Abstract
Objectives
Oral mucositis (OM) is a painful complication of radiation therapy (RT) for head and neck cancer (H&NC). OM can compromise nutrition, require opioid analgesics and hospitalization for pain control, and lead to treatment interruptions. Based on the role of inflammatory pathways in OM pathogenesis, we investigated effect of cyclooxygenase-2 (COX-2) inhibition on severity and morbidity of OM.
Methods
In this double-blind placebo-controlled trial, 40 H&NC patients were randomized to daily use of 200 mg celecoxib or placebo, for the duration of RT. Clinical OM, normalcy of diet, pain scores, and analgesic use were assessed 2–3 times/week by blinded investigators during the 6–7 week RT period, using validated scales.
Results
Twenty subjects were randomized to each arm, which were similar with respect to tumor location, radiation dose, and concomitant chemotherapy. In both arms, mucositis and pain scores increased over course of RT. Intention-to-treat analyses demonstrated no significant difference in mean Oral Mucositis Assessment Scale (OMAS) scores at 5000 cGy (primary endpoint). There was also no difference between the two arms in mean OMAS scores over the period of RT, mean worst pain scores, mean normalcy of diet scores, or mean daily opioid medication use in IV morphine equivalents. There were no adverse events attributed to celecoxib use.
Conclusions
Daily use of a selective COX-2 inhibitor, during period of RT for H&NC, did not reduce the severity of clinical OM, pain, dietary compromise or use of opioid analgesics. These findings also have implications for celecoxib use in H&NC treatment regimens (NCT00698204).
Keywords: Oral Mucositis, Stomatitis, Radiation Therapy, Head and Neck Cancer, Celecoxib, Cyclooxygenase
Introduction
Oral mucositis (OM) refers to inflammatory, erosive/ulcerative oral mucosal lesions caused by chemotherapy or radiation therapy (RT). Patients receiving > 5000 cGy RT for head and neck cancer (H&NC) are more likely to develop OM [1]. In this population, OM typically causes severe pain, requiring use of systemic opioids. The painful ulcerations compromise dietary intake and cause weight loss [2]. Patients may need hospitalization for pain control and nutritional support, including gastrostomy tube placement [3, 4]. OM significantly reduces quality of life and increases healthcare costs [2]. Furthermore, severe OM can necessitate unplanned RT breaks [1, 3], potentially affecting cancer prognosis [5]. Thus, OM is a major dose-limiting toxicity of head and neck RT (H&NRT).
The inflammatory response to RT plays an important role in the pathogenesis of OM [6]. In a hamster cheek-pouch model of radiation mucositis, mRNA levels of tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β) in oral mucosal tissue correlated with OM severity [7]. TNF-α and IL-1β induce cyclooxygenase-2 (COX-2), a key enzyme in the inflammatory process, responsible for increased production of pro-inflammatory prostanoids. These prostanoids, notably prostaglandin E2 (PGE2) and prostacyclin (PGI2), mediate tissue injury and pain.
In patients receiving high-dose chemotherapy, mucositis pain scores were correlated with tissue levels of COX-1 and PGE synthase, and salivary prostaglandins [8]. In another human study, chemotherapy administration caused significant increases in Nuclear Factor kappa B (NFκB) and COX-2 in oral mucosa [9]. In a hamster radiation mucositis model, radiation induced a dramatic increase in COX-2 expression, which paralleled mucositis severity [10]. A similar finding was reported in a rat radiation mucositis model [11]. Thus, COX-2 inhibition appears to be a logical therapeutic target.
Furthermore, preclinical models indicate that celecoxib, a specific COX-2 inhibitor, may selectively increase radiosensitivity of tumor cells (overexpressing COX-2), but not normal cells [12]. There is interest in using celecoxib in treatment regimens for H&NC [13], however, its effects on OM were unclear. This study investigated the anti-inflammatory effect of celecoxib on severity and morbidity of OM.
