Abstract
Objective
Decrements in cognitive function may already be evident in young children with type 1 diabetes (T1D). Here we report prospectively acquired cognitive results over 18 months in a large cohort of young children with and without T1D.
Methods
144 children with T1D (mean HbA1c: 7.9%) and 70 age-matched healthy controls (mean age both groups 8.5 years; median diabetes duration 3.9 yrs; mean age of onset 4.1 yrs) underwent neuropsychological testing at baseline and after 18-months of follow-up. We hypothesized that group differences observed at baseline would be more pronounced after 18 months, particularly in those T1D patients with greatest exposure to glycemic extremes.
Results
Cognitive domain scores did not differ between groups at the 18 month testing session and did not change differently between groups over the follow-up period. However, within the T1D group, a history of diabetic ketoacidosis (DKA) was correlated with lower Verbal IQ and greater hyperglycemia exposure (HbA1c area under the curve) was inversely correlated to executive functions test performance. In addition, those with a history of both types of exposure performed most poorly on measures of executive function.
Conclusions
The subtle cognitive differences between T1D children and nondiabetic controls observed at baseline were not observed 18 months later. Within the T1D group, as at baseline, relationships between cognition (VIQ and executive functions) and glycemic variables (chronic hyperglycemia and DKA history) were evident. Continued longitudinal study of this T1D cohort and their carefully matched healthy comparison group is planned.
Keywords: cognition, early onset, T1D, hyperglycemia, longitudinal, children
Introduction
Children with early onset diabetes (EOD; < 5 years of age at diagnosis) are more likely than those with later onset to have poorer cognitive outcomes in the domains of IQ (Northam et al., 1998; Rovet et al., 1987), executive functions (Bjorgaas et al., 1997; Flykanaka-Gantenbein, 2004; Lin et al., 2010; Ly et al., 2011), learning and memory (Gaudieri et al., 2008; Lin et al., 2010), and processing speed (Lin et al., 2010; Northam et al., 2001; Ryan et al., 1985). One or more severe hypoglycemia episodes (with seizure or loss of consciousness) in childhood has also been associated with poorer cognitive outcomes (Blasetti et al., 2011; Hershey et al., 1997; Hershey et al., 2003, 2004; Hershey et al., 2005; Lin et al., 2010; Naguib et al., 2009; Northam et al., 2001; Perantie et al., 2008; Rovet & Ehrlich, 1999) and brain volume differences (Ferguson et al., 2003; Haumont et al., 1979; Hyllienmark et al., 2005; Musen et al., 2006; Northam et al., 2009; Perantie et al., 2011; Perantie et al., 2007; Perros et al., 1997).
Chronic hyperglycemia may also interfere with brain functioning and cognition. Greater hyperglycemia exposure has been associated with differences in gray and white matter volumes (Perantie et al., 2011; Perantie et al., 2007), lower processing speed (Jacobson et al., 2011) and lower verbal intelligence (Perantie et al., 2008). In our previously reported baseline analyses in the same cohort as reported here, greater hyperglycemia prior to the baseline evaluation was associated with differences in gray matter volumes in frontal, temporal and other posterior cortical regions (Marzelli et al., 2014). Baseline neuroimaging also revealed widespread white matter differences in T1D youth, more so in those with longer disease duration and greater recent exposure to hyperglycemia (Barnea-Goraly et al., 2014).
Investigators have speculated that there may also be interactions between age and degree of exposure to glycemic extremes, with the most vulnerable children being those with early childhood onset of T1D and a history of chronic hyperglycemia or severe hypoglycemia (Arbelaez et al., 2013). Some support for this concept has come from a cross sectional analysis of school-age children, demonstrating that early exposure to severe hypoglycemia was associated with lower memory performance (Hershey et al., 2005). In addition, there has been speculation that early exposure to extreme glycemic states (e.g. DKA at diagnosis) may predispose children to poor outcomes in the case of subsequent prolonged and more severe dysglycemia (e.g. chronic hyperglycemia, Ryan, 2006).
Cross-sectional studies, however, cannot adequately test such complex hypotheses, control for baseline differences or support causal inferences. Thus, prospective longitudinal follow-up is necessary to understand the impact of various degrees of glycemic exposure and their interactions with age. Our ongoing longitudinal study of young children with and without T1D aims to address this question using cognitive and neuroimaging tools. We recently reported that young children with T1D have a different developmental trajectory over 18 months in gray and white matter volumes compared to non diabetic controls and that these differences strongly correlated with the degree of hyperglycemia experienced during an 18-month follow-up period. Specifically, T1D youth had slower grey matter development in widespread regions including left precuneus extending to left parietal and occipital cortex and right frontal, temporal, and parietal lobes. White matter growth was also slower across anterior, inferior frontal, and superior parietal regions. The strongest effect on white matter growth was observed in a right anterior frontal region near the corpus callosum. Among T1D youth, greater extent of overall disease-related exposure to hyperglycemia related inversely to gray matter growth in widespread regions. Higher glucose variability was also associated with slower rates of growth across widespread grey and white matter regions (Mauras et al., 2015).
In the same cohort at baseline, we reported subtle cognitive differences between children (4 to <10 years old) with T1D compared to healthy age-matched controls (Cato et al., 2014). While the group differences did not meet stringent statistical significance thresholds after adjusting for multiple comparisons and correction for parent IQ; child IQ and Executive Functions domain scores trended lower in those with T1D (both p = .02)(Cato et al., 2014)(Cato et al., 2014)(Cato et al., 2014). Further, degree of previous hyperglycemia (as measured by several indices derived by HbA1c values and continuous glucose monitoring [CGM] data) was associated with these differences(Cato et al., 2014)(Cato et al., 2014)(Cato et al., 2014). Chronic hyperglycemia in T1D youth, indexed by averaged A1c area under the curve (AUC) above 6.0% was associated with lower IQ (p = .05). The percentage of time blood glucose level exceeded 180mg/dL was associated with a lower Executive Functions domain score (p = .04). The next logical step, reported in this paper, is to determine how cognitive performance changes over time in T1D vs. controls and whether these changes correlate with glycemic exposure during follow-up. In this ongoing, prospective, well-controlled, large-scale longitudinal study we have a unique opportunity to determine the timeframe, risk factors, and effect of cumulative exposure to glycemic extremes beginning in early childhood. We hypothesized that cognitive differences between groups would become more clearly pronounced over time. We also hypothesized that exposure to dysglycemia and severe metabolic events (severe hypoglycemia [SH], diabetic ketoacidosis [DKA]) during the follow-up period would be associated with worse cognitive outcomes.
