ABSTRACT
Streptococcus suis (SS) is a zoonotic pathogen that can cause systemic infection in pigs and humans. The ingestion of contaminated pig meat is a well-established risk factor for zoonotic S. suis disease. In our studies, we provide experimental evidence that S. suis is capable to translocate across the host gastro-intestinal tract (GIT) using in vivo and in vitro models. Hence, S. suis should be considered an emerging foodborne pathogen. In this addendum, we give an overview of the complex interactions between S. suis and host-intestinal mucosa which depends on the host origin, the serotype and genotype of S. suis, as well as the presence and expression of virulence factors involved in host-pathogen interaction. Finally, we propose a hypothetical model of S. suis interaction with the host-GIT taking in account differences in conditions between the porcine and human host.
KEYWORDS: Streptococcus suis; zoonotic infection; gastro-intestinal infection; food-borne pathogen; intestinal translocation
Zoonotic potential of Streptococcus suis
S. suis is an important bacterial swine pathogen and an emerging zoonotic agent that can cause severe diseases such as meningitis and septic shock in pigs and humans.1 The upper respiratory tract is thought to be the main colonization and entry site of S. suis in its main host, the pig.1 However the gastrointestinal tract (GIT) cannot be excluded as secondary site of infection in piglets as the GIT of weaning piglets is rapidly colonized by S. suis.2
Among the 33 serotypes identified to date, S. suis serotype 2 (SS2) is considered the most virulent as it is the serotype most frequently isolated from both pigs and human cases worldwide.3 It is also the serotype which is globally associated with 75% of zoonotic infections reported to date.3 In contrast, S. suis serotype 9 (SS9) is the most common serotype isolated from diseased pigs in Northern Europe.3,4 SS9 is considered less virulent and with very low zoonotic potential compared to SS2, as only one case of SS9 human infection has been reported to date.3,4 It is not well understood why isolates with serotypes that are highly prevalent among diseased pigs, such as SS9, rarely cause human disease. In addition to serotype, the genotype contributes to virulence of S. suis. Invasive S. suis isolates are limited to certain sequence types (ST), as determined by multi locus sequence typing (MLST).3 Closely related STs cluster into 22 so-called clonal complexes (CC). The majority of the SS2 isolates from human patients belong to ST1 or ST7, which both cluster in CC1.3 A zoonotic clone of SS2 belonging to CC20 and unrelated to CC1, has recently been described in the Netherlands,4 which indicates that multiple zoonotic clones of S. suis are emerging worldwide. In contrast, invasive Dutch SS9 isolates from infected pigs belong mainly to CC16, which is distinct from both CC1 and CC20.4
Epidemiological evidence for an enteric etiology of S. suis infection
Human S. suis infection is considered predominantly an occupational disease in Europe and North America.3 The infection is presumed to occur via skin injuries in people working in direct contact with contaminated pigs and pig-products.1 This is in contrast to South-East Asia where zoonotic infection of S. suis can occur via oral route as well,5-7 due to the consumption of traditional dishes based on undercooked pork meat, such as tie˜t canh (raw pig's blood soup), potentially contaminated with S. suis.8 In a case-control study carried out in Vietnam, ingestion of such high-risk food was independently associated with S. suis meningitis.5 In addition, S. suis was isolated from rectal swab samples of patients with meningitis who reported recent consumption of undercooked pork.5 Finally, a significant number of sporadic cases and 2 outbreaks of S. suis human infections in Thailand, associated with consumption of undercooked pork, have been reported in the last decade.6,7 These data have been summarized in a review by Huong, et al.9 Whether S. suis infection can be associated with a diarrheal syndrome is unknown as this has never been studied systematically. However, Thai patients presented with diarrhea in 20% of the cases after oral S. suis infection.6 Diarrhea was also described in Vietnamese patients.10
S. suis interaction with host-intestinal epithelial cells is serotype and genotype specific
To further elucidate the interaction of S. suis with the intestinal mucosa during natural and zoonotic infection, we designed an in vitro model using porcine (IPEC-J2)11 and human (Caco-2)12 differentiated intestinal epithelial cells (IEC).13
Human and porcine IEC were co-cultured with zoonotic (SS2/CC1, SS2/CC20) and non-zoonotic (SS9/CC16) isolates, and tested for differences in adhesion to and translocation across polarized IEC.13 We found differences in adhesion capacity, depending on the serotype. Zoonotic isolates belonging to serotype 2 (SS2/CC1 and SS2/CC20) exhibited better adhesion capacity to human IEC than non-zoonotic isolates (SS9/CC16) while the latter adhered more efficiently to porcine IEC. The bacterial capsule structure therefore seems an important determinant of adhesion and appears to contribute to differences in binding specificity to host IEC. Across S. suis serotypes, the capsule polysaccharides (CPS) are constituted of a diverse range of carbohydrate units that differ not only by the type of monosaccharides, but also in how these units are joined together.14 Structural analysis of the SS2 capsule revealed that CPS2 is composed of glucose (Glu), rhamnose (Rha), α- and β-galactose (Gal), N-acetylglucosamine and sialic acid (Neu5Ac).15 Based on sequence homology, genes encoding putative glucosyl- (cpsE), rhamnosyl (cps2F), galactosyl- (cps2G, cps2H), N-acetylglucosaminyl- (cps2J), and sialyl transferase (cps2L) could be identified.15 The cps locus of SS2 differs from SS9 in the presence of cps2F and cps2L genes that catalyze the addition of rhamnose and terminal α-2,6-linked sialic acid to the CPS2 respectively.14,15 Thus, differences in capsule composition, including the presence of sialic acid, between SS2 and SS9 are likely to contribute to the distinct adhesive properties to host-IEC observed between the 2 serotypes.