Methods
Study Design
This was a prospective, randomized, double-blind, placebo-controlled, parallel-arm, multi-center study, conducted at UConn Health Center and Hartford Hospital, which serve a predominantly white, middle-class population in Hartford County. We received Institutional Review Board approvals; all subjects provided written informed consent.
Participants
Eligible patients were 18–75 years old and planned to receive a cumulative dose of ≥5000 centigray (cGy) RT (Intensity Modulated RT or Tomotherapy, 2Gy/day), for H&NC, to ≥2 of 14 pre-defined oral sites. Patients were approached by the coordinator or Principal Investigator, who were clinically involved in their pre-radiation dental assessment. Approximately 20% of approached patients declined participation. Key exclusion criteria included history of GI bleeding/ulcers or inflammatory bowel disease, severe hepatic/renal impairment, and allergy to celecoxib. After participation of the first 4 subjects in 2004, this study was suspended in 2005 following market withdrawal of rofecoxib (Vioxx) due to concerns about risk of thrombotic events. Based on new celecoxib labeling, eligibility criteria were modified to exclude patients with history of thromboembolic events, cardiac arrhythmia, or revascularization procedures. Enrollment resumed in 2006 and continued until the planned sample size was reached in 2011. A list of inclusion/exclusion criteria is on clinicaltrials.gov, NCT00698204.
Interventions
Celecoxib and placebo capsules, identical in appearance, were supplied by Pfizer. At study initiation, subjects were randomized to 200 mg oral celecoxib or placebo to be taken twice daily, 7 days/week, starting 5 days before first RT day and continuing until 3 days after RT completion. This regimen was modified in response to the revised celecoxib labeling. For all subjects after the first four, dose of study drug was reduced to 200 mg celecoxib or placebo used once daily only on days of RT, between start and end dates of RT.
Randomization and Blinding
Subjects were randomized by the research pharmacist to celecoxib or placebo using an allocation ratio of 1:1. Randomization assignments in blocks of 10 were generated by the research pharmacist using a web-based pseudo-random number sequence generator specific to number of treatment arms and randomization block size [14]. The research pharmacist dispensed the study drug and kept confidential records of group assignment. Study personnel, subjects and clinical providers were all blinded to subjects’ assignments until the end of the clinical phase of the study.
Clinical Data Collection and Outcome Measures
Subjects were seen for study visits 2–3 times/week during the 6–7 week period of RT. At each visit, we assessed OM, and collected information on diet, mouth pain, and opioid analgesic use. The primary outcome measure was the calibrated examiner’s blinded evaluation of OM clinical severity at 5000 cGy, using the Oral Mucositis Assessment Scale (OMAS) [15]. This validated scale scores ulceration and erythema at nine oral sites. Mucositis severity was also assessed using the World Health Organization (WHO) Oral Mucositis Scale [16] and the National Cancer Institute Common Toxicity Criteria (NCI-CTC) Scale v2 [17]. Ninety-three percent of mucositis assessments were performed by one of two primary examiners (inter-rater reliability 0.94).
Secondary outcome measures included severity of mouth pain, normalcy of diet and opioid analgesic use. Mouth pain was assessed using the severity subscale of the Brief Pain Inventory [18], asking subjects to indicate mouth pain level (worst, least, average, and current) on a validated 11-point scale ranging from 0 (no pain) to 10 (worst pain imaginable). Normalcy of diet was assessed using the normalcy of diet subscale from the Performance Status Scale for H&NC patients [19]. This validated subscale is a ranking of ten food categories arranged from easy-to-eat to hard-to-eat. Ratings are based on the highest ranking food the subject is able to eat ranging from 0 (no alimentation possible) to 10 (full diet). Daily opioid analgesic use data was collected at study visits and validated against the subjects’ daily diary and medical record. Conversion of opioid analgesic use to IV morphine equivalents was performed using the Advanced Opioid Converter [20].
