Abstract
HIV-infected patients receiving antiretroviral therapy have increased risk of metabolic syndrome, including dyslipidemia. In this study, we determined whether individual nutritional counseling reduced dyslipidemia, particularly LDL cholesterol, among HIV-infected patients with dyslipidemia not currently taking lipid-lowering medication. We conducted a randomized 24-week trial among HIV-infected patients with dyslipidemia who were on ART and were eligible to initiate therapeutic lifestyle changes according to the Thai National Cholesterol Education Program. Participants were randomly assigned to an intervention group that received individual counseling with a nutritionist for 7 sessions (baseline, weeks 2, 4, 8, 12, 18 and 24) and a control group that received standard verbal diet information at baseline and nutritional counseling only at week 24. A 24-hr recall technique was used to assess dietary intake for both groups at baseline and week 24. Lipid profile (total cholesterol, LDL, HDL and triglyceride) was measured at baseline and after 12 and 24 weeks of therapy. An intention-to-treat and linear mixed model were used. Seventy-two patients were randomly assigned, and 62 (86%) participants completed their lipid profile test. After 12 weeks of follow-up, there were significant reductions in the intervention group for total cholesterol (−14.4 ± 4.6 mg/dL, P=0.002), LDL cholesterol (−13.7 ± 4.1 mg/dL, P=0.001), and triglyceride (−30.4 ± 13.8 mg/dL, P=0.03). A significant reduction in LDL cholesterol was also observed in the control group (−7.7 ± 3.8 mg/dL, P=0.04), but there were no significant differences in change of mean lipid levels between groups at 12 weeks of follow-up. After 24 weeks, participants assigned to the intervention group demonstrated significantly greater decreases in serum total cholesterol (−19.0 ± 4.6 vs 0.2 ± 4.3 mg/dL, P=0.003) and LDL cholesterol (−21.5 ± 4.1 vs −6.8 ± 3.8 mg/dL, P=0.009). There were no significant changes in HDL cholesterol or triglycerides levels in either group.
Keywords: nutritional counseling, LDL cholesterol, HIV infection, Thailand
Introduction
Globally, more than 34 million people were living with HIV/AIDS at the end of 2011 (UNAIDS 2012). Although receiving antiretroviral therapy has contributed to a decline in deaths among people living with HIV, some treated patients develop dyslipidemia, characterized by an increase of serum total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides, and a low level of high-density lipoprotein cholesterol (HDL-C) (Dube et al., 2003). In Thailand, more than 1.2 million people have been living with HIV/AIDS, a prevalence of nearly 2%. By 2011, antiretroviral therapy (ART) coverage provided through the universal coverage benefit scheme in Thailand was nearly 90% (Thai Ministry of Public Health Bureau of Epidemiology, 2012). The Thai 2010 guidelines for ART for HIV-1-infected adults and adolescents recommends initiating treatment with ART at CD4 count <350 cells/mm3 (Sungkanuparph et al., 2010). Little is known about the effect of individual nutritional counseling on lipid profiles of HIV-infected patients when receiving ART in Thailand. Many previous studies in Western countries have shown that lifestyle modification, particularly diet and exercise, reduced dyslipidemia in HIV-infected patients (Anjos et al., 2011; Barrios et al., 2002; Batterham, Brown, & Workman, 2003; Moyle et al., 2001). The majority of these studies implemented menu plans or provided meals for participants in order to compare two or three groups receiving different diets. Utilization of these menu plans is not practical in places such as Thailand, because of widely varied diets. To date, there are no specific recommendations or guidelines in Thailand to manage HIV-associated dyslipidemia, nor do nutritional interventions (food-based or supplements) for HIV-infected persons with dyslipidemia exist. The objective of this study was to reduce dyslipidemia in HIV-infected persons through a counseling program with a randomized, 24-week trial in HIV-infected patients with dyslipidemia who were on ART. The primary outcome variable was LDL cholesterol level (changes from baseline) at 12 and 24 weeks.
Methods
Study participants
Participants were recruited from HIV-infected patients with abnormal LDL-C who were on ART for at least three months and had visited the outpatient department (OPD) at Bamrasnaradura Infectious Diseases Institute, Ministry of Public Health, Bangkok. One hundred and sixty one HIV-infected patients were assessed for eligibility to take part in a 24-week randomized controlled trial to determine whether nutritional counseling reduces dyslipidemia, particularly LDL-C. Criteria for inclusion in the study included being 18 to 65 years old, on stable ART for more than 3 months, and not currently being treated with lipid-lowering and/or diabetes medication. Subjects were excluded if they had a history of cancer, renal failure, pancreatitis, or liver cirrhosis, were pregnant, or were illiterate and/or unable to complete the food record. Participants who developed cardiovascular events or started lipid-lowering medication during the follow-up period were discontinued. The principal investigator requested infectious disease physicians caring for HIV-infected patients to refer those with LDL-C ≥100 mg/dL to the project. Participants were requested to sign an informed consent form if they were willing to participate the study.
This study was approved by Institutional Review Boards of the University of California, Los Angeles (UCLA), the Thailand Ministry of Public Health Ethical Review Committee for Research in Human Subjects, and the Ethics Board of Bamrasnaradura Infectious Diseases Institute.