Methods
All human data included in this manuscript were obtained after institutional review approval at each of the participating centers and informed written consent obtained from parents or guardians and child assent when appropriate.
Participants
A total of 144 children with T1D and 72 non-diabetic controls participated in the study at baseline (Cato et al., 2014). From this cohort, all 144 children with T1D and 70 of the non-diabetic participants completed at least a portion of the 18-month cognitive testing and follow-up. The T1D and control groups had similar gender distributions, parental education and family income levels. At 18-months follow-up, average age was 8.5 years for both groups (range 5.4 to 11.5 years). The T1D cohort had a median duration of diabetes of 3.9 years, ranging from 1.6 to 9.5 years. Mean age of onset was 4.1 years (range 0.9 to 8.0 years), with 67% (N=97) diagnosed before age five. A comprehensive summary of demographic characteristics of each group was summarized in the baseline study. The T1D and control groups had similar gender distributions, parent education and income levels (Cato et al., 2014, Table 2). Among children with T1D, 16% (n = 23) had a history of at least one episode of severe hypoglycemia and 36% (n = 51) had a history of diabetic ketoacidosis (DKA) prior to the baseline study, while 4% (n = 6) had at least one episode of severe hypoglycemia and 3% (n = 4) had a history of DKA between the baseline study and the 18-month follow-up study.
Table 2.
Cognitive Outcomes Changes from Baseline to 18 Months
| Changes (18 Month – Baseline)
|
||||||
|---|---|---|---|---|---|---|
| N | T1D | Control | P-valueb | |||
|
| ||||||
| Mean ± SD | P-valuea | Mean ± SD | P-valuea | |||
| Z- Scores | ||||||
| IQ c | 137/65 | +0.27 ± 0.69 | <0.001 | +0.13 ± 0.82 | 0.21 | 0.44 |
| Verbal IQ | 137/65 | +0.22 ± 0.82 | 0.002 | +0.06 ± 0.98 | 0.63 | 0.46 |
| Performance IQ | 137/65 | +0.16 ± 0.76 | 0.02 | +0.04 ± 0.78 | 0.72 | 0.89 |
| Executive Functions c | 131/68 | +0.77 ± 0.64 | <0.001 | +0.65 ± 0.61 | <0.001 | 0.68 |
| Learning and Memory c | 137/66 | +1.08 ± 0.65 | <0.001 | +1.04 ± 0.66 | <0.001 | 0.67 |
| Processing Speed c | 135/69 | −0.10 ± 0.82 | 0.14 | −0.15 ± 0.86 | 0.14 | 0.50 |
| BRIEF (Behavior Rating Inventory of Executive Function by Parent)d | 140/67 | −0.12 ± 0.82 | 0.08 | −0.21 ± 0.73 | 0.02 | 0.26 |
| Externalizing (Behavior Assessment by Parent) d | 142/68 | −0.08 ± 0.84 | 0.24 | −0.03 ± 0.72 | 0.71 | 0.44 |
| Internalizing (Behavior Assessment by Parent) d | 142/67 | −0.21 ± 0.77 | 0.002 | −0.04 ± 0.76 | 0.68 | 0.91 |
P-values from paired t- test for within-group changes.
Nominal P-value uncorrected for multiple comparisons. Obtained from repeated measure least squares regression models, adjusted for baseline cognitive scores, siblings from same family, age, gender, and parent IQ.
Positive changes are better.
Positive changes are worse
Glycemic Control
Methods to ascertain glycemic control among the T1D participants were previously described (Cato et al., 2014). HbA1c values were collected every 3 months for the 18 months of the study. For participants using an unblinded personal CGM as part of their diabetes care data were collected every 3 months for the 18 months of the study. Otherwise, participants were asked to use a blinded CGM (iPro2®, Medtronic MiniMed, Northridge, CA or DexCom SEVEN Plus®, DexCom, San Diego, CA) for 6 days every 3 months in order to collect at least 72 hours of CGM data with at least 24 hours of data overnight (10 p.m. to 6 a.m.).
Neurocognitive Testing
The Neurocognitive testing methods have been previously described in detail (Cato et al., 2014). Table 1 lists test battery by domains assessed. In keeping with the literature, the battery was selected to cover the cognitive domains of interest: IQ, Executive Functions, Learning and Memory, and Processing Speed using age-appropriate measures. The assignment of tasks to each cognitive domain was based on clinical experience. At baseline, one of the parents completed an IQ measure to be used as a planned covariate. Parent-reported externalizing behavior, internalizing mood symptoms and executive functioning ratings were also obtained.
Table 1.
Neurocognitive Test Battery: Domains and Measures
| Cognitive Domain | Measure a | Test | Battery b |
|---|---|---|---|
| IQ | Scaled score e | Block design | WPPSI-3 / WASI |
| Scaled score e | Similarities | ||
| Scaled score e | Vocabulary | ||
| Scaled score e | Matrix reasoning | ||
| Executive Functions | Detectability | CPT-2 | Connor’s |
| Total Correct | Auditory Attn | NEPSY-2 | |
| Total Correct | Concept Formation | WJ-3 Cognitive | |
| Total Correct | Numbers | CMS | |
| Learning & Memory | Total items recalled | Word Lists c | CMS |
| Total items recalled | Dot Locations c | CMS | |
| Processing Speed | Standard score d | Visual Match I / II | WJ-3 Cognitive |
| Decision Speed | WJ-3 Cognitive | ||
| Mood/Behavior | Measure | Scale | Battery |
| Executive functioning | Raw score | Global Executive Composite | BRIEF Parent |
| Externalizing symptoms | T score | Externalizing | BASC-2 PRS |
| Internalizing symptoms | T score | Internalizing | BASC-2 PRS |
| Covariate | Measure | Test | Battery |
| Parent IQ | Scaled score e | Vocabulary | WASI |
| Scaled score e | Matrix Reasoning |
z score was calculated for each measure using mean and SD from the current study pooling all participants (N=216) at both baseline and 18 month visits. For domains with more than one test, the average was taken giving equal weight to each z score.