Sample Size and Statistical Methods
Sample size calculations were performed to achieve high power for detection of a one-point difference in mean OMAS score (range 0–5) at 5000cGy RT. Published data for peak OMAS scores in RT patients (no intervention) indicate a standard deviation of +/− 1.1 points [21]. Based on this information, 20 subjects per group were needed to obtain 80% power when applying a two-tailed, two-sample t-test at the 5% level of significance.
The primary endpoint was assessment of OM severity as measured by the OMAS at 5000 cGy RT. The mean OMAS score was derived by dividing the total OMAS score (range 0–45) by the number of sites measured (9). Mean OMAS score at this time-point was compared between the celecoxib and placebo groups using two-sample t-test. Linear mixed models were used to compare OMAS scores, average pain scores, and normalcy of diet scores between groups over the study duration. Since the two groups were similar with respect to important confounders that influence mucositis severity, only treatment assignment was included in these models. To compare adjusted pain scores, daily opioid analgesic use, in IV morphine equivalents, was used as a covariate in linear mixed models. Mean daily opioid analgesic use was compared between groups using two-sample t-test.
For each comparison, two analyses were conducted: an intention to treat (ITT) analysis, in which all data is included regardless of study medication use status, and a per-protocol (PP) analysis in which assessment data are included through the last date of compliance with study medication use.
Results
Participant flow is summarized in Figure 1. Randomization resulted in a balanced distribution of subjects between the celecoxib and placebo arms with regard to factors that influence OM severity (Table 1).
Figure 1.
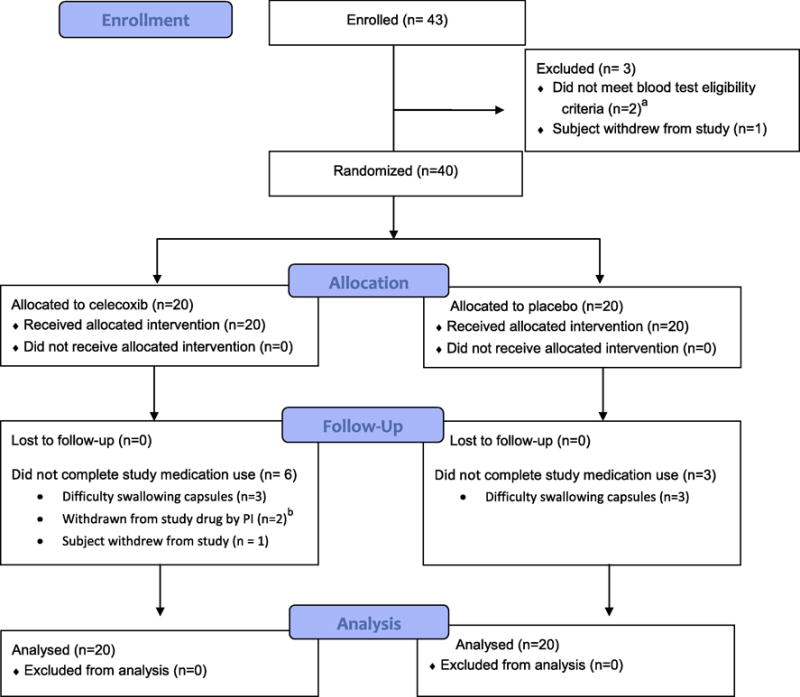
Table 1.