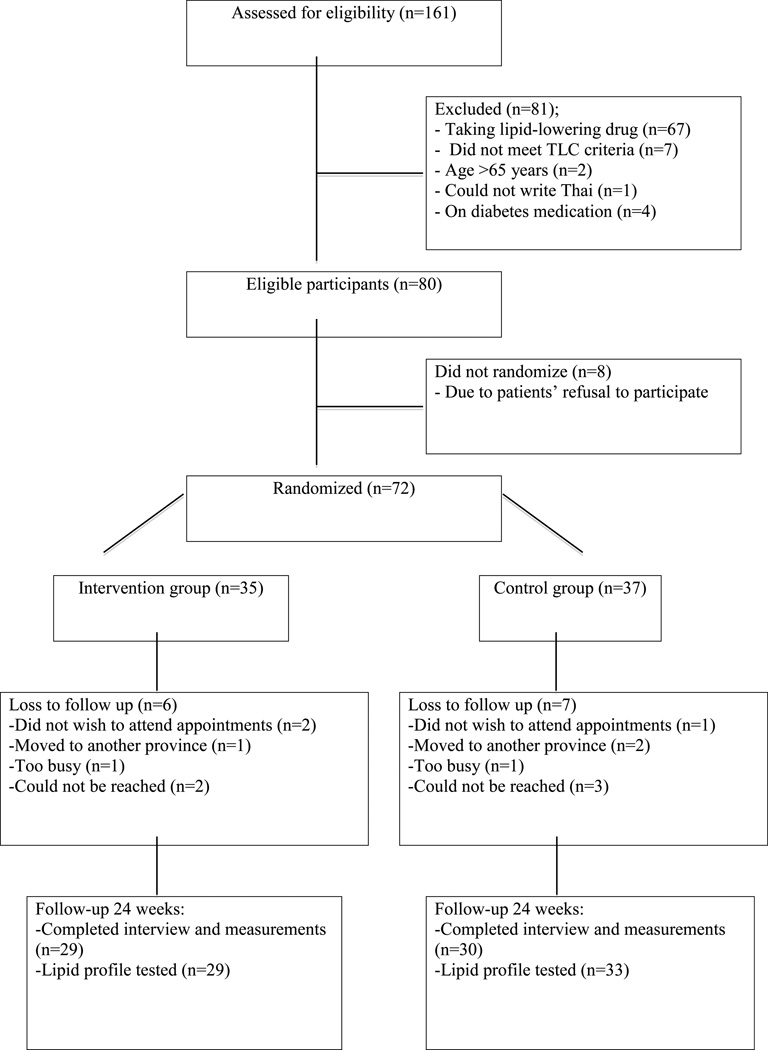
Of the 161 potential participants screened, 81 were ineligible, eight refused, 13 were lost to follow-up, and 59 completed the study (Fig.1). The reasons for not participating included the inconvenience of visiting the hospital frequently and/or being unwilling to come if they did not have an appointment with their own physicians at the follow-up times.
Figure 1.
Diagram demonstrating flow of participants through the study
Study design
We conducted a 24-week follow-up randomized controlled trial of HIV-infected patients stable (at least 12 weeks) on ART who had dyslipidemia and were eligible to initiate therapeutic lifestyle changes according to the National Cholesterol Education Program (NCEP) (Third Report of the NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III), 2002). Data for this study were collected between March 2012 and March 2013. At baseline, all participating participants had fasting serum lipid profiles assessed, and had blood pressure and anthropometric data collected, including weight, height, waist and hip circumference. At baseline and 24 weeks, participants’ dietary intakes were assessed by 24-hour recall with nutritionists. Participants self-administered a questionnaire about their demographic characteristics, lifestyle and behaviors, physical activity, alcohol consumption, and risk of cardiovascular diseases. Self–reported physical activity was assessed using the short interview version of the International Physical Activity Questionnaire (IPAQ) (Booth, 2002). Physical activity levels were calculated as the product of the frequency and duration of each activity in minutes per week, and weighted by an estimate of the metabolic equivalent (MET) of the activity. The results were reported as the mean MET-min per week (Kriska et al., 1990).
Fasting serum lipid profiles were reassessed at 12 and 24 weeks of follow-up. Participants were asked to fast 12 hours overnight before providing venous blood the next morning. Fasting serum lipid profiles were measured using enzymatic colorimetric methods at the laboratory of Bamrasnaradura Infectious Diseases Institute.
Study intervention
Eligible participants were randomly assigned to either the intervention group (individual counseling with nutritionist) or the control group (standard care). For participants in the intervention group, the diet was individualized to participants based on their nutritional needs (according to Thai Food-Based Dietary Guidelines), socio-economic status and dietary habits of each participant. The participants in the intervention group attended a total of 7 sessions with a professional nutritionist during follow-up: at baseline, and at 2, 4, 8, 12, 18, and 24 weeks. The dietary intervention goals followed the Therapeutic Lifestyle Change diet guidelines, which focus on LDL cholesterol changes according to the NCEP Adult Treatment Panel III (ATP III) as follows: less than 25% of daily calories from fat, less than 200 mg/day of cholesterol, less than 7% of total calories from saturated fat, up to 10% of total calories from polyunsaturated fat, up to 20% of total calories from monounsaturated fat, and 20–30 g total fiber per day. We adapted the NCEP guideline for percent fat to make it appropriate for Thais. Food models were used to assess diet quantity. Participants’ dietary intake was assessed by a 24-hr recall administered by a nutritionist at each visit. Participants were asked to record food they consumed during the week prior to their next visit. Dietary advice was provided based on the participants’ one-week food records.
Participants in the control group received standard general verbal diet information at baseline and at subsequent annual visits. Participants were assessed for their dietary intake with 24-hr recall by a nutritionist at baseline and week 24 of the study. All participants received 200 Baht (approximately $7 U.S.) for their participation at each visit.