Abbreviations: CMS – Children’s Memory Scale (Cohen, 1997); CPT-2 – Continuous Performance Test, Second Edition (Connors, 1994); NEPSY-2 – Neuropsychological Battery for Children, Second Edition (Korkman et al., 2007); WJ-3 Cognitive – Woodcock-Johnson Test of Cognitive Abilities, Third Edition (Woodcock et al., 2001); WPPSI3–Wechsler Preschool and Primary Scales of Intelligence, Third Edition (Wechsler, 2002); WASI-Wechsler Adult Scale of Intelligence (Wechsler, 1999); BRIEF – Behavior Rating Inventory of Executive Functions (Gioia et al., 2000); BASCII PRS – Behavior Assessment System for Children, Second Edition Parent Rating Scales (Reynolds & Kamphaus, 2004).
Same version given regardless of age.
Age-based standard score from WJIII Cognitive normative update (NU) sample
Age-based scaled score derived from Wechsler normative sample
The same monitoring of BG concentrations was conducted for the 18-month follow-up. Acceptable BG concentration range was between 70 and 300 mg/dL during testing. Ketones were evaluated in cases of BG > 300 mg/dL and if more than trace urine ketones or blood ketones >0.6 mmol/l were present, testing was postponed. Testing was also suspended if BG dropped below 70 mg/dL. BG levels were checked at least twice during the evaluation at regular, planned intervals, by fingerstick on a home glucose meter. Test protocols were double scored at a centralized location (Washington University in St. Louis), and the results were analyzed at the DirecNet Coordinating Center (Jaeb Center for Health Research, Tampa, FL). The median (interquartile range) duration between baseline testing and 18 months testing was 18 months with range 15 to 23 months.
Statistical Methods
Raw scores were transformed to Z scores for each measure using mean and SD from the current study pooling all participants (N = 216) at baseline and 18-months as described in the prior paper reporting baseline cognitive results (Manschot et al., 2006; van den Berg et al., 2010). Note that some measures administered at baseline were given outside of standardized range to maintain consistency across both timepoints and age range (Cato et al. 2014). Domain scores were omitted in participants who did not complete all sub-domain measures. Supplemental Table 1 summarizes source test scores for T1D and control participants used to produce domain Z scores for cognition, behavior and mood. Repeated measure least squares regression models were used to account for the possibility that outcomes from siblings may be correlated. These models compared children with T1D versus those without on each of the domains and subdomain measures adjusting for age, gender, and parent IQ. The parent-reported child depression score obtained from the BASC 2 PRS was used as an additional covariate for subdomain analyses as presence of depressive symptoms can have a deleterious effect on cognition (Murrough et al., 2011).
Primary outcome domains were pre-defined as learning and memory, executive functions, processing speed and IQ. Secondary outcomes included parent ratings of executive functioning, externalizing behavior symptoms and internalizing mood symptoms. For primary domains, the Hochberg step up approach (Hochberg, 1988) was used to adjust the threshold-defining statistical significance to account for multiple comparisons. No formal correction for multiple comparisons was made for the other secondary domains.
Within the T1D cohort, all glycated hemoglobin levels since diagnosis, and those collected quarterly for 18-months, were used to compute a life-long cumulative index of hyperglycemia exposure based on average amount >6% (HbA1CAUC6%) using the trapezoidal rule. Glycemic variables from continuous glucose monitoring data included: mean glucose (GluMean), percent time when glucose values >180mg/dL, >250 mg/dL and <70 mg/dL, standard deviation (SD), and coefficient of variation (CV). CGM indices were calculated from data downloads at the enrollment visit and each of the follow-up visits every 3 months through 18 months. The average CGM indices across all 7 visits for each participant were calculated giving equal weight to each visit. Participants who had 4 or fewer visits with at least 48 hours of CGM data were not included in the analyses. Other diabetes-specific variables included age of onset, duration of diabetes, number of severe hypoglycemia events and DKA events. Spearman partial correlations were conducted between these variables and each of the cognitive domains, adjusting for age, gender and parent IQ. Only p-values below .01 were considered statistically significant.
Results
Cross-Sectional Data
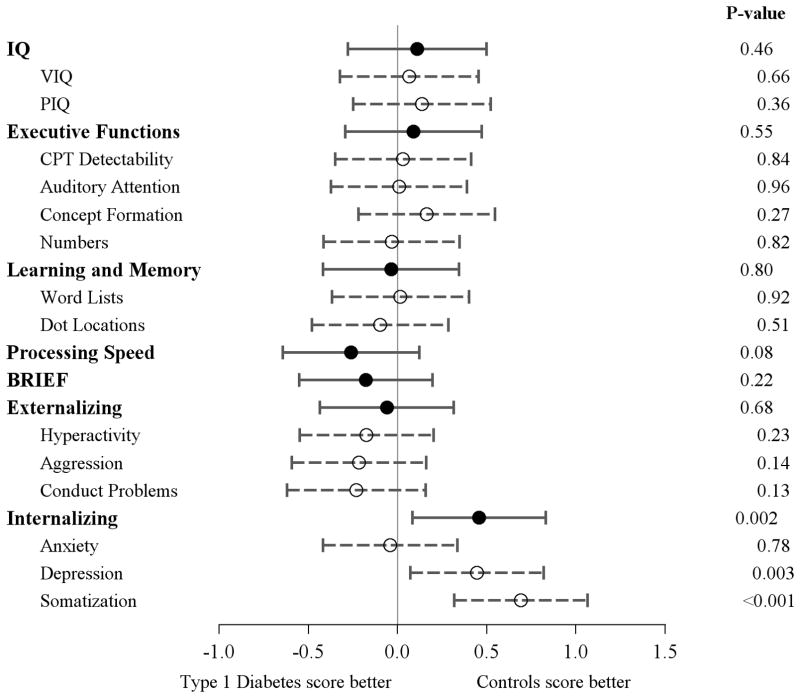
Even though symptom improvement was noted over time, at 18 months T1D subjects still had significantly more internalizing mood symptoms (p=0.002) than controls, replicating what was observed at baseline. None of the cognitive domains were significantly different between the groups at 18 months (Figure 1).
Figure 1. Estimated effect sizes for cognitive domains at 18 months.
The dot represents the point estimate and the width of the bars represents a 99% confidence interval. The confidence intervals are not otherwise corrected for multiple comparisons. For the domains of Executive Functions, IQ, Learning and Memory, and Processing Speed, effect sizes to the right of the vertical line indicate that the control group scored higher. For BRIEF, Externalizing and Internalizing domains, scores are reversed such that effect sizes to the right of the vertical line indicate that the control group had less symptoms.