Baseline Characteristics of 40 Randomized Study Participants receiving Radiation Therapy for Head and Neck (H&N) Cancer
| CHARACTERISTIC | CELECOXIB ARM | PLACEBO ARM |
|---|---|---|
| Number of Participants (n) | 20 | 20 |
| Demographics | ||
| Mean Age in Years (minimum-maximum) | 53.2 (34–71) | 56.0 (36–69) |
| Male (n, %) | 17 (85%) | 15 (75%) |
| Race and Ethnicity (n, %) | ||
| White, non-Hispanic | 20 (100%) | 17 (85%) |
| White, Hispanic | 0 (0%) | 2 (10%) |
| Black, non-Hispanic | 0 (0%) | 1 (5%) |
| Enrollment Site (n, %) | ||
| UConn Health Center | 18 (90%) | 20 (100%) |
| Hartford Hospital | 2 (10%) | 0 (0%) |
| Cancer Treatment | ||
| Mean Radiation Dose to Primary Site in Centigray (SD) | 6873 (266.9) | 6857 (270.9) |
| Bilateral radiation dosing (n, %) | 16 (80%) | 17 (85%) |
| Concomitant Chemotherapy (n, %) | 17 (85%) | 15 (75%) |
| Received surgery before radiation therapy | 9 (45%) | 10 (50%) |
| History of prior H&N radiation therapy | 1 (5%) | 0 (0%) |
| Primary Tumor Site by Category (n, %) | ||
| Oral cavity region | 5 (25%) | 6 (30%) |
| Soft palate/nasopharynx/tonsillar region | 6 (30%) | 4 (20%) |
| Pharynx and adjacent | 5 (25%) | 5 (25%) |
| Larynx and neck | 4 (20%) | 5 (25%) |
| H&N Cancer Stage (n, %) | ||
| I | 1 (5%) | 1 (5%) |
| II | 2 (10%) | 3 (15%) |
| III | 5 (25%) | 8 (40%) |
| IV | 12 (60%) | 8 (40%) |
Severity of Clinical OM
For the primary endpoint ITT analysis, scores from 39 subjects (19 celecoxib, 20 placebo) were compared at the first OMAS assessment after 5000 cGy RT was reached (conducted at a mean of 0.87 days after 5000cGy reached, SD 1.08 days), since all subjects participated in study assessments at this dose point except one in the celecoxib arm who withdrew at 4000 cGy. The primary endpoint PP analysis included scores from the 35 subjects (16 celecoxib, 19 placebo) who were still taking study medication at 5000 cGy RT. No significant difference was seen in the primary endpoint or other OMAS measures in either the ITT or PP analysis (Table 2). The mean cumulative radiation dose on the date of primary endpoint assessment was similar between the groups (5120 vs. 5126 cGy, p=0.91). Two additional OM scales, WHO and NCI-CTC, were also compared with no difference between groups detected in either the ITT or PP analyses (Table 2). No center effects were found (data not shown).
Table 2.
Clinical Oral Mucositis Scores
| Oral Mucositis Assessment Scale (OMAS) | ||||||
|---|---|---|---|---|---|---|
| Intention to Treat Analysis | Per Protocol Analysis (period of study medication use only) | |||||
| Celecoxib n=20 |
Placebo n=20 |
P value | Celecoxib n=20 |
Placebo n=20 |
P value | |
| Mean OMAS Score (range 0–5) (SD) | .98 (.77) |
.97 (.86) |
.8402 | .86 (.72) |
.93 (.86) |