Statistical analysis
Descriptive analysis was used to describe qualitative variables. A comparison of demographic characteristics between groups was made at baseline, using the two-sample t-test for continuous variables and Chi-square test or Fisher’s exact tests for categorical variables. Nutrient intake was analyzed from dietary intake according to the Thai Fatty Acid program, using a Thai computerized dietary database. Participants had LDL-C levels measured at baseline (week 0), and weeks 12 and 24. The primary outcome was change in LDL-C over time. Linear mixed models, which assume a correlation between repeated measurements, were used. With 72 participants, the study provided a power of 50% to detect a 1 mmol/L (39 mg/dL) change in LDL-C level, with P=0.05 significance level. Statistical analyses were performed using the SAS 9.1.3. Statistical software package (SAS Institute Inc., Cary, NC).
Results
Of the 72 participants, 62 (86.1%) completed lipid profile testing at baseline and 12 and 24 weeks, and 59 (81.9%) completed the dietary interview and measurements (blood pressure, body weight, and waist and hip circumferences) at week 24. There were no significant differences in baseline characteristics between participants in the intervention and control groups (Table 1).
Table 1.
Baseline characteristics of participants (N=72)
| Characteristics | Intervention group (N=35) Mean (s.d.*); N (%) |
Control group (N=37) Mean (s.d.*); N (%) |
p-value |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 42.3 (6.2) | 43.9 (7.5) | 0.263 |
| Female gender – N (%) | 23 (65.7) | 19 (51.4) | 0.217 |
| Body mass index (kg/m2) | 23.0 (3.0) | 23.5 (4.2) | 0.517 |
| Waist circumference (cm) | 81.2 (8.0) | 84.2 (9.8) | 0.161 |
| Hip circumference (cm) | 93.0 (5.3) | 94.6 (7.7) | 0.306 |
| Waist:hip ratio | 0.87 (0.05) | 0.89 (0.06) | 0.24 |
| Smokes cigarettes – N (%) | 4 (11.4%) | 5 (13.5%) | 1.000 |
| Consumes alcohol – N (%) | 11 (31.4%) | 10 (27.0%) | 0.681 |
| Duration known as HIV-positive (yrs) | 10.1 (5.0) | 9.2 (3.9) | 0.399 |
| Current ARV treatment – N (%) | |||
| Treatment with protease | 5 (14.3%) | 7 (18.9%) | 0.598 |
| Treatment with NRT | 34 (97.1%) | 36 (97.3%) | 1.000 |
| Treatment with NNRT | 30 (85.7%) | 32 (86.5%) | 1.000 |
| CD4 count (cells/mm3) | 517.8 (183.6) | 475.4 (171.9) | 0.315 |
| Lipid profile | |||
| Total cholesterol (mg/dL) | 229.2 (30.7) | 226.4 (21.8) | 0.653 |
| LDL-C (mg/dL) | 161.0 (24.2) | 157.3 (21.6) | 0.502 |
| HDL-C (mg/dL) | 56.1 (18.4) | 54.2 (17.2) | 0.652 |
| Triglycerides (mg/dL) | 152.1 (89.8) | 166.2 (99.1) | 0.531 |
| Dietary parameters | |||
| Energy (kcal) | 1362.9 (543.3) | 1366.7 (434.8) | 0.974 |
| Protein (%) | 14.1 (5.1) | 15.2 (4.0) | 0.322 |
| Carbohydrate (%) | 58.6 (11.3) | 56.9 (8.9) | 0.478 |
| Fat (%) | 27.3 (10.3) | 28.0 (7.3) | 0.763 |
| Saturated fat (%) | 4.8 (3.9) | 4.9 (5.1) | 0.929 |
| Polyunsaturated fat (%) | 2.5 (2.2) | 1.6 (1.7) | 0.072 |
| Monounsaturated fat (%) | 4.6 (3.9) | 4.3 (3.8) | 0.741 |
| Cholesterol (mg) | 173.0 (144.8) | 200.6 (154.0) | 0.437 |
| Total fiber (g) | 7.2 (3.8) | 6.3 (4.5) | 0.332 |
| Soluble fiber (mg) | 0.5 (0.9) | 0.3 (0.7) | 0.281 |
| Physical activity parameters | |||
| Total PA (Met-min/week)** | 491.9 (477.2) | 412.9 (393.0) | 0.460 |
s.d. = standard deviation
Metabolic equivalent: Met-min calculated by multiplying minutes of the respective activity in the past week by 8 (vigorous), 4 (moderate), and 3.3 (walking). Total PA (physical activity) is the sum of vigorous, moderate and walking Met-min.
Of the 35 participants in intervention group, 66% were female. The average age was 42 years, mean BMI was 23 kg/m2, and average waist and hip circumferences were 81.2 and 93.0 centimeters, respectively. The majority of participants (97.1%) received ART with a combination of a NRTI regimen. Participants received antiviral treatments with a non-nucleoside reverse transcriptase inhibitor (NNRTI) (85.7%) or a protease inhibitor (PI) regimen (14.3%). The means of total cholesterol, LDL-C, HDL-C and triglycerides were 229.2 mg/dL, 161.0 mg/dL, 56.1 mg/dL, and 152.1 mg/dL, respectively.
Over half (51%) of 37 participants in the control group were female, average age was 44 years, mean BMI was 23.5 kg/m2, and average waist and hip circumferences were 84.2 and 94.6 cm, respectively. The majority of participants (97.3%) received ART with a combination of NRTI regimen, and 86.5% and 18.9% received antiviral treatments with NNRTI and PI regimens, respectively. The means of total cholesterol, LDL-C, HDL-C and triglycerides were 226.4 mg/dL, 157.3 mg/dL, 54.2 mg/dL and 166.2 mg/dL, respectively.