While we observed low invasion for all isolates, the majority of zoonotic SS2/CC1 isolates were found to transmigrate actively across polarized human and porcine IEC within 1-6 hours using a paracellular route.13 However, SS2 and SS9 isolates belonging to other CCs showed poor translocation, indicating that bacterial translocation capability depends on the genetic background of S. suis. The ability of SS2/CC1 isolates to translocate across IEC in vitro correlates with the SS2/CC1 disease pathogenesis through passage of the human and porcine intestinal barrier. Zoonotic SS2/CC20 isolates showed poor translocation ability, consistent with disease epidemiology as human SS2/CC20 disease in the Netherlands is less likely to result from intestinal infection, but mostly is the result of direct blood stream invasion, in particular through skin injuries.1
Virulence factors of S. suis potentially involved in interaction with host intestinal mucosa
The presence of capsule negatively affected the bacterial adhesion and translocation in our in vitro model.13 It is remarkable that unencapsulated mutants of SS2/CC1 strain 10 and SS9/CC16 strain 8067, both generated by insertional mutagenesis in the cpsE gene, appeared to conserve the host-restriction of their encapsulated parental strains when interacting with human and porcine IEC. Thus, in addition to the capsule, we hypothesized that other virulence factors may play a role in host specific interactions of S. suis with IEC (Table 1, Fig. 1). Streptococcal adhesin P (SadP) is an adhesin of S. suis that was shown to bind to galactosyl-α1-4galactose (galabiose), present as a terminal epitope on eukaryotic glycolipid cell receptors.16 Analysis of SadP binding specificity of glycolipid receptors containing galabiose revealed the highest specificity for globotriaosylceramide (Gb3/CD77), a receptor that is abundant in various eukaryotic tissues including the human and porcine intestine.17,18 Analysis of an alignment of available amino acid sequences of SadP showed substantial variation across genotypes (data not shown).
Table 1.
Virulence factors of S. suis potentially involved in interaction with host-intestinal mucosa.
| Annotation S. suis serotype 2 strain P1/7 | Protein | Function | Interaction with Host-IEC1 | Virulence In vivo | |
|---|---|---|---|---|---|
| Galactosyl/rhamnosyl transferase - SSU0520 | CpsE/F | CPS biosynthesis | Adhesion/Invasion/Immune evasion | Attenuated pig | [15] |
| N-acetylneuraminic acid synthase - SSU0535 | NeuB | Sialic acid synthesis | Adhesion/Invasion/Immune evasion | Attenuated pig | [15] |
| Streptococcal adhesin P - SSU0253 | SadP | Binds to Galα1–4Galβ1 Globo-series2 glycolipids | Adhesion epithelium | Not tested | [16] |
| Amylopullulanase – SSU1849 | ApuA | Degradation α-glucans | Adhesion epithelium | Not tested | [19] |
| Hp0197-heparinase - SSU1048 | Hp0197 | GAGs3 and heparin binding | Adhesion ECM4 | No mutant | [22] |
| Enolase - SSU1320 | Eno | Fibronectin and plasminogen binding | Adhesion ECM4 | No mutant | [23] |
| Di-peptidyl-peptidase IV - SSU0187 | DppIV | Fibronectin binding | Adhesion ECM4 | Attenuated mouse | [24] |
| Amynoacyl histidine peptidase - SS1215 | PepD | Peptidase - protease | Invasion | No mutant | [26] |
| Pilus- srtBCD SSU1883 - srtF SSU0428 | Srt-pilus | Pilus-backbone structure | Invasion | Not tested | [29] |
| Suilysin - SSU1231 | Sly | Pore-form toxin | Invasion | Unaffected pig | [30] |
| Hyaluronidase -SSU1050 | Hyl | break down hyaluronan | Invasion | Not tested | [31] |
| Metallo-serine protease - SSU1773 | IgA1 | IgA1 protease | Immune evasion | Attenuated-pig | [32] |
| Cell envelope proteinase - SSU0757 | SspA | Degradation Interleukin-8 | Immune evasion | Attenuated mouse | [33] |
| Anchored DNA nuclease - SSU1760 | SsnA | Host DNA degradation | Immune evasion | Not tested | [34] |
No mutant = No gene knock-out mutant strain published; Not tested = Virulence factor revealed by the construction of gene knockout mutant whose mutant strain has not been tested for virulence in animal models.
IEC = Intestinal Epithelium Cells;2Globo-series = globotriaosylceramide (Gb3/CD77);3GAGs = host-cell glycosaminoglycans; 4ECM = Extra Cellular Matrix.
Figure 1.