.6742 |
| Mean OMAS Ulceration (range 0–3), (SD) | .52 (.53) |
.50 (.53) |
.6682 | .45 (.49) |
.47 (.52) |
.7632 |
| Mean OMAS Erythema (range 0–2) (SD) | .46 (.28) |
.47 (.38) |
.8402 | .42 (.27) |
.46 (.38) |
.5802 |
| Mean OMAS Score (0–5) immediately after 5000 centigray radiation (SD) | 1.42 (.67) n=19 |
1.36 (.88) |
.8311 | 1.31 (.68) n=16 |
1.41 (.87) n=19 |
.7211 |
| Mean Peak OMAS Score (range 0–5) (SD) | 1.71 (.59) |
1.70 (.95) |
.9821 | 1.65 (.58) |
1.69 (.95) |
.8771 |
| World Health Organization (WHO) Oral Mucositis Scale | ||||||
|---|---|---|---|---|---|---|
| Intention to Treat Analysis | Per Protocol Analysis (period of study medication use only) | |||||
| Celecoxib n=20 |
Placebo n=20 |
P value | Celecoxib n=20 |
Placebo n=20 |
P value | |
| Mean WHO Score (range 0–4) (SD) | 1.94 (1.40) |
1.85 (1.43) |
.6542 | 1.74 (1.35) |
1.80 (1.43) |
.8882 |
| WHO Score immediately after 5000 cGy radiation (SD) | 2.47 (1.17) n=19 |
2.55 (1.00) |
.8281 | 2.31 (1.20) n=16 |
2.58 (1.02) n=19 |
.4812 |
| Peak WHO Score (SD) | 3.40 (.68) |
2.95 (1.15) |
.1391 | 3.05 (.76) |
2.95 (1.15) |
.7471 |
| Number of Days with WHO Grade 3 or 4 Mucositis (SD) | 15.85 (13.48) |
17.05 (15.16) |
.7931 | 10.05 (10.79) |
13.85 (12.76) |
.3161 |
| National Cancer Institute Common Toxicity Criteria (NCI-CTC) Oral Mucositis Scale | ||||||
|---|---|---|---|---|---|---|
| Intention to Treat Analysis | Per Protocol Analysis (period of study medication use only) | |||||
| Celecoxib n=20 | Placebo n=20 | P value | Celecoxib n=20 | Placebo n=20 | P value | |
| Mean NCI-CTC Score (Range 0–4) (SD) | 2.00 (1.07) |
1.85 (1.12) |
.3242 | 1.86 (1.07) |
1.81 (1.13) |
.6542 |
| NCI-CTC immediately after 5000 cGy Radiation (SD) | 2.63 (.60) n=19 |
2.50 (.83) |
.5741 | 2.56 (.63) n=16 |
2.53 (.84) n=19 |
.8881 |
| Peak NCI-CTC (SD) | 2.85 (.37) |
2.55 (.37) |
.0931 | 2.84 (.38) |
2.55 (.69) |
.1101 |
| Number of Days with NCI- | 19.60 | 18.20 | .7671 | 14.35 | 16.15 | .6671 |
| CTC Grade 3 or higher (SD) | (14.12) | (15.50) | (12.27) | (13.96) | ||
Two-sample t-test,
linear mixed models
Pain Scores
Mean pain scores across the period of RT were compared between groups and no difference was found in worst pain, least pain, average pain, or pain now, in either ITT or PP analyses. To assess a standardized time point during peak OM, mean worst pain scores at 5000 cGy were evaluated using ITT and PP analyses, again with no significant difference (Table 3). Since all subjects, except 1 placebo subject, used opioid analgesics, ITT and PP analyses were also performed on pain scores adjusted for opioid analgesic use. However, there remained no difference in pain scores between groups (data not shown).
Table 3.
Pain Scores, Opioid Analgesic Use, and Diet Scores.