No significant differences between groups were seen in terms of dietary intake parameters. Overall, the percentage of mean total calories obtained from fat was about 28%, 7% from saturated fat (SFA), 2% from polyunsaturated fat (PUFA), and 4% from monounsaturated fat (MUFA). Daily mean total fiber intake was 7 grams. There was no significant difference in baseline average total physical activity between the two groups measured in terms of met-min/week.
As seen in Table 2, participants in the intervention group demonstrated a significant reduction in energy intake (−244.1 ±100.2 kcal/d, p=0.02) and in carbohydrate intake (−38.3 ± 16.7 g/day, p=0.03) over the 24 weeks of follow-up, which was not seen among the controls. Controls significantly increased their total fiber intake in (3.2 ± 1.1 g/day, p=0.004) more than the intervention group (1.5 ± 1.1 g/day, p=0.175). No significant differences in changes were seen between the two groups in any diet parameters. Participants in the intervention group demonstrated a significant reduction in body weight (−1.2 ± 0.4 kgs, p=0.007). Both intervention and control groups demonstrated a significant reduction in hip circumference (−1.7 ± 0.5 cm, p=0.002 versus −1.1 ± 0.5 cm, p=0.02, respectively). A slight increase in waist:hip ratio was observed in the intervention group (from 0.88 to 0.89, p=0.01). There was no overall change in blood pressure in either group. No significant changes were seen between the two groups in any anthropometric or blood pressure parameters.
Table 2.
Differences in mean (S.E.) change in scores between the groups at baseline and 24 weeks of follow-up
| Variable | Difference in mean (S.E.) between week 24 and baseline | Change between two groups at 24 weeks |
p value |
|||
|---|---|---|---|---|---|---|
| Intervention group (n=29) |
p value |
Control group (n=30) |
p value |
|||
| Body weight (kg) | −1.2 (0.4) | 0.007 | −0.5 (0.4) | 0.156 | 0.7 (0.6) | 0.254 |
| Body mass index (kg/m2) | −0.5 (0.2) | 0.005 | −0.2 (0.2) | 0.120 | 0.3 (0.2) | 0.265 |
| Waist circumference (cm) | 0.01 (0.7) | 0.991 | −0.4 (0.6) | 0.556 | −0.4 (1.0) | 0.688 |
| Hip circumference (cm) | −1.7 (0.5) | 0.002 | −1.1 (0.5) | 0.019 | 0.5 (0.7) | 0.444 |
| Waist:hip ratio | 0.02 (0.01) | 0.011 | 0.01 (0.01) | 0.170 | −0.01 (0.01) | 0.306 |
| Dietary parameters | ||||||
| Energy (kcal) | −244.1 (100.2) | 0.018 | −28.3 (98.5) | 0.775 | 215.8 (140.5) | 0.129 |
| Protein (g) | −3.7 (3.7) | 0.318 | −4.1 (3.6) | 0.269 | −0.3 (5.2) | 0.95 |
| Protein (%) | 1.1 (0.9) | 0.206 | −0.8 (0.9) | 0.362 | −1.9 (1.2) | 0.125 |
| Fat (g) | −8.2 (4.7) | 0.085 | −1.4 (4.6) | 0.756 | 6.8 (6.6) | 0.307 |
| Fat (%) | −0.09 (2.2) | 0.968 | −1.4 (2.1) | 0.525 | −1.3 (3.1) | 0.676 |
| Carbohydrate (g) | −38.3 (16.7) | 0.026 | −0.04 (16.4) | 0.998 | 38.3 (23.5) | 0.108 |
| Carbohydrate (%) | −1.1 (2.4) | 0.661 | 2.2 (2.3) | 0.357 | 3.2 (3.3) | 0.338 |
| SFA (g) | −0.3 (1.6) | 0.879 | −0.7 (1.6) | 0.689 | −0.4 (2.3) | 0.863 |
| SFA (%) | 1.3 (1.0) | 0.214 | −0.7 (1.0) | 0.464 | −2.0 (1.4) | 0.163 |
| PUFA (g) | 0.6 (1.1) | 0.580 | 1.8 (1.1) | 0.111 | 1.2 (1.6) | 0.463 |
| PUFA (%) | 1.3 (0.8) | 0.090 | 1.1 (0.7) | 0.128 | −0.2 (1.0) | 0.884 |
| MUFA (g) | −0.4 (1.5) | 0.781 | 0.7 (1.5) | 0.640 | 1.1 (2.1) | 0.599 |
| MUFA (%) | 1.1 (0.9) | 0.246 | 0.1 (0.9) | 0.876 | −1.0 (1.3) | 0.471 |
| Cholesterol (mg) | −17.3 (34.5) | 0.619 | −19.6 (34.0) | 0.566 | −2.3 (48.4) | 0.962 |
| Total fiber (g) | 1.5 (1.1) | 0.175 | 3.2 (1.1) | 0.004 | 1.7 (1.5) | 0.270 |
|
Systolic blood pressure (mmHg) |
2.2 (2.2) | 0.306 | −1.5 (2.1) | 0.485 | −3.7 (3.1) | 0.224 |
|
Diastolic blood pressure (mmHg) |
1.3 (1.7) | 0.432 | 0.5 (1.6) | 0.775 | −0.8 (2.3) | 0.718 |
p value from linear mixed models
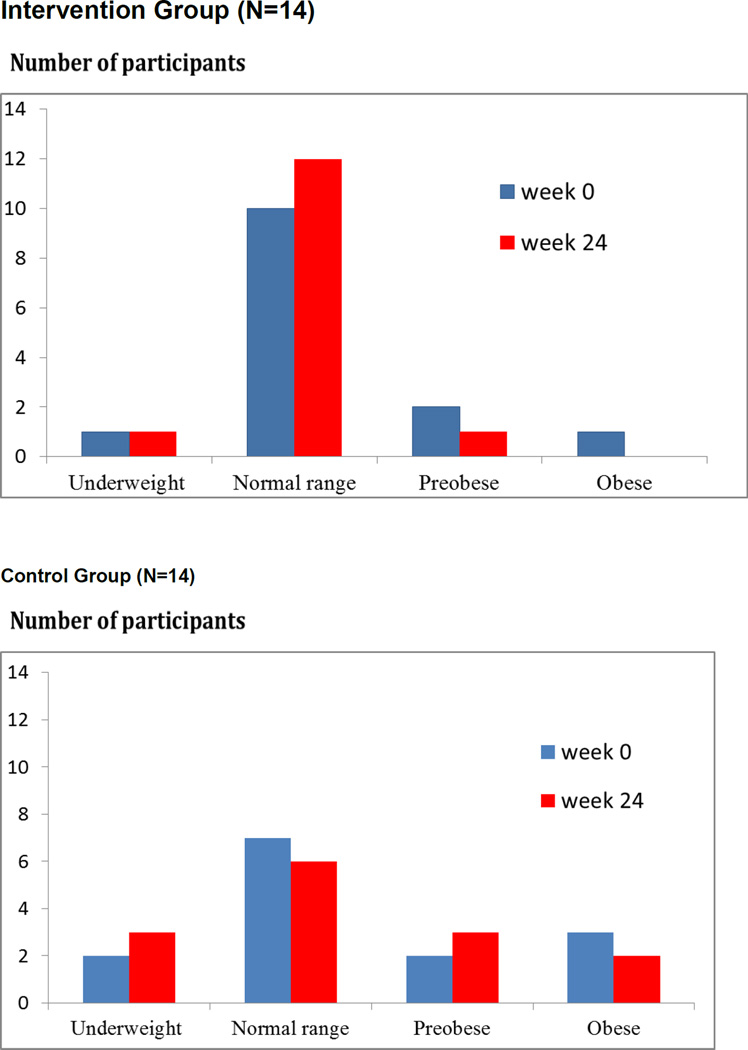