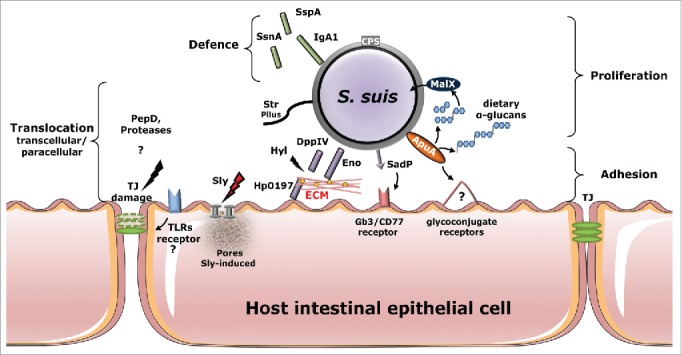
Virulence factors and their (confirmed or putative) role during adhesion to and invasion of host intestinal epithelia. Several S. suis adhesins (ApuA and SadP) have been reported to contribute to bacterial adhesion to unknown (?) or characterized (Gb3/CD77) host-cells receptors. The bacteria can proliferate upon degradation by ApuA of dietary α-glucans and use maltodextrin-specific transport (MalX) to uptake the maltodextrin products from the degradation of α-glucans. Secreted suilysin promotes S. suis invasion, possibly by damaging host epithelial barriers forming pore protein on the eukaryotic membrane cell. Surface-expressed proteins DppIV, Eno and Hp0197-heparinase promote binding and degradation to ECM components, such as fibronectin, plasminogen and GAGs, present between intestinal cells or underlying to the intestinal submucosa. Translocation of the intestinal epithelium may occur via paracellular route through the rearrange of TJ mediated by bacterial protease (PepD) and by activation of TLRs signaling which promotes the reduction of the expression of TJ components whose mechanisms remain to be confirmed. Other secreted or cell wall-anchored proteins (IgA1, SsnA and SspA) protect the bacteria against the mucosal immune response. Abbreviations: ECM, extracellular matrix; TLRs Toll-like receptors; TJ, tight junctions; GAGs glycosaminoglycans Sly, suilysin; Hyl, hyaluronidase; DppIV, peptidyl peptidases; Eno, enolase; ApuA, amylopullulanase; SadP, streptococcal adhesin P; Hp0197-heparinase GAGs-heparin binding; SspA, cell envelope proteinase; SsnA, (extracellular) DNA nuclease; Iga1, metallo-serine protease; CPS, capsule; PepD protease; Srt-pilus structure.
In addition to SadP, several adhesins with metabolic activity may contribute to S. suis adhesion to host mucosal epithelium, by binding to unknown glycoconjugate receptors. An example is amylopullulanase (ApuA), a bifunctional cell wall-anchored enzyme that permits microbial growth through the metabolism of α-glucans (i.e.: starch) derived from the host diet and that binds different host carbohydrates through carbohydrate-binding domains.19 S. suis also interacts with components of the mucosal extracellular matrix (ECM) such as glycosaminoglycans (GAGs), fibronectin, and plasminogen, present in the intestinal submucosa.20,21 A protein potentially involved in host-IEC adhesion is Hp0197-heparinase,22 which contains a GAGs-binding domain found on the surface of microvilli of diverse mucosal epithelia. Finally, enolase (Eno)23 and di-peptidyl-peptidase IV (DppIV)24 are considered major ECM binding enzymes of streptococcal pathogens and both enzymes in S. suis were shown to bind both plasminogen and fibronectin and might be important for adhesion to and translocation through IEC.
Using confocal microscopy, we observed paracellular translocation of SS2/CC1 isolates between IEC with re-arrangement of tight junction (TJ) proteins after only 2 hours of co-incubation with human IEC, without any macroscopic damage to the monolayer.13 The translocation mechanism of S. suis is still unknown. Several proteases produced by S. suis may affect TJ integrity.25,26 The alteration of TJ might also be an indirect effect due to the stimulation of Toll-like receptors (TLRs), which recognize highly conserved bacterial structures. For example, Streptococcus pneumoniae was shown to activate TLRs signaling, resulting in a reduction of TJ proteins expression required for maintenance of epithelial integrity with consequent opening of the paracellular space between epithelium cells.27
Other S. suis factors that may support the translocation across the host mucosal epithelia include the presence of pilus-like structures and the damaging effect of toxins on the epithelium. The presence of pili may facilitate paracellular translocation across IEC as observed for group B streptococci (GBS). Using high-resolution confocal imaging, translocating GBS were targeted in the intercellular space of Caco-2 cells with pili heading toward the basolateral cell surface.28 Analysis of S. suis whole genomes revealed the presence of 4 gene clusters (srtBCD, srtE, srtF, and srtG) encoding for sortase enzymes and pilin elements involved in pili assembly,29 although their role in adhesion and translocation remains to be investigated. Suilysin (Sly) is an extracellular pore-forming toxin involved in invasion of various host cells.30 In our study, the Sly production was not essential during the early stage of translocation across IEC,13 but Sly may contribute to subsequent damage to the epithelium after initial infection is settled at mucosa level.30 Synergistically to suilysin, the hyaluronidase (Hyl)31 of S. suis might degrade hyaluronan present in the ECM, thereby contributing to subsequent blood stream invasion and spread of S. suis.