| Evaluation of Pain Severity (0–10 scale, 0 = no pain, 10 = worst pain imaginable) | ||||||
|---|---|---|---|---|---|---|
| Parameter | Intention to Treat Analysis | Per Protocol Analysis* | ||||
| Celecoxib n=20 | Placebo n=20 | P value | Celecoxib n=20 | Placebo n=20 | P value | |
| Mean Average Pain Score (SD) | 2.44 (2.41) |
2.20 (2.39) |
.5762 | 2.23 (2.39) |
2.14 (2.39) |
.9072 |
| Mean Worst Pain Score (SD) | 3.38 (3.07) |
3.31 (3.32) |
.8302 | 3.01 (2.95) |
3.18 (3.27) |
.7332 |
| Mean Least Pain Score (SD) | 1.75 (2.15) |
1.31 (1.91) |
.3242 | 1.63 (2.16) |
1.28 (1.93) |
.4722 |
| Mean Pain Now Score (SD) | 2.30 (2.46) |
2.05 (2.48) |
.5822 | 2.08 (2.38) |
1.96 (2.44) |
.7982 |
| Mean Worst Pain at 5000 cGy RT (SD) | 4.47 (2.82) n=19 |
3.70 (3.20) | .4291 | 4.25 (2.89) n=19 |
3.89 (3.16) |
.7331 |
| Opioid Analgesic Use in IV Morphine Equivalents | ||||||
| Mean Daily Opioid Analgesic Use In IV morphine equivalents in mg (SD) | 19.08 (16.56) |
20.48 (19.07) |
.8061 | 14.06 (11.79) |
17.77 (15.29) |
.3961 |
| Evaluation of Normalcy of Diet (0–10 scale, 0 = no alimentation possible, 10 = can consume a full diet) | ||||||
| Mean diet score (SD) | 5.43 (3.86) |
5.11 (3.94) |
.6542 | 5.81 (3.80) |
5.37 (3.90) |
.3912 |
| Mean lowest score (worst ability to eat) (SD) | 1.15 (1.42) |
1.65 (3.01) |
.5061 | 1.90 (1.68) |
1.80 (3.00) |
.8971 |
| Number of days from start of RT to first reduced diet score due to mucositis (SD) | 15.75 (11.53) |
16.65 (12.81) |
.8172 | 22.20 (10.88) |
21.85 (11.77) |
.9211 |
Per protocol analysis: Data included during period of study medication use only.
Two-sample t-test,
linear mixed models
Opioid Medication Use
Opioid analgesic use in IV morphine equivalents was compared between groups using ITT and PP analyses. Data from 1992 individual opioid analgesic daily use reports were used for ITT analysis and 1620 for PP analysis. There was no difference between groups in the mean daily use of opioid analgesics in IV morphine equivalents (Table 3).
Normalcy of Diet
Normalcy of diet was evaluated by comparing mean diet scores, mean lowest diet scores, and number of days from start of RT to the first reduced diet score, using ITT and PP analysis. There was no significant difference between the celecoxib and placebo groups for any of these measures (Table 3).
Adverse Events
This study was monitored by an independent Data and Safety Monitoring Board (DSMB) composed of a cardiologist, an oral medicine specialist, and a dentist scientist. The DSMB reviewed all adverse events, recruitment status, completions, and drop outs, on an annual and occurrence basis, and determined that there were no adverse events attributable to study participation.
Discussion
In this randomized, double-blind, placebo-controlled trial, no difference was found in OM severity between celecoxib and placebo groups, on any of the 3 scales used. The number of days with severe OM was similar between the groups, indicating that the intervention did not delay onset of severe OM. The rationale for measuring pain scores was that reduction of OM severity by celecoxib would result in a reduction of OM-associated pain. We found no difference in pain scores or opioid analgesic use between the two groups. Since most subjects were on high doses of potent opioids, the relatively small analgesic effect of 200 mg celecoxib daily may not have an appreciable direct analgesic effect. The level of dietary compromise was also similar between the groups, documented by the Normalcy of Diet scale as well as by the WHO OM scale which scores the effect of OM on ability to consume solids and/or liquids.
Strengths of this study include the randomized, double-blind, placebo controlled design which minimized the risk of bias in outcome measurements. The celecoxib and placebo groups were similar with regard to important factors that influence OM severity including tumor site, and radiation dose/fields. Use of concomitant chemotherapy can significantly influence OM severity; however, there was no significant difference between the groups in the proportion of such patients. This increases confidence that the study accurately assessed the impact of the intervention. However, other heterogeneity in the study population may have obscured a clinical effect. Limitations of the study include the small sample size. The sample size was calculated on the basis of a relatively large effect size of a 1 point difference on a 5 point scale. Thus, while this study demonstrates the absence of a large effect of the intervention, a smaller effect, albeit less clinically significant, cannot be ruled out. Another limitation is the change in celecoxib total daily dose from 400 mg to 200 mg after the first 4 subjects. Fortunately, these 4 subjects were evenly distributed between the celecoxib and placebo groups. It is possible that the 200 mg dose was insufficient to have a discernible effect on OM. However, there was no difference in OM severity between the subjects receiving the higher dose of celecoxib and the remaining subjects in the celecoxib or placebo groups (data not shown).