Figure 2 indicates body mass index (BMI) of participants who had a weight loss of at least 1 kilogram at 24 weeks of follow up. In the intervention group, weight loss was from the pre-obese to the normal range, whereas in the control group, weight loss was from the normal to the underweight range.
Figure 2.
Body mass index of participants who lost weight at least one kg; intervention group (upper panel) and control group (lower panel)
Participants in the intervention group demonstrated a significant reduction in serum total cholesterol (−19.0 ± 4.6 vs 0.2 ± 4.3 mg/dL, p=<.0001) and LDL-C (−21.5 ± 4.1 vs −6.8 ± 3.8 mg/dL, p=<.0001) at 24 weeks compared with the control group (Table 3). At 12 weeks follow-up, participants in the intervention group demonstrated a significant reduction in total cholesterol (−14.4 ± 4.6 mg/dL, p=0.002), LDL-C (−13.7 ± 4.1 mg/dL, p=0.001), and triglycerides (−30.4 ± 13.8 mg/dL, p=0.03), whereas participants in the control group showed significant reduction only in LDL-C (−7.7 ± 3.8 mg/dL, p=0.04). No significant differences in changes of mean lipid levels between the intervention and the control groups were noted at 12 weeks of follow-up. The difference in changes between these two groups at 24 weeks was also significant for total cholesterol (19.2 ± 6.3 mg/dL, p=0.003) and LDL-C (14.7 ± 5.5 mg/dL, p=0.009). There were no significant changes in HDL-C and triglyceride levels.
Table 3.
Differences in mean (S.E.) change in scores between groups in lipid profiles at baseline, 12 weeks and 24 weeks of follow-up
| Variable | Intervention group (n=29) | p value | Control group (n=33) | p value | Change between the two groups (S.E.) |
p value | ||
|---|---|---|---|---|---|---|---|---|
| Mean (S.E.) | Difference from baseline |
Mean (S.E.) | Difference from baseline |
|||||
| Total cholesterol | ||||||||
| Week 0 | 234.6 (5.5) | 226.8 (5.1) | ||||||
| Week 12 | 220.3 (5.5) | −14.4 (4.6) | 0.002 | 220.8 (5.1) | −6.0 (4.3) | 0.165 | 8.4 (6.3) | 0.186 |
| Week 24 | 215.7 (5.5) | −19.0 (4.6) | <.0001 | 227.0 (5.1) | 0.2 (4.3) | 0.960 | 19.2 (6.3) | 0.003 |
| LDL-C | ||||||||
| Week 0 | 164.8 (4.9) | 156.7 (4.6) | ||||||
| Week 12 | 151.1 (4.9) | −13.7 (4.1) | 0.001 | 149.0 (4.6) | −7.7 (3.8) | 0.042 | 6.0 (5.5) | 0.285 |
| Week 24 | 143.3 (4.9) | −21.5 (4.1) | <. 0001 | 149.9 (4.6) | −6.8 (3.8) | 0.072 | 14.7 (5.5) | 0.009 |
| HDL-C | ||||||||
| Week 0 | 56.5 (3.3) | 56.6 (3.0) | ||||||
| Week 12 | 55.4 (3.3) | −1.1 (1.6) | 0.475 | 55.8 (3.0) | −0.8 (1.5) | 0.592 | 0.4 (2.2) | 0.870 |
| Week 24 | 54.4 (3.3) | −2.1 (1.6) | 0.189 | 56.6 (3.0) | −0.03 (1.5) | 0.984 | 2.1 (2.2) | 0.340 |
| Triglycerides | ||||||||
| Week 0 | 165.5 (17.3) | 161.8 (15.9) | ||||||
| Week 12 | 135.2 (17.3) | −30.4 (13.8) | 0.030 | 149.9 (15.9) | −11.9 (12.7) | 0.352 | 18.5 (18.8) | 0.326 |
| Week 24 | 148.0 (17.3) | −17.5 (13.8) | 0.206 | 171.3 (15.9) | 9.5 (12.7) | 0.457 | 27.0 (18.8) | 0.152 |
p value from linear mixed models
Compared to baseline, less than half of participants (48%) in the intervention group but 57% of participants in the control group reported adherence to the dietary recommendation regarding percent fat intake according to the Therapeutic Lifestyle Changes of the NCEP (2002) at 24 weeks. Similarly, more participants in the control group (73%) than in the intervention group (59%) reported adherence to the recommendation regarding percent saturated fatty acid (% SFA). Approximately half of participants in both groups reported improvements in percent polyunsaturated fatty acid (% PUFA) and percent mono-unsaturated fatty acid (% MUFA) consumption. A few participants in each group reported consumption of 20–30 grams/day of total fiber at 24 weeks of follow-up. We suspect that the low values of fiber consumption were due to food composition database weakness. Compared to the control group, participants in the intervention group demonstrated better adherence to appropriate consumption of protein (28% vs. 17%) and carbohydrate (35% vs. 23%) at 24 weeks of follow-up (Table 4).