Longitudinal Data
Both groups tended to improve on the cognitive testing as expected with age but did not differ in degree of change over 18 months (Table 2). An inspection of within group changes suggests the largest increase across both groups in learning and memory, followed by executive functions. There was also a modest differential increase in verbal IQ among the T1D group. Level of internalizing mood symptoms improved within the T1D group although group differences remained (see above).
Relationship to Glycemic Variables
Mean HbA1c at the beginning and end of the study was 7.9% (63 mmol/mol). As measured by CGM data across the 7 visits during the 18-month study, 50% of participants had glucose levels >180mg/dL for >12 hours a day and >250 mg for >6 hours a day. Median time <70 mg/dL was 66 minutes per day and <60 mg/dL was 39 minutes per day. Within the T1D group, CGM measures did not correlate with change in cognitive domains over 18 months (Table 3).
Table 3.
Spearman Correlation between CGM indices and Change in Cognitive Outcomes from Baseline to 18 Monthsa
| IQ | Verbal IQ | Performance IQ | Executive Functions | Learning & Memory | Processing Speed | BRIEF | Externalizing | Internalizing | |
|---|---|---|---|---|---|---|---|---|---|
| % Glucose in Target Range (71–180 mg/dL) | |||||||||
| Spearman Correlation | +0.01 | −0.10 | +0.02 | −0.15 | −0.04 | −0.09 | +0.03 | +0.06 | −0.01 |
| p-value | 0.91 | 0.30 | 0.87 | 0.10 | 0.63 | 0.35 | 0.73 | 0.47 | 0.88 |
| N | 124 | 122 | 122 | 118 | 124 | 123 | 129 | 129 | 129 |
| % Glucose in Hypoglycemia (<70 mg/dL) | |||||||||
| Spearman Correlation | +0.14 | +0.05 | +0.12 | −0.00 | +0.22 | +0.01 | −0.02 | −0.11 | −0.01 |
| p-value | 0.12 | 0.59 | 0.18 | 0.99 | 0.01 | 0.92 | 0.84 | 0.24 | 0.94 |
| N | 124 | 122 | 122 | 118 | 124 | 123 | 129 | 129 | 129 |
| % Glucose in Hyperglycemia (above 180 mg/dL) | |||||||||
| Spearman Correlation | −0.04 | +0.07 | −0.02 | +0.14 | −0.03 | +0.08 | −0.00 | +0.00 | +0.03 |
| p-value | 0.68 | 0.45 | 0.81 | 0.14 | 0.74 | 0.37 | 0.97 | 0.97 | 0.77 |
| N | 124 | 122 | 122 | 118 | 124 | 123 | 129 | 129 | 129 |
| Glucose Coefficient of Variation (SD/mean) | |||||||||
| Spearman Correlation | +0.15 | +0.04 | +0.16 | +0.02 | +0.19 | −0.01 | −0.02 | −0.06 | −0.06 |
| p-value | 0.10 | 0.69 | 0.08 | 0.86 | 0.04 | 0.95 | 0.79 | 0.52 | 0.50 |
| N | 124 | 122 | 122 | 118 | 124 | 123 | 129 | 129 | 129 |
| Mean Glucose | |||||||||
| Spearman Correlation | −0.04 | +0.07 | −0.02 | +0.14 | −0.02 | +0.12 | −0.02 | +0.00 | +0.04 |
| p-value | 0.65 | 0.48 | 0.84 | 0.13 | 0.86 | 0.20 | 0.83 | 0.96 | 0.67 |
| N | 124 | 122 | 122 | 118 | 124 | 123 | 129 | 129 | 129 |
CGM indices are averaged values from baseline to 18 month. Spearman partial correlation controlled for age at 18 month testing, gender and parent IQ, not adjusted for multiple comparisons.
There were two moderate DKA events and two DKA events with unknown severity in 4 individuals between baseline and 18 months and no known severe DKA events during this time period. Eight SH events occurred in 6 participants between baseline and 18 months, and one seizure/coma event. At 18 months, Verbal IQ negatively correlated with number of DKA events prior to 18 months (r=−.23, p = .009). Within the T1D group, additional data trends were observed including an inverse relationship between hyperglycemia (averaged A1c AUC above 6.0%) and executive functions (r=−.19, p = .03), and an inverse relationship between number of DKA events prior to 18 months and IQ (r=−.17, p = .05) as well as executive functions (r=−.20, p = .02). There was also an inverse relationship between Performance IQ at 18 months and history of SH events (r=−.19, p = .04) (See Table 5).
Table 5.
Spearman Correlation between Diabetic History and Cognitive Outcomes at 18 Months a
| IQ | Verbal IQ | Performance IQ | Executive Functions | Learning & Memory | Processing Speed | BRIEF | Externalizing | Internalizing | |
|---|---|---|---|---|---|---|---|---|---|
| Averaged A1c AUC above 6.0% | |||||||||
| Spearman Correlation | −0.13 | −0.09 | −0.08 | −0.19 | −0.01 | +0.13 | +0.10 | +0.08 | +0.13 |
| p-value | 0.15 | 0.31 | 0.36 | 0.03 | 0.92 | 0.13 | 0.25 | 0.38 | 0.13 |
| N | 133 | 131 | 131 | 134 | 135 | 135 | 140 | 138 | 138 |
| # of SevHypo Events | |||||||||
| Spearman Correlation | −0.14 | −0.06 | −0.19 | −0.06 | −0.08 | +0.06 | −0.04 | −0.00 | +0.01 |
| p-value | 0.12 | 0.52 | 0.04 | 0.51 | 0.34 | 0.48 | 0.63 | 0.96 | 0.90 |
| N | 133 | 131 | 131 | 134 | 135 | 135 | 140 | 138 | 138 |
| # of DKA Events b | |||||||||
| Spearman Correlation | −0.17 | −0.23 | −0.04 | −0.20 | −0.07 | −0.14 | +0.07 | −0.03 | +0.01 |
| p-value | 0.05 | 0.009 | 0.63 | 0.02 | 0.44 | 0.12 | 0.40 | 0.75 | 0.91 |
| N | 131 | 129 | 129 | 133 | 133 | 133 | 138 | 136 | 136 |
| Diabetes Duration | |||||||||
| Spearman Correlation | +0.03 | +0.04 | −0.02 | +0.02 | −0.08 | +0.03 | −0.05 | −0.01 | −0.04 |
| p-value | 0.69 | 0.64 | 0.79 | 0.82 | 0.36 | 0.71 | 0.59 | 0.91 | 0.65 |
| N | 133 | 131 | 131 | 134 | 135 | 135 | 140 | 138 | 138 |
| Age at onset (yrs) | |||||||||
| Spearman Correlation | −0.07 | −0.05 | −0.02 | −0.03 | +0.13 | −0.11 | +0.02 | −0.02 | +0.04 |
| p-value | 0.46 | 0.57 | 0.85 | 0.76 | 0.14 | 0.23 | 0.81 | 0.85 | 0.68 |
| N | 133 | 131 | 131 | 134 | 135 | 135 | 140 | 138 | 138 |
Spearman partial correlation controlled for age at 18 month testing, gender and parent IQ, not adjusted for multiple comparisons.