In conclusion, daily use of a selective COX-2 inhibitor, during H&NRT, did not reduce severity of clinical OM, mouth pain, dietary compromise, or use of opioid analgesics. Since the initiation of this study, other literature has appeared on the effects of selective inhibitors of inflammation on OM. In a mouse model of radiation mucositis, administration of celecoxib or infliximab (an inhibitor of TNFα) did not change the radiation dose required for ulcer formation, as compared to controls [22]. A pilot study examined the use of a tooth patch containing flurbiprofen (a non-selective COX inhibitor) in 12 patients receiving H&N RT. As compared to historical controls, no differences were found in the severity or duration of OM between the two groups [23]. A recent update of the evidence-based mucositis guidelines developed by the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) suggested against the use of misoprostol (prostaglandinE1 analog) mouthrinse for OM in H&NRT [24]. On the other hand, benzydamine, which has anti-inflammatory effects via multiple pathways, was found to have some benefit in moderate levels of H&NRT, without concomitant chemotherapy [6]. These results suggest that use of agents targeting a single inflammatory pathway may not be an effective strategy for radiation-induced OM. Our findings also have important implications for the use of celecoxib in treatment regimens for H&NC.
Supplementary Material
Highlights.
Oral mucositis (OM) is a painful complication of radiation therapy (RT) for head and neck cancer (H&NC).
We investigated the effect of cyclooxygenase-2 inhibition on severity and morbidity of OM.
In this double-blind placebo-controlled trial, 40 H&NC patients were randomized to daily celecoxib or placebo during RT.
Use of celecoxib did not reduce the severity of clinical OM, pain, dietary compromise or use of opioid analgesics.
These findings also have implications for celecoxib use in H&NC treatment regimens.
Acknowledgments
We thank the subjects who participated in this study; DSMB members Drs. Joseph D’Ambrosio, J. Robert Kelly and Peter Schulman; and research pharmacist Ruth LaCasse Kalish, RPh, BCPP.
Funding Sources
This study was supported by grant K23DE016946 from the US National Institute of Dental and Craniofacial Research, grant M01RR06192 from the US National Center for Research Resources, and by Pfizer, Inc. None of the funding entities had any involvement in study design, data collection/analysis/interpretation, or in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors reported any conflicts of interest relevant to this work.
Drugs and Devices
Celebrex (celecoxib) capsules and placebo capsules, supplied by Pfizer, Inc, New York, USA.
References
- 1.Vera-Llonch M, Oster G, Hagiwara M, Sonis S. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 2006;106(2):329–36. doi: 10.1002/cncr.21622. [DOI] [PubMed] [Google Scholar]
- 2.Elting LS, Cooksley CD, Chambers MS, Garden AS. Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys. 2007;68(4):1110–20. doi: 10.1016/j.ijrobp.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–62. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 4.Murphy BA, Beaumont JL, Isitt J, Garden AS, Gwede CK, Trotti AM, et al. Mucositis-related morbidity and resource utilization in head and neck cancer patients receiving radiation therapy with or without chemotherapy. J Pain Symptom Manage. 2009;38(4):522–32. doi: 10.1016/j.jpainsymman.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal DI. Consequences of mucositis-induced treatment breaks and dose reductions on head and neck cancer treatment outcomes. J Support Oncol. 2007;5(9 Suppl 4):23–31. [PubMed] [Google Scholar]
- 6.Nicolatou-Galitis O, Sarri T, Bowen J, Di Palma M, Kouloulias VE, Niscola P, et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients. Support Care Cancer. 2013;21(11):3179–89. doi: 10.1007/s00520-013-1847-y. [DOI] [PubMed] [Google Scholar]