Table 4.
Number and percentage of participants adhering to each dietary recommendation
| Recommended Dietary Intake* | Intervention (N=29); n (%) | Control (N=30); n (%) | ||
|---|---|---|---|---|
| Week 0 | Week 24 | Week 0 | Week 24 | |
| 1. Reduce percent fat to less than 25% of total calories | 13 (44.8%) | 14 (48.3%) | 13 (43.3%) | 17 (56.7%) |
| 2. Reduce cholesterol to <200 mg/day | 19 (65.5%) | 20 (69.0%) | 17 (56.7%) | 21 (70.0%) |
| 3. Reduce SFA to <7% of total calories | 24 (82.8%) | 17 (58.6%) | 23 (76.7%) | 22 (73.3%) |
| 4. Increase PUFA to 10% of total caloriesŦ | NA | 12 (41.4%) | NA | 16 (53.3%) |
| 5. Increase MUFA to 20% of total caloriesψ | NA | 14 (48.3%) | NA | 16 (53.3%) |
| 6. Increase total fiber to 20–30 grams/day | 0 | 1 (3.4%) | 0 | 1 (3.3%) |
| 7. Protein as approximately 15% of total caloriesж | 2 (6.9%) | 8 (27.6%) | 4 (13.3%) | 5 (16.7%) |
| 8. Carbohydrates as 50–60% of total calories | 10 (34.5%) | 10 (34.5%) | 8 (26.7%) | 7 (23.3%) |
Adapted from the NCEP ATP III guideline (2002)
Increased PUFA consumption, with PUFA less than 10% of total calories at 24 weeks of follow up ((PUFAweek24 − PUFAweek0 > 0) and PUFA at week 24 ≤ 10%)
Increased MUFA consumption, with MUFA less than 20% of total calories at 24 weeks of follow up ((MUFAweek24 − MUFAweek0 > 0) and MUFA at week 24 ≤ 20%)
Consumed protein as 14.5–16.0% of total calorie intake (% protein>14.5% and <16%)
Discussion
This is one of only a few studies to assess the effectiveness of individual nutrition counseling on cardiovascular disease risk factors associated with ART in Thai HIV-infected persons. Our study found a significant difference in mean reduction between baseline and 24 weeks in total cholesterol and LDL-C between the intervention group (receiving individual counseling from a nutritionist) and the control group (receiving standard care). Compared to the control group, patients in the intervention group showed significant reductions in serum total cholesterol (8% vs 0%) and LDL-C (13% vs 4%) at 24 weeks of follow-up. The intervention group showed an 11% reduction in triglycerides at 24 weeks, whereas in the control group, it increased by 6% over the 6 months of the study. At 12 weeks of follow-up, a reduction in total cholesterol (6% vs 3%), LDL-C (8% vs 5%), and triglycerides (18% vs 7%) was also observed in the intervention and the control groups. However, none of these differences between the two groups at 12 weeks of follow-up were significant. Our findings are consistent with a three-month non-randomized study of 57 HIV-infected patients who repeated nutritional counseling based on the NCEP guideline that dyslipidemia induced by ART among HIV-infected individuals can be reduced by significantly lowering lipid levels, particularly total cholesterol and LDL-C levels (de Figueiredo et al., 2013).