Excluded 2 subjects with unknown DKA status
We performed additional exploratory sub-group analyses to determine if there were any interaction effects on cognitive outcomes for exposure to glycemic extremes at an early age and subsequent dysglycemia exposure, as proposed by Ryan (Ryan, 2006). As this analysis was exploratory, we selected two cognitive variables: VIQ and a combination of the 3 executive functioning measures that best discriminated among T1D and non-T1D participants in our cohort at baseline. To test Ryan’s hypothesis we examined whether Moderate/Severe DKA around the time of diagnosis would interact with subsequent greater exposure to hyperglycemia (defined as upper quartile in our T1D cohort for cumulative exposure) to predict worse cognitive outcomes. Before examining the impact of the combination of these risk factors, we looked at each component separately within our T1D cohort. Performance on executive functioning measures trended lower for those T1D individuals (n = 32) with baseline history of moderate to severe DKA (p = .03); mean differences were also observed in this direction for VIQ but with p value of only .09. Similarly, lower performance on executive functioning measures (p = .02) was observed for those T1D individuals in the upper quartile for hyperglycemia exposure, defined as A1c AUC ≥ 11.2. The direction of the means was for worst Verbal IQ for T1D participants in upper quartile but the mean difference was not significant (p = .12). Only 9 participants experienced both Severe/Moderate DKA at baseline and were in the upper quartile of our T1D cohort for hyperglycemia exposure over the 18-month period (defined as A1C AUC ≥ 11.2). By examination of mean performances, the small number of children with this combination of risk factors fared the worst on the executive functions measures, consistent with Ryan’s hypothesis. However, among those with moderate/severe DKA history Verbal IQ was not worst for those T1D cases also in the upper quartile hyperglycemia group.
Discussion
Differences in cognitive function between children with and without T1D did not worsen over the course of 18 months in this longitudinal study. In addition, children with T1D did not differ from controls in the rate of change in cognitive function over 18 months. In fact, comparable improvements in performance over time were seen in both groups. Practice effects may have enhanced the rate of improvement over and above underlying developmental processes but did not appear to differentially affect the groups. Regression to the mean, the tendency for most extreme scores to normalize towards distribution center over time, may also have come into play. Even so, group performances at 18 month follow-up were similar and indicated comparable cognitive functioning across groups. This contrasts with the significant differences in gray and white matter regional volumes and overall brain growth observed in this cohort as compared to controls (Mauras et al., E ahead of print).
This pattern of preserved cognitive function in the face of structural growth differences between baseline and 18 months could suggest cerebral reserve in this young age group. We know from other clinical populations that abnormal changes in brain structure can precede clinical manifestations. In Parkinson’s disease it is well established that depletion of dopaminergic cells in the substantia nigra must reach a certain threshold before clinical problems are manifest (Cato & Crosson, 2006). In multiple sclerosis, structural and functional hippocampal changes have been observed with intact memory function (Roosendaal et al. 2010). In childhood epilepsy, children without impaired language skills have been shown to have abnormal brain development and reorganization of language-related brain regions (Caplan et al., 2009). Hermann et al. (2002) posit that childhood onset temporal lobe epilepsy represents an early acquired vulnerability that places patients at risk for progressive cognitive decline, citing a reduction in cerebral reserve over time in this chronic condition that has established impact on both brain structures and function. In the case of childhood onset T1D, long term exposure to dysglycemia may lead to sub-clinical neural changes that accumulate until they become clinically relevant through standardized cognitive and behavioral testing. Thus, the possibility of delayed cognitive impact should be closely monitored over time.
This is especially likely given what we already know about cognition in childhood onset T1D and disease duration. In several of the studies that report cognitive differences in T1D patients with childhood onset disease, the cohorts were older (teenagers) and/or had longer disease duration (e.g., Lin et al., 2010; Northam et al., 1999; Perantie et al. 2008; Ryan, Vega, & Drash, 1985). Specifically, studies from the labs of E. Northam and T. Hershey, for example, suggest that over longer disease duration, such as 12 years (e.g., Lin et al., 2010; Northam et al. 2009; Perantie et al., 2008), risk factors of EOD, severe hypoglycemia and poor metabolic control (DKA and hyperglycemia) have an additive and cumulative impact on cognition. Particularly in the case of Verbal IQ and within the domain of Executive Functions, long term exposure to these risk factors are associated with performance decrements relative to study comparison groups.
In 2006 Ryan proposed that beyond duration of illness, individuals with childhood onset T1D and a history of chronic hyperglycemia, who then went on to experience severe neuroglycopenia at any point thereafter may be particularly vulnerable to suffering cognitively. We similarly explored this in our study and examined the interaction between history of moderate to severe DKA around the time of diagnosis and subsequent exposure to a high degree of chronic hyperglycemia over 18 months. We operationalized highest exposure to cumulative hyperglycemia as upper quartile of A1c AUC at 18 months. Consistent with Ryan’s hypothesis, we found that those who performed lowest on executive functioning measures was the subgroup of T1D children with a history of DKA at diagnosis and greatest cumulative exposure to hyperglycemia.
Within the adult literature, Nunley et al. (2015) found that among middle-aged adult cases with history of childhood onset T1D, individuals with over a 14-year history of average A1c > 7.5%, demonstrated clinically relevant cognitive impairment (odds ratio [OR] of 3.0). Clinically relevant white matter hyperintensities were also observed earlier relative to non-T1D controls in middle-aged patients with childhood onset T1D (Nunley et al., 2015). In a similar vein, Weinstein et al. (2015) reported that in a cohort of 1,497 middle-aged participants, diabetes and higher fasting blood glucose were associated with worse scores on cognitive measures, increased rate of white matter hyperintensities and reductions in gray matter densities and white matter integrity (measured by fractional anisotropy). The issue of duration of illness and compounding insults warrants additional investigation.
