. The impact of requiring patient authorization for use of data in medical records research. J Fam Pract. 1998;47(5):361–365. [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Xia Z, Campion ME, et al. Potential effect of authorization bias on medical record research. Mayo Clin Proc. 1999;74(4):330–338. doi: 10.4065/74.4.330. [DOI] [PubMed] [Google Scholar]
- 18.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173(9):105910–105968. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, Pankratz JJ, Brue SM, Rocca WA. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012 Dec;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009;84(9):787–794. doi: 10.4065/84.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin M, Yawn BP, Hales CM, Bailek S, Harpaz R. Herpes Zoster Vaccine Effectiveness and Manifestations of Herpes Zoster and Associated Pain by Vaccination Status. Hum Vaccin Immunother. 2015 Mar; doi: 10.1080/21645515.2015.1016681. 25:0. [Epub ahead of print] http://www.ncbi.nlm.nih.gov/pubmed/25806911. [DOI] [PMC free article] [PubMed]
- 24.Yawn BP, Wollan PC, Kurland MJ, St Sauver JL, Saddier P. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yawn BP, Wollan P, St Sauver J. Comparing shingles incidence and complication rates from medical record review and administrative database estimates: how close are they? Am J Epidemiol. 2011;174(9):1054–1061. doi: 10.1093/aje/kwr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yawn BP, Wollan PC, Yawn RA, Jacobsen SJ, Roger V. The gender specific frequency of risk factor and CHD diagnoses prior to incident MI: a community study. BMC Fam Pract. 2007;8:18. doi: 10.1186/1471-2296-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson RW, Rice ASC. Postherpetic neuralgia. N Eng J Med. 2014;371:1526–1533. doi: 10.1056/NEJMcp1403062. [DOI] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services. Multiple Chronic Conditions - A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, D.C.: 2010. Dec, [Google Scholar]
- 29.Cohen JW, Cohen SB, Banthin JS. The medical expenditure panel survey: a national information resource to support healthcare cost research and inform policy and practice. Med Care. 2009;47(7 Suppl 1):S44–S50. doi: 10.1097/MLR.0b013e3181a23e3a. [DOI] [PubMed] [Google Scholar]
- 30.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and measuring chronic conditions: imperatives for research, policy, program, and practice. Prev Chronic Dis. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobo W, Rocca W, St Sauver J, Grosshart B, Yawn BP. Physical and Mental Multi-morbidity in a geographically define community population. 2015. Psychosom Med. In press. [Google Scholar]
- 32.Gustad LT, Laugsand LE, Janszky I, Dalen H, Bjerkeset O. Symptoms of anxiety and depression and risk of acute myocardial infarction: the HUNT 2 study. Eur Heart J. 2014;35(21):1394–1403. doi: 10.1093/eurheartj/eht387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, Mcbean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 34.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 36.Chamberlain AM, Finney Rutten LJ, Manemann SM, Yawn BP, Jacobson DJ, Fan C, Grossardt BR, Roger VL, St. Sauver JL. Frailty trajectories in an elderly population-based cohort, 2015. J Am Geriatr Soc. doi: 10.1111/jgs.13944. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yende S, angus DC, Ali IS, et al. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007;55(4):518–525. doi: 10.1111/j.1532-5415.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 38.Case Records of the Massachusetts General Hospital: Weekly clinicopathological exercises: case 36–1996: a 37-year-old man with AIDS, neurologic deterioration, and multiple hemorrhagic cerebral lesions. N Engl J Med. 1996;335:1587–1595. doi: 10.1056/NEJM199611213352109. [DOI] [PubMed] [Google Scholar]
- 39.Nagel MA, Lenggenhager D, White T, Khmeleva N, Heintzman A, Boyer PJ, Gilden D. Disseminated VZV infection and asymptomatic VZV vasculopathy after steroid abuse. 2015. J. Clin. Virol. 66:72–75. doi: 10.1016/j.jcv.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagel MA, Traktinskiy I, Stenmark KR, Frid MG, Choe A, Gilden D. Varicella-zoster virus vasculopathy: immune characteristics of virus-infected arteries. Neurology. 2013 Jan 1;80(1):62–68. doi: 10.1212/WNL.0b013e31827b1ab9. PMCID:PMC3589199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrales-Medina VF, Alvarez KN, Weissfeld LA, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313(3):264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smeeth L, Thomas Sl, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.