Our findings are consistent with results of multiple randomized controlled trials demonstrating that counseling with a nutritionist can effectively improve lipid levels in HIV-uninfected patients in Western countries (Geil, Anderson, & Gustafson, 1995; Hunninghake et al., 1993; Rhodes, Bookstein, Aaronson, Mercer, & Orringer, 1996). However, our study found a smaller reduction in total cholesterol and LDL-C compared to the Rhodes study (Rhodes et al., 1996), which reported a reduction in total cholesterol (10% vs 7%) and LDL-C (11% vs 9%) at 12 weeks of follow-up between a group that received counseling from a dietitian and a group that did not, respectively. Our study is consistent with other studies showing that counseling effectively reduced lipid levels and other cardiovascular risk factors in patients not receiving lipid-lowering drugs (Barrios et al., 2002; Henkin et al., 2000; Ockene et al., 1999). However, we did not observe significant differences in fat intake (% fat, % SFA, % PUFA, and % MUFA) between groups. This is possibly due to the fact that both groups in our study were already consuming low levels of saturated fat and cholesterol at baseline as compared to Western countries. The significant decrease in total cholesterol and LDL-C we found might be the result of a decline in total energy consumption (−224.1 ± 100.2 kcal/day, p=0.02) and a significant reduction in weight (−1.2 ± 0.4 kgs, p=0.007). Participants in the intervention group decreased their intake of carbohydrates by 24 weeks of follow-up (−38.3 ± 16.7 g/day, p=0.03). In contrast to our study, a 12-month randomized controlled trial in Brazil of 53 HIV-infected patients receiving ART showed a reduction in fat consumption at the end of the study, but no significant differences in lipid levels between the intervention and control groups (Almeida, Segurado, Duran, & Jaime, 2011). Similar to our findings, they demonstrated no significant changes in waist circumference or blood pressure between groups.
Mean body mass indices of both groups were in a normal range (18.5–24.9 kg/m2) at baseline and 24 weeks. Of 14 participants who lost at least one kilogram by 24 weeks of follow-up, one (7%) in the intervention group was underweight according to BMI (<18.5 kg/m2) at baseline but did not lose any more weight at 24 weeks of follow-up, whereas two participants (14%) in the control group were underweight at baseline and an additional participant (totaling 21%) was underweight by 24 weeks. Seventy-two percent of participants in the intervention group were in a normal BMI range, and by 24 weeks, 86% were, whereas half of participants (50%) in the control group were in a normal range of BMI at baseline, but only 43% were at 24 weeks. Individual nutritional counseling resulted in a favorable reduction in weight that improved the nutritional status of participants.
Our study did not find protective effects in terms of reduction in waist circumference and blood pressure, unlike other studies (Fitch et al., 2006; Moyle et al., 2001). It is important to note that participants in those studies presented with high BMI and waist circumferences at baseline, whereas the majority of participants in our study presented with normal BMI and waist circumference at baseline. We found a slight increase in waist-hip ratios for both groups, similar to a previous study (Lazzaretti et al., 2012). In our study, hip circumference was significantly reduced in both groups at 24 weeks; however, this may be due to lipodystrophy (fat redistribution) in HIV-infected individuals receiving ART. Lipodystrophy may present with body changes such as localized accumulation (lipohypertrophy) and/or with loss (lipoatrophy) in body fat (WHO, 2004).
The strengths of our study include the use of a randomized controlled trial with a follow-up period long enough to observe changes in lipid levels. We assessed the effects of nutritional counseling on metabolic changes among HIV-infected individuals not receiving lipid-lowering medications. There has been little evidence of nutritional intervention therapy for managing metabolic abnormalities in HIV-infected patients receiving ART in Thailand. This study demonstrated that a practical nutritional counseling program for Thais can be effective in improving dyslipidemia in HIV-infected patients.
Some limitations in this study should be noted. The study had a relatively small sample size that may have resulted in low statistical power to detect small differences in diet parameters. Neither group reported a significant increase in physical activity during the follow-up period. We could not evaluate the effect of soluble fiber and trans-fat because the Thai food database that we used to analyze this data did not contain these parameters. The values of soluble fiber presented in this study were based on values of certain Thai food items that were analyzed by the Bureau of Nutrition, Thailand Bureau of Nutrition (Ministry of Public Health, 2012). Lastly, we used a single 24-hour recall to assess dietary intakes of participants because it is practical and easily collected (Barrett-Connor, 1991). However, the high variability of this method leads to a lower ability to detect significant differences in dietary intake.
Conclusions
This study confirmed the benefit of intensive dietary counseling with a nutritionist for dyslipidemia among HIV-infected patients receiving ART and not receiving lipid medication treatment. For the first time, we demonstrated the feasibility and benefit of dietary counseling in a Thai population. Despite the small sample size and apparent non-adherence to macronutrient recommendations, our study demonstrated improvement with individual nutritional counseling in lipid profiles in HIV-positive persons receiving ART. Those participants who received nutritional counseling showed an improvement in lipid profile that was not observed in the control group. Although the effect of lowering in LDL-C in our study was small, we found greater differences in mean LDL-C between the intervention and the control groups as time passed, and greater reductions in LDL-C levels were observed in the intervention group. A longer follow-up period and a larger population may demonstrate a clearer effect of nutritional intervention on the lipid profile and dietary parameters.
Acknowledgments
The authors wish to express their sincere appreciation to all of the participants, the nutritionists of the Bureau of Nutrition, Ministry of Public Health, Thailand, and staff members at the Outpatient Department of the HIV clinic at the Bamrasnaradura Infectious Diseases Institute for their dedicated involvement in this study and contributing their time and effort. This study was funded by UCLA Fogarty AIDS International Training and Research Program (NIH D43 TW000013).