In this study few patients evidenced new severe hypoglycemia or DKA events over the 18 month period. This may have underestimated the effect that either may have on cognitive course. Indeed there were only 4 total DKA events in 4 individuals between baseline and 18 months, none of which were classified as severe. Only eight SH events (only one with seizure/coma) occurred in 6 participants (4%) between baseline and 18 months. In contrast, during the 12-year follow-up study by Northam and colleagues, 44 percent of the type 1 diabetes group had 1 or more episodes of severe hypoglycemia with seizure/coma (Lin et al., 2010; Northam et al., 2009). From these prior papers we know that severe hypoglycemia can have an adverse impact on verbal ability, working memory and processing speed.
Further limiting our study is the possibility that our measures may not have been sufficiently sensitive. Most of the cognitive measures were the same used at baseline. Our T1D cohort is comprised of bright young children (average VIQbaseline = 108; average PIQbaseline = 109) from educated parents (% parents postgraduate at baseline= 38%) with high SES (% ≥ 100K = 36%) for whom test selection for subsequent time points has centered on avoiding ceiling effects. Given our high functioning groups, the lack of group differences could in part be explained by excessive practice effects and near-ceiling effects. To address these concerns, for timepoints 3 and 4 (both funded and underway), we have selected several alternative measures within the same domains.
Although we do not see clear group differences in our data at this time, given what we know about cognition in childhood onset T1D following longer disease duration, it is likely that as we continue to study this cohort over time, group differences will emerge or subgroup differences will be more clearly evident. Continued study of this cohort will clarify to what extent persistent exposure to diabetes, dysglycemia, and regional brain changes lead to measurable cognitive differences and whether these differences equate to clinically meaningful deficits. In particular, we will continue to examine impact of prolonged exposure to hyperglycemia over time. Recent findings in the adult literature combined with our findings provide a clear message that more scrupulous control to guard against prolonged exposure to hyperglycemia is needed, especially for those most vulnerable (those with early childhood onset T1D).
Supplementary Material
Table 4.
Spearman Correlation between Diabetic History and Cognitive Outcomes Changes from Baseline to 18 Months a
| IQ | Verbal IQ | Performance IQ | Executive Functions | Learning & Memory | Processing Speed | BRIEF | Externalizing | Internalizing | |
|---|---|---|---|---|---|---|---|---|---|
| Averaged A1c AUC above 6.0% in 18 months | |||||||||
| Spearman Correlation | +0.09 | +0.11 | +0.07 | +0.05 | −0.05 | +0.14 | +0.04 | −0.06 | +0.01 |
| p-value | 0.30 | 0.21 | 0.44 | 0.58 | 0.55 | 0.12 | 0.66 | 0.52 | 0.95 |
| N | 133 | 131 | 131 | 127 | 133 | 131 | 136 | 138 | 138 |
| # of SevHypo Events in 18 months | |||||||||
| Spearman Correlation | −0.01 | +0.07 | −0.12 | −0.13 | +0.03 | −0.03 | −0.06 | +0.07 | +0.04 |
| p-value | 0.89 | 0.44 | 0.16 | 0.14 | 0.73 | 0.70 | 0.47 | 0.41 | 0.68 |
| N | 133 | 131 | 131 | 127 | 133 | 131 | 136 | 138 | 138 |
| Diabetes Duration at 18 months | |||||||||
| Spearman Correlation | −0.02 | −0.02 | −0.04 | +0.04 | +0.01 | 0.00 | +0.06 | −0.01 | +0.09 |
| p-value | 0.84 | 0.86 | 0.65 | 0.68 | 0.89 | 0.99 | 0.52 | 0.90 | 0.32 |
| N | 133 | 131 | 131 | 127 | 133 | 131 | 136 | 138 | 138 |
| Age at onset (yrs) | |||||||||
| Spearman Correlation | −0.02 | 0.00 | −0.02 | −0.05 | +0.01 | −0.05 | −0.07 | +0.03 | −0.06 |
| p-value | 0.79 | 0.96 | 0.79 | 0.56 | 0.94 | 0.59 | 0.40 | 0.72 | 0.50 |
| N | 133 | 131 | 131 | 127 | 133 | 131 | 136 | 138 | 138 |
Spearman partial correlation controlled for age at 18 month testing, gender and parent IQ, not adjusted for multiple comparisons.
Acknowledgments
The authors thank the children and their families as well as the clinical and imaging staff at all of the investigator sites. We also thank our external collaborators for use of their imaging facilities, including University of California at San Francisco, El Camino Hospital, and University of Florida & Shands Jacksonville. We are also grateful to Karen Winer, MD and Ellen Leschek, MD at NIDDK for advice and support. This research was supported by funding from the NIH (DIRECNET U01 HD41890, HD41906-10, HD41908-10, HD41915, HD41918, HD56526) and UL1 RR024992. A. Cato, N. Mauras, P. Mazaika, C. Kollman, P. Cheng, T. Aye, J. Ambrosino, R. Beck, K. Ruedy, A. Reiss, M. Tansey, and T. Hershey report no conflict of interest. N.H. White reports receiving payment for consultancy from Novo Nordisk and Daiichi Sankyo and payments to his institution from Bristol-Myers Squibb for a research grant.
The DirecNet Study Group
The DirecNet Study Group: Clinical Centers: (Personnel are listed as (PI) for Principal Investigator, (I) for co-Investigator, (C) for Coordinators and (PM) for Psychometrician.) Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI); Michael J. Tansey, MD (I); Julie Coffey, MSN (C); Joanne Cabbage (C); Sara Salamati (C); Amy Conrad, PhD (PM); Nemours Children’s Health System, Jacksonville, FL: Nelly Mauras, MD (PI); Larry A. Fox, MD (I); Allison Cato, PhD (I); Kim Englert, RN, BSN, CDE (C); Kaitlin Sikes, ARNP, MSN (C); Tina Ewen (C); Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI); Darrell M. Wilson, MD (I); Tandy Aye, MD (I); Kimberly Caswell, ARNP (C); Kristin Schleifer (PM); Christian Ambler (PM); Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI); William V. Tamborlane, MD (I); Amy Steffen, BS (C); Kate Weyman, MSN (C); Melinda Zgorski, BSN (C); Jodie Ambrosino, PhD (I); Washington University in St. Louis, St. Louis, MO: Neil H. White, MD, CDE (PI); Ana Maria Arbelaez, MD, (I); Lucy Levandoski, PA-C (C); Angie Starnes, RN, BSN, CDE (C), Tamara Hershey, PhD (I); Coordinating Center: Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD; Katrina J. Ruedy, MSPH; Craig Kollman, PhD; Peiyao Cheng, MPH; Beth Stevens; Nelly Njeru; Ryan Chapman, TJ Mouse Image Coordinating Center: Allan L. Reiss, MD; Naama Barnea-Goraly, MD; Matthew J. Marzelli, BS; Paul M. Mazaika, PhD; Daniel X. Peng, BS; Cognitive Core: Tamara Hershey, PhD; Allison Cato, PhD; Emily Bihun, MA; Amal Al-Lozi, BA; Allison Bischoff, BA; Michaela Cuneo, BA; Aiden Bondurant, BA. Data and Safety Monitoring Board: Mark Sperling, MD; Dorothy M. Becker, MBBCh; Patricia Cleary, MS; Carla Greenbaum, MD; Antoinette Moran, MD.