- 7.Sonis ST, Peterson RL, Edwards LJ, Lucey CA, Wang L, Mason L, et al. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36(4):373–81. doi: 10.1016/s1368-8375(00)00012-9. [DOI] [PubMed] [Google Scholar]
- 8.Lalla RV, Pilbeam CC, Walsh SJ, Sonis ST, Keefe DM, Peterson DE. Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis: a pilot study. Support Care Cancer. 2010;18(1):95–103. doi: 10.1007/s00520-009-0635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan RM, Gibson RJ, Sonis ST, Keefe DM. Nuclear factor-kappaB (NF-kappaB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007;43(4):395–401. doi: 10.1016/j.oraloncology.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Sonis ST, O’Donnell KE, Popat R, Bragdon C, Phelan S, Cocks D, et al. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004;40(2):170–6. doi: 10.1016/s1368-8375(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 11.Feng CJ, Guo JB, Jiang HW, Zhu SX, Li CY, Cheng B, et al. Spatiotemporal localization of HIF-1alpha and COX-2 during irradiation-induced oral mucositis in a rat model system. Int J Radiat Biol. 2008;84(1):35–45. doi: 10.1080/09553000701616080. [DOI] [PubMed] [Google Scholar]
- 12.Choy H, Milas L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: a rational advance? J Natl Cancer Inst. 2003;95(19):1440–52. doi: 10.1093/jnci/djg058. [DOI] [PubMed] [Google Scholar]
- 13.Kao J, Genden EM, Chen CT, Rivera M, Tong CC, Misiukiewicz K, et al. Phase 1 trial of concurrent erlotinib, celecoxib, and reirradiation for recurrent head and neck cancer. Cancer. 2011;117(14):3173–81. doi: 10.1002/cncr.25786. [DOI] [PubMed] [Google Scholar]
- 14.Dallal GE. Randomization Sequence Generator. [last accessed 7/18/2014]; Available from: www.randomization.com.
- 15.Sonis ST, Eilers JP, Epstein JB, LeVeque FG, Liggett WH, Jr, Mulagha MT, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group Cancer. 1999;85(10):2103–13. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Handbook for reporting results of cancer treatment. World Health Organization; Geneva: 1979. pp. 15–22. [Google Scholar]
- 17.National Cancer Institute. Common Terminology Criteria for Adverse Events v2 (CTCAE) 1999 [Internet document] [last accessed 7/18/2014]; Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcv2nom-4-30-99-final3.pdf.
- 18.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–38. [PubMed] [Google Scholar]
- 19.List MA, Ritter-Sterr C, Lansky SB. A performance status scale for head and neck cancer patients. Cancer. 1990;66(3):564–9. doi: 10.1002/1097-0142(19900801)66:3<564::aid-cncr2820660326>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.McAuley D. Advanced Opioid Converter. [last accessed 7/18/2014]; Available from: http://www.globalrph.com/opioidconverter2.htm.
- 21.Epstein JB, Gorsky M, Guglietta A, Le N, Sonis ST. The correlation between epidermal growth factor levels in saliva and the severity of oral mucositis during oropharyngeal radiation therapy. Cancer. 2000;89(11):2258–65. doi: 10.1002/1097-0142(20001201)89:11<2258::aid-cncr14>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Haagen J, Krohn H, Rollig S, Schmidt M, Wolfram K, Dorr W. Effect of selective inhibitors of inflammation on oral mucositis: preclinical studies. Radiother Oncol. 2009;92(3):472–6. doi: 10.1016/j.radonc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Stokman MA, Spijkervet FK, Burlage FR, Roodenburg JL. Clinical effects of flurbiprofen tooth patch on radiation-induced oral mucositis. A pilot study. Support Care Cancer. 2005;13(1):42–8. doi: 10.1007/s00520-004-0674-6. [DOI] [PubMed] [Google Scholar]
- 24.Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, Keefe DM, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453–61. doi: 10.1002/cncr.28592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