Once present in the mucosal connective tissues, S. suis appears to produce several factors which may interfere with the mucosal immune system, including 1) IgA1 protease capable of cleaving human secretory IgA132; 2) serine-protease (SspA) which was shown to degrade pro-inflammatory interleukin-8 (IL-8);33 and 3) secreted DNase (SsnA) potentially involved in the breakdown of neutrophil entrapments (NETs).34
It is important to note that the expression of the virulence factors described is influenced by environmental conditions during host mucosa colonization and subsequent infection. Indeed it is known that bacterial pathogens alter the transcription of virulence factors in response to the availability of carbohydrates.26 The GIT is particularly rich in dietary complex carbohydrates such as α-glucans (i.e., starch). When S. suis serotype 2 was grown in the presence of α-glucans, adherence to and cell invasion of porcine tracheal epithelial cells increased significantly and this increase was due to the differential expression of 17 S. suis virulence genes.26 For example, the production of Sly, ApuA and Hp0197-heparinase was highly increased during the growth in presence of α-glucans,26 whereas the expression of the S. suis capsule was induced by high glucose availability.35 Thus, carbohydrates present in the intestinal lumen or mucus layer may affect the expression of virulence factors promoting the translocation of S. suis across the host intestinal barrier.
Different modality of S. suis infection at human and porcine intestinal mucosa
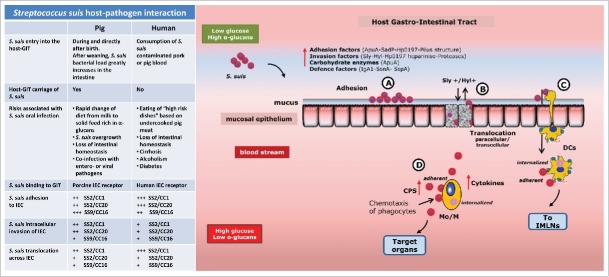
SS2 is capable of translocating across the porcine GIT, after oral challenge and in the absence of any invasive procedures in an in vivo model in piglets.13 Viable SS2 was detected in intestinal mesenteric lymph nodes (IMLNs) of 40% of infected piglets indicating that passage of the intestinal mucosa had occurred.13 Taking together the epidemiological evidence as well as our experimental results, we conclude that S. suis infection can occur via the oral route in both piglets and humans. However, S. suis oral infection occurs under very different conditions in the 2 hosts.
While ingestion of undercooked contaminated pig products is associated with SS2 infection in adults,5 there is no evidence so far that S. suis colonizes permanently the human GIT of healthy individuals as persistent carriage has not been demonstrated.5 In addition, SS2 infection in children appears extremely rare, indicating that risk factors for foodborne infection with SS2 may be related to behavior specific for the adult population such as the consumption of certain “high-risk” dishes.5,6 It has been proposed that certain underlying diseases, such as liver cirrhosis due to alcohol abuse or diabetes could increase the probability to acquire SS2 infection via oral route,36,37 as a result of compromised intestinal homeostasis promoting the intestinal translocation of S. suis,36,37 although these diseases were not confirmed as risk factors in a controlled study.5
The conditions, which may promote S. suis oral infection in piglets, are different from the human situation. Healthy sows carry S. suis in their upper respiratory, genital and intestinal tracts leading to vertical and horizontal transmission to their offspring. During the weaning transition, the composition of the intestinal microbiota changes profoundly with a dramatic increase in S. suis.2 S. suis infection occurs primarily in young pigs between 3 and 12 weeks of age,38 but it is still unclear what triggers the switch from an asymptomatic to pathogenic association of S. suis with the host. Susceptibility to S. suis infection may increase due to weaknesses in weaning piglet defenses through changes in intestinal microbiota composition due to change of diet, infections by other pathogens, or environmental changes.2,39,40 The introduction of solid feed rich in α-glucans may not only influence gut homeostasis and microbiota composition in the piglet,2,39,40 but may also induce a change in the metabolism and virulence gene expression of colonizing S. suis. An excess of α-glucans may also favor intestinal bacterial overgrowth that is often associated with bacterial intestinal translocation.41
Model of S. suis interaction with host-GIT
The initial stages of infection at host epithelium consist of bacterial colonization (adherence and initial multiplication), translocation across the epithelial barrier, followed by evasion of the host immune system. Here, we propose a hypothetical model of how S. suis may interact with the intestinal mucosa, leading to systemic infection, which is shared by the human and porcine host (Fig. 2). It should be noted that the proposed interactions in our hypothetical model, draw from other model studies of S. suis host-pathogen interaction, which used both human and porcine epithelial cells or porcine or mice in vivo models.13,42 The validity of our hypothetical model will be subject of future research.
Figure 2.
A hypothetical model of host-pathogen interaction of S. suis in the gastro-intestinal tract. S. suis translocation model of host -intestinal mucosa. S. suis intestinal infection occurs with 2 different transmissions and circumstances in the 2 hosts as summarized in the table. S. suis isolates belong to clonal complex CC1 and CC20 of serotype 2 (SS2/CC1 and SS2/CC20), and clonal complex CC16 of serotype 9 (SS9/CC16) with low (+) medium (++), high (+++), adhesion and translocation ability, after 2-4 hours of co-incubation with host IEC. The description of interaction steps are found in the text. Sly, suilysin; Hyl, hyaluronidase; CPS, capsular polysaccharides; DCs dendritic cells, Mo/M Monocytes/Macrophages, IMLNs intestinal mesenteric lymph nodes.