References
- Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: The structural and functional integrity of the developing brain. Pediatric Diabetes. 2013;14(8):541–553. doi: 10.1111/pedi.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, Aye T, Buckingham B, Mauras N, White NH, Fox LA, Tansey M, Beck RW, Ruedy KJ, Kollman C, Cheng P, Reiss AL for the Diabetes Research in Children N. Alterations in White Matter Structure in Young Children With Type 1 Diabetes. Diabetes Care. 2014;37(2):332–340. doi: 10.2337/dc13-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorgaas M, Gimse R, Vik T, Sand T. Cognitive function in type 1 diabetic children with and without episodes of severe hypoglycaemia. Acta Paediatr. 1997;86:148–153. doi: 10.1111/j.1651-2227.1997.tb08856.x. [DOI] [PubMed] [Google Scholar]
- Blasetti A, Chiuri RM, Tocco AM, Giulio CD, Mattei PA, Ballone E, Chiarelli F, Verrotti A. The Effect of Recurrent Severe Hypoglycemia on Cognitive Performance in Children With Type 1 Diabetes: A Meta-analysis. J Child Neurol. 2011;26(11):1383–1391. doi: 10.1177/0883073811406730. [DOI] [PubMed] [Google Scholar]
- Cato MA, Crosson B. Stable and slowly progressive dementias. In: Attix DK, Welsh-Bohmer K, editors. Geriatric neuropsychological assessment and intervention. New York: Guilford Publications; 2006. [Google Scholar]
- Cato MA, Mauras N, Ambrosino J, Bondurant A, Conrad AL, Kollman C, Cheng P, Beck RW, Ruedy KJ, Aye T, Reiss AL, White NH, Hershey T. Cognitive Functioning in Young Children with Type 1 Diabetes. Journal of the International Neuropsychological Society. 2014;20(02):238–247. doi: 10.1017/S1355617713001434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MJ. CMS: Children’s Memory Scale. The Psychological Corporation; 1997. [Google Scholar]
- Connors CK. CPT: The Conners Continuous Performance Test. Toronto, Canada: Multi-Health Systems; 1994. [Google Scholar]
- Ferguson SC, Blane A, Perros P, McCrimmon RJ, Best JJ, Wardlaw J, Deary IJ, Frier BM. Cognitive Ability and Brain Structure in Type 1 Diabetes: Relation to Microangiopathy and Preceding Severe Hypoglycemia. Diabetes. 2003;52:149–156. doi: 10.2337/diabetes.52.1.149. [DOI] [PubMed] [Google Scholar]
- Flykanaka-Gantenbein C. Hypoglycemia in Childhood: Long-Term Effects. Pediatr Endocrinol Rev. 2004;1(Suppl 3):530–536. [PubMed] [Google Scholar]
- Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive Function in Children with Type 1 Diabetes: A meta-analysis. Diabetes Care. 2008;31(9):1892–1897. doi: 10.2337/dc07-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Lutz, FL: PAR, Inc; 2000. [Google Scholar]
- Haumont D, Dorchy H, Pelc S. EEG abnormalities in diabetic children: influence of hypoglycemia and vascular complications. Clin Pediatr. 1979;18:750–753. doi: 10.1177/000992287901801205. [DOI] [PubMed] [Google Scholar]
- Hershey T, Craft S, Bhargava N, White NH. Memory and Insulin Dependent Diabetes Mellitus (IDDM): Effects of Childhood Onset and Severe Hypoglycemia. J Int Neuropsychol Soc. 1997;3(6):509–520. [PubMed] [Google Scholar]
- Hershey T, Lillie R, Sadler M, White NH. Severe Hypoglycemia and Long-Term Spatial Memory in Children with Type 1 Diabetes Mellitus: A Retrospective Study. J Int Neuropsychol Soc. 2003;9(5):740–750. doi: 10.1017/S1355617703950077. [DOI] [PubMed] [Google Scholar]
- Hershey T, Lillie R, Sadler M, White NH. A prospective study of severe hypoglycemia and long-term spatial memory in children with type 1 diabetes. Pediatr Diabetes. 2004;5:63–71. doi: 10.1111/j.1399-543X.2004.00045.x. [DOI] [PubMed] [Google Scholar]
- Hershey T, Perantie DC, Warren SL, Zimmerman EC, Sadler M, White NH. Frequency and Timing of Severe Hypoglycemia Affects Spatial Memory in Children with Type 1 Diabetes. Diabetes Care. 2005;10:2372–2377. doi: 10.2337/diacare.28.10.2372. [DOI] [PubMed] [Google Scholar]
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- Hyllienmark L, Maltez J, Dandenell A, Luvigsson J, Brismar T. EEG abnormalities with and without relation to severe hypoglycemi in adolescents with type 1 diabetes. Diabetologia. 2005;48:412–419. doi: 10.1007/s00125-004-1666-2. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Ryan CM, Cleary PA, Waberski BH, Weinger K, Musen G, Dahms W DCCT/EDIC Research Group. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow-up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia. 2011;54(2):245–255. doi: 10.1007/s00125-010-1883-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY-II: Neuropsychological Battery for Children. 2. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- Lin A, Northam EA, Rankins D, Werther GA, Cameron FJ. Neuropsychological profiles of young people with type 1 diabetes 12 yr after disease onset. Pediatr Diabetes. 2010;11:235–243. doi: 10.1111/j.1399-5448.2009.00588.x. [DOI] [PubMed] [Google Scholar]
- Ly TT, Anderson M, McNamara KA, Davis EA, Jones TW. Neurocognitive Outcomes in Young Adults With Early-Onset Type 1 Diabetes: A prospective follow-up study. Diabetes Care. 2011;34(10):2192–2197. doi: 10.2337/dc11-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschot SM, Brands AMA, van der Grond J, Kessels RPC, Algra A, Kappelle LJ, Biessels GJ. Brain Magnetic Resonance Imaging Correlates of Impaired Cognition in Patients With Type 2 Diabetes. Diabetes. 2006;55(4):1106–1113. doi: 10.2337/diabetes.55.04.06.db05-1323. [DOI] [PubMed] [Google Scholar]