References
- 1.Almeida LB, Segurado AC, Duran AC, Jaime PC. Impact of a nutritional counseling program on prevention of HAART-related metabolic and morphologic abnormalities. AIDS Care: Psychological and Socio-medical Aspects of AIDS/HIV. 2011;23:755–763. doi: 10.1080/09540121.2010.525789. [DOI] [PubMed] [Google Scholar]
- 2.Anjos EM, Pfrimer K, Machado AA, Cunha SF, Salomão RG, Monteiro JP. Nutritional and metabolic status of HIV-positive patients with lipodystrophy during one year of follow-up. Clinics (Sao Paulo) 2011;66:407–410. doi: 10.1590/S1807-59322011000300007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett-Connor E. Nutrition epidemiology: how do we know what they ate? American Journal of Clinical Nutrition. 1991;54(1 Suppl):182S–187S. doi: 10.1093/ajcn/54.1.182S. [DOI] [PubMed] [Google Scholar]
- 4.Barrios A, Blanco F, García-Benayas T, Gómez-Viera JM, de la Cruz JJ, Soriano V, González-Lahoz J. Effect of dietary intervention on highly active antiretroviral therapy-related dyslipemia. AIDS. 2002;16:2079–2081. doi: 10.1097/00002030-200210180-00014. [DOI] [PubMed] [Google Scholar]
- 5.Batterham MJ, Brown D, Workman C. Modifying dietary fat intake can reduce serum cholesterol in HIV-associated hypercholesterolemia. AIDS. 2003;17:1414–1416. doi: 10.1097/00002030-200306130-00027. [DOI] [PubMed] [Google Scholar]
- 6.Booth M. Assessment of physical activity: an international perspective. Research Quarterly for Exercise and Sport. 2000;71(2 Suppl):S114–S120. [PubMed] [Google Scholar]
- 7.Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health, Thailand Situation of AIDS patients in Thailand, date 30 September 2012. retrieved at < http://www.boe.moph.go.th/report.php?cat=19&id=1268>. [Google Scholar]
- 8.Bureau of Nutrition, Department of Health, Ministry of Public Health. Dietary Fiber Contents in Thai Foods. 2012 [Google Scholar]
- 9.de Figueiredo SM, Penido MGMG, Guimaraes MMM, de Campos Machado LJ, Filho SAV, Greco DB. Effects of dietary intervention on lipids profile of HIV infected patients on antiretroviral treatment (ART) European Scientific Journal. 2013;9:18. [Google Scholar]
- 10.Dube MP, Stein JH, Berg JA, Fichtenbaum CJ, Gerber JG, Tashima KT, Glesby MJ Adult AIDS Clinical Trials Group Cardiovascular Subcommittee; HIV Medical Association of the Infectious Disease Society of America. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clinical Infectious Diseases. 2003;37:613–627. doi: 10.1086/378131. [DOI] [PubMed] [Google Scholar]
- 11.Fitch KV, Anderson EJ, Hubbard JL, Carpenter SJ, Waddell WR, Caliendo AM, Grinspoon SK. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS. 2006;20:1843–1850. doi: 10.1097/01.aids.0000244203.95758.db. [DOI] [PubMed] [Google Scholar]
- 12.Geil PB, Anderson JW, Gustafson NJ. Women and men with hypercholesterolemia respond similarly to an American Heart Association step 1 diet. Journal of the American Dietetic Association. 1995;95(4):436–441. doi: 10.1016/S0002-8223(95)00118-2. [DOI] [PubMed] [Google Scholar]
- 13.Henkin Y, Shai I, Zuk R, Brickner D, Zuilli I, Neumann L, Shany S. Dietary treatment of hypercholesterolemia: do dietitians do it better? A randomized, controlled trial. American Journal of Medicine. 2000;109:549–555. doi: 10.1016/s0002-9343(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 14.Hunninghake DB, Stein EA, Dujovne CA, Harris WS, Feldman EB, Miller VT, Brewer BK. The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. New England Journal of Medicine. 1993;328:1213–1219. doi: 10.1056/NEJM199304293281701. [DOI] [PubMed] [Google Scholar]
- 15.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Kuller LH. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 16.Lazzaretti RK, Kuhmmer R, Sprinz E, Polanczyk CA, Ribeiro JP. Dietary intervention prevents dyslipidemia associated with highly active antiretroviral therapy in human immunodeficiency virus type 1-infected individuals: a randomized trial. Journal of the American College of Cardiology. 2012;59:979–988. doi: 10.1016/j.jacc.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 17.Moyle GJ, Lloyd M, Reynolds B, Baldwin C, Mandalia S, Gazzard BG. Dietary advice with or without pravastatin for the management of hypercholesterolaemia associated with protease inhibitor therapy. AIDS. 2001;15:1503–1508. doi: 10.1097/00002030-200108170-00007. [DOI] [PubMed] [Google Scholar]
- 18.National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report? Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19.Ockene IS, Hebert JR, Ockene JK, Saperia GM, Stanek E, Nicolosi R, Hurley TG. Effect of physician-delivered nutrition counseling training and an office-support program on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: Worcester Area Trial for Counseling in Hyperlipidemia (WATCH) Archives of Internal Medicine. 1999;159:725–731. doi: 10.1001/archinte.159.7.725. [DOI] [PubMed] [Google Scholar]
- 20.Rhodes KS, Bookstein LC, Aaronson LS, Mercer NM, Orringer CE. Intensive nutrition counseling enhances outcomes of National Cholesterol Education Program dietary therapy. Journal of the American Dietetic Association. 1996;96:1003–1010. doi: 10.1016/S0002-8223(96)00268-4. [DOI] [PubMed] [Google Scholar]
- 21.Sungkanuparph S, Techasathit W, Utaipiboon C, Chasombat S, Bhakeecheep S, Leechawengwongs M, Phanuphak P. Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomedicine. 2010;4:515–528. [Google Scholar]
- 22.UNAIDS. Global Report: UNAIDS report on the global AIDS epidemic 2012. 2012 Retrieved at http://www.unaids.org/en/resources/campaigns/20121120_globalreport2012/globalreport/ [Google Scholar]
- 23.WHO. Nutrition Counselling Care and Support for HIV-infected Women: Guidelines on HIV-related care, treatment and support for HIV-infected women and their children in resource-contrained settings. 2004