We postulate that S. suis translocation across the host-GIT may occur at small intestine level as microbial profiling of porcine and human small intestine displayed abundant populations of Streptococcus spp.2,21 Once S. suis have entered into the lumen of the host-GIT, diverse host factors limit the ability of the bacteria to access to the intestinal cells, such as the mucus layers, antimicrobial peptides produced by specialized enterocytes (Paneth cells present in the intestinal crypts) and secreted IgA1.32 S. suis IgA1 protease, adhesins and pili all mediate the bacterial adhesion to host-enterocytes. During the adhesion stage, the expression of capsule may vary in response to environmental changes, potentially increasing exposure of bacterial surface structures which promote adhesion to host epithelia, including to specific enterocytes receptors (Fig. 2 step-A).35 Carbohydrate degrading enzymes of S. suis may promote the utilization of mucosal carbohydrates such as dietary α-glucans supporting S. suis growth (Fig. 2 step-A). Subsequent passage through the host intestinal mucosa may be a consequence of active bacterial translocation across epithelial cells via paracellular and/or transcellular routes. The production of suilysin by S. suis may facilitate dispersion of bacteria into the deeper tissues due to damage of barrier integrity (Fig. 2 step-B).43 Once bacteria have breached the epithelium and the connective tissues of the lamina propria, adhesins and proteases such as hyaluronidase and Hp0197-heparinase are thought to bind and digest components of the ECM, promoting spread of the infection (Fig. 2 step-B). Bacteria that have translocated across epithelia and have invaded the connective tissue are very likely to be perceived by the mucosal immune system including dendritic cells (DCs) (Fig. 2 step-C).44 DCs are important sentinels in mucosal surfaces that contact the external environment and play a key role in homeostatic control of the intestinal mucosa and in the induction of adaptive immune responses.44 Translocating S. suis bacteria might survive inside the DCs after uptake or may remain attached to their surface, reach IMLNs and rapidly disseminate in the host resulting in invasive disease (Fig. 2 step-C).45 In addition to capsule production, S. suis may use additional virulence factors that modulate DCs functions and escape immune surveillance, mainly by modulating cytokine release and avoiding phagocytosis.45
As previously mentioned, environmental changes such as carbohydrates availability, can influence the expression of bacterial virulence determinants such as the capsule. In the bloodstream, glucose concentration is substantially higher compared to GIT mucosa, because simple carbohydrates from food such as glucose are rapidly absorbed and depleted during transit through the small intestine.21 The higher (around 5 mM) glucose concentrations46,47 will induce a change in metabolic gene expression in order to optimize bacterial metabolism and survival. In presence of high concentrations of glucose, capsule genes will be induced and capsule thickness35 will increase permitting avoidance of opsonization by host antibody and complement components which facilitate the phagocytosis process of S. suis by leukocytes recruited to the site of infection48,49 (Fig. 2 step-D). S. suis may travel in the bloodstream to reach the target organs as internalized by or adherent to monocytes/macrophages (Mo/M) or as free bacteria by a thick capsule layer, causing systemic infection50 (Fig. 2 step-D).
Implications for future research
The identification of the GIT as an entry site of S. suis infection in both humans and pigs creates opportunities for novel approaches toward prevention of S. suis infection.
An in depth understanding of specific host-pathogen interactions, including the identification of human and porcine receptors of S. suis adhesins and of specific components that may interfere with bacterial binding to these receptors, as well as the impact of dietary composition on bacterial gene expression and gut homeostasis in the piglet's intestine, may provide clues to reducing the burden of S. suis infection.
Disclosure of potential conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We thank Niels Willemse for the critical reading of the manuscript.
Funding
This study was supported by EU-FP7 programs ANTIGONE (Project No. FP7-278976) and NADIR (Project No. FP7-228394).