- Marzelli MJ, Mazaika PK, Barnea-Goraly N, Hershey T, Tsalikian E, Tamborlane W, Mauras N, White NH, Buckingham B, Beck RW, Ruedy KJ, Kollman C, Cheng P, Reiss AL for the Diabetes Research in Children N. Neuroanatomical Correlates of Dysglycemia in Young Children With Type 1 Diabetes. Diabetes. 2014;63(1):343–353. doi: 10.2337/db13-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauras N, Mazaika P, Buckingham B, Weinzimer S, White NH, Tsalikian E, Hershey T, Cato A, Cheng P, Kollman C, Beck R, Ruedy K, Aye T, Fox L, Arbelaez AM, Wilson DM, Tansey M, Tamborlane WV, Peng D, Marzelli M, Winer K, Reiss AL Network DRiC. Longitudinal Assessment of Neuroanatomical and Cognitive Differences in Young Children with Type 1 Diabetes: Association with Hyperglycemia. Diabetes. 2015;64(5):1770–1779. doi: 10.2337/db14-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauras N, Mazaika P, Buckingham B, Weinzimer S, White NH, Tsalikian E, Hershey T, Cato A, Cheng P, Kollman C, Beck R, Ruedy K, Aye T, Fox L, Arbelaez AM, Wilson DM, Tansey M, Tamborlane WV, Peng D, Marzelli M, Winer K, Reiss AL Network, D.R.i.C. Longitudinal Assessment of Neuroanatomical and Cognitive Differences in Young Children with Type 1 Diabetes: Association with Hyperglycemia. Diabetes E ahead of print. [Google Scholar]
- Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: Neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96(4):553–563. doi: 10.1016/j.nlm.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Musen G, Lyoo IK, Sparks CR, Weinger K, Hwang J, Ryan CM, Jimerson DC, Hennen J, Renshaw PF, Jacobson AM. Effects of Type 1 Diabetes on Gray Matter Density as Measured by Voxel-Based Morphometry. Diabetes. 2006;55:326–333. doi: 10.2337/diabetes.55.02.06.db05-0520. [DOI] [PubMed] [Google Scholar]
- Naguib JM, Kulinskaya E, Lomax CL, Garralda ME. Neuro-cognitive Performance in Children with Type 1 Diabetes- A Meta-analysis. J Pediatr Psychol. 2009;34(3):271–282. doi: 10.1093/jpepsy/jsn074. [DOI] [PubMed] [Google Scholar]
- Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological Profiles of Children With Type 1 Diabetes 6 Years After Disease Onset. Diabetes Care. 2001;24:1541–1546. doi: 10.2337/diacare.24.9.1541. [DOI] [PubMed] [Google Scholar]
- Northam EA, Anderson PJ, Werther GA, Warne GL, Adler RG, Andrewes D. Neuropsychological Complications of IDDM in Children 2 Years After Disease Onset. Diabetes Care. 1998;21:379–384. doi: 10.2337/diacare.21.3.379. [DOI] [PubMed] [Google Scholar]
- Northam EA, Rankins D, Lin A, Wellard RM, Pell GS, Finch SJ, Werther GA, Cameron FJ. Central Nervous System Function in Youth With Type 1 Diabetes 12 Years After Disease Onset. Diabetes Care. 2009;32(3):445–450. doi: 10.2337/dc08-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perantie DC, Koller JM, Weaver PM, Lugar HM, Black KJ, White NH, Hershey T. Prospectively Determined Impact of Type 1 Diabetes on Brain Volume During Development. Diabetes. 2011;60(11):3006–3014. doi: 10.2337/db11-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9(2):87–95. doi: 10.1111/j.1399-5448.2007.00274.x. [DOI] [PubMed] [Google Scholar]
- Perantie DC, Wu J, Koller JM, Lim A, Warren SL, Black KJ, Sadler M, White NH, Hershey T. Regional Brain Volume Differences Associated with Hyperglycemia and Severe Hypoglycemia in Youth with Type 1 Diabetes. Diabetes Care. 2007;30(9):2331–2337. doi: 10.2337/dc07-0351. [DOI] [PubMed] [Google Scholar]
- Perros P, Deary IJ, Sellar RJ, Best JJ, Frier BM. Brain Abnormalities Demonstrated by Magnetic Resonance Imaging in Adult IDDM Patients with and without a History of Recurrent Severe Hypoglycemia. Diabetes Care. 1997;20:1013–1018. doi: 10.2337/diacare.20.6.1013. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children, Second Edition Parent Rating Scales. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Rovet JF, Ehrlich RM. The Effect of Hypoglycemic Seizures on Cognitive Function in Children with Diabetes: A 7-Year Prospective Study. J Pediatr. 1999;134(4):503–506. doi: 10.1016/s0022-3476(99)70211-8. [DOI] [PubMed] [Google Scholar]
- Rovet JF, Ehrlich RM, Hoppe M. Intellectual Deficits Associated With Early Onset of Insulin-Dependent Diabetes Mellitus in Children. Diabetes Care. 1987;10(4):510–515. doi: 10.2337/diacare.10.4.510. [DOI] [PubMed] [Google Scholar]
- Ryan C, Vega A, Drash A. Cognitive Deficits in Adolescents Who Developed Diabetes Early in Life. Pediatrics. 1985;75:921–927. [PubMed] [Google Scholar]
- van den Berg E, Reijmer YD, de Bresser J, Kessels RPC, Kappelle LJ, Biessels GJ Utrecht Diabetic Encephalopathy Study Group. A 4 year follow-up study of cognitive functioning in patients with type 2 diabetes mellitus. Diabetologia. 2010;53(1):58–65. doi: 10.1007/s00125-009-1571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 3. San Antonio, TX: Psychological Corporation; 2002. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson Test of Cognitive Abilites. 3. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.