References
- [1].Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis 2009; 48:617-625; PMID:19191650; http://dx.doi.org/ 10.1086/596763 [DOI] [PubMed] [Google Scholar]
- [2].Su Y, Yao W, Perez-Gutierrez ON, Smidt H, Zhu WY. Changes in abundance of Lactobacillus spp. and Streptococcus suis in the stomach, jejunum and ileum of piglets after weaning. FEMS Microbiol Ecol 2008; 66:546-555; PMID:18554303; http://dx.doi.org/ 10.1111/j.1574-6941.2008.00529.x [DOI] [PubMed] [Google Scholar]
- [3].Goyette-Desjardins G, Auger JP, Xu J, Segura M, Gottschalk M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect 2014; 3:e45; PMID:26038745; http://dx.doi.org/ 10.1038/emi.2014.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schultsz C, Jansen E, Keijzers W, Rothkamp A, Duim B, Wagenaar JA, van der Ende A. Differences in the population structure of invasive Streptococcus suis strains isolated from pigs and from humans in The Netherlands. PLoS One 2012; 7:e33854; PMID:22563452; http://dx.doi.org/ 10.1371/journal.pone.0033854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nghia HD, Tu le TP, Wolbers M, Thai CQ, Hoang NV, Nga TV, Thao le TP, Phu NH, Chau TT, Sinh DX, et al.. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS One 2011; 6:e17604; PMID:21408132; http://dx.doi.org/ 10.1371/journal.pone.0017604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Takeuchi D, Kerdsin A, Pienpringam A, Loetthong P, Samerchea S, Luangsuk P, Khamisara K, Wongwan N, Areeratana P, Chiranairadul P, et al.. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PLoS One 2012; 7:e31265; PMID:22363601; http://dx.doi.org/ 10.1371/journal.pone.0031265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wertheim HF, Nguyen HN, Taylor W, Lien TT, Ngo HT, Nguyen TQ, Nguyen BN, Nguyen HH, Nguyen HM, Nguyen CT, et al.. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One 2009; 4:e5973; PMID:19543404; http://dx.doi.org/ 10.1371/journal.pone.0005973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Huong VT, Hoa NT, Horby P, Bryant JE, Van Kinh N, Toan TK, Wertheim HF. Raw pig blood consumption and potential risk for Streptococcus suis infection, Vietnam. Emerg Infect Dis 2014; 20:1895-1898; PMID:25340391; http://dx.doi.org/ 10.3201/eid2011.140915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huong VT, Ha N, Huy NT, Horby P, Nghia HD, Thiem VD, Zhu X, Hoa NT, Hien TT, Zamora J, et al.. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis 2014; 20:1105-1114; PMID:24959701; http://dx.doi.org/ 10.3201/eid2007.131594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mai NT, Hoa NT, Nga TV, Linh le D, Chau TT, Sinh DX, Phu NH, Chuong LV, Diep TS, Campbell J, et al.. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis 2008; 46:659-667; PMID:19413493; http://dx.doi.org/ 10.1086/527385 [DOI] [PubMed] [Google Scholar]
- [11].Brosnahan AJ, Brown DR. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol 2012; 156:229-237; PMID:22074860; http://dx.doi.org/ 10.1016/j.vetmic.2011.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lievin-Le Moal V, Servin AL. Pathogenesis of human enterovirulent bacteria: lessons from cultured, fully differentiated human colon cancer cell lines. Microbiol Mol Biol Rev 2013; 77:380-439; PMID:24006470; http://dx.doi.org/ 10.1128/MMBR.00064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferrando ML, de Greeff A, van Rooijen WJ, Stockhofe-Zurwieden N, Nielsen J, Wichgers Schreur PJ, Pannekoek Y, Heuvelink A, van der Ende A, Smith H, et al.. Host-pathogen Interaction at the Intestinal Mucosa Correlates With Zoonotic Potential of Streptococcus suis. J Infect Dis 2015; 212:95-105; PMID:25525050; http://dx.doi.org/ 10.1093/infdis/jiu813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Okura M, Takamatsu D, Maruyama F, Nozawa T, Nakagawa I, Osaki M, Sekizaki T, Gottschalk M, Kumagai Y, Hamada S. Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: potential mechanisms for generation of capsular variation. Appl Environ Microbiol 2013; 79:2796-2806; PMID:23416996; http://dx.doi.org/ 10.1128/AEM.03742-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Van Calsteren MR, Gagnon F, Lacouture S, Fittipaldi N, Gottschalk M. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem Cell Biol 2010; 88:513-525; PMID:20555393; http://dx.doi.org/ 10.1139/O09-170 [DOI] [PubMed] [Google Scholar]
- [16].Kouki A, Haataja S, Loimaranta V, Pulliainen AT, Nilsson UJ, Finne J. Identification of a novel streptococcal adhesin P (SadP) protein recognizing galactosyl-alpha1-4-galactose-containing glycoconjugates: convergent evolution of bacterial pathogens to binding of the same host receptor. J Biol Chem 2011; 286:38854-38864; PMID:21908601; http://dx.doi.org/ 10.1074/jbc.M111.260992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zumbrun SD, Hanson L, Sinclair JF, Freedy J, Melton-Celsa AR, Rodriguez-Canales J, Hanson JC, O'Brien AD. Human intestinal tissue and cultured colonic cells contain globotriaosylceramide synthase mRNA and the alternate Shiga toxin receptor globotetraosylceramide. Infect Immun 2010; 78:4488-4499; PMID:20732996; http://dx.doi.org/ 10.1128/IAI.00620-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Winter KR, Stoffregen WC, Dean-Nystrom EA. Shiga toxin binding to isolated porcine tissues and peripheral blood leukocytes. Infect Immun 2004; 72:6680-6684; PMID:15501802; http://dx.doi.org/ 10.1128/IAI.72.11.6680-6684.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ferrando ML, Fuentes S, de Greeff A, Smith H, Wells JM. ApuA, a multifunctional alpha-glucan-degrading enzyme of Streptococcus suis, mediates adhesion to porcine epithelium and mucus. Microbiology 2010; 156:2818-2828; PMID:20522493; http://dx.doi.org/ 10.1099/mic.0.037960-0 [DOI] [PubMed] [Google Scholar]
- [20].Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect 2015; 17:173-183; PMID:25637951; http://dx.doi.org/ 10.1016/j.micinf.2015.01.004 [DOI] [PubMed] [Google Scholar]
- [21].Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 2015; 14:20-32; PMID:26499895; http://dx.doi.org/ 10.1038/nrmicro3552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yuan ZZ, Yan XJ, Zhang AD, Chen B, Shen YQ, Jin ML. Molecular mechanism by which surface antigen HP0197 mediates host cell attachment in the pathogenic bacteria Streptococcus suis. J Biol Chem 2013; 288:956-963; PMID:23184929; http://dx.doi.org/ 10.1074/jbc.M112.388686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Esgleas M, Li Y, Hancock MA, Harel J, Dubreuil JD, Gottschalk M. Isolation and characterization of alpha-enolase, a novel fibronectin-binding protein from Streptococcus suis. Microbiology 2008; 154:2668-2679; PMID:18757800; http://dx.doi.org/ 10.1099/mic.0.2008/017145-0 [DOI] [PubMed] [Google Scholar]
- [24].Ge J, Feng Y, Ji H, Zhang H, Zheng F, Yin Z, Pan X, Tang J. Inactivation of dipeptidyl-peptidase IV attenuates the virulence of Streptococcus suis serotype 2 that causes streptococcal toxic shock syndrome. Curr Microbiol 2009; 59:248-255; PMID:19484301; http://dx.doi.org/ 10.1007/s00284-009-9425-8 [DOI] [PubMed] [Google Scholar]
- [25].Jobin MC, Grenier D. Identification and characterization of four proteases produced by Streptococcus suis. FEMS Microbiol Lett 2003; 220:113-119; PMID:12644236; http://dx.doi.org/ 10.1016/S0378-1097(03)00088-0 [DOI] [PubMed] [Google Scholar]
- [26].Ferrando ML, van Baarlen P, Orru G, Piga R, Bongers RS, Wels M, De Greeff A, Smith HE, Wells JM. Carbohydrate Availability Regulates Virulence Gene Expression in Streptococcus suis. PLoS One 2014; 9:e89334; PMID:24642967; http://dx.doi.org/ 10.1371/journal.pone.0089334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Clarke TB, Francella N, Huegel A, Weiser JN. Invasive bacterial pathogens exploit TLR-mediated downregulation of tight junction components to facilitate translocation across the epithelium. Cell Host Microbe 2011; 9:404-414; PMID:21575911; http://dx.doi.org/ 10.1016/j.chom.2011.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pezzicoli A, Santi I, Lauer P, Rosini R, Rinaudo D, Grandi G, Telford JL, Soriani M. Pilus backbone contributes to group B Streptococcus paracellular translocation through epithelial cells. J Infect Dis 2008; 198:890-898; PMID:18694342; http://dx.doi.org/ 10.1086/591182 [DOI] [PubMed] [Google Scholar]
- [29].Takamatsu D, Nishino H, Ishiji T, Ishii J, Osaki M, Gottschalk M, Tharavichitkul P, Takai S, Sekizaki T. Genetic organization and preferential distribution of putative pilus gene clusters in Streptococcus suis. Vet Microbiol 2009; 138:132-139; PMID:19303725; http://dx.doi.org/ 10.1016/j.vetmic.2009.02.013 [DOI] [PubMed] [Google Scholar]
- [30].Seitz M, Baums CG, Neis C, Benga L, Fulde M, Rohde M, Goethe R, Valentin-Weigand P. Subcytolytic effects of suilysin on interaction of Streptococcus suis with epithelial cells. Vet Microbiol 2013; 167:584-591; PMID:24095145; http://dx.doi.org/ 10.1016/j.vetmic.2013.09.010 [DOI] [PubMed] [Google Scholar]
- [31].Allen AG, Lindsay H, Seilly D, Bolitho S, Peters SE, Maskell DJ. Identification and characterisation of hyaluronate lyase from Streptococcus suis. Microb Pathog 2004; 36:327-335; PMID:15120159; http://dx.doi.org/ 10.1016/j.micpath.2004.02.006 [DOI] [PubMed] [Google Scholar]
- [32].Zhang A, Mu X, Chen B, Han L, Chen H, Jin M. IgA1 protease contributes to the virulence of Streptococcus suis. Vet Microbiol 2011; 148:436-439; PMID:21041043; http://dx.doi.org/ 10.1016/j.vetmic.2010.09.027 [DOI] [PubMed] [Google Scholar]
- [33].Bonifait L, Grenier D. The SspA subtilisin-like protease of Streptococcus suis triggers a pro-inflammatory response in macrophages through a non-proteolytic mechanism. BMC Microbiol 2011; 11:47; PMID:21362190; http://dx.doi.org/ 10.1186/1471-2180-11-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fontaine MC, Perez-Casal J, Willson PJ. Investigation of a novel DNase of Streptococcus suis serotype 2. Infect Immun 2004; 72:774-781; PMID:14742520; http://dx.doi.org/ 10.1128/IAI.72.2.774-781.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Willenborg J, Fulde M, de Greeff A, Rohde M, Smith HE, Valentin-Weigand P, Goethe R. Role of glucose and CcpA in capsule expression and virulence of Streptococcus suis. Microbiology 2011; 157:1823-1833; PMID:21349980; http://dx.doi.org/ 10.1099/mic.0.046417-0 [DOI] [PubMed] [Google Scholar]
- [36].Nakayama T, Takeuchi D, Matsumura T, Akeda Y, Fujinaga Y, Oishi K. Alcohol consumption promotes the intestinal translocation of Streptococcus suis infections. Microb Pathog 2013; 65:14-20; PMID:24036179; http://dx.doi.org/ 10.1016/j.micpath.2013.08.006 [DOI] [PubMed] [Google Scholar]
- [37].Kerdsin A, Dejsirilert S, Puangpatra P, Sripakdee S, Chumla K, Boonkerd N, Polwichai P, Tanimura S, Takeuchi D, Nakayama T, et al.. Genotypic profile of Streptococcus suis serotype 2 and clinical features of infection in humans, Thailand. Emerg Infect Dis 2011; 17:835-842; PMID:21529392; http://dx.doi.org/ 10.3201/eid1705.100754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vecht U, van Leengoed LA, Verheijen ER. Streptococcus suis infections in pigs in the Netherlands (Part I). Vet Q 1985; 7:315-321; PMID:4071952; http://dx.doi.org/ 10.1080/01652176.1985.9694005 [DOI] [PubMed] [Google Scholar]
- [39].Moeser AJ, Klok CV, Ryan KA, Wooten JG, Little D, Cook VL, Blikslager AT. Stress signaling pathways activated by weaning mediate intestinal dysfunction in the pig. Am J Physiol Gastrointest Liver Physiol 2007; 292:G173-181; PMID:16901995; http://dx.doi.org/ 10.1152/ajpgi.00197.2006 [DOI] [PubMed] [Google Scholar]
- [40].Ulluwishewa D, Anderson RC, McNabb WC, Moughan PJ, Wells JM, Roy NC. Regulation of tight junction permeability by intestinal bacteria and dietary components. J Nutr 2011; 141:769-776; PMID:21430248; http://dx.doi.org/ 10.3945/jn.110.135657 [DOI] [PubMed] [Google Scholar]
- [41].Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol 1999; 473:11-30; PMID:10659341; http://dx.doi.org/ 10.1007/978-1-4615-4143-1_2 [DOI] [PubMed] [Google Scholar]
- [42].Fittipaldi N, Segura M, Grenier D, Gottschalk M. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol 2012; 7:259-279; PMID:22324994; http://dx.doi.org/ 10.2217/fmb.11.149 [DOI] [PubMed] [Google Scholar]
- [43].He Z, Pian Y, Ren Z, Bi L, Yuan Y, Zheng Y, Jiang Y, Wang F. Increased production of suilysin contributes to invasive infection of the Streptococcus suis strain 05ZYH33. Mol Med Rep 2014; 10:2819-2826; PMID:25241621; http://dx.doi.org/ 10.3892/mmr.2014.2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rossi O, van Baarlen P, Wells JM. Host-recognition of pathogens and commensals in the mammalian intestine. Curr Top Microbiol Immunol 2013; 358:291-321; PMID:22179258; http://dx.doi.org/ 10.1007/82_2011_191 [DOI] [PubMed] [Google Scholar]
- [45].Meijerink M, Ferrando ML, Lammers G, Taverne N, Smith HE, Wells JM. Immunomodulatory effects of Streptococcus suis capsule type on human dendritic cell responses, phagocytosis and intracellular survival. PLoS One 2012; 7:e35849; PMID:22558240; http://dx.doi.org/ 10.1371/journal.pone.0035849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Meierhans R, Bechir M, Ludwig S, Sommerfeld J, Brandi G, Haberthür C, Stocker R, Stover JF. Brain metabolism is significantly impaired at blood glucose below 6 mM and brain glucose below 1 mM in patients with severe traumatic brain injury. Crit Care 2010; 14:R13; PMID:20141631; http://dx.doi.org/ 10.1186/cc8869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kim S, Melikyan H, Kim J, Babajanyan A, Lee JH, Enkhtur L, Friedman B, Lee K. Noninvasive in vitro measurement of pig-blood d-glucose by using a microwave cavity sensor. Diabetes Res Clin Pract 2012; 96:379-84; PMID:22305939; http://dx.doi.org/ 10.1016/j.diabres.2012.01.018 [DOI] [PubMed] [Google Scholar]
- [48].Seitz M, Beineke A, Singpiel A, Willenborg J, Dutow P, Goethe R, Valentin-Weigand P, Klos A, Baums CG. Role of capsule and suilysin in mucosal infection of complement-deficient mice with Streptococcus suis. Infect Immun 2014; 82:2460-2471; PMID:24686060; http://dx.doi.org/ 10.1128/IAI.00080-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Benga L, Fulde M, Neis C, Goethe R, Valentin-Weigand P. Polysaccharide capsule and suilysin contribute to extracellular survival of Streptococcus suis co-cultivated with primary porcine phagocytes. Vet Microbiol 2008; 132:211-219; PMID:18565698; http://dx.doi.org/ 10.1016/j.vetmic.2008.05.005 [DOI] [PubMed] [Google Scholar]
- [50].Tenenbaum T, Adam R, Eggelnpohler I, Matalon D, Seibt A, K Novotny GE, Galla HJ, Schroten H. Strain-dependent disruption of blood-cerebrospinal fluid barrier by Streptoccocus suis in vitro. FEMS Immunol Med Microbiol 2005; 44:25-34; PMID:15780575; http://dx.doi.org/ 10.1016/j.femsim.2004.12.006 [DOI] [PubMed] [Google Scholar]